Comparative Study of Growth, Cadmium Accumulation and Tolerance of Three Chickpea (Cicer arietinum L.) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Chickpea Seeds and Growth Conditions
2.2. Plants Materials and Cadmium Treatments
2.3. Measurements of Plant Growth and Cd Accumulation
- Cd accumulation = biomass (DW) × Cd concentration in plant tissues,
- Total Cd accumulation = Cd accumulation in root + Cd accumulation in shoot,
- TF = Cds/Cdr,
- BCF = Cdr/Cdns,
- BAC = Cds/Cdns.
2.4. Growth Tolerance Indices
- TIr = (Cdr/MVrc) × 100,
- TIs = (Cds/MVSc) × 100,
- TItp = (Cdtp/MVtpc) × 100.
2.5. Shoot Water Content Measurement
- WC = (FW − DW)/FW × 100.
2.6. Statistical Analysis
3. Results
3.1. Visual Observation and Cd Toxicity
3.2. Plant Growth Measurement
3.3. Biomass Production
3.3.1. Fresh Biomass
3.3.2. Dry Biomass
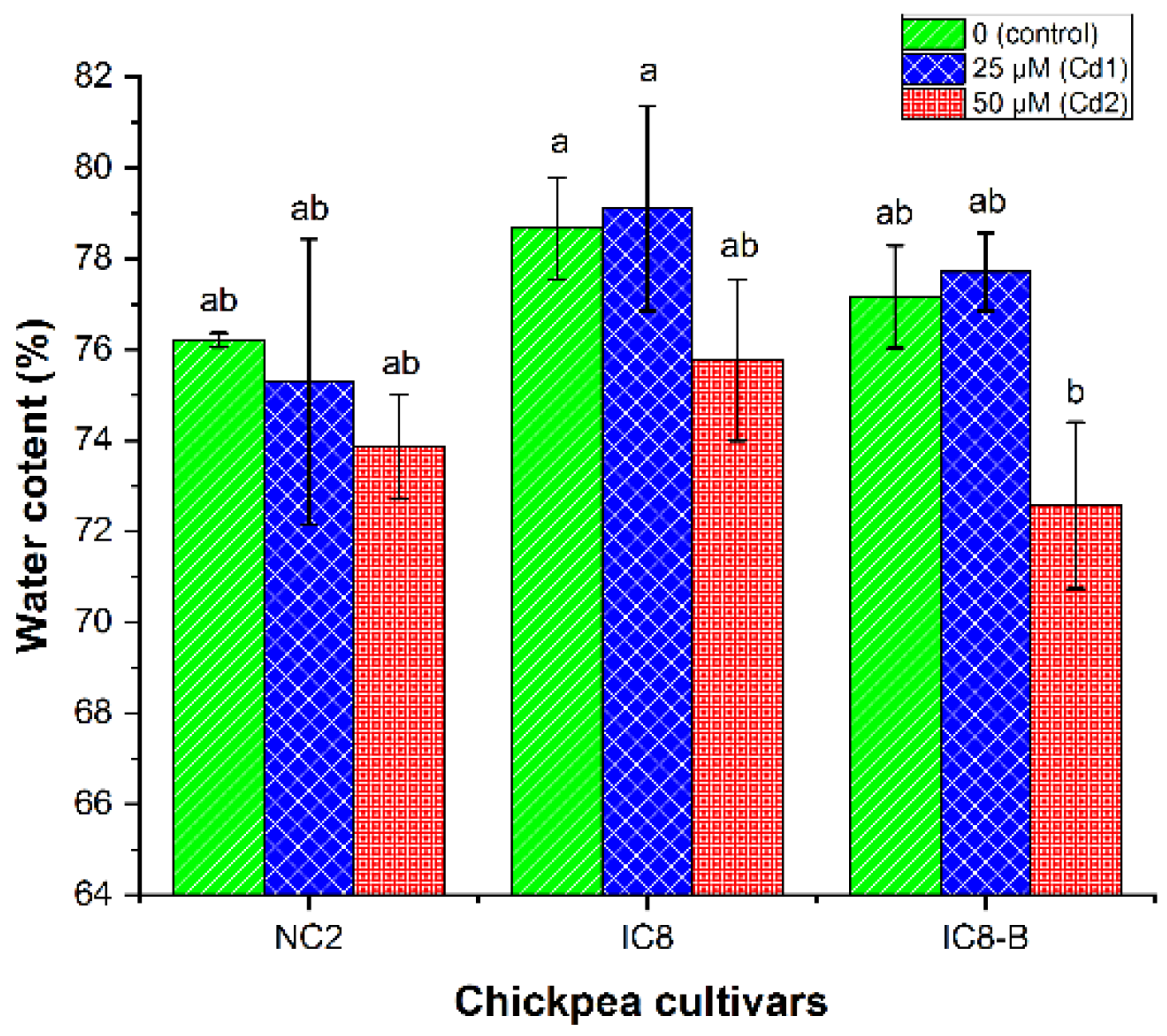
3.4. Shoot Water Content (WC)
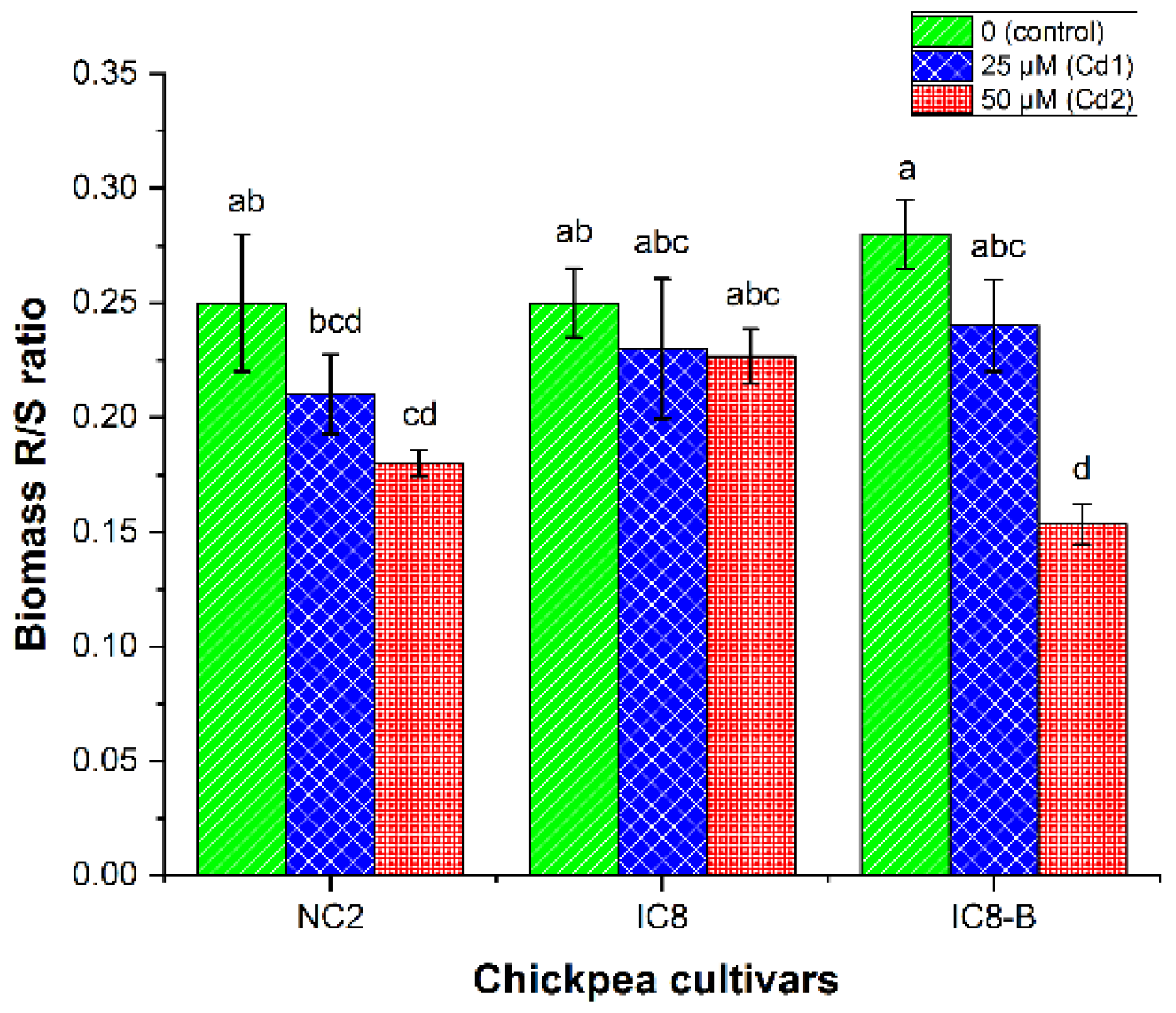
3.5. Biomass and Cd Roots/Shoot (R/S) Ratio
3.6. Tolerance Indices (TI)
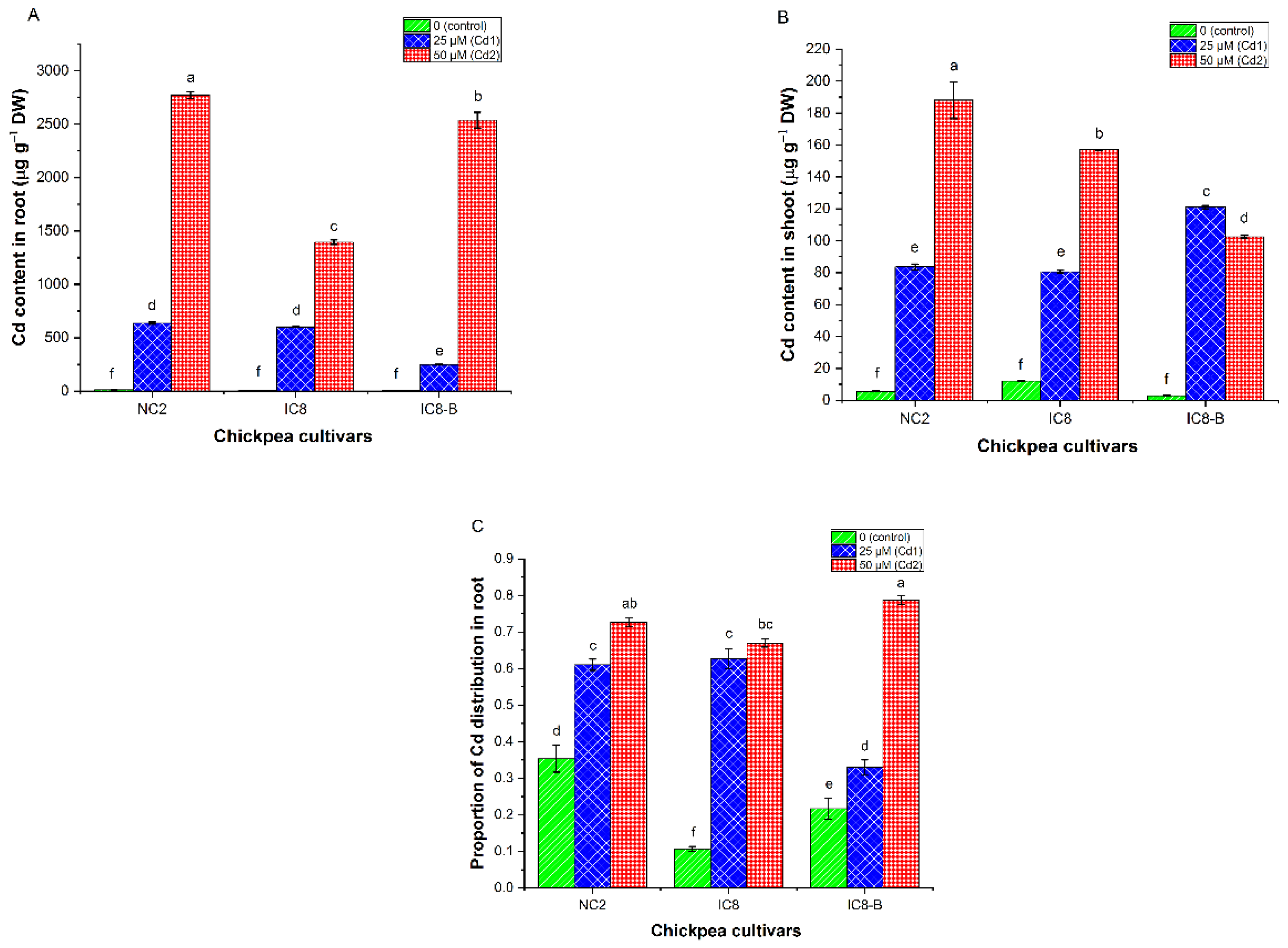
3.7. Cadmium Contents and Distribution Proportion
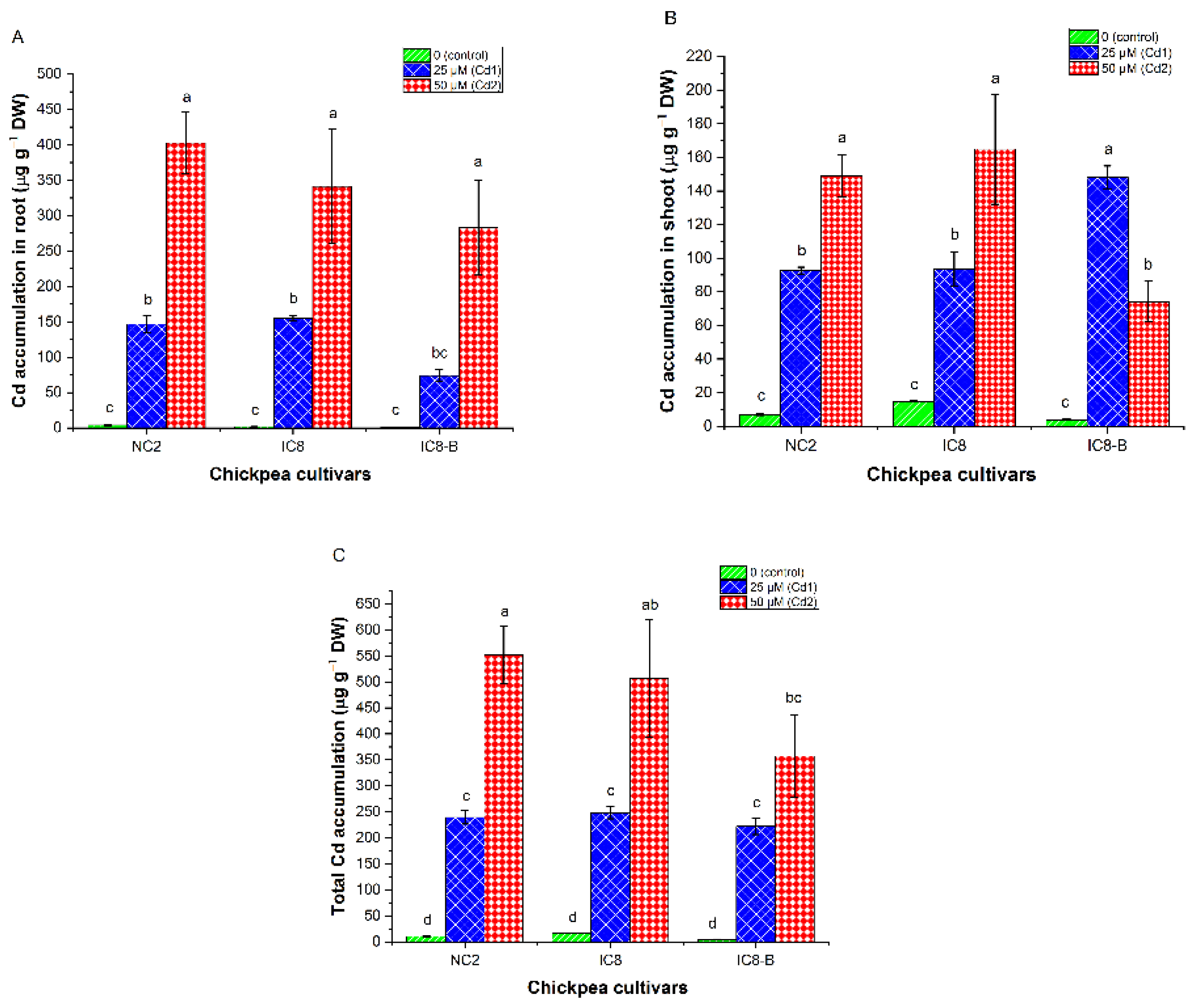
3.8. Cadmium Accumulation
3.9. Translocation Factor (TF), Bioconcentration Factor (BCF) and Bioaccumulation Coefficient (BAC)
4. Discussion
4.1. Growth and Biomass Production
4.2. Water Relation
4.3. Growth Tolerance Indices
4.4. Biomass and Cadmium R/S Ratio
4.5. Cadmium Concentration and Distribution Proportion in Chickpea Cultivars
4.6. Cd Uptake and Accumulation Capacities
5. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anjum, N.A.; Ahmad, I.; Mohmood, I.; Pacheco, M.; Duarte, A.C.; Pereira, E.; Umar, S.; Ahmad, A.; Khan, N.A.; Iqbal, M.; et al. Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—A review. Environ. Exp. Bot. 2012, 75, 307–324. [Google Scholar] [CrossRef]
- Liu, B.; Wu, F.; Dai, X.; Liu, F.; Mi, B. Variations of Cadmium Accumulation and Translocation in Different Pakchoi Cultivars and Screening for Cd-Pollution-Safe Cultivars Using Cluster Analysis. Pol. J. Environ. Stud. 2019, 28, 2215–2222. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; Filho, S.C.V.; Müller, C.; Rodrigues, D.A.; Sales, J.D.F.; Zuchi, J.; Costa, A.C.; Rodrigues, C.L.; Da Silva, A.A.; Barbosa, D.P. Tolerance of Eugenia dysenterica to Aluminum: Germination and Plant Growth. Plants 2019, 8, 317. [Google Scholar] [CrossRef]
- Zoffoli, H.J.O.; Amaral-Sobrinho, N.M.B.D.; Zonta, E.; Luisi, M.V.; Marcon, G.; Tolon-Becerra, A.; Amaral-Sobrinho, N.M.B. Inputs of heavy metals due to agrochemical use in tobacco fields in Brazil’s Southern Region. Environ. Monit. Assess. 2012, 185, 2423–2437. [Google Scholar] [CrossRef]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Guo, H.; Ma, Y.; Wang, L.; Wei, D.; Hua, L. Genotypic variations in the accumulation of Cd exhibited by different vegetables. J. Environ. Sci. 2010, 22, 1246–1252. [Google Scholar] [CrossRef]
- Nocito, F.F.; Lancilli, C.; Dendena, B.; Lucchini, G.; Sacchi, G.A. Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant Cell Environ. 2011, 34, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Afzal, J.; Hu, C.; Imtiaz, M.; Elyamine, A.M.; Rana, M.S.; Imran, M.; Farag, M.A. Cadmium tolerance in rice cultivars associated with antioxidant enzymes activities and Fe/Zn concentrations. Int. J. Environ. Sci. Technol. 2018, 16, 4241–4252. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Ye, X.; Zhang, Q.; Xiao, W. Responses to cadmium stress in two tomato genotypes differing in heavy metal accumulation. Turk. J. Bot. 2015, 39, 615–624. [Google Scholar] [CrossRef]
- Cojocaru, P.; Gusiatin, Z.M.; Cretescu, I. Phytoextraction of Cd and Zn as single or mixed pollutants from soil by rape (Brassica napus). Environ. Sci. Pollut. Res. 2016, 23, 10693–10701. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Latef, A.A.A.; Abd_Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gücel, S. Calcium and Potassium Supplementation Enhanced Growth, Osmolyte Secondary Metabolite Production, and Enzymatic Antioxidant Machinery in Cadmium-Exposed Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 1303. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Bertrand, A.; Reis, R.; Mourato, M.; Martins, L.L.; González, A. Growth and physiological responses to cadmium stress of two populations of Dittrichia viscosa (L.) Greuter. J. Hazard. Mater. 2013, 244, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M. Cadmium toxicity and tolerance in vascular plants. Environ. Exp. Bot. 1995, 35, 525–545. [Google Scholar] [CrossRef]
- Mondal, N.K.; Das, C.; Roy, S.; Datta, J.K.; Banerjee, A. Effect of varying cadmium Stress on chickpea (Cicer arietinum L) seedlings: An Ultrastructural study. Ann. Environ. Sci. 2013, 7, 59–70. [Google Scholar]
- Faizan, S.; Kausar, S.; Perveen, R. Variation in growth, physiology and yield of four chickpea cultivars exposed to cadmium chloride. J. Environ. Biol. 2012, 33, 1137–1142. [Google Scholar]
- Faizan, S.; Kausar, S.; Perveen, R. Varietal differences for cadmium-induced seedling mortality, foliar toxicity symptoms, plant growth, proline and nitrate reductase activity in chickpea (Cicer arietinum L.). Biol. Med. 2011, 3, 196–206. [Google Scholar]
- Júnior, C.A.L.; Mazzafera, P.; Arruda, M.A.Z. A comparative ionomic approach focusing on cadmium effects in sunflowers (Helianthus annuus L.). Environ. Exp. Bot. 2014, 107, 180–186. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef]
- Ramos, I.; Esteban, E.; Lucena, J.J.; Gárate, A. Cadmium uptake and subcellular distribution in plants of Lactuca sp. Cd–Mn interaction. Plant Sci. 2002, 162, 761–767. [Google Scholar] [CrossRef]
- Fang, Z.; Lou, L.; Tai, Z.; Wang, Y.; Yang, L.; Hu, Z.; Cai, Q. Comparative study of Cd uptake and tolerance of two Italian ryegrass (Lolium multiflorum) cultivars. PeerJ 2017, 5, e3621. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.R.; Llugany, M.; Barceló, J.; Poschenrieder, C. Cadmium exclusion a key factor in differential Cd-resistance in Thlaspi arvense ecotypes. Biol. Plant. 2012, 56, 729–734. [Google Scholar] [CrossRef]
- Greger, M.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Pollut. 2016, 211, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Khan, N.A.; Anjum, N.A.; Tuteja, N. Amelioration of cadmium stress in crop plants by nutrients management: Morphological, physiological and biochemical aspects. Plant Stress 2011, 5, 1–23. [Google Scholar]
- Hasan, S.A.; Fariduddin, Q.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium: Toxicity and tolerance in plants. J. Environ. Biol. 2009, 30, 165–174. [Google Scholar]
- Verbruggen, N.; Juraniec, M.; Baliardini, C.; Meyer, C.-L. Tolerance to cadmium in plants: The special case of hyperaccumulators. BioMetals 2013, 26, 633–638. [Google Scholar] [CrossRef]
- Imtiaz, M.; Tu, S.; Xie, Z.; Han, D.; Ashraf, M.; Rizwan, M.S. Growth, V uptake, and antioxidant enzymes responses of chickpea (Cicer arietinum L.) genotypes under vanadium stress. Plant Soil 2015, 390, 17–27. [Google Scholar] [CrossRef]
- Metwally, A.; Safronova, V.I.; Belimov, A.A.; Dietz, K.-J. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J. Exp. Bot. 2004, 56, 167–178. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Hasan, S.A.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium-induced changes in the growth and carbonic anhydrase activity of chickpea. Turk. J. Biol. 2007, 31, 137–140. [Google Scholar]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, M.S.; Amin, F. Accumulation and distribution of lead (Pb) in plant tissues of guar (Cyamopsis tetragonoloba L.) and sesame (Sesamum indicum L.): Profitable phytoremediation with biofuel crops. Geol. Ecol. Landsc. 2018, 2, 51–60. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Hanus-Fajerska, E.; Muszynska, E.; Smolen, S. Comparative Assessment of Response to Cadmium in Heavy Metal-Tolerant Shrubs Cultured In Vitro. Water Air Soil Pollut. 2017, 228, 304. [Google Scholar] [CrossRef]
- Zhou, W.; Qiu, B. Effects of cadmium hyperaccumulation on physiological characteristics of Sedum alfredii Hance (Crassulaceae). Plant Sci. 2005, 169, 737–745. [Google Scholar] [CrossRef]
- Daud, M.; Ali, S.; Variath, M.; Zhu, S. Differential physiological, ultramorphological and metabolic responses of cotton cultivars under cadmium stress. Chemosphere 2013, 93, 2593–2602. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Chen, X.; Chen, H. Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 108, 114–119. [Google Scholar] [CrossRef]
- Rai, V.; Khatoon, S.; Bisht, S.; Mehrotra, S. Effect of cadmium on growth, ultramorphology of leaf and secondary metabolites of Phyllanthus amarus Schum. and Thonn. Chemosphere 2005, 61, 1644–1650. [Google Scholar] [CrossRef]
- Chen, L.; Long, X.-H.; Zhang, Z.-H.; Zheng, X.-T.; Rengel, Z.; Liu, Z.-P. Cadmium Accumulation and Translocation in Two Jerusalem Artichoke (Helianthus tuberosus L.) Cultivars. Pedosphere 2011, 21, 573–580. [Google Scholar] [CrossRef]
- He, S.; Yang, X.; He, Z.; Baligar, V.C. Morphological and Physiological Responses of Plants to Cadmium Toxicity: A Review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Zhang, G.; Fukami, M.; Sekimoto, H. Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage. Field Crop. Res. 2002, 77, 93–98. [Google Scholar] [CrossRef]
- Adhikari, T.; Tel-Or, E.; Libal, Y.; Shenker, M. Effect of cadmium and iron on rice (Oryza sativa L.) plant in chelator-buffered nutrient solution. J. Plant Nutr. 2006, 29, 1919–1940. [Google Scholar] [CrossRef]
- Wahid, A.; Ghani, A. Varietal differences in mungbean (Vigna radiata) for growth, yield, toxicity symptoms and cadmium accumulation. Ann. Appl. Biol. 2008, 152, 59–69. [Google Scholar] [CrossRef]
- Al Mahmud, J.; Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Fujita, M. Relative tolerance of different species of Brassica to cadmium toxicity: Coordinated role of antioxidant defense and glyoxalase systems. Plant Omics 2017, 10, 107–117. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water relations in plants subjected to heavy metal stresses. Acta Physiol. Plant. 2016, 38, 257. [Google Scholar] [CrossRef]
- Costa, G.; Spitz, E. Influence of cadmium on soluble carbohydrates, free amino acids, protein content of in vitro cultured Lupinus albus. Plant Sci. 1997, 128, 131–140. [Google Scholar] [CrossRef]
- Belimov, A.A.; Malkov, N.V.; Puhalsky, J.V.; Tsyganov, V.E.; Bodyagina, K.B.; Safronova, V.I.; Dietz, K.-J.; Tikhonovich, I.A. The crucial role of roots in increased cadmium-tolerance and Cd-accumulation in the pea mutant SGECdt. Biol. Plant. 2018, 62, 543–550. [Google Scholar] [CrossRef]
- Vijendra, P.D.; Huchappa, K.M.; Lingappa, R.; Basappa, G.; Jayanna, S.G.; Kumar, V. Physiological and Biochemical Changes in Moth Bean (Vigna aconitifolia L.) under Cadmium Stress. J. Bot. 2016, 2016, 1–13. [Google Scholar] [CrossRef][Green Version]
- Barceló, J.; Poschenrieder, C.; Andreu, I.; Gunsé, B. Cadmium-Induced Decrease of Water Stress Resistance in Bush Bean Plants (Phaseolus vulgaris L. cv. Contender) I. Effects of Cd on Water Potential, Relative Water Content, and Cell Wall Elasticity. J. Plant Physiol. 1986, 125, 17–25. [Google Scholar] [CrossRef]
- Costa, G.; Michaut, J.-C.; Morel, J. Influence of cadmium on water relations and gas exchanges, in phosphore deficient Lupinus albus. Plant Physiol. Biochem. (Paris) 1994, 32, 105–114. [Google Scholar]
- Aery, N.C.; Rana, D.K. Growth and cadmium uptake in barley under cadmium stress. J. Environ. Biol. 2003, 24, 117–123. [Google Scholar]
- Wang, Y.; Yan, A.; Dai, J.; Wang, N.; Wu, D. Accumulation and tolerance characteristics of cadmium in Chlorophytum comosum: A popular ornamental plant and potential Cd hyperaccumulator. Environ. Monit. Assess. 2011, 184, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Barzanti, R.; Colzi, I.; Arnetoli, M.; Gallo, A.; Pignattelli, S.; Gabbrielli, R.; Gonnelli, C. Cadmium phytoextraction potential of different Alyssum species. J. Hazard. Mater. 2011, 196, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, I.H.; Zhang, G.; Hu, H.; Xue, Q.; Hussain, N.; Ali, E.; Shen, Q.; Zheng, W.; Zhang, Q.; Liu, X.; et al. Assessment of the hazardous effects of Cd on physiological and biochemical characteristics of soybean genotypes. Int. J. Agric. Biol. 2014, 16, 41–48. [Google Scholar]
- Inouhe, M.; Ninomiya, S.; Tohoyama, H.; Joho, M.; Murayama, T. Different characteristics of roots in the cadmium-tolerance and Cd-binding complex formation between mono- and dicotyledonous plants. J. Plant Res. 1994, 107, 201–207. [Google Scholar] [CrossRef]
- Lux, A.; Šottníková, A.; Opatrná, J.; Greger, M. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol. Plant. 2004, 120, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Belz, R.G.; Kitao, M.; Koike, T.; Calabrese, E.J. Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J. For. Res. 2018, 30, 1569–1580. [Google Scholar] [CrossRef]
- Nikolić, N.; Kojic, D.; Pilipovic, A.; Pajevic, S.; Krstic, B.; Borisev, M.; Orlovic, S. Responses of hybrid poplar to cadmium stress: Photosynthetic characteristics, cadmium and proline accumulation, and antioxidant enzyme activity. Acta Biol. Crac. Bot. 2008, 50, 95–103. [Google Scholar]
- Hawrylak-Nowak, B.; Dresler, S.; Matraszek, R. Exogenous malic and acetic acids reduce cadmium phytotoxicity and enhance cadmium accumulation in roots of sunflower plants. Plant Physiol. Biochem. 2015, 94, 225–234. [Google Scholar] [CrossRef]
- Alves, L.R.; Monteiro, C.C.; Carvalho, R.F.; Ribeiro, P.C.; Tezotto, T.; Azevedo, R.A.; Gratão, P.L. Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ. Exp. Bot. 2017, 134, 102–115. [Google Scholar] [CrossRef]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef]
- Wojcik, M.; Vangronsveld, J.; Tukiendorf, A. Cadmium tolerance in Thlaspi caerulescensI. Growth parameters, metal accumulation and phytochelatin synthesis in response to cadmium. Environ. Exp. Bot. 2005, 53, 151–161. [Google Scholar] [CrossRef]
- Solisdominguez, F.; Gonzalezchavez, M.; Carrillogonzalez, R.; Rodriguezvazquez, R. Accumulation and localization of cadmium in Echinochloa polystachya grown within a hydroponic system. J. Hazard. Mater. 2007, 141, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Louati, M.; Ucarli, C.; Arikan, B.; Ghada, B.; Hannachi, A.S.; Kara, T.; Turgut-Kara, N. Genetic, Morphological, and Biochemical Diversity of Argan Tree (Argania spinosa L.) (Sapotaceae) in Tunisia. Plants 2019, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Delpérée, C.; Lutts, S. Growth Inhibition Occurs Independently of Cell Mortality in Tomato (Solanum lycopersicum) Exposed to High Cadmium Concentrations. J. Integr. Plant Biol. 2008, 50, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, I.; Marchal, G.; Meerts, P.; Correal, E.; Lutts, S. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environ. Exp. Bot. 2009, 65, 142–152. [Google Scholar] [CrossRef]
- Belimov, A.; Safronova, V.; Tsyganov, V.; Borisov, A.; Kozhemyakov, A.; Stepanok, V.; Martenson, A.; Gianinazzi-Pearson, V.; Tikhonovich, I. Genetic variability in tolerance to cadmium and accumulation of heavy metals in pea (Pisum sativum L.). Euphytica 2003, 131, 25–35. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Chen, W.; Yuan, F.; Yan, K.; Tao, D. Accumulation and tolerance characteristics of cadmium in a potential hyperaccumulator—Lonicera japonica Thunb. J. Hazard. Mater. 2009, 169, 170–175. [Google Scholar] [CrossRef]
- Pernía, B.; Calabokis, M.; Noris, K.; Bubis, J.; Guerra, M.; Castrillo, M. Effects of cadmium in plants of Sphagneticola trilobata (L.) Pruski. Bioagro 2019, 31, 133–142. [Google Scholar]
- Arifin, A.; Parisa, A.; Hazandy, A.H.; Mahmud, T.M.; Junejo, N.; Fatemeh, A.; Mohsen, S.; Majid, N.M. Evaluation of cadmium bioaccumulation and translocation by Hopea odorata grown in a contaminated soil. Afr. J. Biotechnol. 2012, 11, 7472–7482. [Google Scholar]

| Cultivars | Treatments | Root Length ± SE (cm) | Shoot Length ± SE (cm) | Total Height ± SE (cm) |
|---|---|---|---|---|
| NC2 | control | 10.62 ± 0.23 bc | 18.48 ± 2.08 ab | 29.10 ± 2.15 abc |
| Cd1 | 9.80 ± 0.08 cd | 15.65 ± 0.73 bcd | 25.45 ± 0.66 cde | |
| Cd2 | 9.12 ± 0.32 d | 14.08 ± 1.28 cd | 23.20 ± 1.36 ef | |
| IC8 | control | 11.37 ± 0.31 b | 20.38 ± 1.61 a | 31.74 ± 1.86 a |
| Cd1 | 10.01 ± 0.43 cd | 17.93 ± 0.41 abc | 27.94 ± 0.60 abcd | |
| Cd2 | 9.91 ± 0.28 cd | 16.40 ± 0.70 bcd | 26.31 ± 0.88 bcde | |
| IC8-B | control | 12.62 ± 0.62 a | 17.60 ± 0.57 abc | 30.22 ± 1.20 ab |
| Cd1 | 9.15 ± 0.46 d | 15.17 ± 1.37 bcd | 24.31 ± 1.74 def | |
| Cd2 | 7.80 ± 0.52 e | 13.26 ± 0.47 d | 21.06 ± 0.86 f |
| Cultivars | Treatments | Root Fresh Biomass ± SE (g/plant) | Shoot Fresh Biomass ± SE (g/plant) | Whole Plant Fresh Biomass ± SE (g/plant) |
|---|---|---|---|---|
| NC2 | control | 1.41 ± 0.41 ab | 5.10 ± 0.45 a | 6.51 ± 0.85 ab |
| Cd1 | 1.33 ± 0.01 ab | 4.62 ± 0.54 a | 5.95 ± 0.54 ab | |
| Cd2 | 1.24 ± 0.15 ab | 3.07 ± 0.35 bc | 4.32 ± 0.50 bc | |
| IC8 | control | 1.97 ± 0.43 a | 5.76 ± 0.38 a | 7.73 ± 0.29 a |
| Cd1 | 1.91 ± 0.48 ab | 5.55 ± 0.06 a | 7.47 ± 0.42 a | |
| Cd2 | 1.90 ± 0.50 ab | 4.43 ± 1.03 ab | 6.33 ± 1.53 ab | |
| IC8-B | control | 2.01 ± 0.32 a | 5.80 ± 0.19 a | 7.81 ± 0.50 a |
| Cd1 | 1.91 ± 0.18 ab | 5.48 ± 0.10 a | 7.40 ± 0.23 a | |
| Cd2 | 0.80 ± 0.20 b | 2.65 ± 0.42 c | 3.45 ± 0.55 c |
| Cultivars | Treatments | Root Dry Biomass ± SE (g/plant) | Shoot Dry Biomass ± SE (g/plant) | Whole-Plant Dry Biomass ± SE (g/plant) |
|---|---|---|---|---|
| NC2 | control | 0.31 ± 0.06 ab | 1.21 ± 0.11 a | 1.53 ± 0.17 a |
| Cd1 | 0.23 ± 0.01 bc | 1.11 ± 0.01 ab | 1.34 ± 0.02 ab | |
| Cd2 | 0.15 ± 0.01 cd | 0.79 ± 0.06 bc | 0.94 ± 0.08 bc | |
| IC8 | control | 0.31 ± 0.01 ab | 1.22 ± 0.01 a | 1.52 ± 0.03 a |
| Cd1 | 0.26 ± 0.01 b | 1.17 ± 0.13 a | 1.42 ± 0.13 a | |
| Cd2 | 0.24 ± 0.05 bc | 1.05 ± 0.21 ab | 1.30 ± 0.26 ab | |
| IC8-B | control | 0.37 ± 0.02 a | 1.32 ± 0.02 a | 1.69 ± 0.04 a |
| Cd1 | 0.30 ± 0.03 ab | 1.22 ± 0.05 a | 1.52 ± 0.09 a | |
| Cd2 | 0.11 ± 0.02 d | 0.72 ± 0.11 c | 0.83 ± 0.13 c |
| Treatments | NC2 | IC8 | IC8-B |
|---|---|---|---|
| control | 2.18 ± 0.09 e | 0.50 ± 0.01 f | 0.99 ± 0.16 f |
| Cd1 | 7.62 ± 0.29 d | 7.45 ± 0.41 d | 2.06 ± 0.01 e |
| Cd2 | 14.82 ± 0.74 b | 8.89 ± 0.14 c | 24.74 ± 0.53 a |
| Cultivars | Treatments | (TIr) ± SE (%) | (TIs) ± SE (%) | (TItp) ± SE (%) |
|---|---|---|---|---|
| NC2 | Cd1 | 74.27 ± 6.41 ab | 91.23 ± 0.26 a | 87.77 ± 1.43 ab |
| Cd2 | 46.68 ± 5.08 bc | 65.53 ± 5.51 ab | 61.68 ± 5.40 bc | |
| IC8 | Cd1 | 84.45 ± 2.08 a | 95.4 ± 11.37 a | 93.21 ± 9.12 a |
| Cd2 | 79.53 ± 18.10 a | 86.19 ± 17.23 a | 84.85 ± 17.36 ab | |
| IC8-B | Cd1 | 79.59 ± 9.75 a | 92.64 ± 4.55 a | 89.77 ± 89.77 ab |
| Cd2 | 29.72 ± 6.36 c | 54.72 ± 8.76 b | 49.23 ± 8.19 c |
| Cultivars | Treatments | (BCF) ± SE | (BAC) ± SE | (TF) ± SE |
|---|---|---|---|---|
| NC2 | control | 2.16 ± 0.02 f | 0.97 ± 0.03 d | 0.46 ± 0.02 c |
| Cd1 | 111.41 ± 1.75 d | 14.64 ± 0.32 b | 0.13 ± 0.01 d | |
| Cd2 | 242.81 ± 2.61 a | 16.48 ± 1.00 ab | 0.07 ± 0.00 d | |
| IC8 | control | 1.06 ± 0.03 f | 1.06 ± 0.01 d | 2.01 ± 0.02 a |
| Cd1 | 105.22 ± 0.62 d | 14.12 ± 0.13 b | 0.13 ± 0.00 d | |
| Cd2 | 122.23 ± 1.80 c | 18.32 ± 4.54 ab | 0.11 ± 0.00 d | |
| IC8-B | control | 0.49 ± 0.02 f | 0.26 ± 0.03 d | 1.06 ± 0.17 b |
| Cd1 | 43.79 ± 0.62 e | 21.21 ± 0.17 a | 0.48 ± 0.01 c | |
| Cd2 | 222.33 ± 6.49 b | 8.98 ± 0.06 c | 0.04 ± 0.00 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Khan, J.; Hayat, K.; Abdelfattah Elateeq, A.; Salam, U.; Yu, B.; Ma, Y.; Wang, H.; Tang, Z.-H. Comparative Study of Growth, Cadmium Accumulation and Tolerance of Three Chickpea (Cicer arietinum L.) Cultivars. Plants 2020, 9, 310. https://doi.org/10.3390/plants9030310
Ullah S, Khan J, Hayat K, Abdelfattah Elateeq A, Salam U, Yu B, Ma Y, Wang H, Tang Z-H. Comparative Study of Growth, Cadmium Accumulation and Tolerance of Three Chickpea (Cicer arietinum L.) Cultivars. Plants. 2020; 9(3):310. https://doi.org/10.3390/plants9030310
Chicago/Turabian StyleUllah, Shakir, Jafar Khan, Khizar Hayat, Ahmed Abdelfattah Elateeq, Uzma Salam, Bofan Yu, Yuehua Ma, Hongzheng Wang, and Zhong-Hua Tang. 2020. "Comparative Study of Growth, Cadmium Accumulation and Tolerance of Three Chickpea (Cicer arietinum L.) Cultivars" Plants 9, no. 3: 310. https://doi.org/10.3390/plants9030310
APA StyleUllah, S., Khan, J., Hayat, K., Abdelfattah Elateeq, A., Salam, U., Yu, B., Ma, Y., Wang, H., & Tang, Z.-H. (2020). Comparative Study of Growth, Cadmium Accumulation and Tolerance of Three Chickpea (Cicer arietinum L.) Cultivars. Plants, 9(3), 310. https://doi.org/10.3390/plants9030310







