Morphological Responses and Gene Expression of Grain Amaranth (Amaranthus spp.) Growing under Cd
Abstract
1. Introduction
2. Results
2.1. Amaranth Growth under the Cadmium Exposure
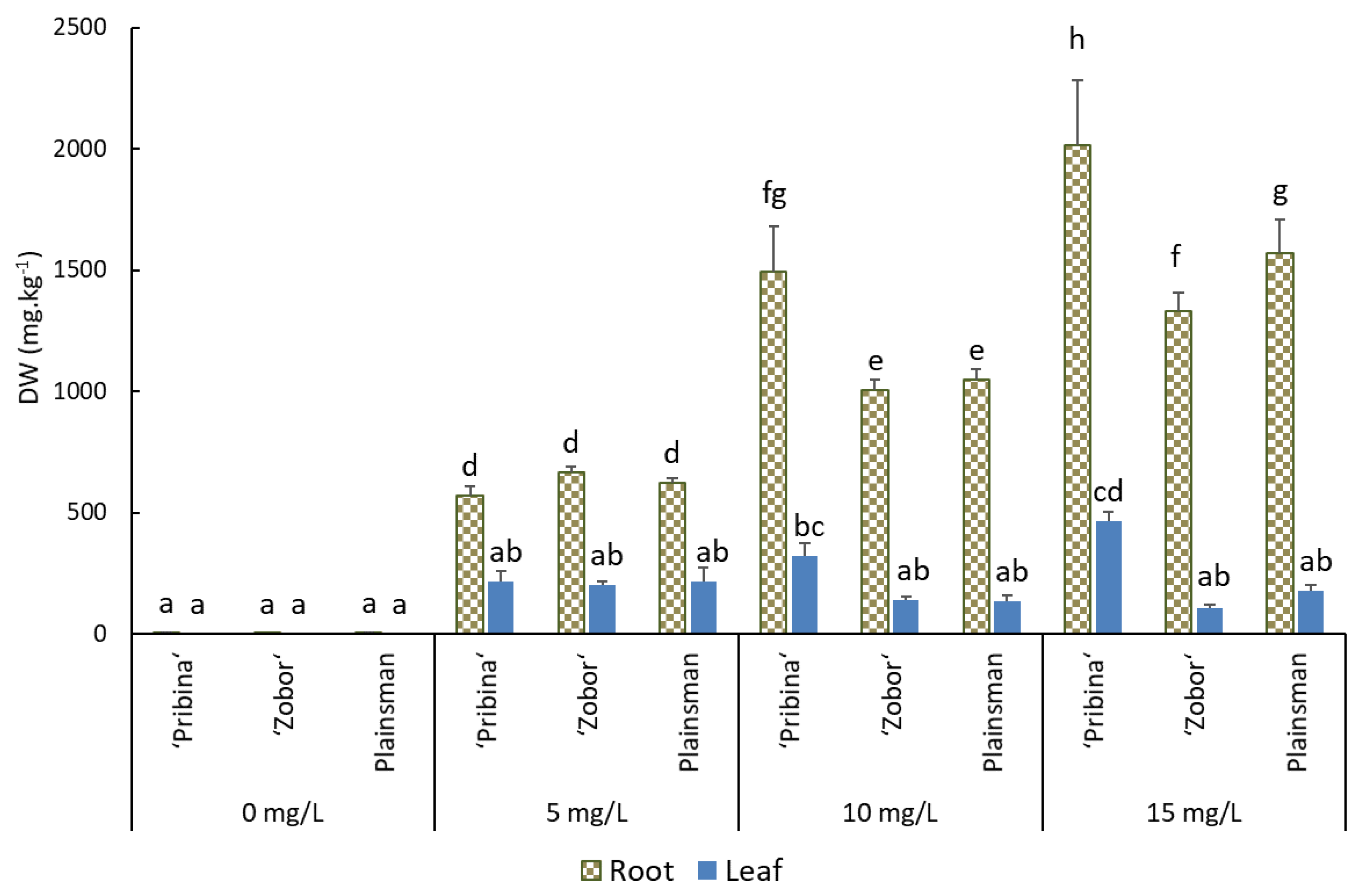
2.2. Cadmium Accumulation in Root and Shoot Tissues Determined by ICP-OES
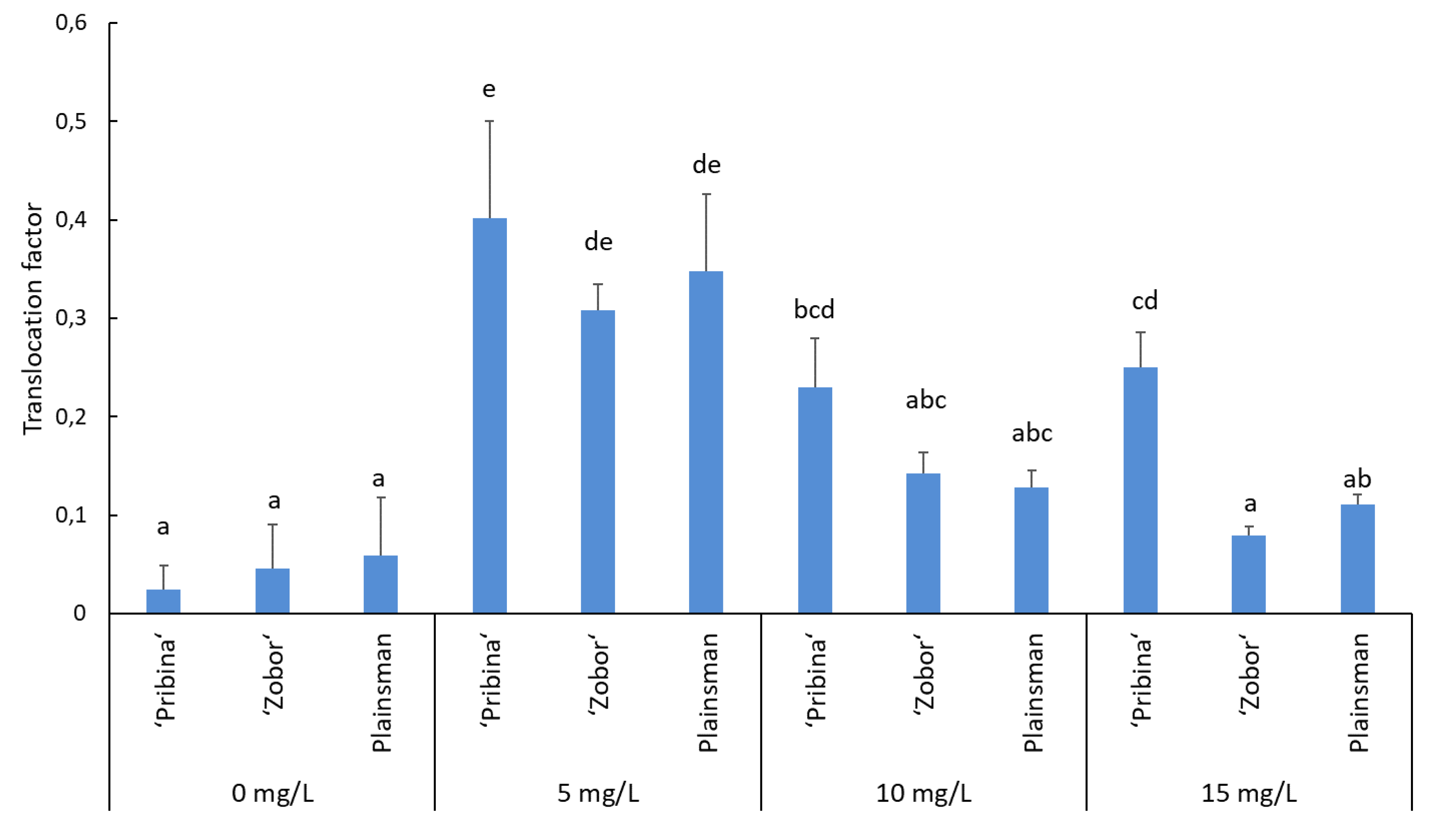
2.3. Translocation of Cadmium from Roots to Shoots
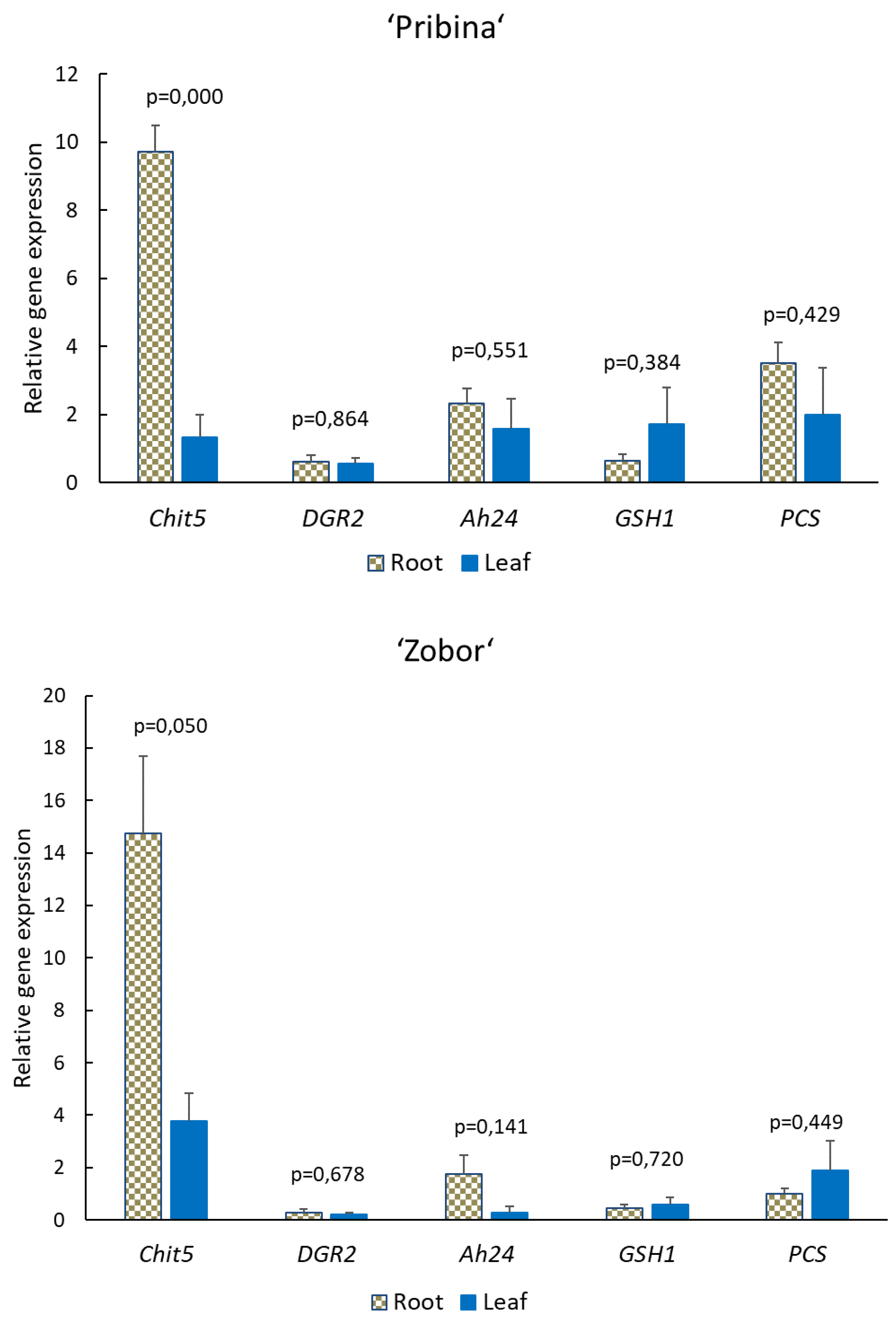
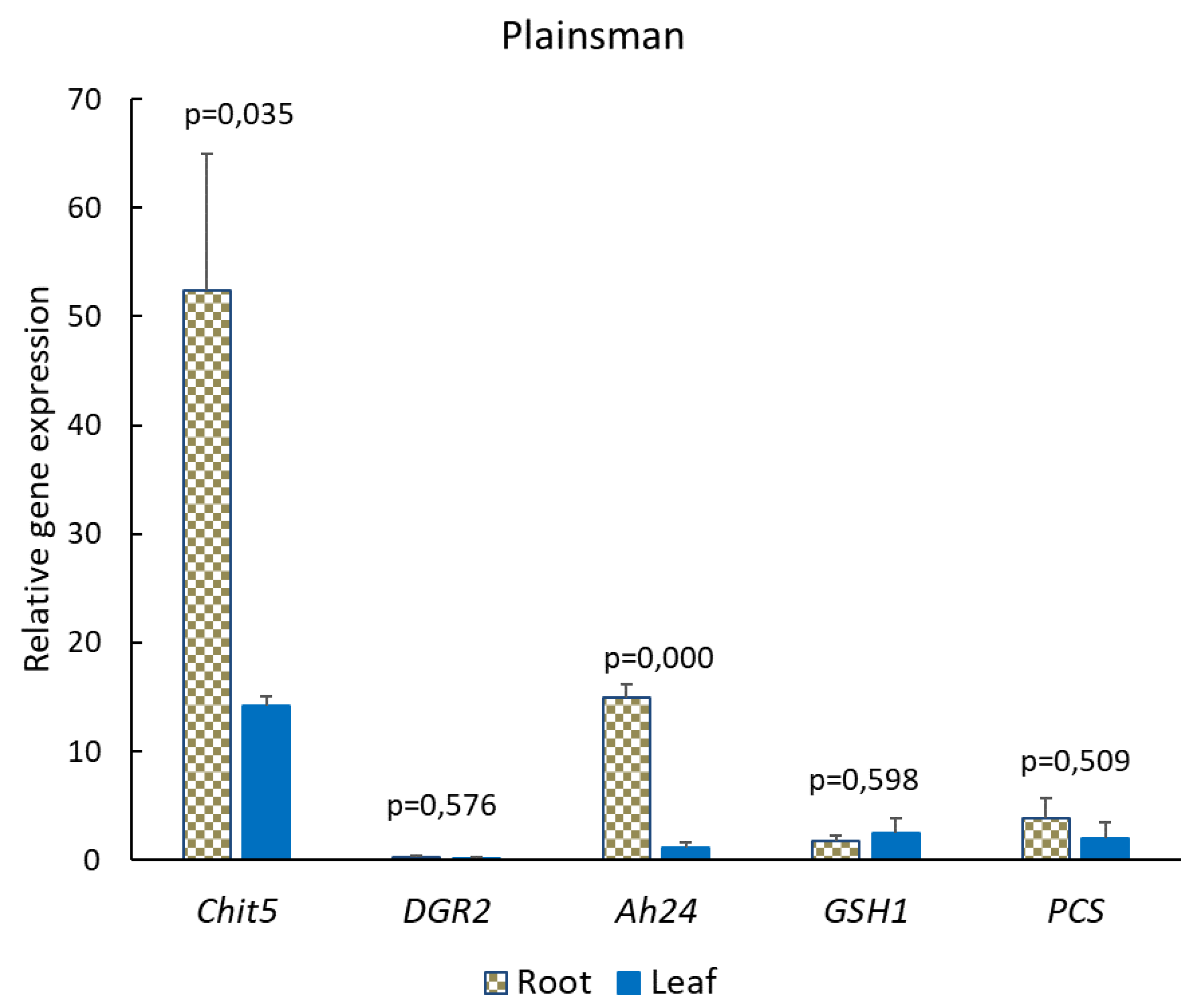
2.4. Expression Analysis of Stress-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Hydroponic Experiments Set Up
4.2. Morphological Measurements
4.3. ICP-OES Analysis
4.4. Gene Expression Analysis
4.4.1. RNA Isolation and Reverse Transcription
4.4.2. qRT-PCR Assay of Stress Responsive Genes
4.5. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.K.; Singh, N.K.; Patel, M.P.; Tiwari, M.R.; Rai, U.N. Metal contamination of soil and translocation in vegetables growing under industrial wastewater irrigated agricultural field of Vadodara, Gujarat, India. Ecotoxicol. Environ. Saf. 2011, 74, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.G.; Lopez, R.B.; Beltran, M. Removal of As, Cd, Cu, Ni, Pb, and Zn from a highly contaminated industrial soil using surfactant enhanced soil washing. Phys. Chem. Earth Parts A/B/C 2012, 37, 30–36. [Google Scholar] [CrossRef]
- Baldantoni, D.; Morra, L.; Zaccardelli, M.; Alfani, A. Cadmium accumulation in leaves of leafy vegetables. Ecotoxicol. Environ. Saf. 2016, 123, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Hinojosa, R.P.; Föllmi, K.B.; Gillet, F.; Matera, V. Cadmium accumulation in six common plant species associated with soils containing high geogenic cadmium concentrations at Le Gurnigel, Swiss Jura Mountains. Catena 2015, 124, 85–96. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Ow, D.W. Promises and Prospects of Phytoremediation. Plant Physiol. 1996, 110, 715–719. [Google Scholar] [CrossRef]
- Li, J.T.; Baker, A.J.M.; Ye, Z.H.; Wang, H.B.; Shu, W.S. Phytoextraction of Cd-contaminated soils: Current status and future challenges. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2113–2152. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Leitenmeier, B. Cadmium-Accumulating Plants. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer Science Business Media: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Leitenmeier, B.; Küpper, H. Compartmentation and complexation of metals in hyperaccumulator plants. Front. Plant Sci. 2013, 4, 374. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Hanikenne, M.; Nouet, C. Metal hyperaccumulation and hypertolerance: A model for plant evolutionary genomics. Curr. Opin. Plant Biol. 2011, 14, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Muszynska, E.; Hanus-Fajerska, E. Why are heavy metal hyperaccumulating plants so amazing? Bio Technol. 2015, 4, 265–271. [Google Scholar] [CrossRef]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A green approach to contaminant containment. Adv. Agron. 2011, 112, 145–204. [Google Scholar]
- Bert, V.; Meerts, P.; Saumitou-Laprade, P.; Salis, P.; Gruber, W.; Verbruggen, N. Genetic basis of Cd tolerance and hyperaccumulation in Arabidopsis Halleri. Plant Soil 2003, 249, 9–18. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Nenzou, G. Studies on arsenic rich mine dumps. II. The heavy element uptake by vegetation. J. Environ. Sci. Health 1997, 32, 455–464. [Google Scholar] [CrossRef]
- Bigaliev, A.; Boguspaev, K.; Znanburshin, E. Phytoremediation potential of Amaranthus sp. for heavy metals contaminated soil of oil producing territory. In Proceedings of the 10th Annual International Petroleum Environmental Conference, Houston, TX, USA, 12–14 November 2013. [Google Scholar]
- Prasad, M. Phytoremediation of Metal-Polluted Ecosystems: Hype for commercialization. Russ. J. Plant Physiol. 2003, 50, 686–700. [Google Scholar] [CrossRef]
- Chinmayee, M.D.; Mahesh, B.; Pradesh, S.; Mini, I.; Swapna, T.S. The assessment of phytoremediation potential of invasive weed Amaranthus spinosus L. Appl. Biochem. Biotechnol. 2012, 167, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Mellem, J.; Baijanth, H.; Odhav, B. Translocation and accumulation of Cr, Hg, As, Pb, Cu and Ni by Amaranthus dubius (Amaranthaceae) from contaminated sites. J. Environ. Sci. Health 2009, 44, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Iori, V.; Pietrini, F.; Cheremisina, A.; Shevyakova, N.I.; Raduykina, N.; Kuznetsov, V.V.; Zacchini, M. Growth responses, metal accumulation and phytoremoval capability in amaranthus plants exposed to nickel under hydroponics. Water Air Soil Pollut. 2013, 224, 1450. [Google Scholar] [CrossRef]
- Fan, H.; Zhou, W. Screening of amaranth cultivars (Amaranthus mangostanus L.) for cadmium hyperaccumulation. Agric. Sci. China 2009, 8, 342–351. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Xu, X.; Li, T.; Gong, G.; Jia, Y.; Li, Y.; Deng, L. Tolerance and accumulation characteristics of cadmium in Amaranthus hybridus L. J. Hazard. Mater. 2010, 180, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; He, H.; Xiao, L.; Zhong, T.; Liu, H.; Li, S.; Deng, P.; Ye, Z.; Jing, Y. Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere 2014, 103, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Chi, G.Y.; Shi, Y.; Chen, X. Plant growth and Cd accumulation characteristics in different planting modes of maize and Amaranthus hypochondriacus. J. Appl. Ecol. 2019, 30, 3164–3174. [Google Scholar]
- Wang, K.; Liu, Y.; Song, Z.; Wang, D.; Qiu, W. Chelator complexes enhanced Amaranthus hypochondriacus L. phytoremediation efficiency in Cd-contaminated soils. Chemosphere 2019, 237, 124480. [Google Scholar] [CrossRef]
- He, C.T.; Zhou, Y.Z.; Huang, Y.Y.; Fu, H.L.; Wang, X.S.; Gong, F.Y.; Tan, X.; Yang, Z.Y. Different proteomic processes related to the cultivar-dependent cadmium accumulation of Amaranthus gangeticus. J. Agric. Food Chem. 2018, 66, 1085–1095. [Google Scholar] [CrossRef]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Pavlović, P. Ecological potential of plants for phytoremediation and ecorestoration of fly ash deposits and mine wastes. Front. Environ. Sci. 2018, 6, 124. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; Walker, P.L. Ecophysiology of Metal Uptake by Tolerant Plants. In Heavy Metal Tolerance in Plants: Evolutionary Aspects; Shaw, A.J., Ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 155–177. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Sricoth, T.; Meeinkuirt, W.; Saengwilai, P.; Pichtel, J.; Taeprayoon, P. Aquatic plants for phytostabilization of cadmium and zinc in hydroponic experiments. Environ. Sci. Pollut. Res. 2018, 25, 14964–14976. [Google Scholar] [CrossRef] [PubMed]
- Phusantisampan, T.; Meeinkuirt, W.; Saengwilai, P.; Pichtel, J.; Chaiyarat, R. Phytostabilization potential of two ecotypes of Vetiveria zizanioides in cadmium-contaminated soils: Greenhouse and field experiments. Environ. Sci. Pollut. Res. 2016, 23, 20027–20038. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhou, Q.; Wang, X. Identification of weed plants excluding the uptake of heavy metals. Environ. Int. 2005, 31, 829–834. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D.; Zhukovskaya, N.V.; Schat, H. Cadmium tolerance and accumulation in excluder Thlaspi arvense and various acessions of hyperaccumulator Noccae caerulescens. Russ. J. Plant Physiol. 2015, 62, 837–846. [Google Scholar] [CrossRef]
- Li, N.; Guo, B.; Li, H.; Fu, Q.; Feng, R.; Ding, Y. Effects of double harvesting on heavy metal uptake by six forage species and the potential for phytoextraction in field. Pedosphere 2016, 26, 717–724. [Google Scholar] [CrossRef]
- Mishra, T.; Pandey, V.C. Phytoremediation of red mud deposits through natural succession. In Phytomanagement of Polluted Sites. Market Opportunities in Sustainable Phytoremediation, 1st ed.; Pandey, V.C., Bauddh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–424. [Google Scholar]
- Thongchai, A.; Meeinkuirt, W.; Taeprayoon, P.; Pichtel, J. Soil amendments for cadmium phytostabilization by five marigold cultivars. Environ. Sci. Pollut. Res. 2019, 26, 8737–8747. [Google Scholar] [CrossRef]
- González-Rodríguez, T.; Cisneros-Hernández, I.; Acosta Bayona, J.; Ramírez-Chavez, E.; Martínez-Gallardo, N.; Mellado-Mojica, E.; López-Pérez, M.G.; Molina-Torres, J.; Délano-Frier, J. Identification of factors linked to higher water-deficit stress tolerance in Amaranthus hypochondriacus compared to other grain amaranths and A. hybridus, their shared ancestor. Plants 2019, 8, 239. [Google Scholar] [CrossRef]
- Hasan, K.; Cheng, Y.; Kanwar, M.; Chu, X.; Ahammed, G.; Qi, Z. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, L.; Xia, Y.; Wang, F.; Zhu, C. Emerging roles of micro RNAs plant heavy metal tolerance and homeostasis. J. Agric. Food Chem. 2020, 68, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Massange-Sanchez, J.A.; Palmeros-Suarez, P.A.; Martinez-Gallardo, N.A.; Castrillon-Arbelaez, P.A.; Avilés-Arnaut, H.; Alatorre-Cobos, F.; Tiessen, A.; Délano-Frier, J.P. The novel and taxonomically restricted Ah24 gene from grain amaranth (Amaranthus hypochondriacus) has a dual role in development and defence. Front. Plant Sci. 2015, 6, 602. [Google Scholar] [CrossRef] [PubMed]
- Palmeros-Suárez, P.A.; Massange-Sánchez, J.A.; Sánchez-Segura, L.; Martínez-Gallardo, N.A.; Espitia Rangel, E.; Gómez-Leyva, J.F.; Délano-Frier, J.P. AhDGR2, an amaranth abiotic stress-induced DUF642 protein gene, modifies cell wall structure and composition and causes salt and ABA hyper-sensibility in transgenic Arabidopsis. Planta 2016, 245, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, C.; Xia, X.; Li, M.; Li, H.; Wang, Y.; Wang, S.; Wang, C.; Ma, Y.; Zhou, C. Comparative transcriptome analysis of duckweed (Landoltia punctata) in response to cadmium provides insights into molecular mechanisms underlying hyperaccumulation. Chemosphere 2018, 190, 154–165. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Ďurechová, D.; Jopčík, M.; Rajninec, M.; Moravčíková, J.; Libantová, J. Expression of Drosera rotundifolia chitinase in transgenic tobacco plants enhanced their antifungal potential. Mol. Biotechnol. 2019, 61, 916–928. [Google Scholar] [CrossRef]
- Grover, A. Plant chitinases: Genetic diversity and physiological roles. Crit. Rev. Plant Sci. 2013, 31, 57–73. [Google Scholar] [CrossRef]
- Békésiová, B.; Hraška, Š.; Libantová, J.; Moravčíková, J.; Matušíková, I. Heavy-metal stress induced accumulation of chitinase isoforms in plants. Mol. Biol. Rep. 2008, 35, 579–588. [Google Scholar] [CrossRef]
- Mészáros, P.; Rybanský, L.; Spiess, N.; Socha, P.; Kuna, R.; Libantová, J.; Moravčíková, J.; Piršelová, B.; Hauptvogel, P.; Matušíková, I. Plant chitinase responses to different metal-type stresses reveal specificity. Plant Cell Rep. 2014, 33, 1789–1799. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, Y.S.; Cheng, H.; Zhang, J.P.; Yeok, F.S. Cloning of the Aegiceras corniculatum class I chitinase gene (AcCHI I) and the response of AcCHI I mRNA expression to cadmium stress. Ecotoxicology 2015, 24, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Wang, Y.S.; Cheng, H.; Zhang, J.P.; Gu, J.D. Molecular cloning of class III chitinase gene from Avicennia marina and its expression analysis in response to cadmium and lead stress. Ecotoxicology 2015, 24, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Chen, F.; Sun, H.; Zhang, G.; Chen, Z.H.; Wu, F. Genome-wide transcriptome and functional analysis of two contrasting genotypes reveals key genes for cadmium tolerance in barley. BMC Genom. 2014, 15, 611. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.; Safronova, V.; Belimov, A.A.; Dietz, K.J. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J. Exp. Bot. 2005, 56, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Becerril, F.; Van Tuinen, D.; Martin-Laurent, F.; Metwally, A.; Dietz, K.J.; Gianinazzi-Pearson, V. Molecular changes in Pisum sativum L. roots during arbuscular mycorrhiza buffering of cadmium stress. Mycorrhiza 2005, 16, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Meléndez, A.L. Análisis Proteómico del Amaranto, Sometido a Herbivoría (Spodoptera exigua) y/o Evocadores Relacionados con la Resistencia a Insectos. Master’s Thesis, Centro de Investigación y de Estudios Avanzados del IPN, Mexico City, Mexico, 2009. [Google Scholar]
- Huerta-Ocampo, J.A.; Barrera-Pacheco, A.; Mendoza-Hernández, C.S.; Espitia Rangel, E.; Mock, H.P.; Barba de la Rosa, A.P. Salt stress-induced alterations in the root proteome of Amaranthus cruentus L. J. Proteome Res. 2014, 13, 3607–3627. [Google Scholar] [CrossRef]
- Délano-Frier, J.P.; Avilés-Arnaut, H.; Casarrubias-Castillo, K.; Casique-Arroyo, G.; Castrillón-Arbeláez, P.A.; Herrera-Estrella, L.; Massange-Sánchez, J.; Martínez-Gallardo, N.A.; Parra-Cota, F.I.; Vargas-Ortiz, E.; et al. Transcriptomic analysis of grain amaranth (Amaranthus hypochondriacus) using 454 pyrosequencing: Comparison with A. tuberculatus, expression profiling in stems and in response to biotic and abiotic stress. BMC Genom. 2011, 12, 363. [Google Scholar] [CrossRef]
- Jez, J.M.; Cahoon, R.E.; Chen, S. Arabidopsis thaliana glutamate-cysteine ligase, functional properties, kinetic mechanism, and regulation of activity. J. Biol. Chem. 2004, 279, 33463–33470. [Google Scholar] [CrossRef]
- Schnaubelt, D.; Schulz, P.; Hannah, M.A.; Yocgo, R.E.; Foyer, C.H. A phenomics approach to the analysis of the influence of glutathione on leaf area and abiotic stress tolerance in Arabidopsis thaliana. Front. Plant Sci. 2013, 4, 416. [Google Scholar] [CrossRef]
- Wei, H.; Rowe, M.; Riethoven, J.; Grove, R.; Adamec, J.; Jikumaru, Y.; Staswick, P. Overaccumulation of γ-glutamylcysteine in a jasmonate-hypersensitive Arabidopsis mutant causes jasmonate-dependent growth inhibition. Plant Physiol. 2015, 169, 1371–1381. [Google Scholar] [CrossRef]
- Dubreuil-Maurizi, C.; Poinssot, B. Role of glutathione in plant signaling under biotic stress. Plant Signal. Behav. 2012, 7, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Li, Y.; Rosen, B.P.; Shi, J.; Salt, D.; Senecoff, J.F.; Sashti, N.A.; Meagher, R.B. Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ-glutamylcysteine synthetase expression. Nat. Biotechnol. 2002, 20, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Steffens, J.C. The heavy-metal binding peptides of plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 553–575. [Google Scholar] [CrossRef]
- Ding, Y.; Jian, H.; Wang, T.; Di, F.; Wang, J.; Li, J.; Liu, L. Screening of candidate gene responses to cadmium stress by RNA sequencing in oilseed rape (Brassica napus L.). Environ. Sci. Pollut. Res. 2018, 25, 32433–32446. [Google Scholar] [CrossRef] [PubMed]
- Kisa, D. Responses of phytochelatin and proline-related genes expression associated with heavy metal stress in Solanum lycopersicum. Acta Bot. Croat. 2019, 78, 9–16. [Google Scholar] [CrossRef]
- Gajdošová, A.; Libiaková, G.; Fejér, J. Improvement of selected Amaranthus cultivars by means of mutation induction and biotechnological approaches. In Breeding of Neglected and Under-Utilized Crops, Spices and Herbs; Science Publishers: Enfield, UK, 2007; pp. 151–169. [Google Scholar]
- Hricová, A.; Fejér, J.; Libiaková, G.; Szabóová, M.; Gažo, J.; Gajdošová, A. Characterization of phenotypic and nutritional properties of valuable Amaranthus cruentus L. mutants. Turk. J. Agric. For. 2016, 40, 761–771. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plant without Soil; University of California Berkley Press: Berkeley, CA, USA; California Agricultural Experiment Station: Davis, CA, USA, 1950; Volume 347, p. 32. [Google Scholar]
- Chomczynski, P.; Mackey, K. Short technical report. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lancíková, V.; Tomka, M.; Žiarovská, J.; Gažo, J.; Hricová, A. Morphological Responses and Gene Expression of Grain Amaranth (Amaranthus spp.) Growing under Cd. Plants 2020, 9, 572. https://doi.org/10.3390/plants9050572
Lancíková V, Tomka M, Žiarovská J, Gažo J, Hricová A. Morphological Responses and Gene Expression of Grain Amaranth (Amaranthus spp.) Growing under Cd. Plants. 2020; 9(5):572. https://doi.org/10.3390/plants9050572
Chicago/Turabian StyleLancíková, Veronika, Marián Tomka, Jana Žiarovská, Ján Gažo, and Andrea Hricová. 2020. "Morphological Responses and Gene Expression of Grain Amaranth (Amaranthus spp.) Growing under Cd" Plants 9, no. 5: 572. https://doi.org/10.3390/plants9050572
APA StyleLancíková, V., Tomka, M., Žiarovská, J., Gažo, J., & Hricová, A. (2020). Morphological Responses and Gene Expression of Grain Amaranth (Amaranthus spp.) Growing under Cd. Plants, 9(5), 572. https://doi.org/10.3390/plants9050572



