Isatis tinctoria L. (Woad): A Review of Its Botany, Ethnobotanical Uses, Phytochemistry, Biological Activities, and Biotechnological Studies
Abstract
1. Introduction
2. Botanical Description
3. Ethnobotanical Uses
4. Chemical Composition
4.1. Alkaloids
4.2. Phenolic Compounds
4.3. Glucosinolates
4.4. Carotenoids
4.5. Monolignols and Oligolignols
4.6. Volatile Constituents
4.7. Other Constituents
5. Biological Activities
5.1. Anti-Inflammatory Activity
5.2. Anti-Tumor Activity
5.3. Antimicrobial and Antiviral Activities
5.4. Antioxidant Activity
6. Plant Biotechnological Studies on I. tinctoria
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ball, P.W. Isatis L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Valentine, D.H., Walters, S.M., Webb, D., Eds.; Cambridge University Press: Cambridge, UK, 1964; Volume I, pp. 268–269. [Google Scholar]
- Akeroid, J.R. Isatis Linnaeus. In The European Garden Flora; Cullen, J., Alexander, J.C.M., Brady, A., Brickell, C.D., Green, P.S., Heywood, V.H., Jørgensen, P.M., Jury, S.L., Knees, S.G., Leslie, A.C., et al., Eds.; Cambridge University Press: Cambridge, UK, 2002; Volume IV, p. 135. [Google Scholar]
- Al-Shehbaz, I.A.; Beilstein, M.A.; Kellogg, E.A. Systematics and phylogeny of the Brassicaceae (Cruciferae): An overview. Plant Syst. Evol. 2006, 259, 89–120. [Google Scholar] [CrossRef]
- Spataro, G.; Taviani, P.; Negri, V. Genetic variation and population structure in a Eurasian collection of Isatis tinctoria L. Genet. Resour. Crop Evol. 2007, 54, 573–584. [Google Scholar] [CrossRef]
- Spataro, G.; Negri, V. Adaptability and variation in Isatis tinctoria L.: A new crop for Europe. Euphytica 2008, 163, 89–102. [Google Scholar] [CrossRef]
- Hamburger, M. Isatis tinctorial—From the rediscovery of an ancient medicinal plant towards a novel anti-inflammatory phytopharmaceutical. Phytochem. Rev. 2002, 1, 333–344. [Google Scholar] [CrossRef]
- Aichele, D. Che fiore è? Biblioteca Universale Rizzoli: Milano, Italy, 1987; p. 114. [Google Scholar]
- Poli Marchese, E. Piante e fiori dell’Etna; Sellerio: Palermo, Italy, 1991; p. 76. [Google Scholar]
- Monograph. Isatis tinctoria. Altern. Med. Rev. 2002, 7, 523–524.
- Branca, F. Isatis tinctoria L.: An ancient dye plant of interest as a multifunctional crop. Chron. Horticult. 2015, 55, 20–24. [Google Scholar]
- Zech-Matterne, V.; Leconte, L. New archaeobotanical finds of Isatis tinctoria L. (woad) from Iron Age Gaul and a discussion of the importance of woad in ancient time. Veg. Hist. Archaeobot. 2010, 19, 137–142. [Google Scholar] [CrossRef]
- De Melo, J.S.; Rondão, R.; Burrows, H.D.; Melo, M.J.; Navaratnam, S.; Edge, R.; Voss, G. Spectral and photophysical studies of substituted indigo derivatives in their keto forms. ChemPhysChem 2006, 7, 2303–2311. [Google Scholar] [CrossRef]
- Guarino, C.; Casoria, P.; Menale, B. Cultivation and use of Isatis tinctoria L. (Brassicaceae) in Southern Italy. Econ. Bot. 2000, 54, 395–400. [Google Scholar] [CrossRef]
- Meijer, L.; Shearer, J.; Bettayeb, K.; Ferandin, Y. Diversity of the intracellular mechanisms underlying the anti-tumor properties of indirubins. Int. Congr. Ser. 2007, 1304, 60–74. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Schwarzländer, M.; Gibson, R.D.; Simpson, H.; Marshall, D.L.; Gerber, E.; Hinz, H. Geographic population structure in an outcrossing plant invasion after centuries of cultivation and recent founding events. AoB Plants 2018, 10. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Jamali, A.; Grand, E.; Morreel, K.; Marcelo, P.; Gontier, E.; Dauwe, R. Phenylpropanoid profiling reveals a class of hydroxycinnamoyl glucaric acid conjugates in Isatis tinctoria leaves. Phytochemistry 2017, 144, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Danz, H.; Stoyanova, S.; Wippich, P.; Brattström, A.; Hamburger, M. Identification and isolation of the cyclooxygenase-2 inhibitory principle in Isatis tinctoria. Planta Med. 2001, 67, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.-C.; Cerdá-Nicolás, M.; Potterat, O.; Hamburger, M.; Ríos, J.L. Anti-inflammatory and antiallergic activity in vivo of lipophilic Isatis tinctoria extracts and tryptanthrin. Planta Med. 2006, 72, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Condurso, C.; Verzera, A.; Romeo, V.; Ziino, M.; Trozzi, A.; Ragusa, S. The leaf volatile constituents of Isatis tinctoria by solid-phase microextraction and gas chromatography/mass spectrometry. Planta Med. 2006, 72, 924–928. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines. Isatis root (Isatidis radix). In European Pharmacopoeia, 7th ed.; Suppl. 7.3 (2566); European Directorate for the Quality of Medicines: Strasburg, Germany, 2011; pp. 3866–3867. [Google Scholar]
- Cosmetic IngredientDatabase (CosIng). Available online: https://ec.europa.eu/growth/sectors/cosmetics/cosing (accessed on 15 January 2020).
- Sales, E.; Kanhonou, R.; Baixauli, C.; Giner, A.; Cooke, D.; Gilbert, K.; Arrillaga, I.; Segura, J.; Ros, R. Sowing date, transplanting, plant density and nitrogen fertilization affect indigo production from Isatis species in a Mediterranean region of Spain. Ind. Crops Prod. 2006, 23, 29–39. [Google Scholar] [CrossRef]
- Galletti, S.; Bagatta, M.; Iori, R.; Ragusa, L.; Branca, F.; Argento, S. Nutraceutical value of woad (Isatis tinctoria) flower buds of ecotypes from Sicily, Italy. Acta Hortic. 2013, 1005, 349–353. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines. Isatis root (Isatidis radix). In European Pharmacopoeia, 9th ed.; European Directorate for the Quality of Medicines: Strasburg, Germany, 2017; pp. 1399–1400. [Google Scholar]
- Angelini, L.G.; Tozzi, S.; Nassi o Di Nasso, N. Differences in leaf yield and indigo precursors production in woad (Isatis tinctoria L.) and Chinese woad (Isatis indigotica Fort.) genotypes. Field Crop. Res. 2007, 101, 285–295. [Google Scholar] [CrossRef]
- Gilbert (nee Stoker), G.; Garton, S.; Karam, A.; Arnold, M.; Karp, A.; Edwards, J.; Cooke, T.; Barker, A. A high degree of genetic diversity is revealed in Isatis spp. (dyer’s woad) by amplified fragment length polymorphism (AFLP). Theor. Appl. Genet. 2002, 104, 1150–1156. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Pang, X.H. Discussion on the botanical origin of Isatidis radix and Isatidis folium based on DNA barcoding. Yao Xue Xue Bao 2013, 48, 1850–1855. [Google Scholar]
- Buhner, S.H. Herbal Antivirals: Natural Remedies for Emerging, Resistant and Epidemic Viral Infections; Storey Publishing: North Adams, MA, USA, 2013; pp. 192–208. [Google Scholar]
- Oberthür, C.; Jaggi, R.; Hamburger, M. HPLC based activity profiling for 5-lipoxygenase inhibitory activity in Isatis tinctoria leaf extracts. Fitoterapia 2005, 76, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.M. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; MIT Press: Cambridge, UK, 1980; p. 111. [Google Scholar]
- Garland, S. Il Giardino Delle Aromatiche; Centro Botanico: Milano, Italy, 1988; pp. 145–146. [Google Scholar]
- Palma, L. Le Piante Medicinali d’Italia; SEI: Torino, Italy, 1964; p. 226. [Google Scholar]
- Mohn, T.; Plitzko, I.; Hamburger, M. A comprehensive metabolite profiling of Isatis tinctoria leaf extracts. Phytochemistry 2009, 70, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jiang, X.; Zhang, L. Optimisation of extraction conditions for polysaccharides from the roots of Isatis tinctoria L. by response surface methodology and their in vitro free radicals scavenging activities and effects on IL-4 and IFN-γ mRNA expression in chicken lymphocytes. Carbohydr. Polym. 2011, 86, 1320–1326. [Google Scholar] [CrossRef]
- Duke, J.A.; Ayensu, E.S. Medicinal Plants of China; Reference Publications, Inc.: Algonac, MI, USA, 1985; p. 210. [Google Scholar]
- Zhu, Y.-P. Chinese Materia Medica: Chemistry, Phamacology and Applications; Harwood Academic Publishers: Amsterdam, The Netherlands, 1998; pp. 185–189. [Google Scholar]
- Zhang, D.; Du, K.; Zhao, Y.; Shi, S.; Wu, Y.; Jia, Q.; Chen, K.; Li, Y.; Wang, R. Indole alkaloid glycosides from Isatis tinctoria roots. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Wen, T.; Bradshaw, J.P.; Zhou, J.; Rao, P. Antiviral decoction of Isatidis radix (bǎn lán gēn) inhibited influenza virus adsorption on MDCK cells by cytoprotective activity. J. Tradit. Complement. Med. 2012, 2, 47–51. [Google Scholar] [CrossRef][Green Version]
- Huang, K.C. The Pharmacology of Chinese Herbs; CRC Press: Boca Raton, FL, USA, 1993; pp. 304–305. [Google Scholar]
- Tang, W.; Eisenbrand, G. Qingdai. In Chinese Drugs of Plant Origin; Springer: Berlin, Germany, 1992; pp. 805–806. [Google Scholar]
- Kim, K.-S.; Hwang, W.-G.; Jang, H.-G.; Heo, B.-G.; Suhaj, M.; Leontowicz, H.; Leontowicz, M.; Jastrzebski, Z.; Tashma, Z.; Gorinstein, S. Assessment of Indigo (Polygonum tinctorium Ait.) water extracts’ bioactive compounds, and their antioxidant and antiproliferative activities. LWT-Food Sci. Technol. 2012, 46, 500–510. [Google Scholar] [CrossRef]
- Yeung, H.C. Handbook of Chinese Herbs and Formulas; Institute of Chinese Medicine: Los Angeles, CA, USA, 1985. [Google Scholar]
- Honda, G.; Tosirisuk, V.; Tabata, M. Isolation of an antidermatophytic, tryptanthrin, from indigo plants, Polygonum tinctorium and Isatis tinctoria. Planta Med. 1980, 38, 275–276. [Google Scholar] [CrossRef]
- Maugard, T.; Enaud, E.; Choisy, P.; Legoy, M.D. Identification of an indigo precursor from leaves of Isatis tinctoria (Woad). Phytochemistry 2001, 58, 897–904. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Marcelo, P.; Gontier, E.; Dauwe, R. Metabolic markers for the yield of lipophilic indole alkaloids in dried woad leaves (Isatis tinctoria L.). Phytochemistry 2019, 163, 89–98. [Google Scholar] [CrossRef]
- Miceli, N.; Filocamo, A.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Celano, M.; Maggisano, V.; Taviano, M.F. Chemical characterization and biological activities of phenolic-rich fraction from cauline leaves of Isatis tinctoria L. (Brassicaceae) growing in Sicily, Italy. Chem. Biodivers. 2017, 14, e1700073. [Google Scholar] [CrossRef]
- Taviano, M.F.; Filocamo, A.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Paterniti Mastrazzo, G.; De Rose, R.F.; Celano, M.; Lombardo, G.E.; et al. Phenolic profile, antioxidant and cytotoxic properties of polar extracts from leaves and flowers of Isatis tinctoria L. (Brassicaceae) growing in Sicily. Plant Biosyst. 2018, 152, 795–803. [Google Scholar] [CrossRef]
- Hartleb, I.; Seifert, K. A novel anthranilic acid derivate from Isatis tinctoria. Planta Med. 1995, 60, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Mohn, T.; Cutting, B.; Ernst, B.; Hamburger, M. Extraction and analysis of intact glucosinolates—A validated pressurized liquid extraction/liquid chromatography–mass spectrometry protocol for Isatis tinctoria, and qualitative analysis of other cruciferous plants. J. Chromatogr. A 2007, 1166, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Mohn, T.; Hamburger, M. Glucosinolate pattern in Isatis tinctoria and I. indigotica seeds. Planta Med. 2008, 74, 885–888. [Google Scholar] [CrossRef]
- Verzera, A.; Condurso, C.; Dima, G.; Ziino, M.; Ragusa, S. Volatile constituents in dried roots of Isatis tinctoria L. (Brassicaceae). J. Essent. Oil Res. 2010, 22, 483–485. [Google Scholar] [CrossRef]
- Kizil, S.; Turk, M.; Çakmak, Ö.; Özgüven, M.; Khawar, K.M. Microelement contents and fatty acid compositions of some Isatis species seeds. Not. Bot. Horti Agrobot. Cluj Napoca 2009, 37, 175–178. [Google Scholar] [CrossRef]
- Oberthür, C.; Schneider, B.; Graf, H.; Hamburger, M. The elusive Indigo Precursors in Woad (Isatis tinctoria L.)–Identification of the Major Indigo Precursor, Isatan A and a Structure Revision of Isatan B. Chem. Biodivers. 2004, 1, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Nabors, M.W.; Stowe, B.B. Origin of Indigo of Woad. Nature 1967, 216, 547–549. [Google Scholar] [CrossRef]
- Oberthür, C.; Graf, H.; Hamburger, M. The content of indigo precursors in Isatis tinctoria leaves—A comparative study of selected accessions and post-harvest treatments. Phytochemistry 2004, 65, 3261–3268. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, Y.; Shi, S.; Wu, X.; Zhang, L.; Chen, K.; Li, Y.; Wang, R. Isatisindigoticanine A, a novel indole alkaloid with an unpresented carbon skeleton from the roots of Isatis tinctoria. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Danz, H.; Baumann, D.; Hamburger, M. Quantitative determination of the dual COX-2/5-LOX inhibitor tryptanthrin in Isatis tinctoria by ESI-LC-MS. Planta Med. 2002, 68, 152–157. [Google Scholar] [CrossRef]
- Oberthür, C.; Hamburger, M. Tryptanthrin content in Isatis tinctoria leaves—A comparative study of selected strains and post-harvest treatments. Planta Med. 2004, 70, 642–645. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, A.J.; Panesar, S.S.; Gil, V. Thermal degradation of glucosinolates. Phytochemistry 1981, 20, 977–980. [Google Scholar] [CrossRef]
- Miceli, N.; Cavò, E.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Marino, A.; Cincotta, F.; Condurso, C.; Taviano, M.F. Phytochemical characterization and biological activities of a hydroalcoholic extract obtained from the aerial parts of Matthiola incana (L.) R. Br. subsp. incana (Brassicaceae) growing wild in Sicily (Italy). Chem. Biodivers. 2019, 16, e1800677. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Johansen, H.K.; Moser, C.; Høiby, N. Effects of Chinese medicinal herbs on a rat model of chronic Pseudomonas aeruginosa lung infection. APMIS 1996, 104, 350–354. [Google Scholar] [CrossRef]
- Oberthür, C.; Heinemann, C.; Elsner, P.; Benfeldt, E.; Hamburger, M. A comparative study on the skin penetration of pure tryptanthrin and tryptanthrin in Isatis tinctoria extract by dermal microdialysis coupled with isotope dilution ESI-LC-MS. Planta Med. 2003, 69, 385–389. [Google Scholar] [CrossRef]
- Heinemann, C.; Schliemann-Willers, S.; Oberthür, C.; Hamburger, M.; Elsner, P. Prevention of experimentally induced irritant contact dermatitis by extracts of Isatis tinctoria compared to pure tryptanthrin and its impact on UVB-induced erythema. Planta Med. 2004, 70, 385–390. [Google Scholar] [CrossRef]
- Recio, M.C.; Cerdá-Nicolás, M.; Hamburger, M.; Ríos, J.-L. Anti-arthritic activity of a lipophilic woad (Isatis tinctoria) extract. Planta Med. 2006, 72, 715–720. [Google Scholar] [CrossRef][Green Version]
- Brattström, A.; Schapowal, A.; Kamal, M.A.; Maillet, I.; Ryffel, B.; Moser, R. The plant extract Isatis tinctoria L. extract (ITE) inhibits allergen-induced airway inflammation and hyperreactivity in mice. Phytomedicine 2010, 17, 551–556. [Google Scholar] [CrossRef]
- Cooperative Group of Clinical Therapy of Indirubin. Clinical studies of 314 cases of CML treated with indirubin. Chin. J. Int. Med. 1980, 1, 132–135. [Google Scholar]
- Kimoto, T.; Yamamoto, Y.; Hino, K.; Koay, S.; Aga, H.; Hashimoto, T.; Hanaya, T. Cytotoxic effects of substances in indigo plant (Polygonum tinctorium Lour.) on maligant tumour cells. Nat. Med. 1999, 53, 72–79. [Google Scholar]
- Kimoto, T.; Hino, K.; Koya-Miyata, S.; Yamamoto, Y.; Takeuchi, M.; Nishizaki, Y.; Micallef, M.J.; Ushio, S.; Iwaki, K.; Ikeda, M.; et al. Cell differentiation and apoptosis of monocytic and promyelocytic leukemia cells (U-937 and HL-60) by tryptanthrin, an active ingredient of Polygonum tinctorium Lour. Pathol. Int. 2001, 51, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Koya-Miyata, S.; Kimoto, T.; Micallef, M.J.; Hino, K.; Taniguchi, M.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Prevention of azoxymethane-induced intestinal tumors by a crude ethyl acetate-extract and tryptanthrin extracted from Polygonum tinctorium Lour. Anticancer Res. 2001, 21, 13295–13300. [Google Scholar]
- Marko, D.; Schätzle, S.; Friedel, A.; Genzlinger, A.; Zankl, H.; Meijer, L.; Eisenbrand, G. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br. J. Cancer 2001, 84, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.T.; Chen, T.M.; Tseng, S.Y.; Chen, Y.H. Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2007, 358, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.T.; Chen, T.M.; Chern, J.W.; Tseng, S.Y.; Chen, Y.H. Downregulation of GSTpi expression by tryptanthrin contributing to sensitization of doxorubicin-resistant MCF-7 cells through c-jun NH2-terminal kinase-mediated apoptosis. Anticancer Drugs 2009, 20, 382–388. [Google Scholar] [CrossRef]
- Chan, H.-L.; Yip, H.-Y.; Mak, N.-K.; Leung, K.N. Modulatory effects and action mechanisms of tryptanthrin on murine myeloid leukemia cells. Cell Mol. Immunol. 2009, 6, 335–342. [Google Scholar] [CrossRef]
- Miao, S.; Shi, X.; Zhang, H.; Wang, S.; Sun, J.; Hua, W.; Miao, Q.; Zhao, Y.; Zhang, C. Proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on human chronic myeloid leukemia K562 cell line in vitro. Int. J. Mol. Sci. 2011, 12, 3831–3845. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Wu, Y.; Dong, Y.; Lai, L.; Zhang, J.; Lu, B.; Dai, F.; He, L.; Liu, M.; et al. Indirubin inhibits tumor growth by antitumor angiogenesis via blocking VEGFR2-mediated JAK/STAT3 signaling in endothelial cell. Int. J. Cancer 2011, 129, 2502–2511. [Google Scholar] [CrossRef]
- Liao, X.; Leung, K.N. Tryptanthrin induces growth inhibition and neuronal differentiation in the human neuroblastoma LA-N-1 cells. Chem.-Biol. Interact. 2013, 203, 512–521. [Google Scholar] [CrossRef]
- Liao, X.; Zhou, X.; Mak, N.-k.; Leung, K.-n. Tryptanthrin inhibits angiogenesis by targeting the VEGFR2-mediated ERK1/2 signalling pathway. PLoS ONE 2013, 8, e82294. [Google Scholar] [CrossRef]
- Chang, C.F.; Hsu, Y.L.; Lee, C.Y.; Wu, C.H.; Wu, Y.C.; Chuang, T.H. Isolation and cytotoxicity evaluation of the chemical constituents from Cephalantheropsis gracilis. Int. J. Mol. Sci. 2015, 16, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Alex, V.V.; Nisthul, A.A.; Bava, S.V.; Sundaram, S.; Retnakumari, A.P.; Chittalakkottu, S.; Anto, R.J. Pre-clinical evidences for the efficacy of tryptanthrin as a potent suppressor of skin cancer. Cell Prolif. 2019, e12710. [Google Scholar] [CrossRef] [PubMed]
- Honda, G.; Tabata, M.; Tsuda, M. The antimicrobial specificity of tryptanthrin. Planta Med. 1979, 37, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Dornberger, K.; Lich, H. Screening for antimicrobial and presumed cancerostatic plant metabolites. Pharmazie 1982, 37, 215–221. [Google Scholar] [PubMed]
- Yang, Z.C.; Wang, B.C.; Yang, X.S.; Wang, Q.; Ran, L. The synergistic activity of antibiotics combined with eight traditional Chinese medicines against two different strains of Staphylococcus aureus. Colloids Surf. B Biointerfaces 2005, 41, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.R.; Li, A.; Leu, Y.L.; Fang, J.Y.; Lin, Y.K. An in vitro study of the antimicrobial effects of Indigo naturalis prepared from Strobilanthes formosanus Moore. Molecules 2013, 18, 14381–14396. [Google Scholar] [CrossRef]
- Costa, D.C.M.; Azevedo, M.M.B.; Silva, D.O.E.; Romanos, M.T.V.; Souto-Padrón, T.C.B.S.; Alviano, C.S.; Alviano, D.S. In vitro anti-MRSA activity of Couroupita guianensis extract and its component tryptanthrin. Nat. Prod. Res. 2017, 31, 2077–2080. [Google Scholar] [CrossRef]
- Ullah, I.; Wakeel, A.; Shinwari, Z.K.; Jan, S.A.; Khalil, A.T.; Ali, M. Antibacterial and antifungal activity of Isatis tinctoria L. (Brassicaceae) using the micro-plate method. Pak. J. Bot. 2017, 49, 1949–1957. [Google Scholar]
- Mak, N.-K.; Leung, C.-Y.; Wei, X.-Y.; Shen, X.L.; Wong, R.N.; Leung, K.N.; Fung, M.C. Inhibition of RANTES expression by indirubin in influenza virus-infected human bronchial epithelial cells. Biochem. Pharmacol. 2004, 67, 167–174. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Zhou, H.; Zeng, L.; Chen, T.; Chen, Q.; Zhou, B.; Wang, Y.; Chen, Q.; Hu, P.; et al. Radix Isatidis polysaccharides inhibit Influenza A virus and Influenza A virus-induced inflammation via suppression of host TLR3 signaling in vitro. Molecules 2017, 22, 116. [Google Scholar] [CrossRef]
- Wang, C.; Ruan, S.; Gu, X.; Zhu, B. Antiviral activities of Radix Isatidis polysaccharide against type II herpes simplex virus in vitro. Food Sci. Technol. 2018, 38, 180–183. [Google Scholar] [CrossRef]
- Fialova, S.; Valigura, R.; Tekelova, D.; Grancai, D. Isatis tinctoria L. (dyer’s woad) from the standpoint of phenolic compound content and scavenging activity. Farm Obz. 2009, 78, 54–58. [Google Scholar]
- Yang, C.-H.; Chang, H.-W.; Lin, H.-Y.; Chuang, L.-Y. Evaluation of antioxidant and antimicrobial activities from 28 Chinese Herbal Medicines. J. Pharmacogn. Phytochem. 2013, 2, 294–305. [Google Scholar]
- Zhao, G.; Li, T.; Qu, X.; Zhang, N.; Lu, M.; Wang, J. Optimization of ultrasound-assisted extraction of indigo and indirubin from Isatis indigotica Fort and their antioxidant capacities. Food Sci. Biotechnol. 2017, 26, 1313–1323. [Google Scholar] [CrossRef]
- Danz, H.; Stoyanova, S.; Thomet, O.A.; Simon, H.U.; Dannhardt, G.; Ulbrich, H.; Hamburger, M. Inhibitory activity of tryptanthrin on prostaglandin and leukotriene synthesis. Planta Med. 2002, 68, 875–880. [Google Scholar] [CrossRef]
- Ishihara, T.; Kohno, K.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Tryptanthrin inhibits nitric oxide and prostaglandin E2 synthesis by murine macrophages. Eur. J. Pharmacol. 2000, 407, 197–204. [Google Scholar] [CrossRef]
- Pergola, C.; Jazzar, B.; Rossi, A.; Northoff, H.; Hamburger, M.; Sautebin, L.; Werz, O. On the inhibition of 5-lipoxygenase product formation by tryptanthrin: Mechanistic studies and efficacy in vivo. Br. J. Pharmacol. 2012, 165, 765–776. [Google Scholar] [CrossRef]
- Micallef, M.J.; Iwaki, K.; Ishihara, T.; Ushio, S.; Aga, M.; Kunikata, K.; Koya-Miyata, S.; Kimoto, T.; Ikeda, M.; Kurimoto, M. The natural plant product tryptanthrin ameliorates dextran sodium sulfate-induced colitis in mice. Int. Immunopharmacol. 2002, 2, 565–578. [Google Scholar] [CrossRef]
- Rüster, G.U.; Hoffmann, B.; Hamburger, M. Inhibitory activity of indolin-2-one derivatives on compound 48/80-induced histamine release from mast cells. Pharmazie 2004, 59, 236–237. [Google Scholar]
- Kiefer, S.; Mertz, A.C.; Koryakina, A.; Hamburger, M.; Küenzi, P. (E,Z)-3-(3’,5’-Dimethoxy-4’-hydroxy-benzylidene)-2-indolinone blocks mast cell degranulation. Eur. J. Pharm. Sci. 2010, 40, 143–147. [Google Scholar] [CrossRef]
- Polychronopoulos, P.; Magiatis, P.; Skaltsounis, A.L.; Myrianthopoulos, V.; Mikros, E.; Tarricone, A.; Musacchio, A.; Roe, S.M.; Pearl, L.; Leost, M.; et al. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J. Med. Chem. 2004, 47, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Kunikata, T.; Tatefuji, T.; Aga, H.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur. J. Pharmacol. 2000, 410, 93–100. [Google Scholar] [CrossRef]
- Qi, T.; Li, H.; Li, S. Indirubin improves antioxidant and anti-inflammatory functions in lipopolysaccharide-challenged mice. Oncotarget 2017, 8, 36658–36663. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Hao, Y.; Liu, B. Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in China. Leuk. Lymphoma 2002, 43, 1763–1768. [Google Scholar] [CrossRef]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.M.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D.; et al. Indirubin, the active constituent of a Chinese antileukemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef]
- Wu, G.Y.; Liu, J.Z.; Fang, F.D.; Zuo, J. Studies on the mechanism of indirubin action in the treatment of chronic granulocytic leukemia. V. Binding between indirubin and DNA and identification of the type of binding. Sci. Sin. B 1982, 25, 1071–1079. [Google Scholar]
- Eisenbrand, G.; Hippe, F.; Jakobs, S.; Muehlbeyer, S. Molecular mechanisms of indirubin and its derivatives: Novel anticancer molecules with their origin in traditional Chinese phytomedicine. J. Cancer Res. Clin. Oncol. 2004, 130, 627–635. [Google Scholar] [CrossRef]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.-Z.; Mandelkow, E.-M.; et al. Indirubins inhibit glycogen synthase kinase-3β and CDK5/p25, two kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most CDK inhibitors? J. Biol. Chem. 2001, 276, 251–260. [Google Scholar] [CrossRef]
- Damiens, E.; Baratte, B.; Marie, D.; Eisenbrand, G.; Meijer, L. Anti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: Induction of endoreplication following prophase arrest. Oncogene 2001, 20, 3786–3797. [Google Scholar] [CrossRef]
- Lin, C.W.; Tsai, F.J.; Tsai, C.H.; Lai, C.C.; Wan, L.; Ho, T.Y.; Hsieh, C.C.; Chao, P.D. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005, 68, 36–42. [Google Scholar] [CrossRef]
- Taviano, M.F.; Rashed, K.; Filocamo, A.; Cacciola, F.; Dugo, P.; Mondello, L.; Bisignano, C.; Acquaviva, R.; D’Arrigo, M.; Miceli, N. Phenolic profile and biological properties of the leaves of Ficus vasta Forssk. (Moraceae) growing in Egypt. BMC Complement. Altern. Med. 2018, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, S.; Huang, X.; Hong, J.; Du, L.; Zhang, L.; Ye, L. Environmental factors affecting growth and development of Banlangen (Radix Isatidis) in China. Afr. J. Plant Sci. 2015, 9, 421–426. [Google Scholar] [CrossRef]
- Hammond, J.; McGarvey, P.; Yusibov, V. Plant Biotechnology: New Products and Applications; Springer: Berlin, Germany, 2000. [Google Scholar]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Gaj, M.D. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004, 43, 27–47. [Google Scholar] [CrossRef]
- Ramawat, K.; Mathur, M. Factors affecting the production of secondary metabolites. In Biotechnology: Secondary Metabolites, plants and Microbes; Ramawat, K., Merillon, J., Eds.; Science Publishers: Enfield, NH, USA, 2007; pp. 59–102. [Google Scholar]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Luczkiewicz, M.; Ekiert, H. Schisandra lignans production regulated by different bioreactor type. J. Biotechnol. 2017, 247, 11–17. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Luo, M.; Wang, W.; Ma, W.; Zu, Y.-G.; Fu, Y.-J. Establishment of high-productive Isatis tinctoria L. hairy root cultures: A promising approach for efficient production of bioactive alkaloids. Biochem. Eng. J. 2015, 95, 37–47. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Luo, M.; Wei, Z.F.; Zu, Y.G.; Ma, W.; Fu, Y.J. Establishment of hairy root cultures by Agrobacterium rhizogenes mediated transformation of Isatis tinctoria L. for the efficient production of flavonoids and evaluation of antioxidant activities. PLoS ONE 2015, 10, e0119022. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, X.; Qin, Q.-P.; Wang, Z.-Y.; Liu, J.; Fu, Y.-J. Chitosan elicitation of Isatis tinctoria L. hairy root cultures for enhancing flavonoid productivity and gene expression and related antioxidant activity. Ind. Crops Prod. 2018, 124, 28–35. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Yao, L.-P.; Niu, L.-L.; Zang, Y.-P.; Fu, Y.-J. Ultraviolet radiation for flavonoid augmentation in Isatis tinctoria L. hairy root cultures mediated by oxidative stress and biosynthetic gene expression. Ind. Crops Prod. 2018, 118, 347–354. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.-Y.; Wang, W.; Zang, Y.-P.; Niu, L.-L.; Fu, Y.-J.; Wang, X. Remarkable enhancement of flavonoid production in a co-cultivation system of Isatis tinctoria L. hairy root cultures and immobilized Aspergillus niger. Ind. Crops Prod. 2018, 112, 252–261. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Wang, X.; Zang, Y.-P.; Niu, L.-L.; Fu, Y.-J. Elicitation of Isatis tinctoria L. hairy root cultures by salicylic acid and methyl jasmonate for the enhanced production of pharmacologically active alkaloids and flavonoids. Plant Cell Tissue Org. Cult. 2019, 137, 77–86. [Google Scholar] [CrossRef]
- Saglam, S.; Ciftci, C.Y. Effects of agar and ısubgol on adventitous shoot regeneration of woad (Isatis tinctoria). Int. J. Agric. Biol. 2010, 12, 281–285. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Leal, F.; Cipriano, J.; Carnide, V.; Pinto-Carnide, O. In vitro culture establishment of woad (Isatis tinctoria L.). Acta Hortic. 2009, 812, 121–124. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]

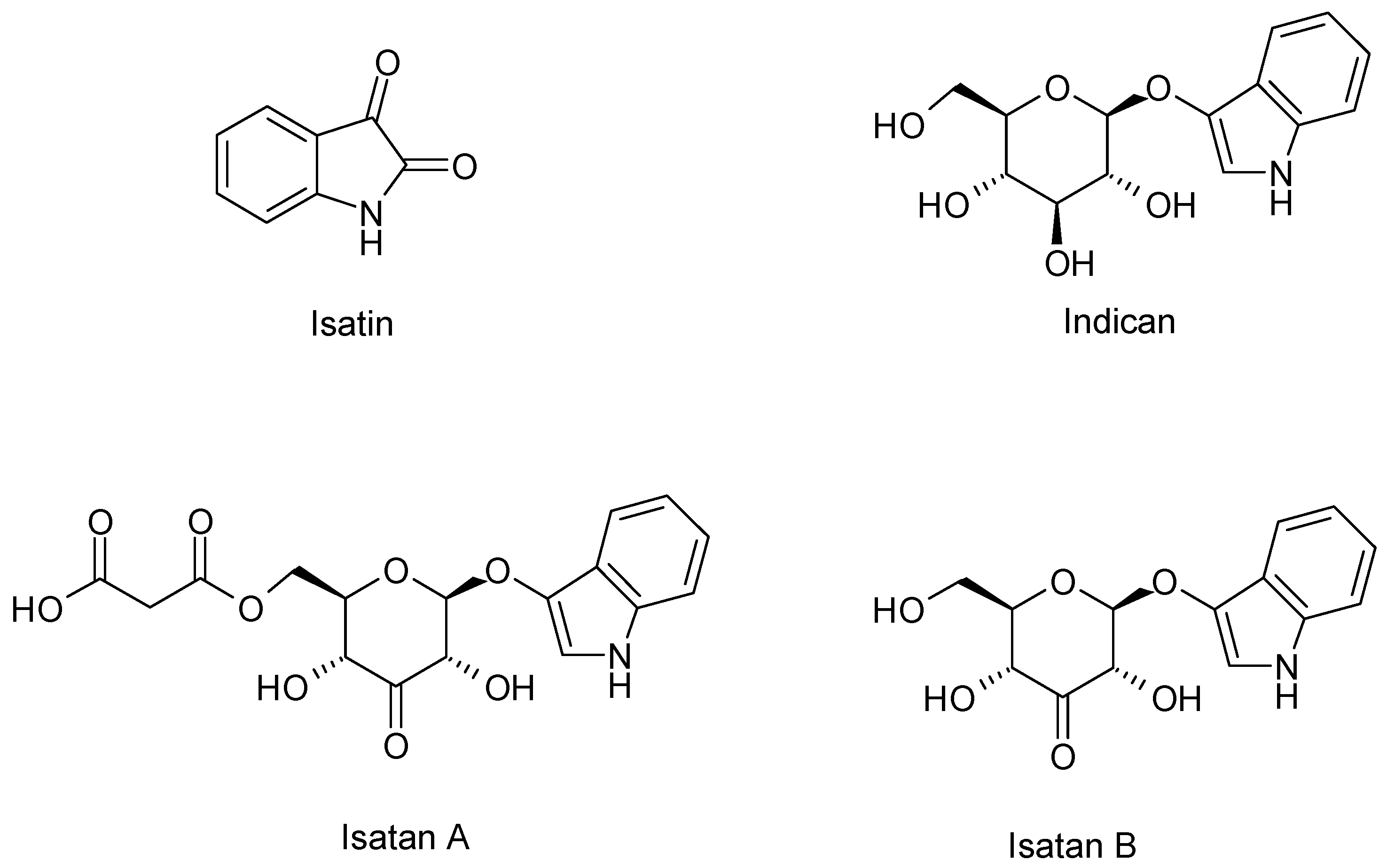
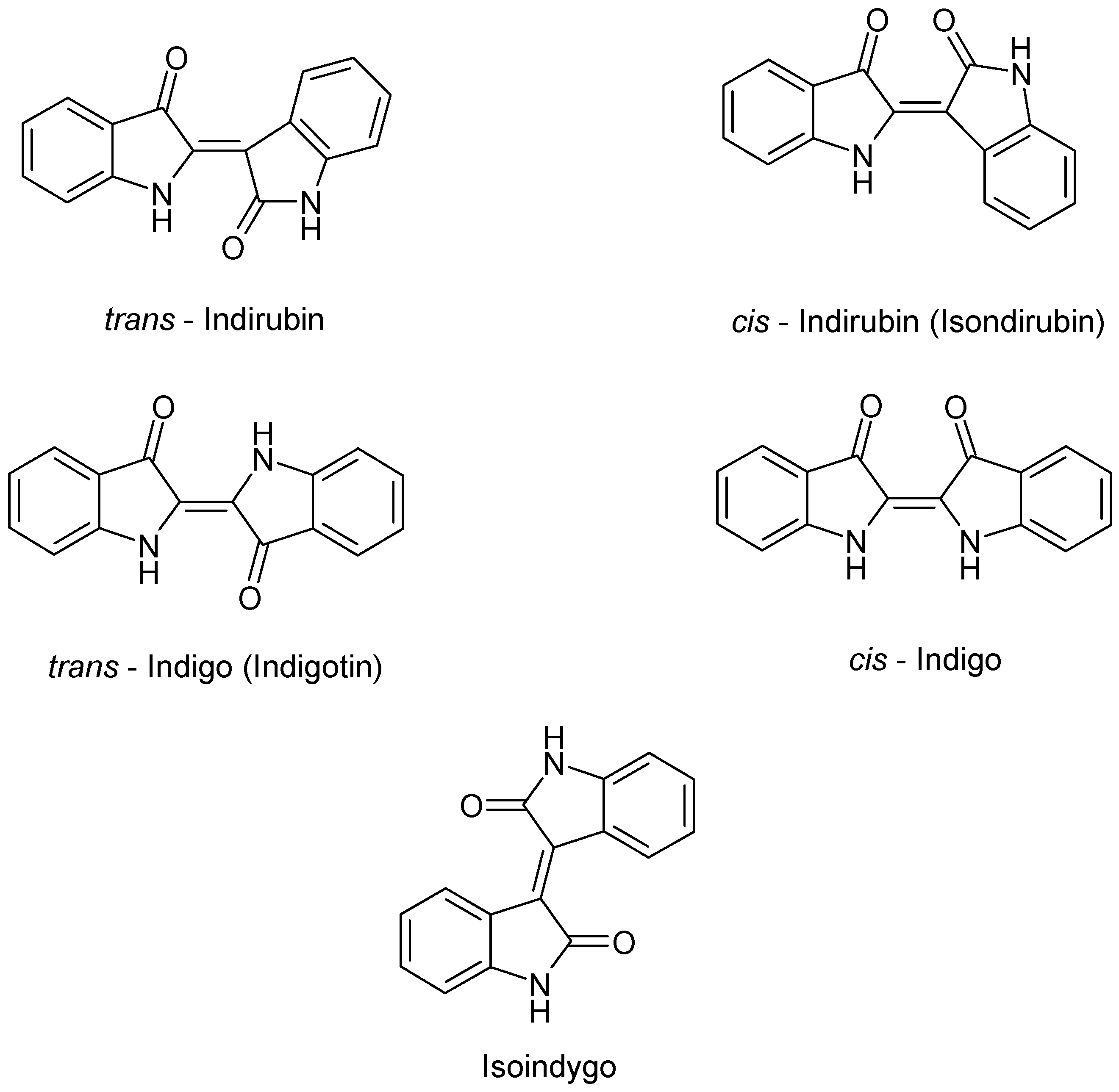
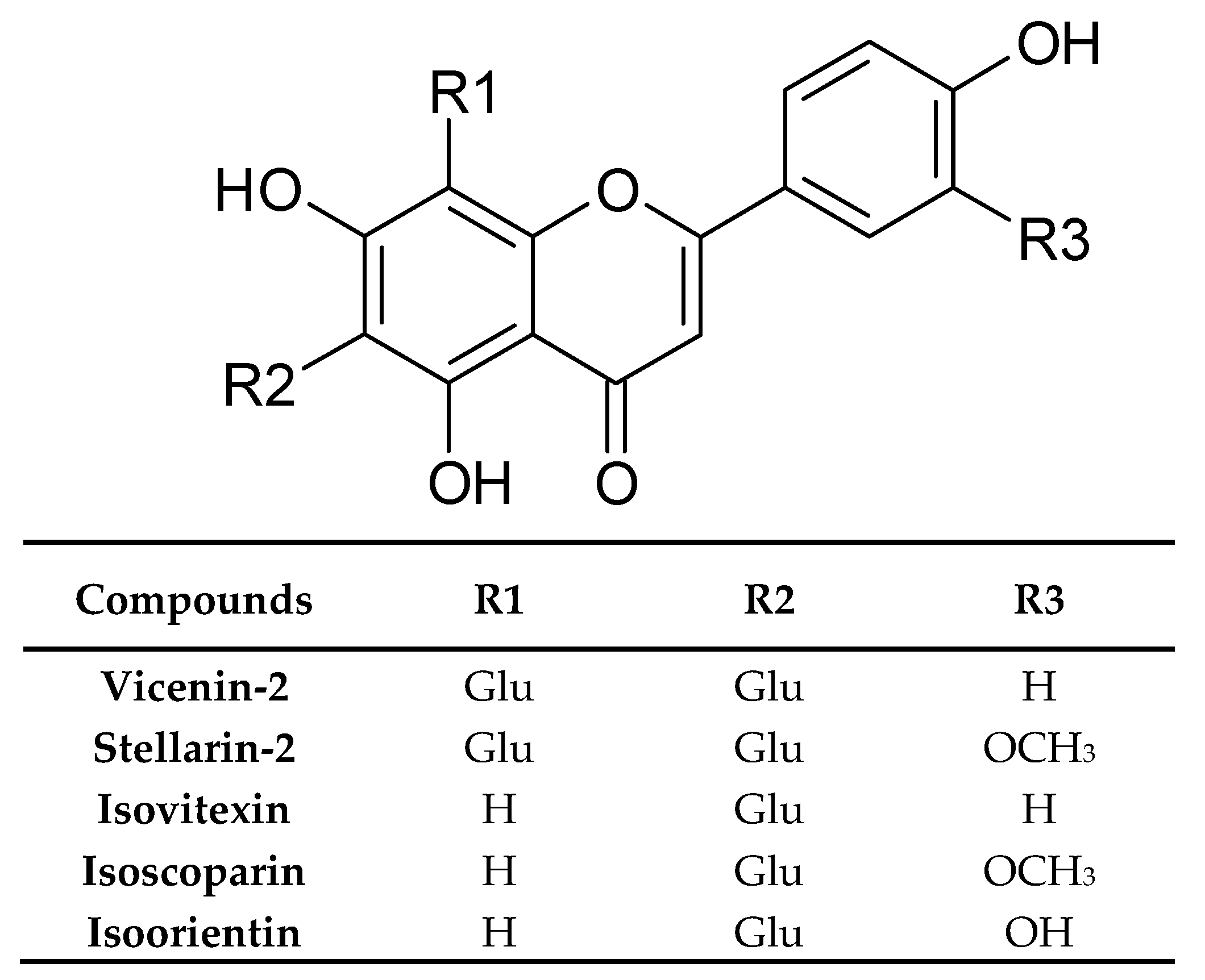

| Chemical Class | Compound | Site of Collection | Plant Part/Extract | Ref. |
|---|---|---|---|---|
| Alkaloids | Tryptanthrin | Japan | Dried rosetta leaves Chloroform | [43] |
| Isatin | France | Fresh Leaves Acetone/acetic acid 1% v/v | [44] | |
| Isatan A – B – C | ||||
| Isoindigo | ||||
| Indoxyl | ||||
| Indicant | ||||
| cis/trans Indirubin | ||||
| cis/trans Indigo | ||||
| (E)-3-(3’,5’-Dimethoxy-4’-hydroxy-benzylidene)-2-Indolinone | Germany | Dried rosette leaves Dichloromethane | [33] | |
| 5-Hydroxyoxindole | ||||
| 3-(2’-Carboxyphenyl)quinazolin-4-one | ||||
| Bisindigotin | ||||
| Deoxyvasicinone | ||||
| N-formyl anthranilic acid | Germany | Dried rosette leaves Methanol | [33] | |
| Acetylindican-carboxyl acid | France | Frozen and lyophilized rosette leaves Methanol | [16] | |
| 6-Hydroxyindolone-3-carboxylic acid 6-O-glucoside | France | Frozen and lyophilized/dried rosette leaves Methanol and Dichloromethane | [45] | |
| 6-Hydroxyindolone-3-carboxylic acid glucose ester | ||||
| Acetylindican | ||||
| Malonylindican | ||||
| Dioxindole glucoside | ||||
| Dyhydroascorbigen | ||||
| Flavonoids and their conjugates | Vicenin-2 | Germany | Dried rosette leaves, Methanol | [33] |
| Stellarin-2 | ||||
| Isoorientin | ||||
| Isovitexin | ||||
| Isoscoparin | ||||
| Isoorientin-3”-O-glucoside | ||||
| Isovitexin-3”-O-glucoside | ||||
| Isoscoparin-3”-O-glucoside | ||||
| Isoscoparine | Germany | Dried rosette leaves Dichloromethane | [33] | |
| Luteolin-6-C-glucoside-7-O-glucoside | France | Frozen and lyophilized rosette leaves Methanol | [16] | |
| Vicenin-2 | ||||
| Stellarin-2 | ||||
| Isovitexin | ||||
| Isovitexin-3”-O-glucoside | ||||
| Isovitexin-3”-O-glucoside-7-O-glucoside | ||||
| Isoorientin | ||||
| Isoorientin-3”-O-glucoside | ||||
| Iscoscoparin | ||||
| Isoscoparin-3”-O-glucoside-7-O-glucoside | ||||
| Iscoscoparin-3”-O-glucoside | ||||
| 4’-O-Feruloyl iscoscoparin-3”-O-glucoside-7-O-glucoside | ||||
| 2”-O-Feruloyl isoscoparin-3”-O-glucoside-7-O-glucoside | ||||
| Isoscoparin-3”-O-glucoside-7-O-feruloylglucoside | ||||
| Isoscoparin-3”-O-p-coumaroylglucoside | ||||
| Isoscoparin-3”-O-sinapoylglucoside | ||||
| Isoscoparin-3”-O-feruloylglucoside | ||||
| Chrysoeriol-7-O-glucoside | ||||
| Luteolin glucuronide | Italy | Lyophilized cauline leaves 70% Methanol | [46] | |
| Rutin | ||||
| Vicenin-2 | ||||
| Bluddleoside | ||||
| Stellarin-2 | ||||
| Flavone-di-glucoside | ||||
| Apigenin-di-glucoside | ||||
| Isovitexin | ||||
| Quercetin | ||||
| Isoscoparin | ||||
| Isoscoparin-di-glucoside | ||||
| Kaempferol | ||||
| Apigenin-glucoside | ||||
| Luteolin- glucuronide | Italy | Lyophilized rosette leaves 70% Methanol | [47] | |
| Vicenin-2 | ||||
| Stellarin-2 | ||||
| Flavone-di-glucoside | ||||
| Apigenin-glucoside | ||||
| Apigenin-di-glucoside | ||||
| Isovitexin | ||||
| Quercetin | ||||
| Isoscoparin | ||||
| Luteolin-glucuronide | Italy | Lyophilized cauline leaves 70% Methanol | [47] | |
| Flavone-di-glucoside | ||||
| Vicenin-2 | ||||
| Buddleoside | ||||
| Stellarin-2 | ||||
| Apigenin-glucoside | ||||
| Apigenin-di-glucoside | ||||
| Isovitexin | ||||
| Quercetin | ||||
| Isoscoparin | ||||
| Luteolin-glucuronide | Italy | Lyophilized flowers 70% Methanol | [47] | |
| Vicenin-2 | ||||
| Stellarin-2 | ||||
| Apigenin-di-glucoside | ||||
| Isovitexin | ||||
| Quercetin | ||||
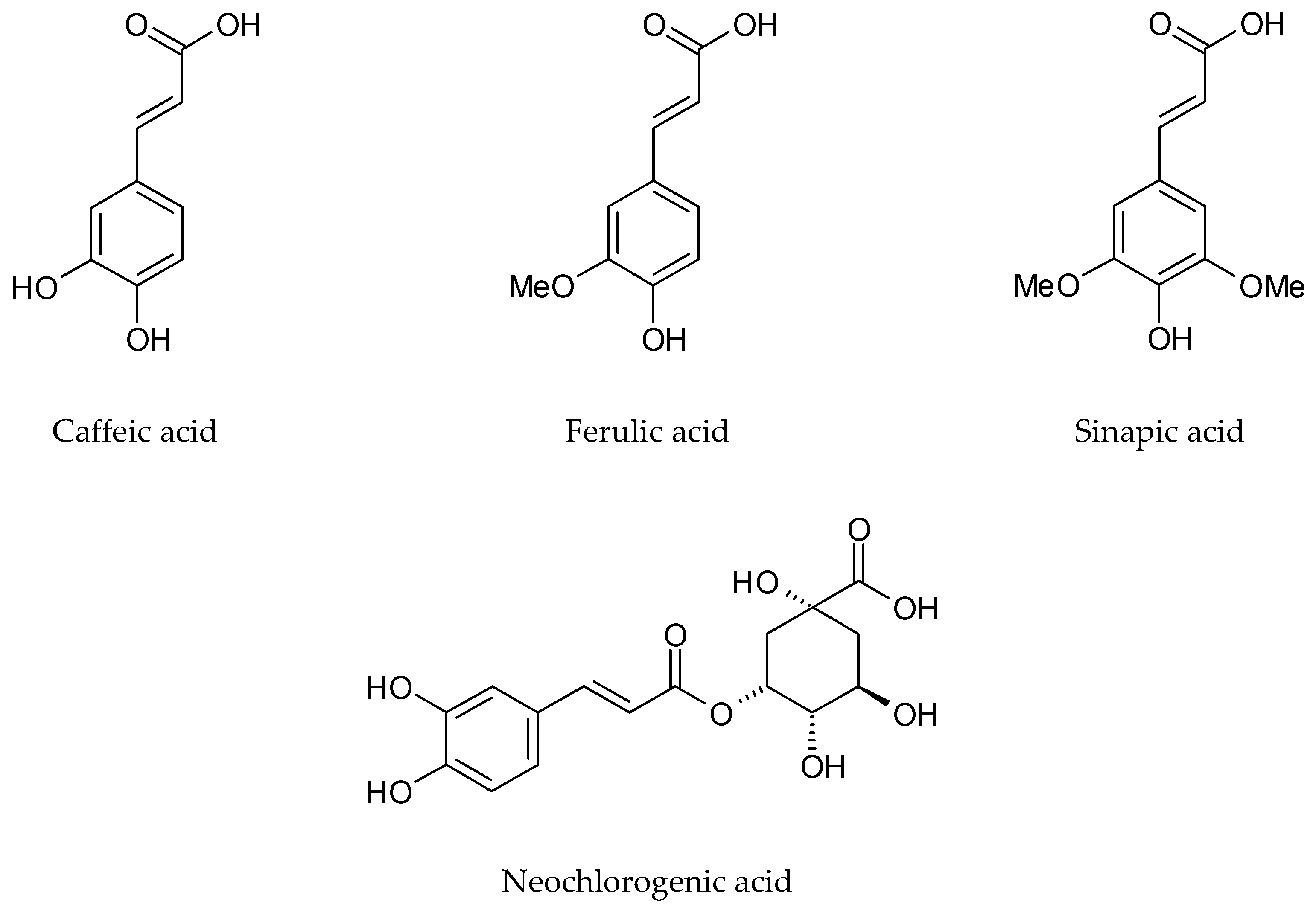
| Phenolic acids and their conjugates | p-Hydroxybenzoic acid | Dried leaves, Methanol | [48] | |
| o-Methoxybenzoic acid | ||||
| p-Methoxybenzoic acid | ||||
| Dihydrocaffeic acid | ||||
| 4-Hydroxy-3-methoxyphenylpropanoic acid | ||||
| Sinapic acid | Germany | Dried rosette leaves, Dichloromethane | [33] | |
| Ferulic acid | ||||
| Neochlorogenic acid | Italy | Lyophilized cauline leaves 70% Methanol | [46] | |
| Chlorogenic acid | ||||
| Caffeic acid | ||||
| Coumarylquinic acid | ||||
| Sinapic acid | ||||
| Ferulic acid | ||||
| p-Coumaric acid | ||||
| Protocatechuic acid hexoside | France | Frozen and lyophilized rosette leaves, Methanol | [16] | |
| Protocatechoyl glucose | ||||
| p-Coumaroyl glucaric acid | ||||
| p-Coumaric acid hexoside | ||||
| p-Coumaroyl dihexoside | ||||
| p-Coumaroyl hexoside | ||||
| p-Coumaroyl sinapoyl glucaric acid | ||||
| di-p-Coumaroyl glucaric acid | ||||
| Feruloyl dihexoside | ||||
| Feruloyl glucaric acid | ||||
| Diferuloyl glucaric acid | ||||
| Feruloyl glicerate | ||||
| Feruloyl sinapoyl glucaric acid | ||||
| Feruloyl p-coumaroyl glucaric acid | ||||
| Sinapoyl hexoside | ||||
| Sinapoyl gentiobioside | ||||
| Sinapoyl glucaric acid | ||||
| Disinapoyl glucaric acid | ||||
| Sinapoyl malate | ||||
| Disinapoyl methoxyglucaric acid | ||||
| Disinapoyl hexoside | ||||
| Guaiacyl(8-O-4)feruloyl sinapoyl glucarid acid | ||||
| Neochlorogenic acid | Italy | Lyophilized rosette leaves 70% Methanol | [47] | |
| Caffeic acid | ||||
| Sinapic acid | ||||
| Ferulic acid | ||||
| Neochlorogenic acid | Italy | Lyophilized cauline leaves 70% Methanol | [47] | |
| Caffeic acid | ||||
| Sinapic acid | ||||
| Ferulic acid | ||||
| Caffeic acid | Italy | Lyophilized flowers 70% Methanol | [47] | |
| Ferulic acid | ||||
| Monolignols and oligolignols | Syringe | France | Frozen and lyophilized rosette leaves Methanol | [16] |
| Coniferin | ||||
| Pinoresinol dihexoside | ||||
| Syringaresinol hexoside | ||||
| Isodihydrodehydrodiconiferyl alcohol hexoside | ||||
| Isodihydrodehydrodiconiferyl alcohol dihexoside | ||||
| 5-Hydroxy-coniferyl alcohols hexoside | ||||
| Syringyl(8-5)guaiacyl hexoside | ||||
| Guaiacyl(8-5)guaiacyl hexoside | ||||
| Guaiacyl(erythro8-O-4)guaiacyl hexoside | ||||
| Guaiacyl(threo8-O-4)guaiacyl hexoside | ||||
| Guaiacyl(threo8-O-4)dihydroguaiacyl hexoside | ||||
| Guaiacyl(threo 8-O-4)syringyl(8-5)guaiacyl hexoside | ||||
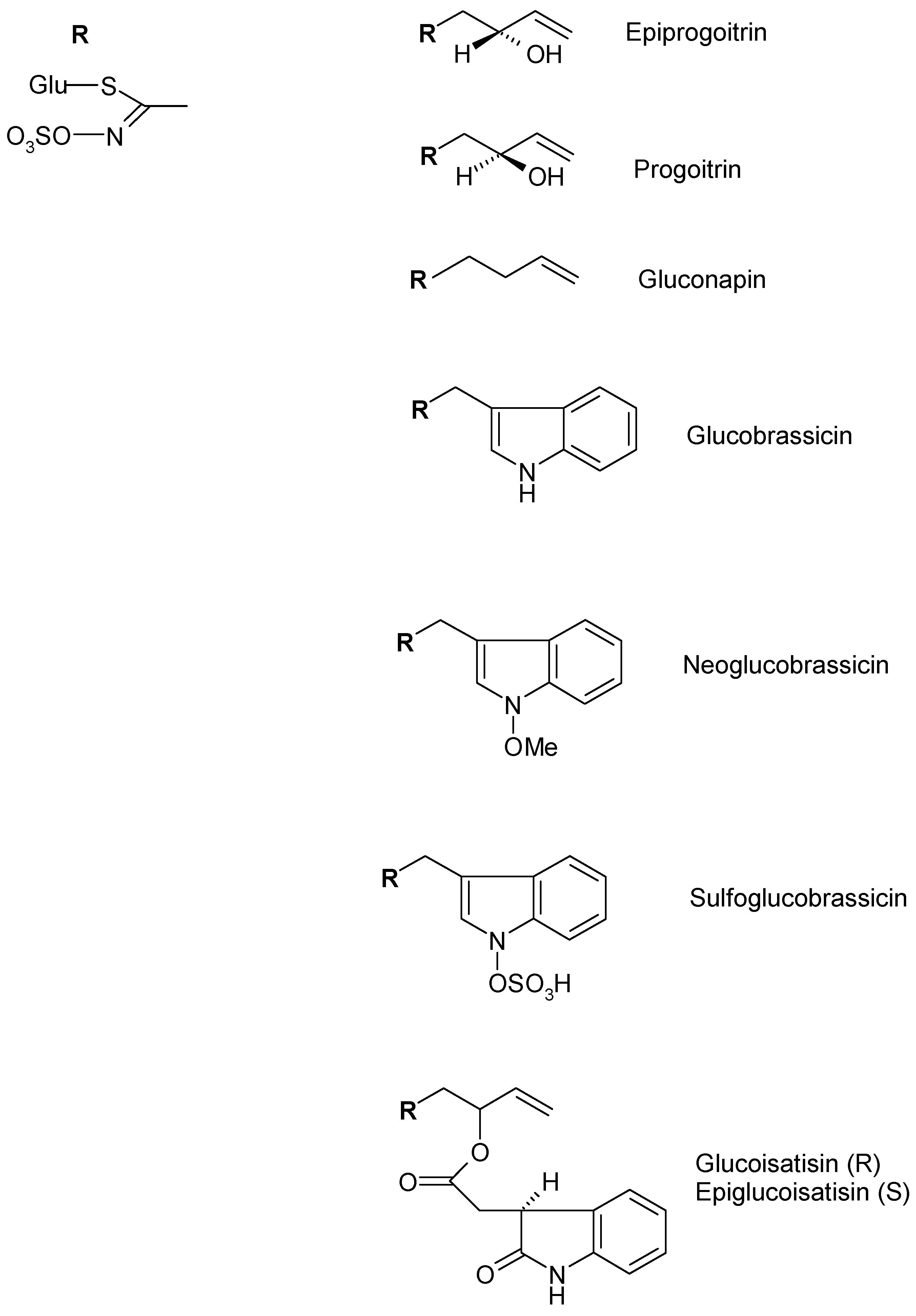
| Alifatic Glucosinolate | Epiprogoitrin | Germany | Frozen and lyophilized rosette leaves 70% Methanol | [49] |
| Progoitrin | ||||
| Gluconapin | ||||
| Indolic Glucosinolates | Glucobrassicin | Germany | Frozen and lyophilized rosette leaves 70% Methanol | [49] |
| Neoglucobrassicin | ||||
| Sulfoglucobrassicn | ||||
| 4-Hydroxyglucobrassicin | ||||
| Glucotropaeolin | ||||
| Glucoisatisin/epiglucoisatisin | Germany | Seed Aqueous | [50] | |
| Gluconapoleiferin | France | Frozen and lyophilized rosette leaves Methanol | [16] | |
| Glucoibericin | Italy | Lyophilized rosette and cauline leaves, flowers 70% Methanol | [47] | |
| 4-Methoxyglucobrassicin | Italy | Flower 70% Methanol | [47] | |
| Carotenoids | (all-E)-β-Carotene | Germany | Dried rosette leaves Dichloromethane | [33] |
| (13Z)-or (13’Z)- Lutein mixture | Germany | Dried rosette leaves Hexane/Acetone (1:1) | [33] | |
| (all-E)-Lutein | ||||
| (9Z)-Lutein | ||||
| (9’Z)-Lutein | ||||
| (15Z)-β-Carotene | ||||
| (9Z)-β-Carotene | ||||
| (Z)-Neochrome | ||||
| (15Z)-Violaxantin | ||||
| (all-E)-Neochrome | ||||
| (di-Z)-Violaxantin | ||||
| Porphyrins | 10-Hydroxy phaeophorbide | Germany | Dried rosette leaves Dichloromethane | [33] |
| Phaephorbide a | ||||
| Phaephorbide a’ | ||||
| Pyrophaeophorbide a | ||||
| Isothiocyanates and thiocyanates | 2-Hydroxy-3-butenyl isothiocyanate | Italy | Fresh leaves HS-SPME | [19] |
| 3-Butenyl isothiocyanate | ||||
| Allyl isothiocyanate | ||||
| Pentyl isothiocyanate | ||||
| 3-Methylthiopropyl isothiocyanate | ||||
| Hexyl isothiocyanate | ||||
| Benzyl isothiocyanate | ||||
| Methyl thiocyanate | ||||
| 3-Butenyl isothiocyanate | Italy | Dried roots HS-SPME | [51] | |
| Ciclopentyl isothiocyanate | ||||
| Methyl thiocyanate | ||||
| Aldehydes | 3-Methylbutanal | Italy | Fresh leaves HS-SPME | [19] |
| But-2-enal | ||||
| Hexenal | ||||
| trans-Pent-2-enal | ||||
| trans-Hex-2-enal | ||||
| Nonanal | ||||
| trans, trans-Hexa-2,4-dienal | ||||
| trans-Oct-2-enal | ||||
| trans, trans-Hepta-2,4-dienal | ||||
| Benzenecarbaldehyde | ||||
| cis, trans-Nona-2,6-dienal | ||||
| 4-Ethylbenzenecarbaldehyde | ||||
| Tetradecanal | ||||
| Furfural | Italy | Dried roots HS-SPME | [51] | |
| Benzaldehyde | ||||
| Sulfurated compounds | 2-Ethylthiophene | Italy | Fresh leaves HS-SPME | [19] |
| Carbonyl sulphide | ||||
| Carbon disulphide | ||||
| Cyclopenthanethiol | ||||
| Thiophene | ||||
| Alcohols | Tetradecan-1-ol | Italy | Fresh leaves HS-SPME | [19] |
| 2-Cyclopentylethanol | ||||
| Butan-1-ol | ||||
| cis-Pent-2-en-1-ol | ||||
| trans-Hex-3-en-1-ol | ||||
| 2-Butyloctan-1-ol | ||||
| Pentadecan-1-ol | ||||
| Heptadecan-1-ol | ||||
| 2-Methylexadecan-1-ol | ||||
| Nonadecan-1-ol | ||||
| Hexanol | Italy | Dried roots HS-SPME | [51] | |
| 1-Octen-3-ol | ||||
| Heptanol | ||||
| Furfuryl alcohol | ||||
| 2-Penylethyl alcohol | ||||
| Phenol | ||||
| Terpenes and Sesquiterpenes | Limonene | Italy | Fresh leaves HS-SPME | [19] |
| Sabinene | ||||
| δ-3-Carene | ||||
| Eucalyptol | ||||
| γ-Terpinene | ||||
| p-Cymene | ||||
| Terpinolene | ||||
| Myrtenal | ||||
| p-Cymenene | ||||
| β-Cyclocitral | ||||
| Valencene | ||||
| δ-Cadinene | ||||
| Geranyl acetone | ||||
| 6-Methyl-5-hepten-2-one | Italy | Dried roots HS-SPME | [51] | |
| Camphor | ||||
| Geranyl acetone | ||||
| Guaiacol | ||||
| Acids | Acetic acid | Italy | Fresh leaves HS-SPME | [19] |
| Octanoid acid | ||||
| Butyric | Italy | Dried roots HS-SPME | [51] | |
| Octanoic acid | ||||
| Esters | Methyl-2-hydroxybenzoate | Italy | Fresh leaves HS-SPME | [19] |
| Butyl tetradecanoate | ||||
| Methyl butyrate | Italy | Dried roots HS-SPME | [51] | |
| Ethers | 1-Methoxy-4-prop-2-enylbenzene | Italy | Fresh leaves HS-SPME | [19] |
| Dyphenil ether | ||||
| Furans | 2-Ethylfuran | Italy | Fresh leaves Leaf HS-SPME | [19] |
| Hydrocarbons | trans-1,5-Heptadiene | Italy | Fresh leaves HS-SPME | [19] |
| Heptadecene | ||||
| Nonadecene | ||||
| Eicosene | ||||
| Heneicosene | ||||
| Tetracosene | ||||
| Decane | Italy | Fresh leaves HS-SPME | [51] | |
| Tridecane | ||||
| Pentadecane | ||||
| Heptadecane | ||||
| Ketones | 1-Penten-3-one | Italy | Fresh leaves HS-SPME | [19] |
| Octan-2,5-dione | ||||
| trans-β-Ionone | ||||
| 2-Heptanone | Italy | Dried roots HS-SPME | [51] | |
| 2-Nonanone | ||||
| (E,E)-3,5-Octadien-2-one | ||||
| 1-Phenyl-1-propanone | ||||
| Nitriles | 4-Pentenenitrile | Italy | Fresh leaves HS-SPME | [19] |
| 3-Hydroxy-4-pentenenitrile | ||||
| Heptanenitrile | ||||
| Octanenitrile | ||||
| 2-Phenylacetonitrile | ||||
| 2-Pentenenitrile | Italy | Dried roots HS-SPME | [51] | |
| 4-Pentenenitrile | ||||
| 2,4-Pentadiene nitrile | ||||
| Fatty acids | Palmitic acid | Turkey | Seed Chloroform-Methanol (2:1 v/v) | [52] |
| Linoleic acid | ||||
| Oleic acid | ||||
| Linolenic acid | ||||
| Stearic acid | ||||
| 11-Eicosenoic acid | ||||
| Arachidic acid | ||||
| Erucic acid | ||||
| Behenic acid | ||||
| 15-Tetracosanoic acid | ||||
| Tetracosanoic acid | ||||
| Ursolic acid | Germany | Dried rosette leaves Dichloromethane | [33] | |
| Palmitoleic acid | ||||
| α-Lysolecithin | ||||
| (7Z, 10Z, 13Z)-Hexadecatrienoic acid | ||||
| Corchorifatty acid B | ||||
| 9-Hydroxy-(10E, 12E, 14E)-octadecatrienoic acid | ||||
| 9-oxo-(10E, 12Z, 15Z)-Octadecatrienoic acid | ||||
| Polysaccharides | Root | [34] |
| Biological Activity | Experimental Model | Site of Collection | Plant Part/ Extract or Compound | Mode of Administration and Doses | Ref. |
|---|---|---|---|---|---|
| Anti-inflammatory Activity | Chronic P. aeruginosa lung infection mimicking cystic fibrosis in rat | Pharmac. factory of Sichuan Yaan | Aqueous | s.c. 400 mg/kg | [61] |
| Micro-dialysis assay in the ex vivo pig foreleg skin | Germany | Dried rosette leaf Supercritical fluid Tryptanthrin | topical 0.5 g/10 mL + Try 0.115–1.84 mg/mL | [62] | |
| SLS-induced irritant contact dermatitis and UVB-induced erythema in healthy human volunteers | Germany | Dried rosette leaf Supercritical fluid Tryptanthrin | topical 50 µL 50 µL | [63] | |
| Carrageenan-induced paw oedema in mouse | Germany | Dried rosette leaf Dichloromethane Supercritical fluid Tryptanthrin | per os 75–125 mg/kg 125–175 mg/kg 70–40 mg/kg | [18] | |
| TPA-induced ear oedema in mouse | Germany | Dried rosette leaf Dichloromethane Supercritical fluid Tryptanthrin | per os 125 mg/kg 100 mg/kg 70 mg/kg | [18] | |
| Dried rosette leaf Dichloromethane Supercritical fluid Tryptanthrin | topical 0.5 mg/ear 0.5 mg/ear 0.25 mg/ear | ||||
| TPA-induced ear oedema in mouse (sub-chronic inflammation) | Germany | Dried rosette leaf Dichloromethane | per os 150 mg/kg topical 1 mg/ear | [18] | |
| Delayed-type hypersensitivity induced by DNFB in mouse | Germany | Dried rosette leaf Dichloromethane | per os 150 mg/kg topical 1 mg/ear | [18] | |
| Adjuvant-induced arthritis in rats | Germany | Dried rosette leaf Dichloromethane | per os 150–250 mg/kg | [64] | |
| TNF-α and IL-1β production in RAW 264.7 macrophages | Germany | Dried rosette leaf Dichloromethane | 25–100 µg/mL | [64] | |
| OVA-induced allergic airway disease (asthma) in mouse | Dried leaf Supercritical fluid | intranasal 10–100 μg/mouse | [65] | ||
| Analgesic Activity | Writhing test in mice | Germany | Dried rosette leaf Dichloromethane Tryptanthrin | per os 150–200 mg/kg 40 mg/kg | [18] |
| Anti-tumour Activity | Clinical trials (patients with chronic myelocytic and chronic granulocytic leukaemia) | Indirubin | per os 150–450 mg/day | [66] | |
| Human gastric cancer cells (HGC) Lung cancer cells (HLC) Promyelocytic leukaemia cells (HL-60) | Tryptanthrin | IC50 = 1.5 µg/mL 2.2 µg/mL 4.2 µg/mL | [67] | ||
| Human monocytic (U-937) and promyelocytic (HL-60) leukaemia cells | Tryptanthrin | 0.78–25 μg/mL IC50 = 3.1–6.3 μg/mL | [68] | ||
| Azoxymethane-induced intestinal tumor in F344 rats | Tryptanthrin | per os 50 mg/kg | [69] | ||
| Mammary carcinoma cell line (MCF-7) and Large cell lung tumour xenograft cell line (LXFL529L) | Indirubin | IC50 = 4.0 ± 2.0 μM 9.9 ± 0.1 μM | [70] | ||
| MCF-7 cells and Doxorubicin-resistant breast cancer (MCF-7/adr) cells | Tryptanthrin | 10-6 M | [71,72] | ||
| Myelomonocytic leukaemia induced in BALB/c mice by WEHI-3B JCS cells | Tryptanthrin | i.p. 0.04–0.16 mg/kg/day | [73] | ||
| Murine myeloid leukaemia (WEHI-3B JCS) cells | Tryptanthrin | 0–5 µM IC50 = 1.5 µM | [73] | ||
| Human chronic myeloid leukaemia K562 cells | Tryptanthrin | 0.39–25 μg/mL IC50 = 8.8 μg/mL | [74] | ||
| Xenograft human prostate tumour in BALB/c nude mouse model | Indirubin | Intralesionally injected 10 mg/kg/day | [75] | ||
| Human umbilical vein endothelial cell (HUVEC) and Human prostate cancer cells (PC-3) | Indirubin | 0–100 μM | [75] | ||
| N-myc amplified human neuroblastoma LA-N-1 cells | Tryptanthrin | 0–30 μM IC50 = 15.8 ± 1.41 μM | [76] | ||
| Matrigel plug assay in BALB/c mice | Tryptanthrin | 0–20 μM | [77] | ||
| Human microvascular endothelial HMEC-1 cells | Tryptanthrin | 0–20 μM | [77] | ||
| Non-small cell lung cancer NCI-H460, human glioblastoma SF-268, and human breast cancer MCF-7 cells | Tryptanthrin | IC50 = 8.5 ± 0.8 μM 22.6 ± 1.1 μM 9.4 ± 0.3 μM | [78] | ||
| Human anaplastic thyroid cancer cell lines CAL-62 and 8505C cells | Italy | Frozen and lyophilized cauline leaf Phenolic-rich fraction | 0.01–0.1 mg/mL | [46] | |
| Human anaplastic thyroid cancer cell lines CAL-62, 8505C and C-643 | Italy | Frozen and lyophilized Rosette leaf Cauline leaf Flower 70% Methanol | 0.1–1 mg/mL | [47] | |
| DMBA/PMA-induced skin carcinogenesis model in Swiss albino mice | Tryptanthrin | topical 0.5–1 mg | [79] | ||
| Antimicrobial activity | Agar dilution test for bacteria, yeasts, and dermatophytes | Tryptanthrin | 3.1–400 µg/mL | [80] | |
| Paper disc method for phytopathogenic microorganisms | Tryptanthrin | 1–500 µg/mL | [80] | ||
| Agar diffusion test for 23 micro-organisms. Sensitive strains: Bacillus mycoides, B. subtilis, tetracycline resistant Micrococcus luteus, and Saccharomyces cerevisiae | Germany | Fresh whole plant Water/Ethanol | ‒ | [81] | |
| Agar dilution test for synergistic activity with antibiotics against Methicillin-resistant (MRSA) and standard Staphylococcus aureus | China | Dried leaf 75% Ethanol | 500 µg/mL | [82] | |
| Agar diffusion test for 15 micro-organisms. Sensitive strains: Staphylococcus epidermis, S. aureus, and MRSA | Tryptanthrin | 12.5–100 µg/mL | [83] | ||
| Microdilution broth method for MRSA | Tryptanthrin | 15–1000 µg/mL | [84] | ||
| Micro-titter plate method for 14 micro-organisms. Most sensitive strains: B. subtilis, M. luteus and S. aureus | Not indicated | Branches, flowers, leaves and roots Extracted with 14 different solvents | 3.7–100 µg/mL for bacterial strains | [85] | |
| Antiviral activity | Production of RANTES by Human bronchial epithelial cells H292 infected with influenza virus A/NWS/33 and B/Lee/40 – ELISA | Indirubin | 100–200 μM | [86] | |
| Human influenza viruses (H1N1 and H3N2) and avian influenza viruses (H6N2 and H9N2) – MTT assay | Polysaccharides | IC50 = from 4.35 ± 0.07 to 28.20 ± 0.49 mg/mL | [87] | ||
| Vero cells infected with Herpes simplex virus type II (HSV-2) - Cytopathic effect and MTT assay | Polysaccharides | 25–800 mg/L | [88] | ||
| Antioxidant activity | 1,1-diphenyl-2-picrylhydrazyl (DPPH) test | Leaf Hydroalcoholic | SC50 = 103.9 μg/mL | [89] | |
| 2,2-Azino-bis-3-ethylbenzothiazoline—6-sulfonic Acid (ABTS) assay | Polysaccharides | Scavenging effect at 0.3 mg/mL= 64.3% | [34] | ||
| 1,1-diphenyl-2-picrylhydrazyl (DPPH) test | Dried I. tinctoria (plant part not specified) 95% Ethanol | IC50 = 1583.45 ± 23.69 mg/mL | [90] | ||
| Trolox Equivalent Antioxidant Capacity (TEAC) | mM Trolox/g = 589 ± 0.51 | [90] | |||
| Reducing power assay | Abs700 = 0.32 ± 0.004 | [90] | |||
| 1,1-diphenyl-2-picrylhydrazyl (DPPH) test | Indigo Indirubin | EC50 = > 0.26 mg/mL > 0.26 mg/mL | [91] | ||
| Superoxide anion radical scavenging activity | Indigo Indirubin | EC50 = 0.61 mg/mL 0.74 mg/mL | [91] | ||
| Hydroxyl radical scavenging activity | Indigo Indirubin | Not active Not active | [91] | ||
| Reducing power | Indigo Indirubin | Not active Not active | [91] | ||
| 1,1-diphenyl-2-picrylhydrazyl (DPPH) test | Italy | Frozen and lyophilized cauline leaf Phenolic-rich fraction | IC50 = 0.6657 ± 0.0024 mg/mL | [46] | |
| Reducing power | Italy | Frozen and lyophilized cauline leaf Phenolic-rich fraction | ASE/mL = 3.87 ± 0.71 | [46] | |
| Ferrous ions (Fe2+) chelating activities assay | Italy | Frozen and lyophilized cauline leaf Phenolic-rich fraction | Not active | [46] | |
| Protective effect on Escherichia coli under H2O2 stress | Italy | Frozen and lyophilized cauline leaf Phenolic-rich fraction | Not active | [46] | |
| 1,1-diphenyl-2-picrylhydrazyl (DPPH) test | Italy | Frozen and lyophilized Rosette leaf Cauline leaf Flower 70% Methanol | IC50 = 1.151 ± 0.004 mg/mL 0.581 ± 0.001 mg/mL 0.437 ± 0.003 mg/mL | [47] | |
| Reducing power | Italy | Frozen and lyophilized Rosette leaf Cauline leaf Flower 70% Methanol | ASE/mL = 2.775 ± 0.163 1.546 ± 0.006 2.799 ± 0.042 | [47] | |
| Ferrous ions (Fe2+) chelating activities assay | Italy | Frozen and lyophilized Rosette leaf Cauline leaf Flower 70% Methanol | IC50 = 1.234 ± 0.010 mg/mL 0.564 ± 0.011 mg/mL 0.856 ± 0.002 mg/mL | [47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, J.; Miceli, N.; Taviano, M.F.; Ragusa, S.; Kwiecień, I.; Szopa, A.; Ekiert, H. Isatis tinctoria L. (Woad): A Review of Its Botany, Ethnobotanical Uses, Phytochemistry, Biological Activities, and Biotechnological Studies. Plants 2020, 9, 298. https://doi.org/10.3390/plants9030298
Speranza J, Miceli N, Taviano MF, Ragusa S, Kwiecień I, Szopa A, Ekiert H. Isatis tinctoria L. (Woad): A Review of Its Botany, Ethnobotanical Uses, Phytochemistry, Biological Activities, and Biotechnological Studies. Plants. 2020; 9(3):298. https://doi.org/10.3390/plants9030298
Chicago/Turabian StyleSperanza, Jasmine, Natalizia Miceli, Maria Fernanda Taviano, Salvatore Ragusa, Inga Kwiecień, Agnieszka Szopa, and Halina Ekiert. 2020. "Isatis tinctoria L. (Woad): A Review of Its Botany, Ethnobotanical Uses, Phytochemistry, Biological Activities, and Biotechnological Studies" Plants 9, no. 3: 298. https://doi.org/10.3390/plants9030298
APA StyleSperanza, J., Miceli, N., Taviano, M. F., Ragusa, S., Kwiecień, I., Szopa, A., & Ekiert, H. (2020). Isatis tinctoria L. (Woad): A Review of Its Botany, Ethnobotanical Uses, Phytochemistry, Biological Activities, and Biotechnological Studies. Plants, 9(3), 298. https://doi.org/10.3390/plants9030298








