In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.)
Abstract
1. Introduction
2. Results
2.1. Polyphenolic Composition of the Pistachio Extract
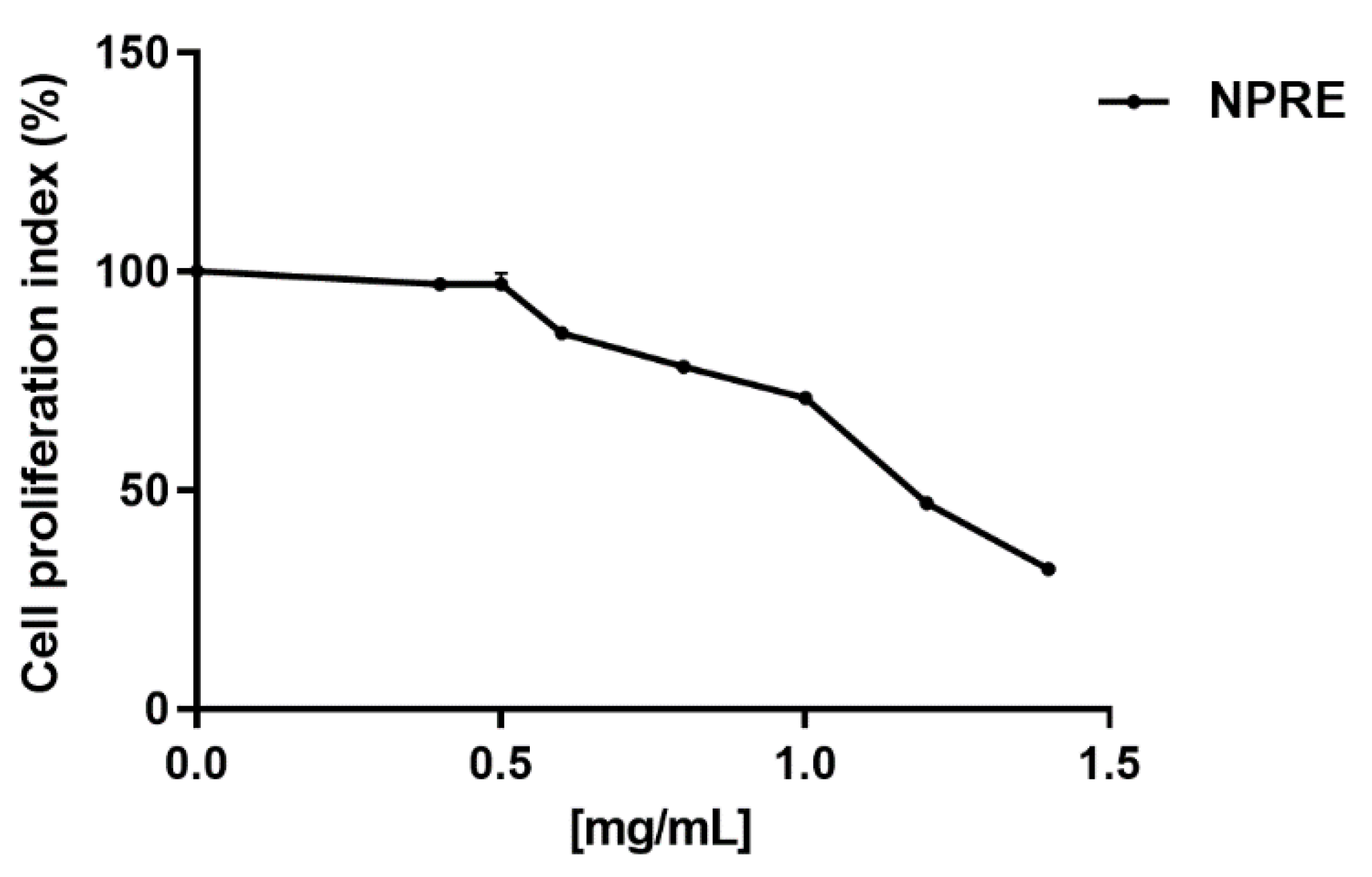
2.2. Cytotoxicity
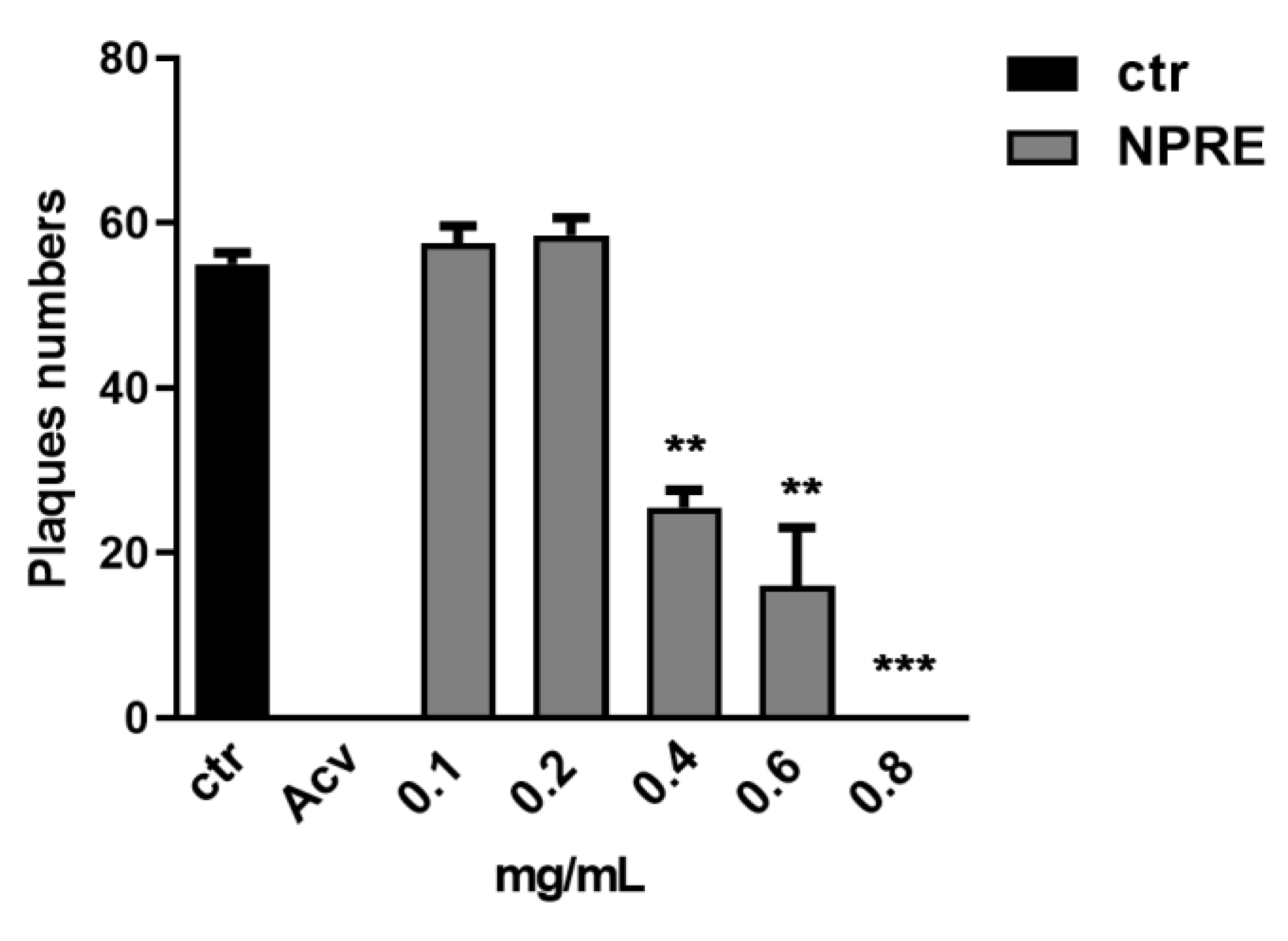
2.3. Plaque Reduction Assay
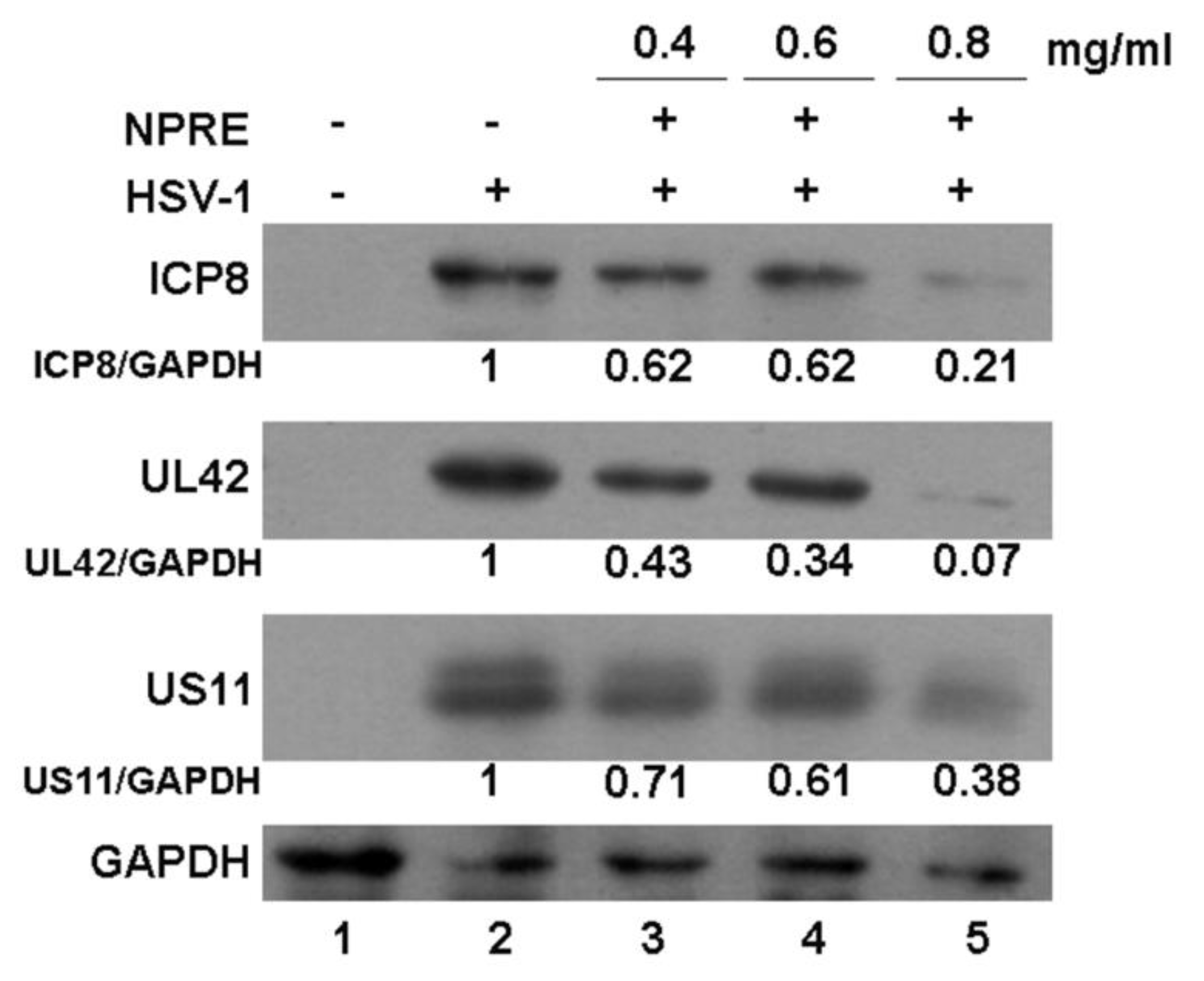
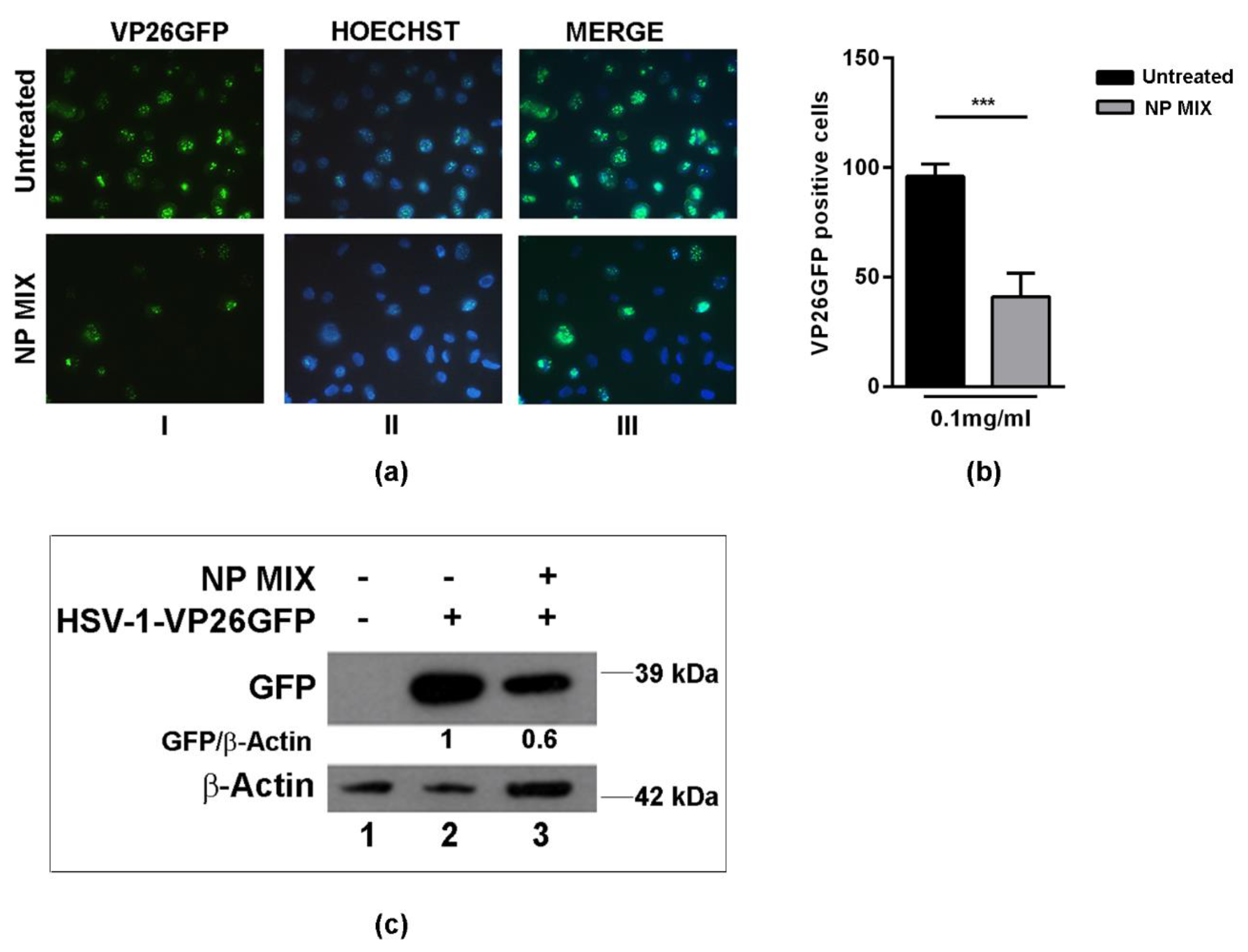
2.4. Analysis of Viral Protein Expression
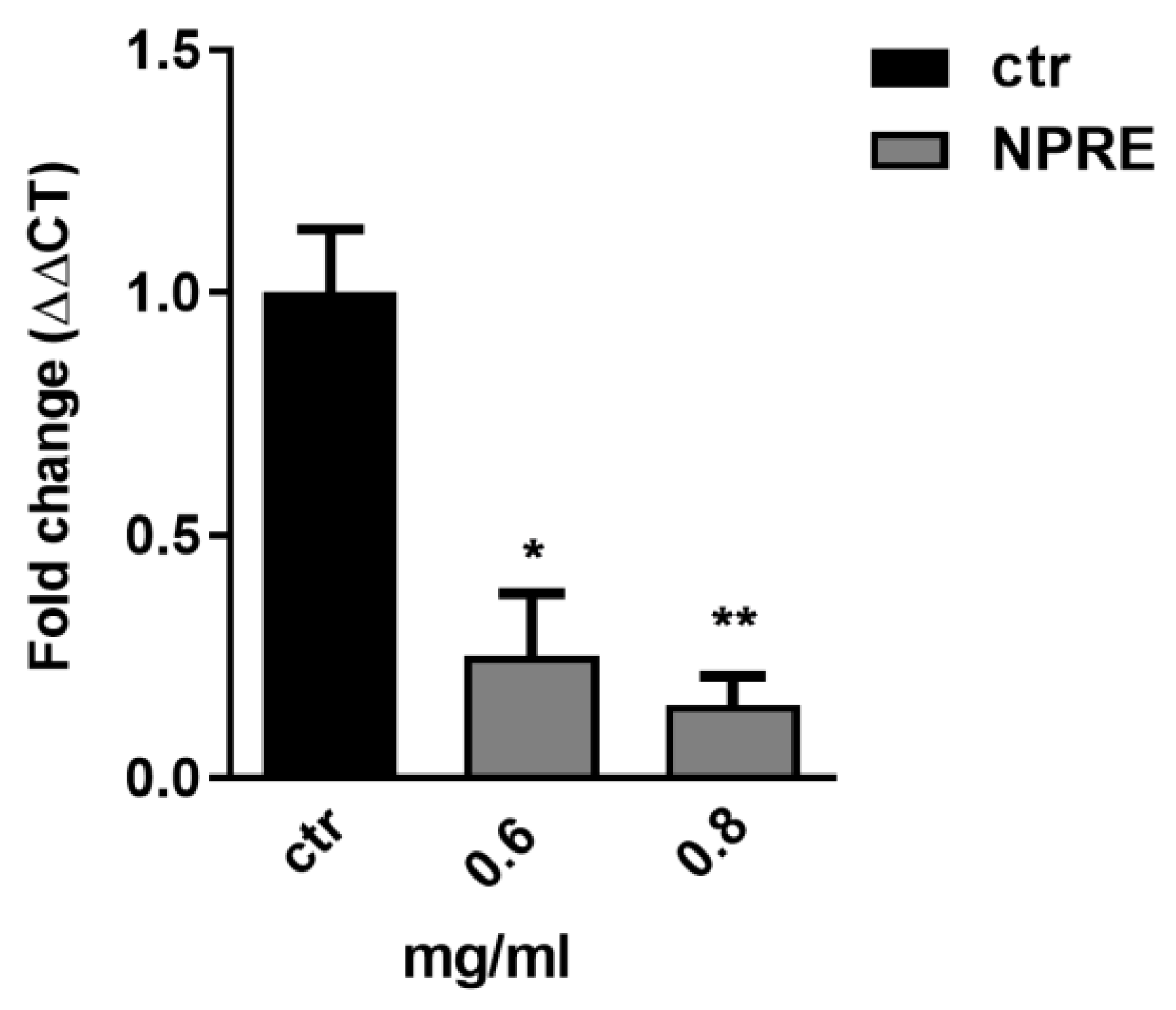
2.5. Quantification of Viral DNA
2.6. Binding Assay Using NPRE and NP MIX
3. Discussion
4. Materials and Methods
4.1. Plant Material, Extraction, and NP MIX Preparation
4.2. Cell Culture and Virus
4.3. Cell Viability to Test the Cytotoxicity of NPRE and NP MIX
4.4. Plaque Reduction Assay
4.5. Binding Assay to Test the Antiviral Activity of NPRE and NP MIX
4.6. Western Blot Analysis
4.7. Antibodies
4.8. DNA Extraction and Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tyler, K.L. Herpes simplex virus infections of the central nervous system: Encephalitis and meningitis, including Mollaret’s. Herpes 2004, 11 (Suppl. 2), 57A–64A. [Google Scholar] [PubMed]
- Whitley, R. New approaches to the therapy of HSV infections. Herpes 2006, 13, 53–55. [Google Scholar] [PubMed]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Pillay, D.; Ratcliffe, D.; Cane, P.A.; Cllinghan, K.E.; Milligan, D.W. Resistance to antiviral drugs in herpes simplex virus infections among allogenic stem cell transplant recipients: Risk factors and prognostic significance. J. Infect. Dis. 2000, 181, 2055–2058. [Google Scholar] [CrossRef]
- Chen, Y.; Scieux, V.; Garrait, V.; Socie, G.; Rocha, V.; Molina, J.M.; Thouvenot, D.; Morfin, F.; Hocqueloux, I.; Garderei, L.; et al. Resistant herpes simplex virus type 1 infection: An emerging concern after allogenetic stem cell transplantation. Clin. Infect. Dis. 2000, 31, 927–935. [Google Scholar] [CrossRef]
- Levin, M.J.; Bacon, T.H.; Leary, J.J. Resistance of herpes simplex virus infections to nucleoside analogues in HIV-infected patients. Clin. Infect Dis. 2004, 39 (Suppl. 5), S248–S257. [Google Scholar] [CrossRef]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 24 (Suppl. 1), S20–S28. [Google Scholar] [CrossRef]
- Son, M.; Lee, M.; Sung, G.H.; Lee, T.; Shin, Y.S.; Cho, H.; Lieberman, P.M.; Kang, H. Bioactive activities of natural products against herpesvirus infection. J. Microbiol. 2013, 51, 545. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Chiang, W.; Chang, M.Y.; Lin, C.C. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 2003, 69, 600–604. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef]
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat. Prod. Commun. 2010, 5, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T.C. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013, 52, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, L.; Huleihel, M.; Zaccai, M.; Ben-Shabat, S. Potent antiviral flavone glycosides from Ficus benjamina leaves. Fitoterapia 2012, 83, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.Y.; Ho, T.Y. Emodin is a novel alkaline nuclease inhibitor that suppresses herpes simplex virus type 1 yields in cell cultures. Br. J. Pharmacol. 2008, 155, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.R.; Luo, J.; Hou, W.; Xiao, H.; Yang, Z.Q. The effect of emodin, an anthraquinone derivative extracted from the roots of Rheum tanguticum, against herpes simplex virus in vitro and in vivo. J. Ethnopharmacol. 2011, 133, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, F.; Moshaverinia, M.; Motamedifar, M.; Alyaseri, M. Assessment of Anti HSV-1 Activity of Aloe Vera Gel Extract: An In Vitro Study. J. Dent. 2016, 17, 49–54. [Google Scholar]
- Bisignano, C.; Filocamo, A.; Faulks, R.M.; Mandalari, G. In vitro antimicrobial activity of pistachio (Pistacia vera L.) polyphenols. FEMS Microbiol. Lett. 2013, 341, 62–67. [Google Scholar] [CrossRef] [PubMed]
- La Camera, E.; Bisignano, C.; Crisafi, G.; Smeriglio, A.; Denaro, M.; Trombetta, D.; Mandalari, G. Biochemical Characterization of Clinical Strains of Staphylococcus spp. and Their Sensitivity to Polyphenols-Rich Extracts from Pistachio (Pistacia vera L.). Pathogens 2018, 7, 82. [Google Scholar] [CrossRef]
- Ozçelik, B.; Aslan, M.; Orhan, I.; Karaoglu, T. Antibacterial, antifungal, and antiviral activities of the lipophylic extracts of Pistacia vera. Microbiol. Res. 2005, 160, 159–164. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Casto, A.M.; Roychoudhury, P.; Xie, H.; Selke, S.; Perchetti, G.A.; Wofford, H.; Huang, M.L.; Verjans, G.M.; Gottlieb, G.S.; Wald, A.; et al. Large, Stable, Contemporary Interspecies Recombination Events in Circulating Human Herpes Simplex Viruses. J. Infect. Dis. 2019, jiz199. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Pfister, J.R.; Spear, S.J. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm. Dis. 2003, 30, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.H.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Schneider, S.; Reichling, J.; Stintzing, F.; Carle, R.; Schnitzler, P. Efficacy of an aqueous Pelargonium sidoides extract against herpesvirus. Planta Med. 2008, 74, PA322. [Google Scholar] [CrossRef]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Viruses 2017, 9, 178. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Saro, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio (Pistacia vera L.) polyphenols, xanthophylls and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef]
| Extracts | CC50 (mg/mL) a | EC50 (mg/mL) b | SI c |
|---|---|---|---|
| NPRE | 1.2 | 0.4 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Smeriglio, A.; Mandalari, G.; Sciortino, M.T. In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.). Plants 2020, 9, 267. https://doi.org/10.3390/plants9020267
Musarra-Pizzo M, Pennisi R, Ben-Amor I, Smeriglio A, Mandalari G, Sciortino MT. In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.). Plants. 2020; 9(2):267. https://doi.org/10.3390/plants9020267
Chicago/Turabian StyleMusarra-Pizzo, Maria, Rosamaria Pennisi, Ichrak Ben-Amor, Antonella Smeriglio, Giuseppina Mandalari, and Maria Teresa Sciortino. 2020. "In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.)" Plants 9, no. 2: 267. https://doi.org/10.3390/plants9020267
APA StyleMusarra-Pizzo, M., Pennisi, R., Ben-Amor, I., Smeriglio, A., Mandalari, G., & Sciortino, M. T. (2020). In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.). Plants, 9(2), 267. https://doi.org/10.3390/plants9020267










