Flexibility in the Energy Balancing Network of Photosynthesis Enables Safe Operation under Changing Environmental Conditions
Abstract
1. Introduction
2. Energy Balancing is Essential for Safe and Optimal Photosynthetic Systems
3. The Structure of the Energy Network Simplifies ATP and NADPH Balancing
4. Introduction to Supply-Side Mechanisms for Energy Balancing
4.1. Cyclic Electron Flux around Photosystem I
4.2. The Malate Valve
4.3. The Mehler Peroxidase Reaction (Water–Water Cycle)
5. Metabolic Demand for ATP and NADPH
6. Determining the Efficiency of Energy Balancing Mechanisms
7. With an Efficient Malate Valve, Why is CEF Important?
8. Demand-Side Energy Balancing Processes
9. The Acclimation of Energy Balancing Networks to Long-Term Change in Energy Demand
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| ΔpH | The gradient potential component of the proton motive force |
| Δψ | Electric force component of the proton motive force |
| A | Net CO2 assimilation |
| AOX | Alternative oxidase |
| CEF | Cyclic electron flux around photosystem I |
| Fd | Ferredoxin |
| Fd-Trx | Ferredoxin-thioredoxin system |
| FQR | Ferredoxin:plastoquinone reductase |
| GS | glutamine synthetase |
| GOGAT | glutamine-2-oxoglutarate aminotransferase |
| LEF | Linear electron flow |
| NDH | NADPH/Ferredoxin:plastoquinone dehydrogenase (NDH) |
| pmf | Proton motive force |
| MDH | Malate dehydrogenase |
| NiR | Nitrite reductase |
| NPQ | Non-photochemical quenching |
| NR | Nitrate reductase |
| PQ | Plastoquinone |
| PQH2 | Plastoquinol |
| PQR | Ferredoxin:plastoquinone reductases |
| PSI | Photosystem I |
| PSII | Photosystem II |
| qE | Energy-dependent quenching |
| Rl | Respiration in the light |
| Vc | Rate of rubisco carboxylation |
| Vo | Rate of rubisco oxygenation |
Appendix A
Calculating ATP and NADPH Ratios from Gas Exchange Data
References
- Miyake, C.; Miyata, M.; Shinzaki, Y.; Tomizawa, K.-I. CO2 Response of Cyclic Electron Flow around PSI (CEF-PSI) in Tobacco Leaves-Relative Electron fluxes through PSI and PSII Determine the Magnitude of Non-photochemical Quenching (NPQ) of Chl Fluorescence. Plant Cell Physiol. 2005, 46, 629–637. [Google Scholar] [CrossRef]
- Kramer, D.M.; Avenson, T.J.; Edwards, G.E. Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci. 2004, 9, 349–357. [Google Scholar] [CrossRef]
- Ort, D.R.; Yocum, C.F. Light reactions of oxygenic photosynthesis. In Oxygenic Photosynthesis: The Light Reactions; Ort, D.R., Yocum, C.F., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 1–9. [Google Scholar]
- Cape, J.L.; Bowman, M.K.; Kramer, D.M. Understanding the cytochrome bc complexes by what they don’t do. The Q-cycle at 30. Trends Plant Sci. 2006, 11, 46–55. [Google Scholar] [CrossRef]
- Fischer, S.; Graber, P. Comparison of DpH- and Dy-driven ATP synthesis catalyzed by the H(+)-ATPases from Escherichia coli or chloroplasts reconstituted into liposomes. FEBS Lett. 1999, 457, 327–332. [Google Scholar] [CrossRef]
- Hangarter, R.P.; Good, N.D. Energy thresholds for ATP synthesis in chloroplasts. Biochim. Biophys. Acta 1982, 681, 396–404. [Google Scholar] [CrossRef]
- Kramer, D.M.; Evans, J.R. The importance of energy balance in improving photosynthetic productivity. Plant Phys. 2011, 155, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Avenson, T.J.; Kanazawa, A.; Cruz, J.A.; Takizawa, K.; Ettinger, W.E.; Kramer, D.M. Integrating the proton circuit into photosynthesis: Progress and challenges. Plant Cell Environ. 2005, 28, 97–109. [Google Scholar] [CrossRef]
- Avenson, T.J.; Cruz, J.A.; Kanazawa, A.; Kramer, D.M. Regulating the proton budget of higher plant photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9709–9713. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.A.; Avenson, T.J.; Kanazawa, A.; Takizawa, K.; Edwards, G.E.; Kramer, D.M. Plasticity in light reactions of photosynthesis for energy production and photoprotection. J. Exp. Bot. 2005, 56, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Amthor, J.S. From sunlight to phytomass: On the potential efficiency of converting solar radiation to phyto-energy. New Phytol. 2010, 188, 939–959. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Barber, J. Mechanisms of photodamage and protein degradation during photoinhibition of photosystem II. In Photosynthesis and the Environment; Baker, N.R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 101–121. [Google Scholar]
- Styring, S.; Virgin, I.; Ehrenberg, A.; Andersson, B. Strong light photoinhibition of electron transport in photosystem II. Impairment of the function of the first quinone acceptor, QA. Biochim. Biophys. Acta 1990, 1015, 269. [Google Scholar] [CrossRef]
- Aro, E.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Baker, N.R.; Bowyer, J.R. Photoinhibition of photosynthesis from molecular mechanisms to the field. In Environmental Plant Biology Series; Davies, W.J., Ed.; Bios Scientific Publishers: Institute of Environmental and Biological Sciences; Bios Scientific Publishers: Institute of Environmental and Biological Sciences, Division of Biological Sciences, University of Lancaster: Oxford, UK, 1994; pp. 1–471. [Google Scholar]
- Demmig-Adams, B.; Adams-III, W.W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Eskling, M.; Emanuelsson, A.; Akerlund, H.-E. Enzymes and mechanisms for violaxanthin-zeaxanthin conversion. In Regulation of Photosynthesis; Aro, E.-M., Anderson, B., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; Volume 100, pp. 806–816. [Google Scholar]
- Li, X.P.; Muller-Moule, P.; Gilmore, A.M.; Niyogi, K.K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 2002, 99, 15222–15227. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Li, X.-P.; Rosenberg, V.; Jung, H.-S. Is PsbS the site of non-photochemical quenching in photosynthesis? J. Exp. Bot. 2004, 56, 375–382. [Google Scholar] [CrossRef]
- Takizawa, K.; Kanazawa, A.; Cruz, J.A.; Kramer, D.M. In vivo thylakoid proton motive force. Quantitative non-invasive probes show the relative lumen pH-induced regulatory responses of antenna and electron transfer. Biochim. Biophys. Acta 2007, 1767, 1233–1244. [Google Scholar] [CrossRef]
- Tikhonov, A.N. The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. 2014, 81, 163–183. [Google Scholar] [CrossRef]
- Cruz, J.A.; Avenson, T.J.; Takizawa, K.; Kramer, D.M. The contribution of cyclic electron flux (CEF1) to formation of proton motive force (pmf). In Proceedings of the 13th International Congress of Photosynthesis: Fundamental Aspects to Global Perspectives, Lawrence, KA, USA, 29 August–3 September 2005; pp. 1033–1035. [Google Scholar]
- Kanazawa, A.; Kramer, D.M. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc. Natl. Acad. Sci. USA 2002, 99, 12789–12794. [Google Scholar] [CrossRef]
- Avenson, T.; Cruz, J.; Kramer, D. Modulation of CFO-CF1 ATP synthase conductivity and proton motive force (pmf) partitioning regulate light capture. In Proceedings of the 13th International Congress of Photosynthesis: Fundamental Aspects to Global Perspectives, Lawrence, KA, USA, 29 August–3 September 2005; pp. 575–577. [Google Scholar]
- Avenson, T.; Cruz, J.A.; Kramer, D. Modulation of energy dependent quenching of excitons (qE) in antenna of higher plants. Proc. Natl. Acad. Sci. USA 2004, 101, 5530–5535. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.A.; Sacksteder, C.A.; Kanazawa, A.; Kramer, D.M. Contribution of Electric Field (Δψ) to Steady-State Transthylakoid Proton Motive Force (pmf) in Vitro and in Vivo. Control of pmf Parsing into Δψ and ΔpH by Ionic Strength. Biochemistry 2001, 40, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.M.; Cruz, J.A.; Kanazawa, A. Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci. 2003, 8, 27–32. [Google Scholar] [CrossRef]
- Zhang, R.; Cruz, J.A.; Kramer, D.M.; Magallanes-Lundback, M.; DellaPenna, D.; Sharkey, T.D. Heat stress reduces the pH component of the transthylakoid proton motive force in light-adapted intact tobacco leaves. Plant Cell Environ. 2009, 32, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef]
- Li, X.P.; Gilmore, A.M.; Caffarri, S.; Bassi, R.; Golan, T.; Kramer, D.; Niyogi, K.K. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 2004, 279, 22866–22874. [Google Scholar] [CrossRef]
- Scheibe, R. Malate valves to balance cellular energy supply. Physiol. Plant 2004, 120, 21–26. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Alric, J.; Johnson, X. Alternative electron transport pathways in photosynthesis: A confluence of regulation. Curr. Opin. Plant Biol. 2017, 37, 78–86. [Google Scholar] [CrossRef]
- Strand, D.D.; Fisher, N.; Kramer, D.M. Distinct Energetics and Regulatory Functions of the Two Major Cyclic Electron Flow Pathways in Chloroplasts; Caister Academic Press: Poole, UK, 2016. [Google Scholar]
- Ishikawa, N.; Endo, T.; Sato, F. Electron transport activities of Arabidopsis thaliana mutants with impaired chloroplastic NAD(P)H dehydrogenase. J. Plant Res. 2008, 121, 521–526. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 Is Involved in Cyclic Electron Flow around Photosystem I and Is Essential for Photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- DalCorso, G.; Pesaresi, P.; Masiero, S.; Aseeva, E.; Schünemann, D.; Finazzi, G.; Joliot, P.; Barbato, R.; Leister, D. A Complex Containing PGRL1 and PGR5 Is Involved in the Switch between Linear and Cyclic Electron Flow in Arabidopsis. Cell 2008, 132, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Hertle, A.P.; Blunder, T.; Wunder, T.; Pesaresi, P.; Pribil, M.; Armbruster, U.; Leister, D. PGRL1 Is the Elusive Ferredoxin-Plastoquinone Reductase in Photosynthetic Cyclic Electron Flow. Mol. Cell 2013, 49, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Nandha, B.; Finazzi, G.; Joliot, P.; Hald, S.; Johnson, G.N. The role of PGR5 in the redox poising of photosynthetic electron transport. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Joliot, P.; Johnson, G.N. Regulation of cyclic and linear electron flow in higher plants. Proc. Natl. Acad. Sci. USA 2011, 108, 13317–13322. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.; Kramer, D.M. Non-photochemical reduction of thylakoid photosynthetic redox carriers in vitro: Relevance to cyclic electron flow around photosystem I? Biochim. Biophys. Acta Bioenerg. 2014, 1837, 1944–1954. [Google Scholar] [CrossRef]
- Mosebach, L.; Heilmann, C.; Mutoh, R.; Gäbelein, P.; Steinbeck, J.; Happe, T.; Ikegami, T.; Hanke, G.; Kurisu, G.; Hippler, M. Association of Ferredoxin:NADP+ oxidoreductase with the photosynthetic apparatus modulates electron transfer in Chlamydomonas reinhardtii. Photosynth. Res. 2017, 134, 291–306. [Google Scholar] [CrossRef]
- Burrows, P.A.; Sazanov, L.A.; Svab, Z.; Maliga, P.; Nixon, P.J. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 1998, 17, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, T. Chloroplast NDH: A different enzyme with a structure similar to that of respiratory NADH dehydrogenase. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1015–1022. [Google Scholar] [CrossRef]
- Strand, D.D.; Fisher, N.; Kramer, D.M. The higher plant plastid NAD(P)H dehydrogenase-like complex (NDH) is a high efficiency proton pump that increases ATP production by cyclic electron flow. J. Biol. Chem. 2017, 292, 11850–11860. [Google Scholar] [CrossRef]
- Laughlin, T.G.; Bayne, A.N.; Trempe, J.-F.; Savage, D.F.; Davies, K.M. Structure of the complex I-like molecule NDH of oxygenic photosynthesis. Nature 2019, 566, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Joliot, P.; Joliot, A. Cyclic electron transfer in plant leaf. Proc. Natl. Acad. Sci. USA 2002, 99, 10209–10214. [Google Scholar] [CrossRef] [PubMed]
- Stroebel, D.; Choquet, Y.; Popot, J.-L.; Picot, D. An atypical haem in the cytochrome b6f complex. Nature 2003, 426, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Alric, J.; Pierre, Y.; Picot, D.; Lavergne, J.; Rappaport, F. Spectral and redox characterization of the heme ci of the cytochrome b6f complex. Proc. Natl. Acad. Sci. USA 2005, 102, 15860–15865. [Google Scholar] [CrossRef]
- Strand, D.D.; D’Andrea, L.; Bock, R. The plastid NAD(P)H dehydrogenase-like complex: Structure, function and evolutionary dynamics. Biochem. J. 2019, 476, 2743–2756. [Google Scholar] [CrossRef]
- Joliot, P.; Béal, D.; Joliot, A. Cyclic electron flow under saturating excitation of dark-adapted Arabidopsis leaves. Biochim. Biophys. Acta Bioenerg. 2004, 1656, 166–176. [Google Scholar] [CrossRef]
- Kohzuma, K.; Cruz, J.A.; Akashi, K.; Hoshiyasu, S.; Munekage, Y.N.; Yokota, A.; Kramer, D.M. The long-term responses of the photosynthetic proton circuit to drought. Plant Cell Environ. 2009, 32, 209–219. [Google Scholar] [CrossRef]
- Kubicki, A.; Funk, E.; Westhoff, P.; Steinmüller, K. Differential expression of plastome-encoded ndh genes in mesophyll and bundle-sheath chloroplasts of the C4 plant Sorghum bicolor indicates that the complex I-homologous NAD(P)H-plastoquinone oxidoreductase is involved in cyclic electron transport. Planta 1996, 199, 276–281. [Google Scholar] [CrossRef]
- Takabayashi, A.; Kishine, M.; Asada, K.; Endo, T.; Sato, F. Differential use of two cyclic electron flows around photosystem I for driving CO2-concentration mechanism in C4 photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 16898–16903. [Google Scholar] [CrossRef]
- Lucker, B.; Kramer, D.M. Regulation of cyclic electron flow in Chlamydomonas reinhardtii under fluctuating carbon availability. Photosynth. Res. 2013, 117, 449–459. [Google Scholar] [CrossRef]
- Scheibe, R. Maintaining homeostasis by controlled alternatives for energy distribution in plant cells under changing conditions of supply and demand. Photosynth. Res. 2019, 139, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Selinski, J.; Scheibe, R. Malate valves: Old shuttles with new perspectives. Plant Biol. 2019, 21, 21–30. [Google Scholar] [CrossRef]
- Fickenscher, K.; Scheibe, R. Limited proteolysis of inactive tetrameric chloroplast NADP-Malate dehydrogenase produces active dimers. Arch. Biochem. Biophys. 1988, 260, 771–779. [Google Scholar] [CrossRef]
- Ocheretina, O.; Harnecker, J.; Rother, T.; Schmid, R.; Scheibe, R. Effects of N-terminal truncations upon chloroplast NADP-malate dehydrogenases from pea and spinach. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1993, 1163, 10–16. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Feilke, K. The Dual Role of the Plastid Terminal Oxidase PTOX: Between a Protective and a Pro-oxidant Function. Front. Plant Sci. 2016, 6, 1147. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyeva, Y.; Suorsa, M.; Tikkanen, M.; Aro, E.-M. Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 2014, 66, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Ruuska, S.A.; Badger, M.R.; Andrews, T.J.; von Caemmerer, S. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: Little evidence for significant Mehler reaction. J. Exp. Bot. 2000, 51, 357–368. [Google Scholar] [CrossRef]
- Heber, U. Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants. Photosynth. Res. 2002, 73, 223–231. [Google Scholar] [CrossRef]
- Driever, S.M.; Baker, N.R. The water–water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ. 2011, 34, 837–846. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C. Review article. A re-evaluation of the ATP:NADPH budget during C3 photosynthesis: A contribution from nitrate assimilation and its associated respiratory activity? J. Exp. Bot. 1998, 49, 1895–1908. [Google Scholar] [CrossRef]
- Edwards, G.E.; Walker, D.A. C3, C4: Mechanisms, and Cellular and Environmental Regulation, of Photosynthesis; Blackwell Scientific Publications: Oxford, UK, 1983. [Google Scholar]
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Estimating the rate of photorespiration in leaves. Physiol. Plant 1988, 73, 147–152. [Google Scholar] [CrossRef]
- Walker, B.J.; Strand, D.D.; Kramer, D.M.; Cousins, A.B. The response of cyclic electron flow around photosystem I to changes in photorespiration and nitrate assimilation. Plant Phys. 2014, 165, 453–462. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Reed, A.J.; Canvin, D.T. Light and Dark Controls of Nitrate Reduction in Wheat (Triticum aestivum L.) Protoplasts. Plant Phys. 1982, 69, 508–513. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Lack of malate valve capacities lead to improved N-assimilation and growth in transgenic A. thaliana plants. Plant Signal. Behav. 2014, 9, e29057. [Google Scholar] [CrossRef]
- Rachmilevitch, S.; Cousins, A.B.; Bloom, A.J. Nitrate assimilation in plant shoots depends on photorespiration. Proc. Natl. Acad. Sci. USA 2004, 101, 11506–11510. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Asensio, J.S.R.; Cousins, A.B. Carbon Dioxide Enrichment Inhibits Nitrate Assimilation in Wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Ponce, M.A.; Cao, X.; Yang, Z.; Ohlrogge, J.B. Lipid turnover during senescence. Plant Sci. 2013, 205–206, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Focke, M.; Pollard, M.; Ohlrogge, J. Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J. 2000, 22, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Ohlrogge, J. Testing Models of Fatty Acid Transfer and Lipid Synthesis in Spinach Leaf Using in Vivo Oxygen-18 Labeling. Plant Phys. 1999, 121, 1217–1226. [Google Scholar] [CrossRef]
- Bonaventure, G.; Bao, X.; Ohlrogge, J.; Pollard, M. Metabolic responses to the reduction in palmitate caused by disruption of the FATB gene in Arabidopsis. Plant Phys. 2004, 135, 1269–1279. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.; Ohlrogge, J.B. Turnover of Fatty Acids during Natural Senescence of Arabidopsis, Brachypodium, and Switchgrass and in Arabidopsis β-Oxidation Mutants. Plant Phys. 2009, 150, 1981–1989. [Google Scholar] [CrossRef]
- Geigenberger, P.; Kolbe, A.; Tiessen, A. Redox regulation of carbon storage and partitioning in response to light and sugars. J. Exp. Bot. 2005, 56, 1469–1479. [Google Scholar] [CrossRef]
- Shastri, A.A.; Morgan, J.A. Flux Balance Analysis of Photoautotrophic Metabolism. Biotechnol. Prog. 2005, 21, 1617–1626. [Google Scholar] [CrossRef]
- Von Ballmoos, C.; Cook, G.M.; Dimroth, P. Unique Rotary ATP Synthase and Its Biological Diversity. Annu. Rev. Biophys. 2008, 37, 43–64. [Google Scholar] [CrossRef]
- Von Ballmoos, C.; Wiedenmann, A.; Dimroth, P. Essentials for ATP Synthesis by F1F0 ATP Synthases. Annu. Rev. Biochem. 2009, 78, 649–672. [Google Scholar] [CrossRef]
- Junge, W.; Sielaff, H.; Engelbrecht, S. Torque generation and elastic power transmission in the rotary FOF1-ATPase. Nature 2009, 459, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.J. ATP synthase: From sequence to ring size to the P/O ratio. Proc. Natl. Acad. Sci. USA 2010, 107, 16755–16756. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, T. The mitochondrial phosphate-to-oxygen ratio is not an integer. Biochem. Mol. Biol. Educ. 2005, 33, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Watt, I.N.; Montgomery, M.G.; Runswick, M.J.; Leslie, A.G.W.; Walker, J.E. Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc. Natl. Acad. Sci. USA 2010, 107, 16823–16827. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.J.; Sorgato, M.C. Proton Electrochemical Gradients and Energy-Transduction Processes. Annu. Rev. Biochem. 1982, 51, 185–217. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial ion circuits. Essays Biochem. 2010, 47, 25–35. [Google Scholar]
- Bhatla, S.C.; Lal, M.A. ATP Synthesis. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2018; pp. 315–337. [Google Scholar]
- Lambers, H.; Oliveira, R.S. Photosynthesis, Respiration, and Long-Distance Transport: Respiration. In Plant Physiological Ecology; Springer International Publishing: Cham, Switzerland, 2019; pp. 115–172. [Google Scholar]
- Haupt-Herting, S.; Klug, K.; Fock, H.P. A new approach to measure gross CO2 fluxes in leaves. Gross CO2 sssimilation, photorespiration, and mitochondrial respiration in the light in tomato under drought stress. Plant Phys. 2001, 126, 388–396. [Google Scholar] [CrossRef]
- Villar, R.; Held, A.A.; Merino, J. Comparison of methods to estimate dark respiration in the light in leaves of two woody species. Plant Phys. 1994, 105, 167–172. [Google Scholar] [CrossRef]
- Kromer, S. Respiration During Photosynthesis. Annu Rev. Plant Physiol Plant Mol. Biol 1995, 46, 45–70. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Aleksandrova, A.; King, M.S.; Majd, H.; Ashton, V.L.; Cerson, E.; Springett, R.; Kibalchenko, M.; Tavoulari, S.; Crichton, P.G.; et al. The transport mechanism of the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta BBAMol. Cell Res. 2016, 1863, 2379–2393. [Google Scholar] [CrossRef]
- Palmieri, F.; Monné, M. Discoveries, metabolic roles and diseases of mitochondrial carriers: A review. Biochim. Biophys. Acta BBAMol. Cell Res. 2016, 1863, 2362–2378. [Google Scholar] [CrossRef]
- Heldt, H.W. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969, 5, 11–14. [Google Scholar] [CrossRef]
- Schunemann, D.; Borchert, S.; Flugge, U.I.; Heldt, H.W. ADP/ATP Translocator from Pea Root Plastids (Comparison with Translocators from Spinach Chloroplasts and Pea Leaf Mitochondria). Plant Phys. 1993, 103, 131–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, W.; Yang, Y.-J.; Hu, H.; Zhang, S.-B. Different roles of cyclic electron flow around photosystem I under sub-saturating and saturating light intensities in tobacco leaves. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.M.; Ratcliffe, R.G.; Sweetlove, L.J. A Method of Accounting for Enzyme Costs in Flux Balance Analysis Reveals Alternative Pathways and Metabolite Stores in an Illuminated Arabidopsis Leaf. Plant Phys. 2015, 169, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Shameer, S.; Ratcliffe, R.G.; Sweetlove, L.J. Leaf Energy Balance Requires Mitochondrial Respiration and Export of Chloroplast NADPH in the Light. Plant Phys. 2019, 180, 1947–1961. [Google Scholar] [CrossRef] [PubMed]
- Munekage, Y.; Hashimoto, M.; Miyake, C.; Tomizawa, K.-I.; Endo, T.; Tasaka, M.; Shikanai, T. Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 2004, 429, 579–582. [Google Scholar] [CrossRef]
- Strand, D.D.; Fisher, N.; Davis, G.A.; Kramer, D.M. Redox regulation of the antimycin A sensitive pathway of cyclic electron flow around photosystem I in higher plant thylakoids. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1–6. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.E.; Francia, F.; Danon, A.; Marchand, C.; Fermani, S.; Trost, P.; et al. Redox regulation of the Calvin–Benson cycle: Something old, something new. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Bloom, A.J.; Caldwell, R.M.; Finazzo, J.; Warner, R.L.; Weissbart, J. Oxygen and Carbon Dioxide Fluxes from Barley Shoots Depend on Nitrate Assimilation. Plant Physiol. 1989, 91, 352–356. [Google Scholar] [CrossRef]
- Bloom, A.J.; Smart, D.R.; Nguyen, D.T.; Searles, P.S. Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc. Natl. Acad. Sci. USA 2002, 99, 1730–1735. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Hebbelmann, I.; Selinski, J.; Wehmeyer, C.; Goss, T.; Voss, I.; Mulo, P.; Kangasjärvi, S.; Aro, E.-M.; Oelze, M.-L.; Dietz, K.-J.; et al. Multiple strategies to prevent oxidative stress in Arabidopsis plants lacking the malate valve enzyme NADP-malate dehydrogenase. J. Exp. Bot. 2011, 63, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B. The Ferredoxin/Thioredoxin System: A Key Element in the Regulatory Function of Light in Photosynthesis. Bioscience 1984, 34, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, B.B.; Balmer, Y. REDOX REGULATION: A Broadening Horizon. Annu. Rev. Plant Biol. 2005, 56, 187–220. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, R. Thioredoxinm in pea chloroplasts: Concentration and redox state under light and dark conditions. FEBS Lett. 1981, 133, 301–304. [Google Scholar] [CrossRef]
- Scheibe, R.; Stitt, M. Comparison of NADP-malate dehydrogenase activation, QA reduction and O2 evolution in spinach leaves. Plant Physiol. Biochem. 1988, 26, 473–481. [Google Scholar]
- Joliot, P.; Joliot, A. Cyclic electron flow in C3 plants. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 362–368. [Google Scholar] [CrossRef]
- Becker, B.; Holtgrefe, S.; Jung, S.; Wunrau, C.; Kandlbinder, A.; Baier, M.; Dietz, K.-J.; Backhausen, J.E.; Scheibe, R. Influence of the photoperiod on redox regulation and stress responses in Arabidopsis thaliana L. (Heynh.) plants under long- and short-day conditions. Planta 2006, 224, 380–393. [Google Scholar] [CrossRef]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef]
- Tomaz, T.; Bagard, M.; Pracharoenwattana, I.; Linden, P.; Lee, C.P.; Carroll, A.J.; Stroher, E.; Smith, S.M.; Gardestrom, P.; Millar, A.H. Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Phys. 2010, 154, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Lindén, P.; Keech, O.; Stenlund, H.; Gardeström, P.; Moritz, T. Reduced mitochondrial malate dehydrogenase activity has a strong effect on photorespiratory metabolism as revealed by 13C labelling. J. Exp. Bot. 2016, 67, 3123–3135. [Google Scholar] [CrossRef] [PubMed]
- Cousins, A.B.; Pracharoenwattana, I.; Zhou, W.; Smith, S.M.; Badger, M.R. Peroxisomal malate dehydrogenase is not essential for photorespiration in Arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release. Plant Phys. 2008, 148, 786–795. [Google Scholar] [CrossRef]
- Cousins, A.B.; Walker, B.J.; Pracharoenwattana, I.; Smith, S.M.; Badger, M.R. Peroxisomal hydroxypyruvate reductase is not essential for photorespiration in arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release. Photosynth. Res. 2011, 108, 91–100. [Google Scholar] [CrossRef]
- Anderson, J.M.; Chow, W.S.; Park, Y.-I. The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental cues. Photosynth. Res. 1995, 46, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y. A study on the dynamic features of photosystem stoichiometry: Accomplishments and problems for future studies. Photosynth. Res. 1997, 53, 83–93. [Google Scholar] [CrossRef]
- Melis, A.; Murakami, A.; Nemson, J.A.; Aizawa, K.; Ohki, K.; Fujita, Y. Chromatic regulation inChlamydomonas reinhardtii alters photosystem stoichiometry and improves the quantum efficiency of photosynthesis. Photosynth. Res. 1996, 47, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Pfannschmidt, T.; Allen, J.F.; Oelmüller, R. Principles of redox control in photosynthesis gene expression. Physiol. Plant 2001, 112, 1–9. [Google Scholar] [CrossRef]
- Dietzel, L.; Bräutigam, K.; Pfannschmidt, T. Photosynthetic acclimation: State transitions and adjustment of photosystem stoichiometry—Functional relationships between short-term and long-term light quality acclimation in plants. FEBS J. 2008, 275, 1080–1088. [Google Scholar] [CrossRef]
- Allen, J.F. Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta Bioenerg. 1992, 1098, 275–335. [Google Scholar] [CrossRef]
- Gupta, P.; Duplessis, S.; White, H.; Karnosky, D.F.; Martin, F.; Podila, G.K. Gene expression patterns of trembling aspen trees following long-term exposure to interacting elevated CO2 and tropospheric O3. New Phytol. 2005, 167, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A.; Vodkin, L.O.; Walter, A.; Schurr, U. The Effects of Elevated CO2 Concentration on Soybean Gene Expression. An Analysis of Growing and Mature Leaves. Plant Phys. 2006, 142, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; CSIRO: Collingwood, Australia, 2000; Volume 2. [Google Scholar]
- Von Caemmerer, S. Steady-state models of photosynthesis. Plant Cell Environ. 2013, 36, 1617–1630. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.J.; Ort, D.R. Improved method for measuring the apparent CO2 photocompensation point resolves the impact of multiple internal conductances to CO2 to net gas exchange. Plant Cell Environ. 2015, 38, 2462–2474. [Google Scholar] [CrossRef] [PubMed]
- Laisk, A. Kinetics of photosynthesis and photorespiration in C3 plants. Nauka Mosc. 1977. (In Russian) [Google Scholar]
- Laisk, A.; Loreto, F. Determining photosynthetic parameters from leaf CO2 exchange and chlorophyll fluorescence. Plant Physiol. 1996, 110, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Bernacchi, C.J.; Portis, A.R.; Nakano, H.; von Caemmerer, S.; Long, S.P. Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Phys. 2002, 130, 1992–1998. [Google Scholar] [CrossRef]
- Tazoe, Y.; von Caemmerer, S.; Estavillo, G.M.; Evans, J.R. Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant Cell Environ. 2011, 34, 580–591. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Pou, A.; Zwieniecki, M.A.; Holbrook, N.M. On measuring the response of mesophyll conductance to carbon dioxide with the variable J method. J. Exp. Bot. 2012, 63, 413–425. [Google Scholar] [CrossRef]
- Tholen, D.; Ethier, G.; Genty, B.; Pepin, S.; Zhu, X.-G. Variable mesophyll conductance revisited: Theoretical background and experimental implications. Plant Cell Environ. 2012, 35, 2087–2103. [Google Scholar] [CrossRef]
- Evans, J.R.; von Caemmerer, S. Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ. 2013, 36, 745–756. [Google Scholar] [CrossRef]
- Jahan, E.; Amthor, J.S.; Farquhar, G.D.; Trethowan, R.; Barbour, M.M. Variation in mesophyll conductance among Australian wheat genotypes. Funct. Plant Biol. 2014, 41, 568–580. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Evans, J.R. Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 2014, 38, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Harley, P.C.; Di Marco, G.; Sharkey, T.D. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Phys. 1992, 98, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
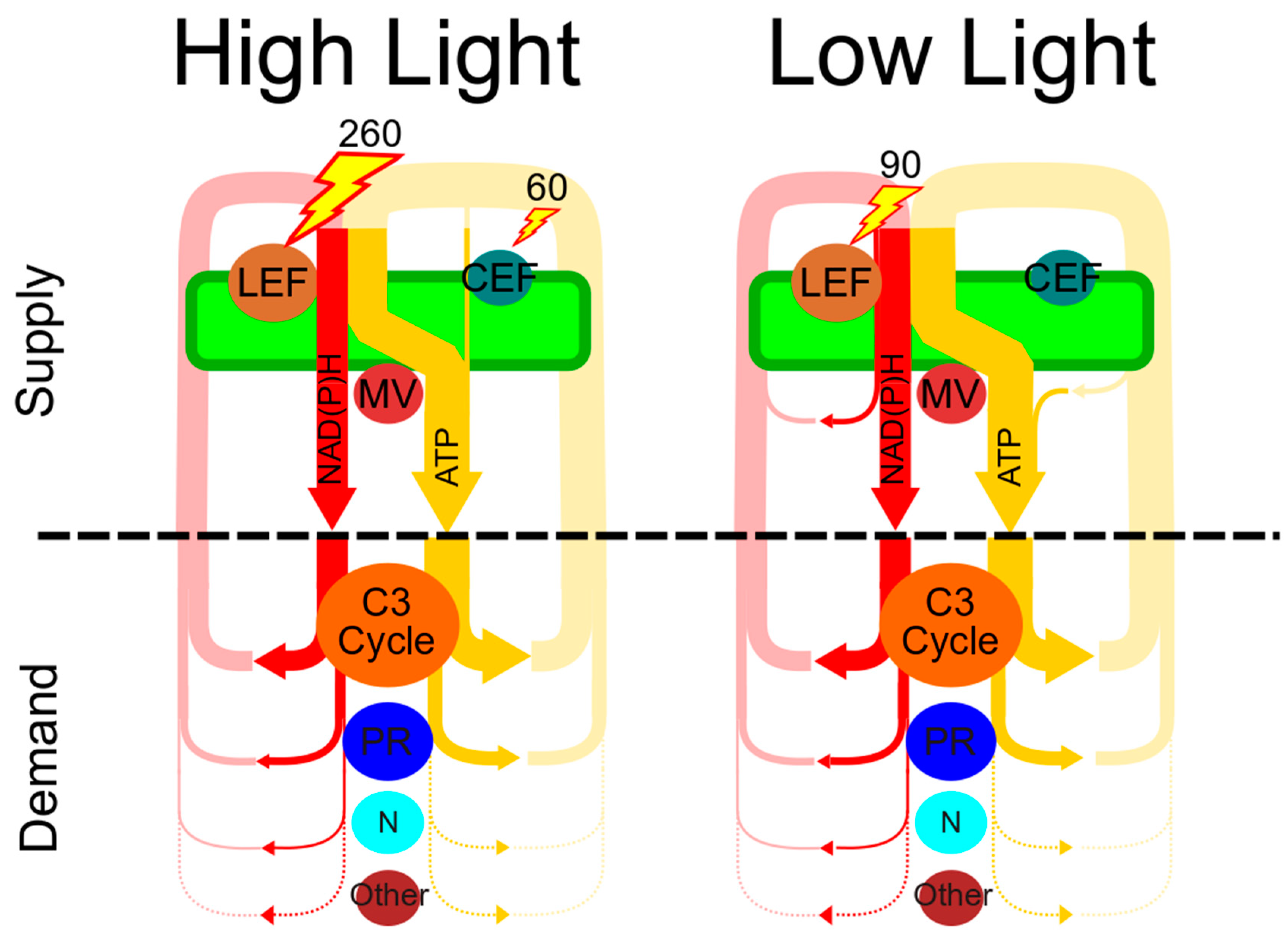
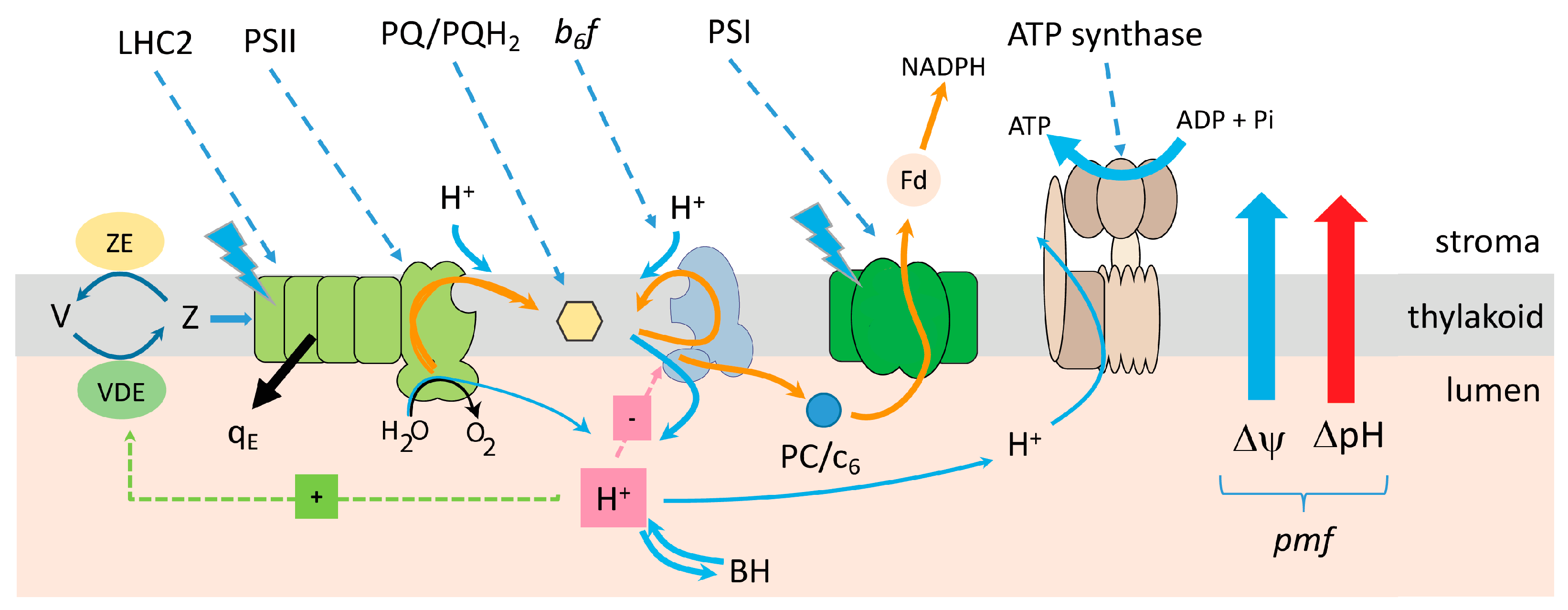
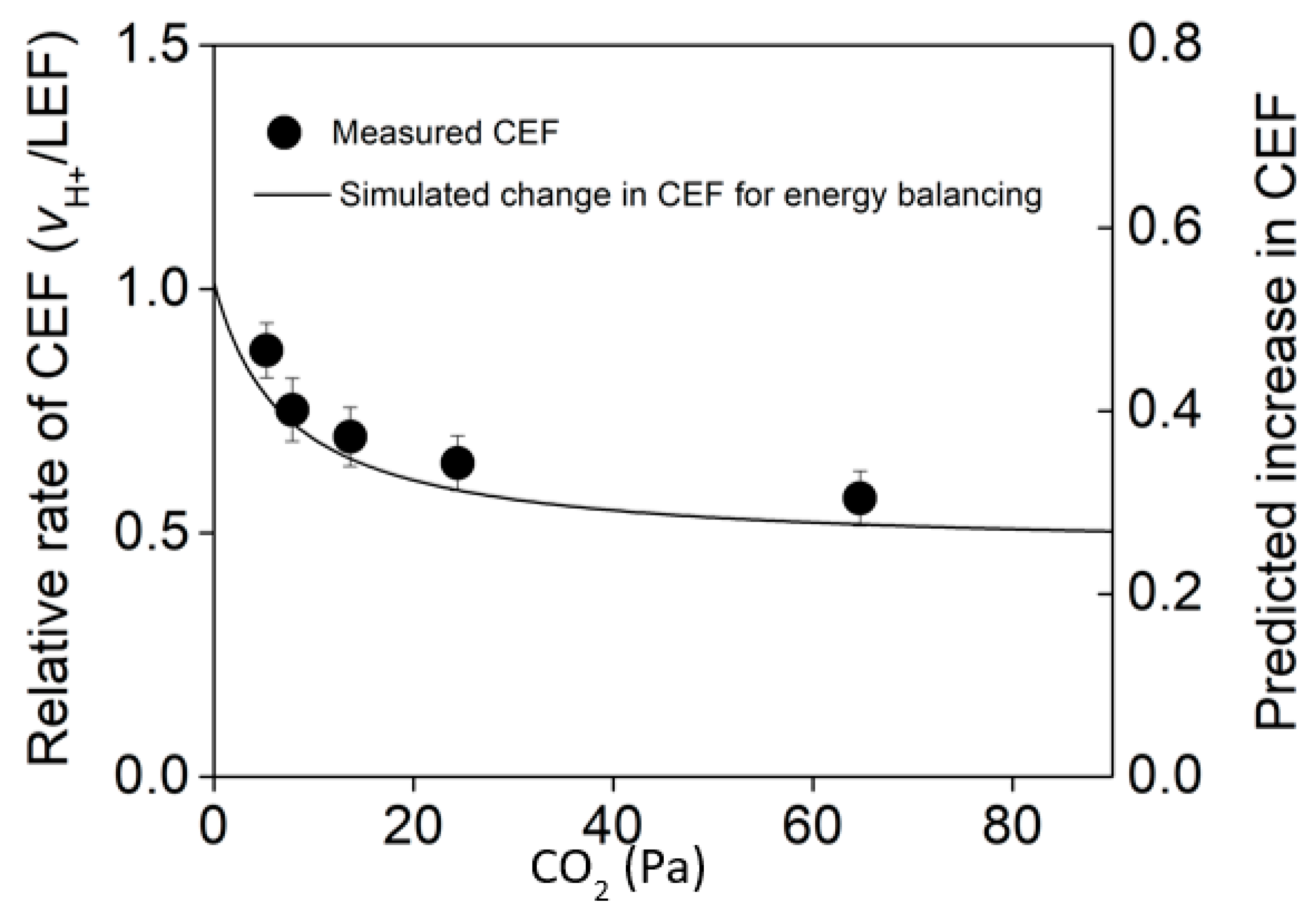
| High Light (1100 PAR) | Low Light (150 PAR) | |
|---|---|---|
| Metabolic demand | ||
| A (μmol CO2 m−2 s−1) | 21.3 * | 5.7 * |
| Intercellular CO2 (Pa) | 23.0 * | 25.0 * |
| Chloroplastic CO2 (Pa) | 19.5 | 24.1 |
| vc (μmol CO2 m−2 s−1) | 30.1 | 8.9 |
| vo (μmol O2 m−2 s−1) | 14.5 | 3.5 |
| vn (μmol N m−2 s−1) | 1.5 | 0.5 |
| vl (μmol N m−2 s−1) | 0.3 | 0.1 |
| Total ATP demand (μmol ATP m−2 s−1) | 143 | 40 |
| Total NADPH demand (μmol NADPH m−2 s−1) | 97 | 27 |
| Total ATP/NADPH ratio | 1.47 | 1.45 |
| Energy supply | ||
| LEF (μmol m−2 s−1) | 132.0 * | 45.6 * |
| JPSI (μmol m−2 s−1) | 192.0 * | 56.4 * |
| LEFpred (μmol m−2 s−1) | 193.9 | 54.4 |
| ATPLEF (μmol m−2 s−1) | 124.1 | 34.8 |
| ATP deficit (μmol m−2 s−1) | 18.6 | 4.7 |
| Energy balancing requirements via CEF | ||
| NDH e− (μmol m−2 s−1) | 43.3 | 11.1 |
| NDH photons (μmol m−2 s−1) | 43.3 | 11.1 |
| FQR/b6f e− (μmol m−2 s−1) | 86.6 | 22.2 |
| FQR/b6f photons (μmol m−2 s−1) | 86.6 | 22.2 |
| Energy balancing requirements via malate valve | ||
| e− (μmol m−2 s−1) | 9.3 | 2.4 |
| Photons (μmol m−2 s−1) | 18.5 | 4.7 |
| CEF Pathways | ||||
|---|---|---|---|---|
| NDH | FQR | b6f | Malate Valve | |
| Chloroplast | ||||
| e-/photons | 1 | 1 | 1 | 0.5 |
| H+/e− | 2 | 1 | 1 | 3 |
| ATP/H+ | 0.21 | 0.21 | 0.21 | 0.21 |
| ATP/photon in chloroplast | 0.43 | 0.21 | 0.21 | 0.32 |
| Mitochondria | ||||
| H+/e− | - | - | - | 5 |
| ATP/H+ | - | - | - | 0.27 |
| ATP/photon in mitochondria | - | - | - | 0.68 |
| Total ATP/photon | 0.43 | 0.21 | 0.21 | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, B.J.; Kramer, D.M.; Fisher, N.; Fu, X. Flexibility in the Energy Balancing Network of Photosynthesis Enables Safe Operation under Changing Environmental Conditions. Plants 2020, 9, 301. https://doi.org/10.3390/plants9030301
Walker BJ, Kramer DM, Fisher N, Fu X. Flexibility in the Energy Balancing Network of Photosynthesis Enables Safe Operation under Changing Environmental Conditions. Plants. 2020; 9(3):301. https://doi.org/10.3390/plants9030301
Chicago/Turabian StyleWalker, Berkley J., David M. Kramer, Nicholas Fisher, and Xinyu Fu. 2020. "Flexibility in the Energy Balancing Network of Photosynthesis Enables Safe Operation under Changing Environmental Conditions" Plants 9, no. 3: 301. https://doi.org/10.3390/plants9030301
APA StyleWalker, B. J., Kramer, D. M., Fisher, N., & Fu, X. (2020). Flexibility in the Energy Balancing Network of Photosynthesis Enables Safe Operation under Changing Environmental Conditions. Plants, 9(3), 301. https://doi.org/10.3390/plants9030301




