Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees
Abstract
1. Introduction
2. Results
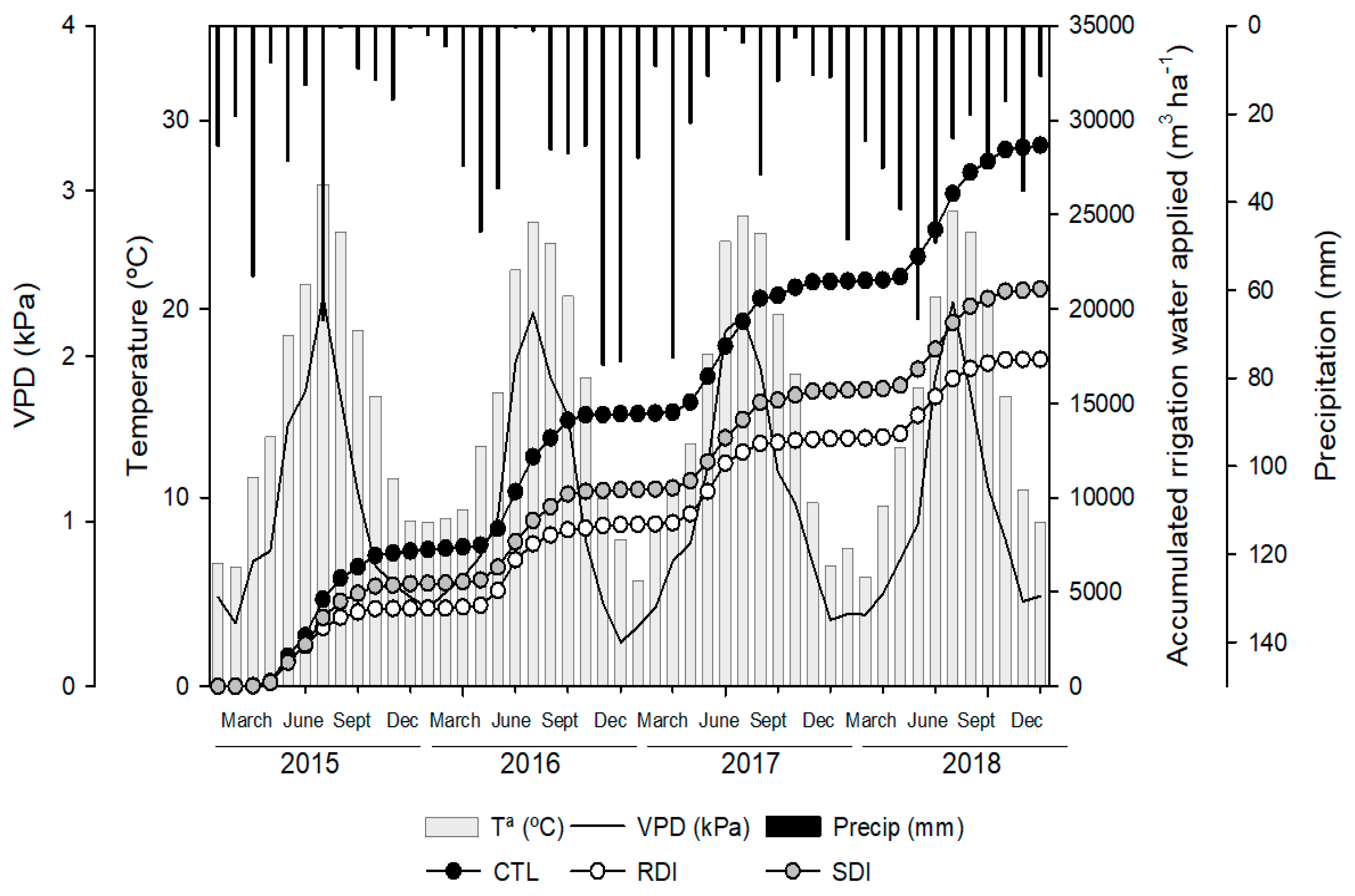
2.1. Water Applied and Meteorological Conditions
2.2. Chilling Requirements and Heat Accumulation until Full Bloom
2.3. Flower Density, Number of Flower Buds per Spur and Fruit Setting
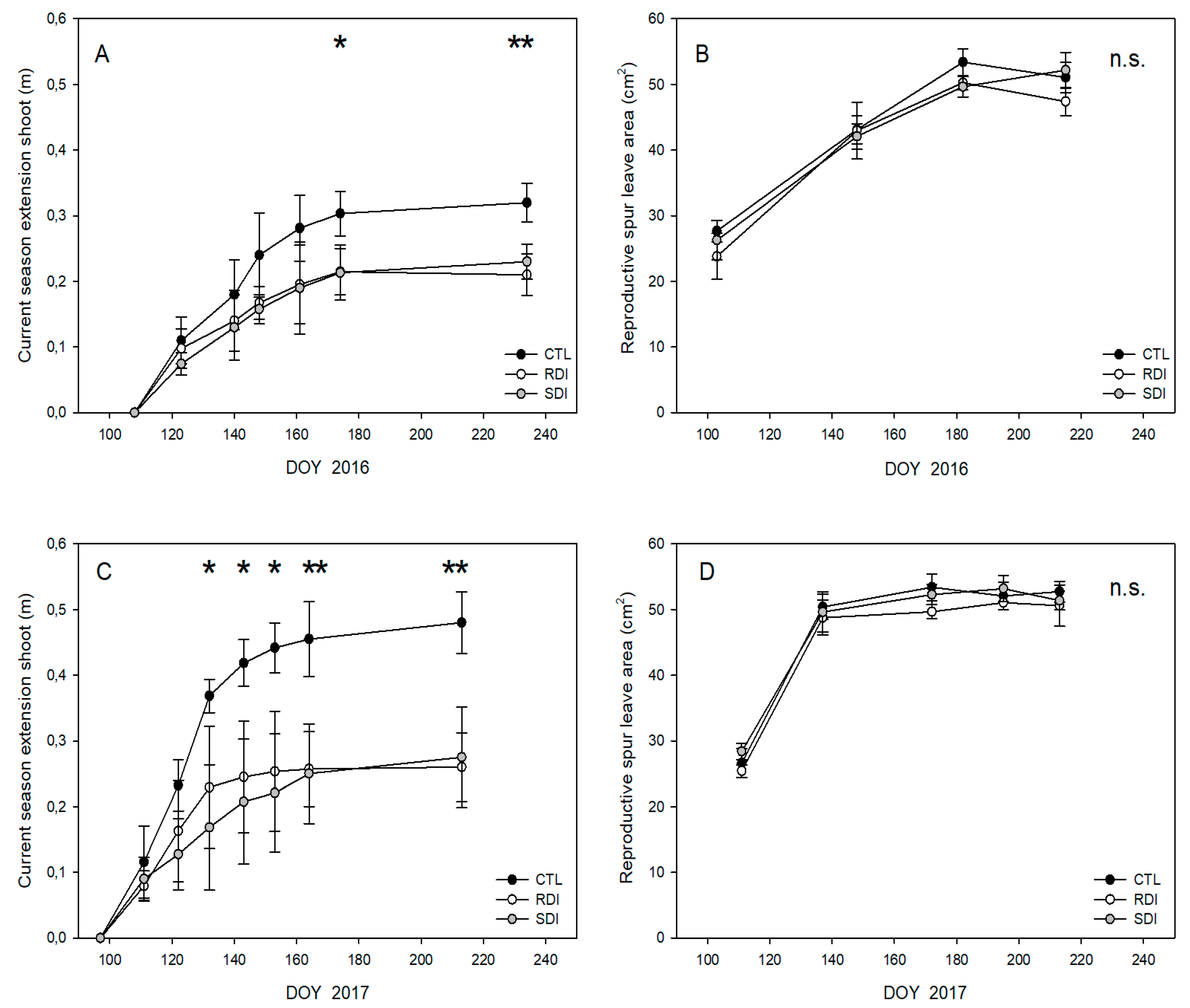
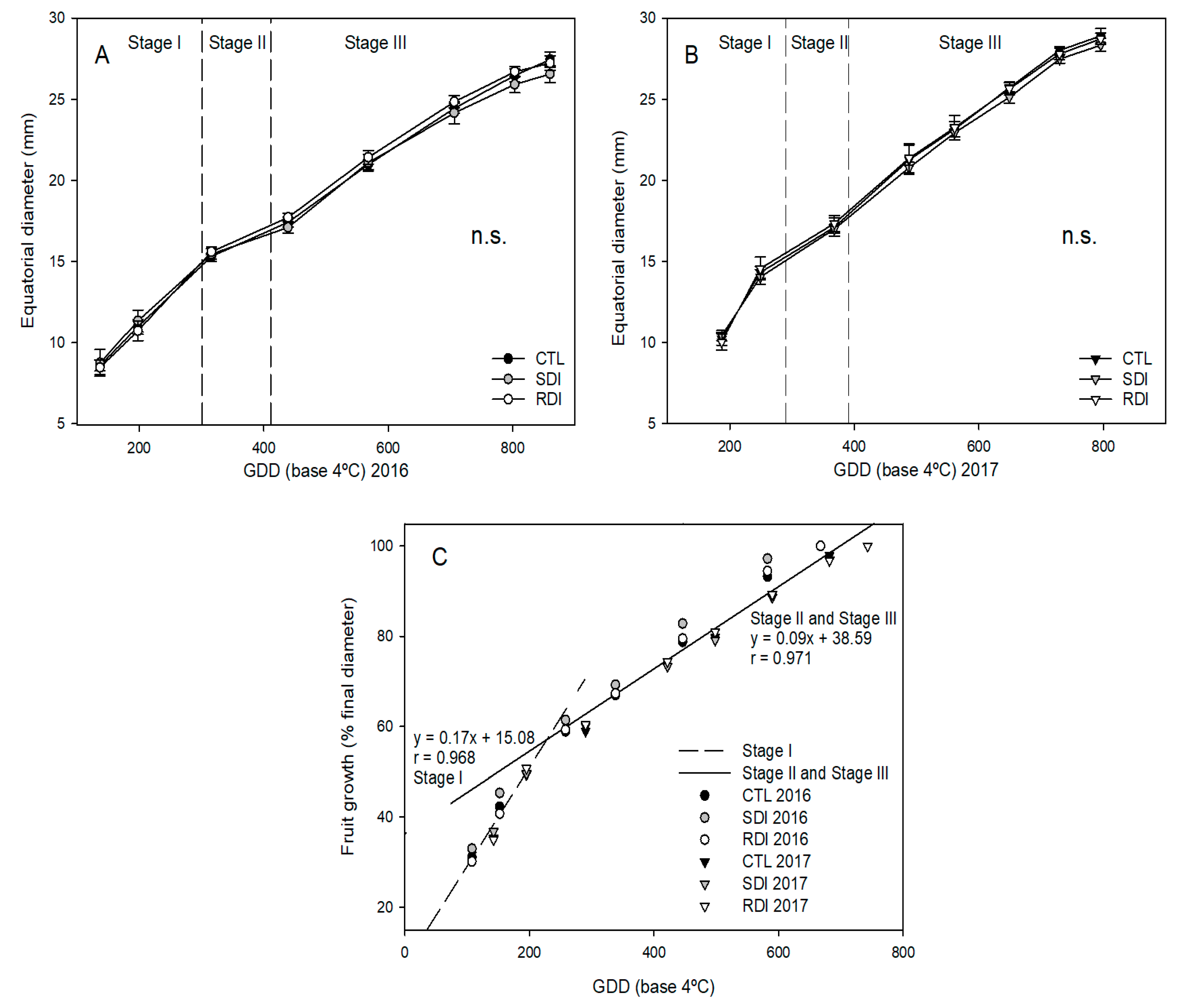
2.4. Fruit and Vegetative Growth
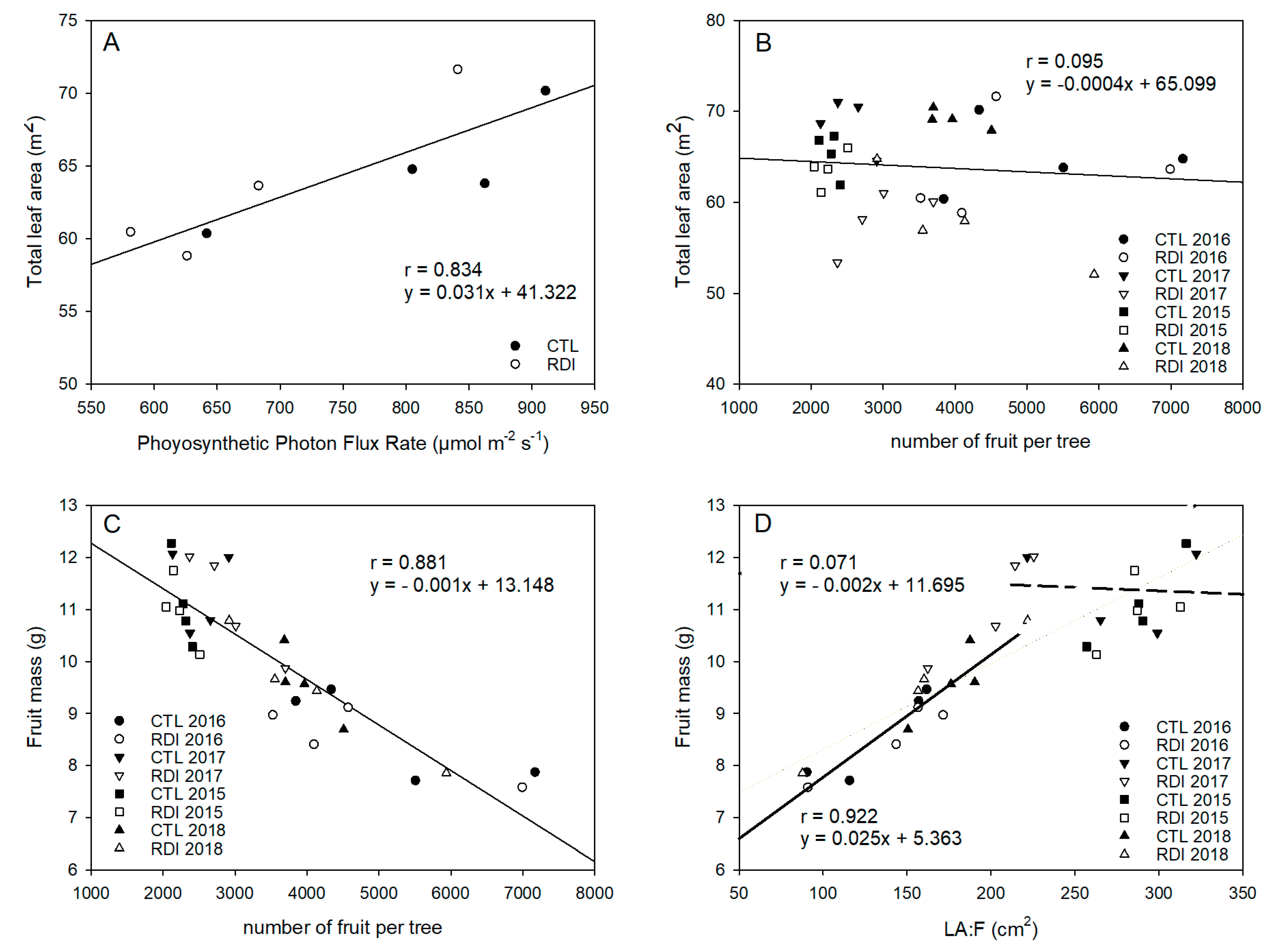
2.5. Leaf Area and LA:F Ratio
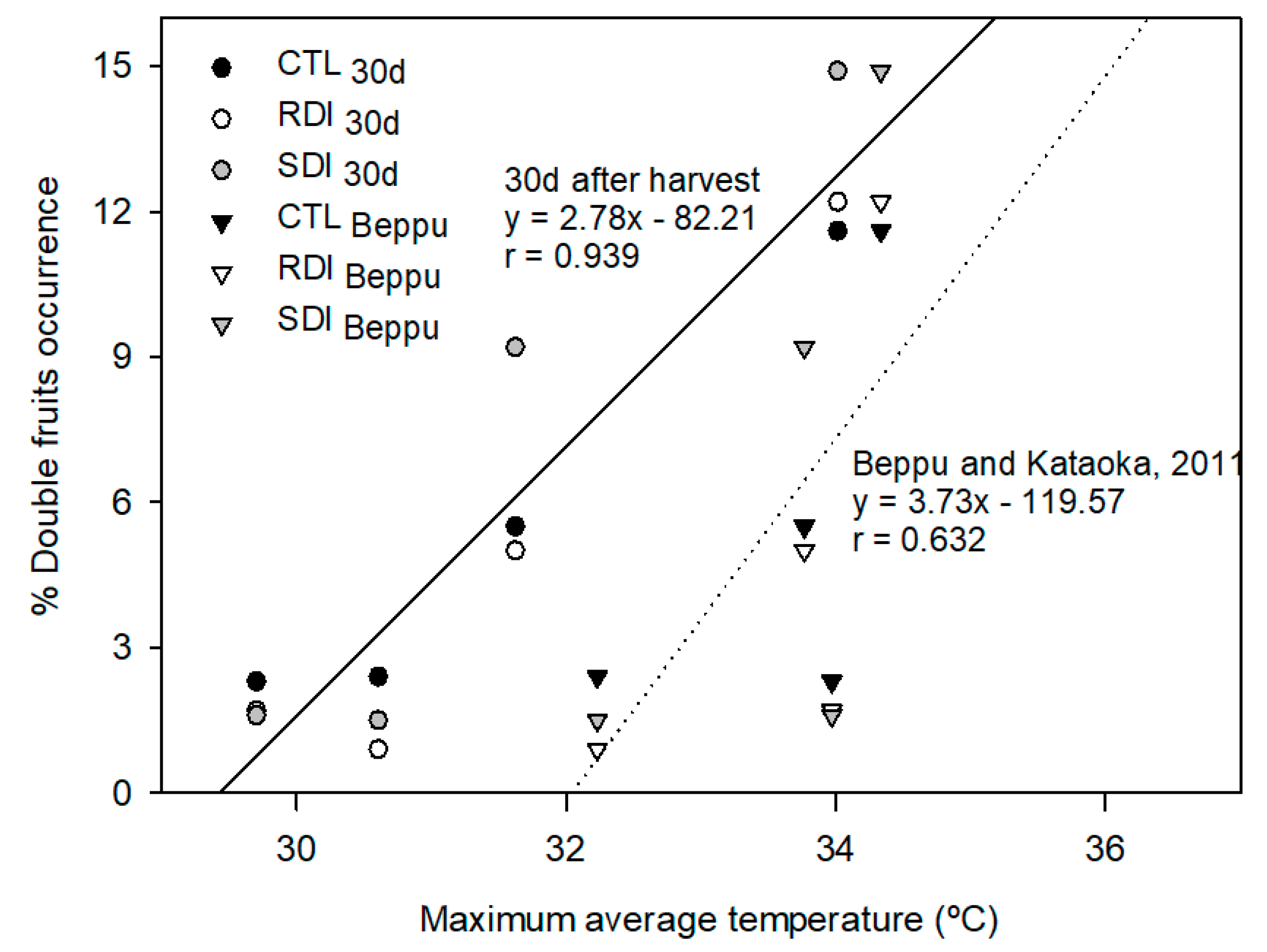
2.6. Double Fruits Incidence
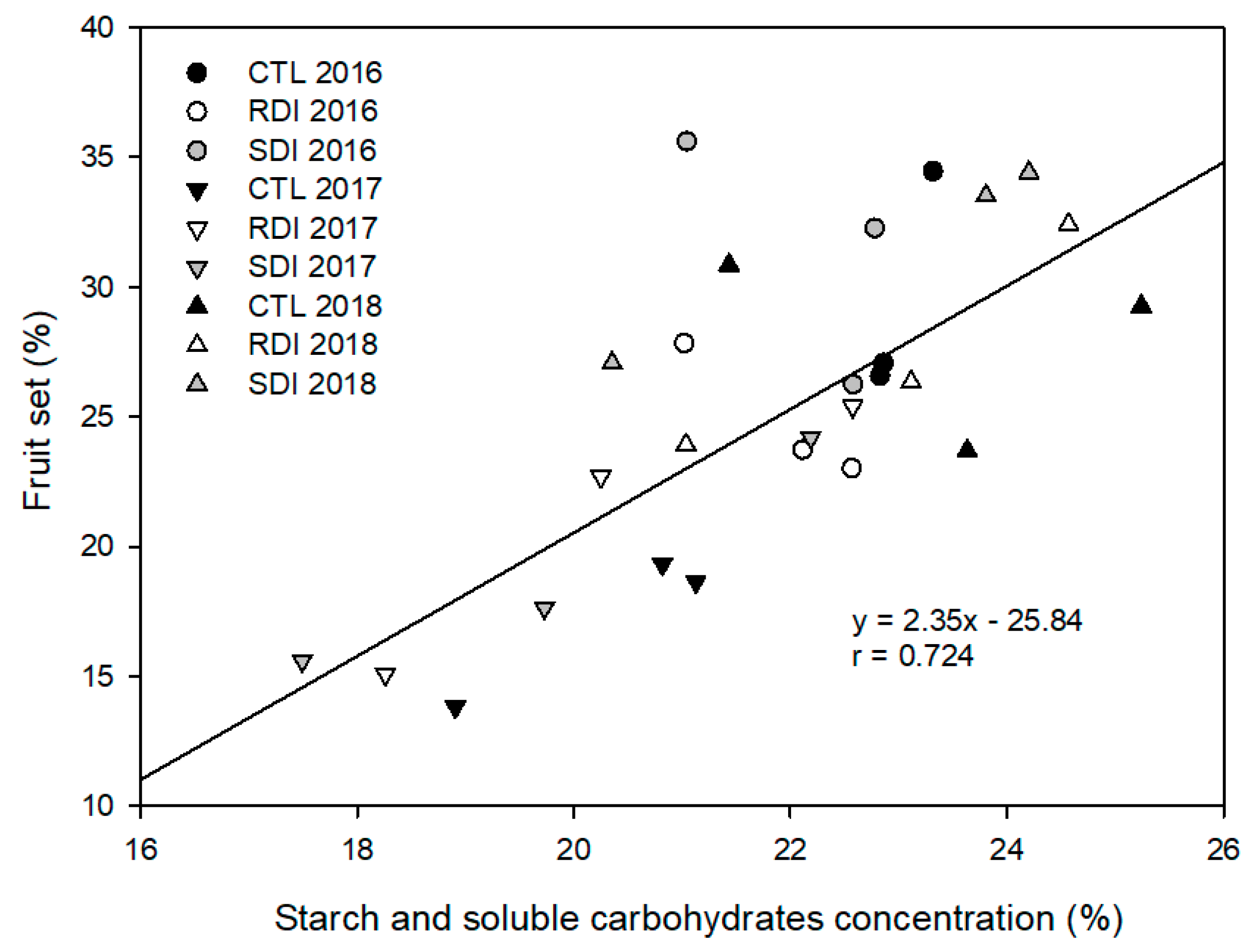
2.7. Starch and Soluble Carbohydrates Concentration (SSCC)
3. Discussion
4. Materials and Methods
4.1. Experimental Site and Plant Material
4.2. Irrigation Treatments
4.3. Weather Conditions
4.4. Flowering and Fruiting
4.5. Fruit and Vegetative Growth
4.6. Yield
4.7. Starch and Soluble Carbohydrates Concentration
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luedeling, E.; Girvetz, E.H.; Semenov, M.A.; Brown, P.H. Climate change affects winter chill for temperate fruit and nut trees. PLoS ONE 2011, 6, e20155. [Google Scholar] [CrossRef]
- Choo, Y.M.; Jo, D.J.; Yun, G.S.; Lee, E.H. A Study on the improvement of flood forecasting techniques in urban areas by considering rainfall intensity and duration. Water 2019, 11, 1883. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, M.C.; Domingo, R.; Castel, J.R. Deficit irrigation in fruit trees and vines in Spain. Span. J. Agric. Res. 2010, 2, 5–20. [Google Scholar] [CrossRef]
- Conesa, M.R.; Falagán, N.; de la Rosa, J.M.; Aguayo, E.; Domingo, R.; Pérez-Pastor, A. Post-veraison deficit irrigation regimes enhance berry coloration and health-promoting bioactive compounds in ‘Crimson Seedless’ table grapes. Agric. Water Manag. 2016, 163, 9–18. [Google Scholar] [CrossRef]
- Blanco, V.; Martínez-Hernández, G.B.; Artés-Hernández, F.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Water relations and quality changes throughout fruit development and shelf life of sweet cherry grown under regulated deficit irrigation. Agric. Water Manag. 2019, 217, 243–254. [Google Scholar] [CrossRef]
- Pérez-Pastor, A.; Ruiz-Sánchez, M.C.; Martínez, J.A.; Nortes, P.A.; Artés-Calero, F.; Domingo, R. Effect of deficit irrigation on apricot fruit quality at harvest and during storage. J. Sci. Food Agric. 2007, 87, 2409–2415. [Google Scholar] [CrossRef]
- EUROSTAT. Structure of Orchards in 2017; Newsrelease 32/2019; Eurostat Press Office: Luxembourg City, Luxembourg, 2019. [Google Scholar]
- Quero-García, J.; Schuster, M.; López-Ortega, G.; Charlot, G. Chapter 4: Sweet cherry varieties and improvement. In Cherries: Botany, Production and Uses, 1st ed.; Quero-García, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Oxfordshire, UK, 2017; pp. 60–94. [Google Scholar]
- Selahvarzi, Y.; Zamani, Z.; Fatahi, R.; Talaei, A.R. Effect of deficit irrigation on flowering and fruit properties of pomegranate (Punica granatum cv. Shahvar). Agric. Water Manag. 2017, 192, 189–197. [Google Scholar] [CrossRef]
- Cuevas, J.; Romero, I.M.; Fernández, M.D.; Hueso, J.J. Deficit irrigation schedules to promote early flowering in ’Algerie’ loquat. Acta Hortic. 2007, 750, 281–286. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Shackel, K. Irrigation cutoff and drought irrigation strategy effects on almond. In Proceedings of the 18th Almond Research Conference, Fresno, CA, USA, December 1990; pp. 30–35. [Google Scholar]
- Alburquerque, N.; Burgos, L.; Egea, J. Apricot flower bud development and abscission related to chilling, irrigation and type of shoots. Sci. Hortic. 2003, 98, 265–276. [Google Scholar] [CrossRef]
- Whiting, M.D.; Lang, G.A. ″Bing″ sweet cherry on the dwarfing rootstock ″Gisela 5″: Thinning affects fruit quality and vegetative growth but not net CO2 exchange. J. Am. Soc. Hortic. Sci. 2004, 129, 407–415. [Google Scholar] [CrossRef]
- Brown, D.S. The effects of irrigation on flower bud development and fruiting in the apricot. Proc. Am. Soc. Hortic. Sci. 1953, 61, 119–124. [Google Scholar]
- Oukabli, A.; Mahhou, A. Dormancy in sweet cherry (Prunus avium L.) under Mediterranean climatic conditions. Biotechnol. Agron. Soc. Environ. 2007, 11, 133–139. [Google Scholar]
- Alburquerque, N.; García-Montiel, F.; Carrillo-Navarro, A.; Burgos, L. Chilling and heat requirements of sweet cherry cultivars and the relationship between altitude and the probability of satisfying the chill requirements. Environ. Exp. Bot. 2008, 64, 162–170. [Google Scholar] [CrossRef]
- Beppu, K.; Ikeda, T.; Kataoka, I. Effect of high temperature exposure time during flower bud formation on the occurrence of double pistils in ‘Satohnishiki’ sweet cherry. Sci. Hortic. 2001, 87, 77–84. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Warm temperatures at bloom reduce fruit set in sweet cherry. J. Appl. Bot. Food Qual. 2007, 81, 158–164. [Google Scholar]
- Beppu, K.; Kataoka, I.J. Studies on pistil doubling and fruit set of sweet cherry in warm climate. Japan. J. Soc. Hortic. Sci. 2011, 80, 1–13. [Google Scholar] [CrossRef]
- Parker, L.E.; Abatzoglou, J.T. Warming winters reduce chill accumulation for peach production in the Southeastern United States. Climate 2019, 7, 94. [Google Scholar] [CrossRef]
- Ault, T.R.; Henebry, G.M.; de Beurs, K.M.; Schwartz, M.D.; Betancourt, J.L.; Moore, D. The false spring of 2012, earliest in North American record. Eos Trans. Am. Geophys. Union 2013, 94, 181–182. [Google Scholar] [CrossRef]
- Mitchell, P.D.; Chalmers, D.J.; Jerie, P.H.; Burge, G. The use of initial withholding of irrigation and tree spacing to enhance the effect of regulated deficit irrigation on pear trees. J. Am. Soc. Hortic. Sci. 1986, 111, 858–861. [Google Scholar]
- Nuñez-Elisea, R.; Davenport, T. Flowering of mango trees in containers as influenced by seasonal temperature and water stress. Sci. Hortic. 1994, 58, 57–66. [Google Scholar] [CrossRef]
- Arzani, K.; Wood, D.; Lawes, G.S. Influence of first season application of Paclobutrazol, root-pruning and regulated deficit irrigation on second season flowering and fruiting of mature ‘Sundrop’ apricot trees. Acta Hortic. 2000, 516, 75–82. [Google Scholar] [CrossRef]
- Johnson, R.S.; Handley, D.F.; Day, K.R. Postharvest water stress of an early maturing plum. J. Hortic. Sci. 1994, 69, 1035–1041. [Google Scholar] [CrossRef]
- Koutinas, N.; Pepelyankov, G.; Lichev, V. Flower induction and flower bud development in apple and sweet cherry. Biotechnol. Biotechnol. Equip. 2010, 24, 1549–1558. [Google Scholar] [CrossRef]
- Ayala, M.; Lang, G. Chapter 12: Morphology, cropping physiology and canopy training. In Cherries: Botany, Production and Uses, 1st ed.; Quero-García, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Oxfordshire, UK, 2017; pp. 269–304. [Google Scholar]
- Li, B.; Xie, Z.; Zhang, A.; Xu, W.; Zhang, C.; Liu, Q.; Liu, C.; Wang, S. Tree growth characteristics and flower bud differentiation of sweet cherry (Prunus avium L.) under different climate conditions in China. Hortic. Sci. 2010, 37, 6–13. [Google Scholar] [CrossRef]
- Mounzer, O.H.; Conejero, W.; Nicolás, E.; Abrisqueta, I.; García-Orellana, Y.V.; Tapia, L.M.; Vera, J.; Abrisqueta, J.M.; Ruiz-Sánchez, M. Growth pattern and phenological stages of early-maturing peach trees under a Mediterranean climate. HortScience 2008, 43, 1813–1818. [Google Scholar] [CrossRef]
- Johnson, R.S.; Handley, D.F. Using water stress to control vegetative growth and productivity of temperate fruit trees. HortScience 2000, 35, 1048–1050. [Google Scholar] [CrossRef]
- Podesta, L.; Sanchez, E.E.; Vallone, R.; Morábito, J. Effect of water deficit irrigation on vegetative growth of young cheery trees (Prunus avium L.). Revista de la Facultad de Ciencias Agrarias Universidad Nacional de Cuyo 2010, 42, 73–91. [Google Scholar]
- Blanco, V.; Torres-Sánchez, R.; Blaya-Ros, P.J.; Pérez-Pastor, A.; Domingo, R. Vegetative and reproductive response of ‘Prime Giant’ sweet cherry trees to regulated deficit irrigation. Sci. Hortic. 2019, 249, 478–489. [Google Scholar] [CrossRef]
- Candogan, B.N.; Yazgan, S. The effects of different irrigation levels on vegetative growth of young dwarf cherry trees in a sub-humid climate. Pak. J. Bot. 2010, 42, 3399–3408. [Google Scholar]
- Nieto, E.; Prieto, M.H.; Fortes, R.; Gonzalez, V.; Campillo, C. Response of a long-lived cherry cultivar to contrasting irrigation strategies in the Jerte Valley, Extremadura, Spain. Acta Hortic. 2017, 1161, 197–204. [Google Scholar] [CrossRef]
- Koumanov, K.; Staneva, I.; Kornov, G.D.; Germanova, D.R. Intensive sweet cherry production on dwarfing rootstocks revisited. Sci. Hortic. 2018, 229, 193–200. [Google Scholar] [CrossRef]
- Von Bennewitz, E.; Sanhueza, S.; Elorriaga, A. Effect of different crop load management strategies on fruit production and quality of sweet cherries (Prunus avium L.) ‘Lapins’ in Central Chile. J. Fruit Ornam Plant Res. 2010, 18, 51–57. [Google Scholar]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. High tunnel cultivation of sweet cherry (Prunus avium L.): Physiological and production variables. Sci. Hortic. 2019, 251, 108–117. [Google Scholar] [CrossRef]
- Usenik, V.; Orazem, P.; Stampar, F. Low leaf to fruit ratio delays fruit maturity of ’Lapins’ sweet cherry on Gisela5. Sci. Hortic. 2010, 126, 33–36. [Google Scholar] [CrossRef]
- Whiting, M.D.; Lang, G.; Ophardt, D. Rootstock and training system affect cherry growth, yield, and fruit quality. HortScience 2005, 40, 582–586. [Google Scholar] [CrossRef]
- Patten, K.; Nimr, G.; Neuendorff, E. Fruit doubling of peaches as affected by water stress. Acta Hortic. 1989, 254, 319–321. [Google Scholar] [CrossRef]
- Naor, A. Timing and severity of postharvest water stress affect following-year productivity and fruit quality of field-grown ‘Snow Queen’ nectarine. J. Am. Soc. Hortic. Sci. 2005, 130, 806–812. [Google Scholar] [CrossRef]
- Marsal, J.; Lopez, G.; del Campo, J.; Mata, M.; Arbones, A.; Girona, J. Postharvest regulated deficit irrigation in ’Summit’ sweet cherry fruit yield and quality in the following season. Irrig. Sci. 2010, 28, 181–189. [Google Scholar] [CrossRef]
- Dichio, B.; Xiloyannis, C.; Nuzzo, V.; Montanaro, G.; Palese, A.M. Postharvest regulated deficit irrigation of peach tree in a Mediterranean environment: Effects on vegetative growth and yield. Acta Hortic. 2004, 664, 169–174. [Google Scholar] [CrossRef]
- Marsal, J. FAO Irrigation and Drainage Paper 66. In Crop Yield Response Water; FAO: Rome, Italy, 2012; pp. 449–457. [Google Scholar]
- Fereres, E.; Martinich, D.A.; Aldrich, T.M.; Castel, J.R.; Holzapfel, E.; Schulbach, H. Drip irrigation saves money in young almond orchards. Calif. Agric. 1982, 36, 12–13. [Google Scholar]
- Weinberger, J.H. Chilling requirements of peach varieties. Proc. Am. Soc. Hortic. Sci. 1950, 56, 122–128. [Google Scholar]
- Gilreath, P.; Buchanan, D. Rest prediction model for low chilling Sungold nectarine. J. Am. Soc. Hortic. Sci. 1981, 106, 426–429. [Google Scholar]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Mathematical analysis of a two-step model involving cooperative transition. J. Theor. Biol. 1987, 124, 473–483. [Google Scholar] [CrossRef]
- Luedeling, E.; Zhang, M.; Luedeling, V.; Girvetz, E.H. Sensitivity of winter chill models for fruit and nut trees to climatic changes expected in California’s Central Valley. Agric. Ecosyst. Environ. 2009, 133, 23–31. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar]
| Chill Hours | Chill Units | Chill Portions | GDH | Blooming Date | |
|---|---|---|---|---|---|
| 2015 | 1299 | 1123 | 94 | 6853 | April 6th |
| 2016 | 1181 | 1038 | 93 | 7014 | April 16th |
| 2017 | 1326 | 1269 | 96 | 6363 | April 3rd |
| 2018 | 1274 | 1087 | 92 | 6708 | April 12th |
| Mean | 1270 | 1129 | 94 | 6735 | |
| SE | 31.4 | 49.6 | 0.8 | 139 | |
| CV | 0.02 | 0.04 | 0.01 | 0.02 |
| Spurs per Meter | Flower Buds per Spur | Flowers per Bud | N0 Fruits per 100 Flowers | ||
|---|---|---|---|---|---|
| Treatment | CTL | 15.77 | 5.17 | 3.04 | 21.93 |
| RDI | 17.43 | 5.18 | 3.10 | 22.06 | |
| SDI | 18.33 | 5.39 | 3.19 | 24.87 | |
| Year | 2015 | - | 4.79 | 2.92 | 15.08 |
| 2016 | 18.09 | 5.40 | 3.48 | 28.53 | |
| 2017 | 14.93 | 5.16 | 3.04 | 19.16 | |
| 2018 | 18.51 | 5.63 | 3.00 | 29.05 | |
| ANOVA | Treatment | n.s. | n.s. | n.s. | n.s. |
| Year | n.s. | n.s. | n.s. | *** | |
| T × Y | n.s. | n.s. | n.s. | n.s. |
| DOY | DAFB | Duration | GDD | ||
|---|---|---|---|---|---|
| 2015 | Full Bloom | 96 | 0 | 8 | 74 |
| Fruit Set | 104 | 8 | 9 | 98 | |
| Stage I | 113 | 17 | 10 | 128 | |
| Stage II | 123 | 27 | 7 | 107 | |
| Stage III | 130 | 34 | 24 | 370 | |
| Harvest | 154 | 58 | 58 | 777 | |
| 2016 | Full Bloom | 107 | 0 | 7 | 72 |
| Fruit Set | 114 | 7 | 10 | 89 | |
| Stage I | 124 | 17 | 13 | 140 | |
| Stage II | 137 | 30 | 9 | 111 | |
| Stage III | 146 | 39 | 23 | 392 | |
| Harvest | 169 | 62 | 62 | 805 | |
| 2017 | Full Bloom | 93 | 0 | 8 | 75 |
| Fruit Set | 95 | 8 | 7 | 94 | |
| Stage I | 105 | 15 | 14 | 121 | |
| Stage II | 116 | 29 | 8 | 101 | |
| Stage III | 126 | 37 | 23 | 352 | |
| Harvest | 153 | 60 | 60 | 743 | |
| 2018 | Full Bloom | 102 | 0 | 8 | 76 |
| Fruit Set | 110 | 8 | 7 | 89 | |
| Stage I | 117 | 15 | 12 | 128 | |
| Stage II | 129 | 27 | 8 | 93 | |
| Stage III | 137 | 35 | 28 | 405 | |
| Harvest | 165 | 63 | 63 | 791 | |
| Total duration | Mean | 160 | 61 | 779 | |
| SE | 8.0 | 2.2 | 26.6 | ||
| CV | 0.050 | 0.036 | 0.034 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees. Plants 2020, 9, 94. https://doi.org/10.3390/plants9010094
Blanco V, Blaya-Ros PJ, Torres-Sánchez R, Domingo R. Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees. Plants. 2020; 9(1):94. https://doi.org/10.3390/plants9010094
Chicago/Turabian StyleBlanco, Victor, Pedro José Blaya-Ros, Roque Torres-Sánchez, and Rafael Domingo. 2020. "Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees" Plants 9, no. 1: 94. https://doi.org/10.3390/plants9010094
APA StyleBlanco, V., Blaya-Ros, P. J., Torres-Sánchez, R., & Domingo, R. (2020). Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees. Plants, 9(1), 94. https://doi.org/10.3390/plants9010094





