Simalikalactone D, a Potential Anticancer Compound from Simarouba tulae, an Endemic Plant of Puerto Rico
Abstract
1. Introduction
2. Results and Discussion
2.1. Assessment of the Antiproliferative Potential of Extracts in Cancer Cell Lines
2.1.1. Preparation of Plant Extracts
2.1.2. Determination of the Biological Activity of Plant Extracts in Cancer Cells
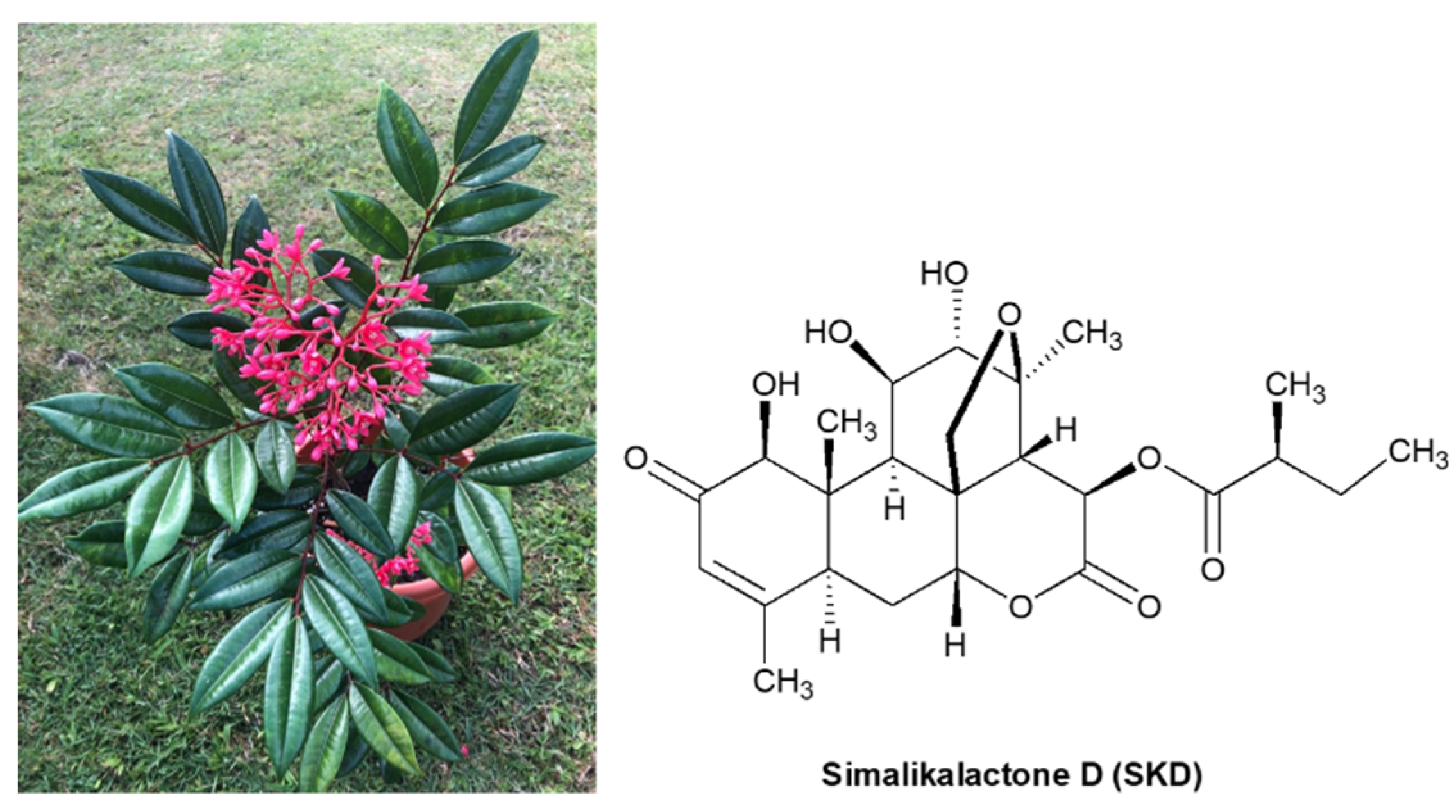
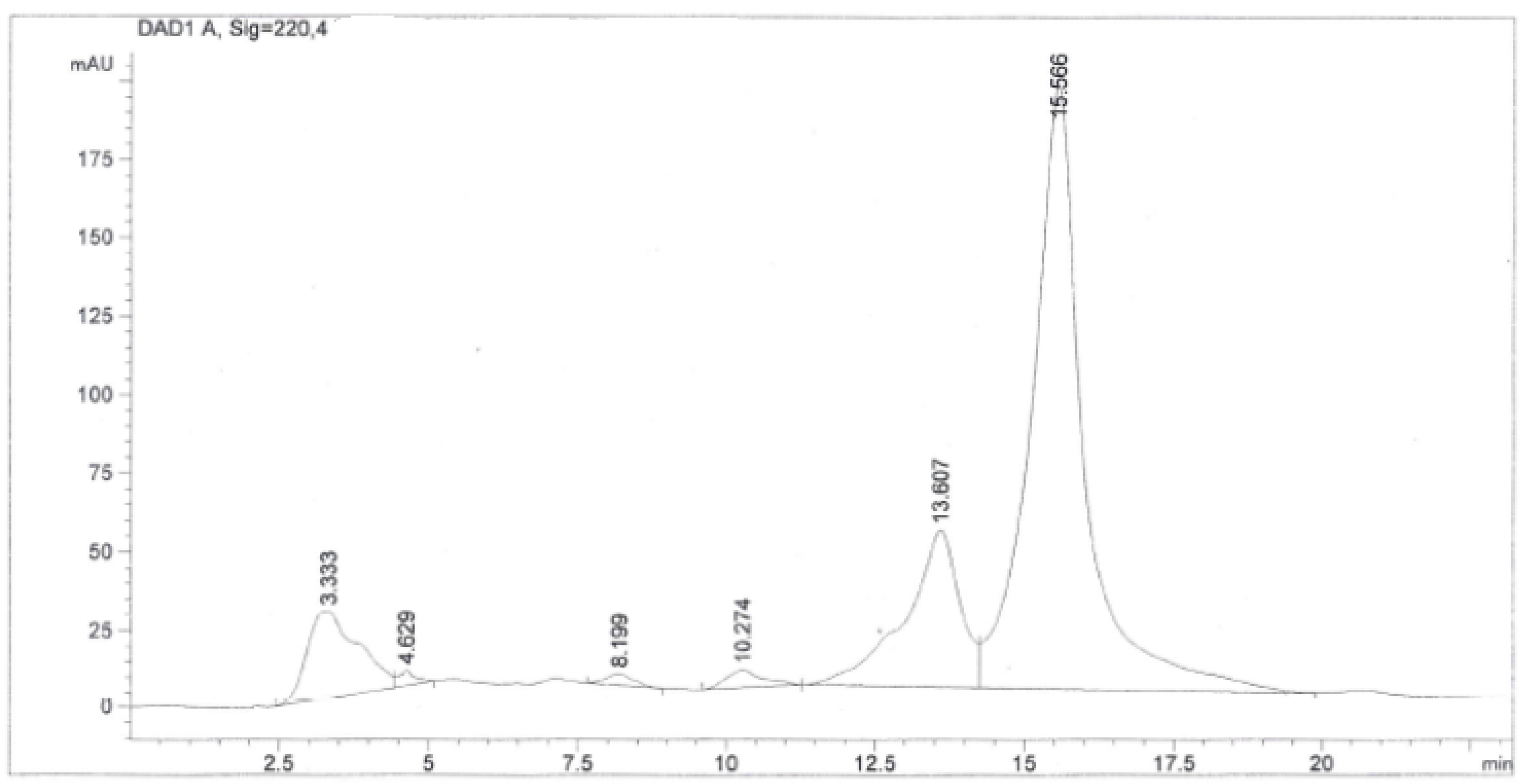
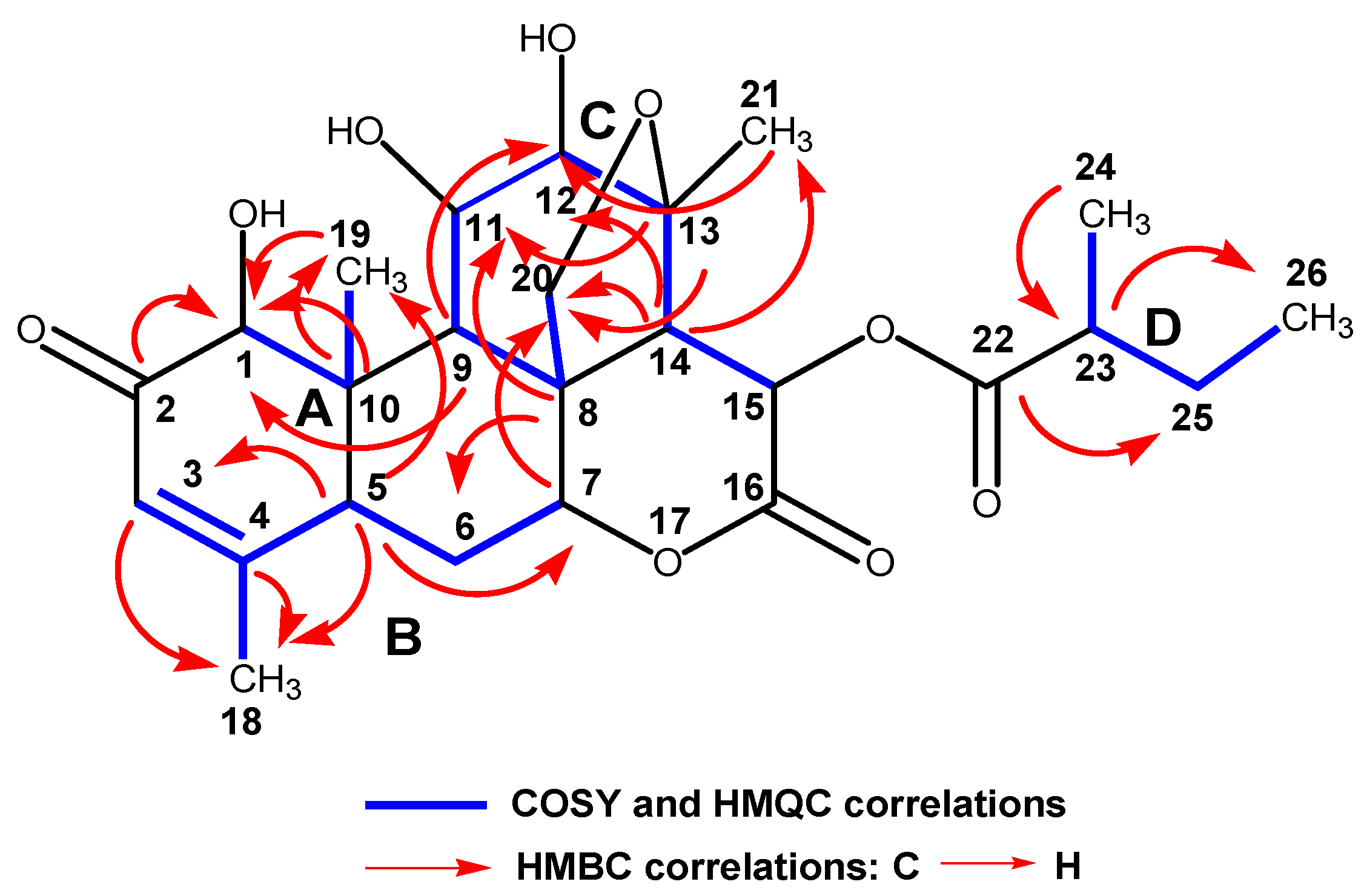
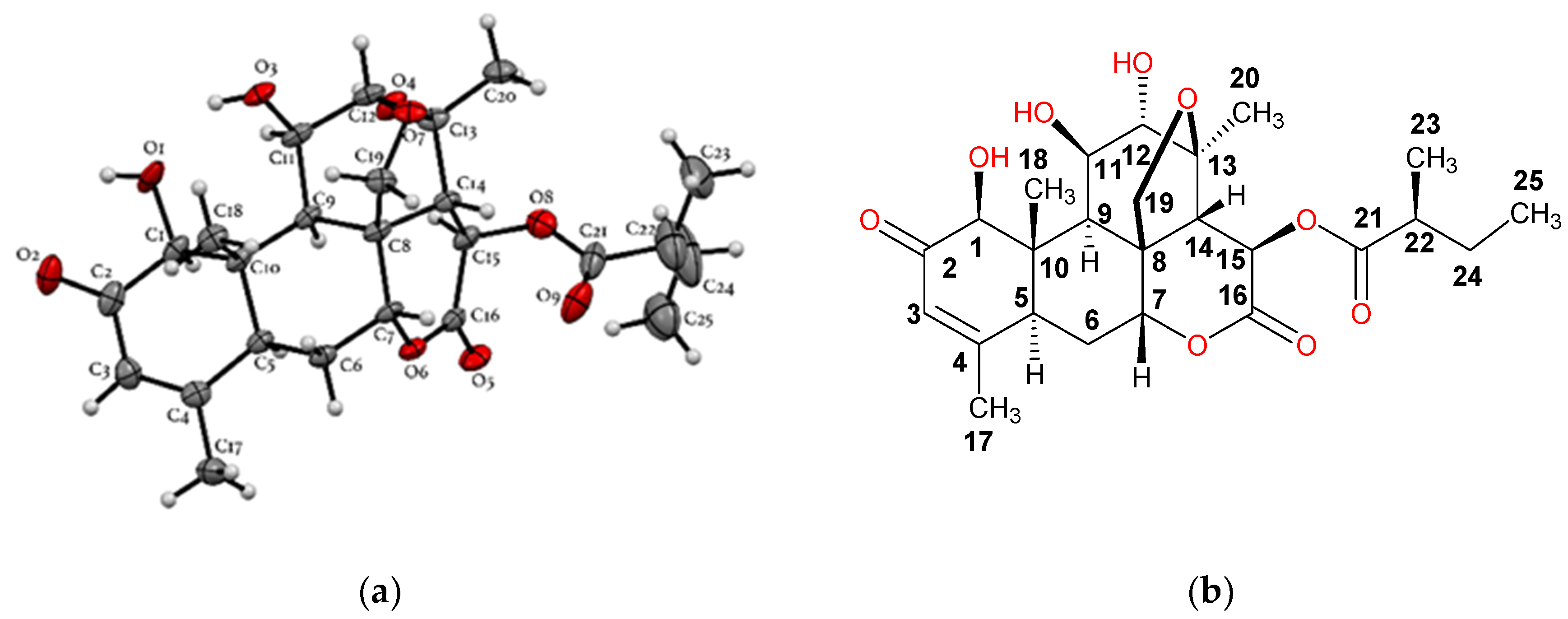
2.2. Purification, Isolation, and Characterization of Simalikalactone D (SKD)
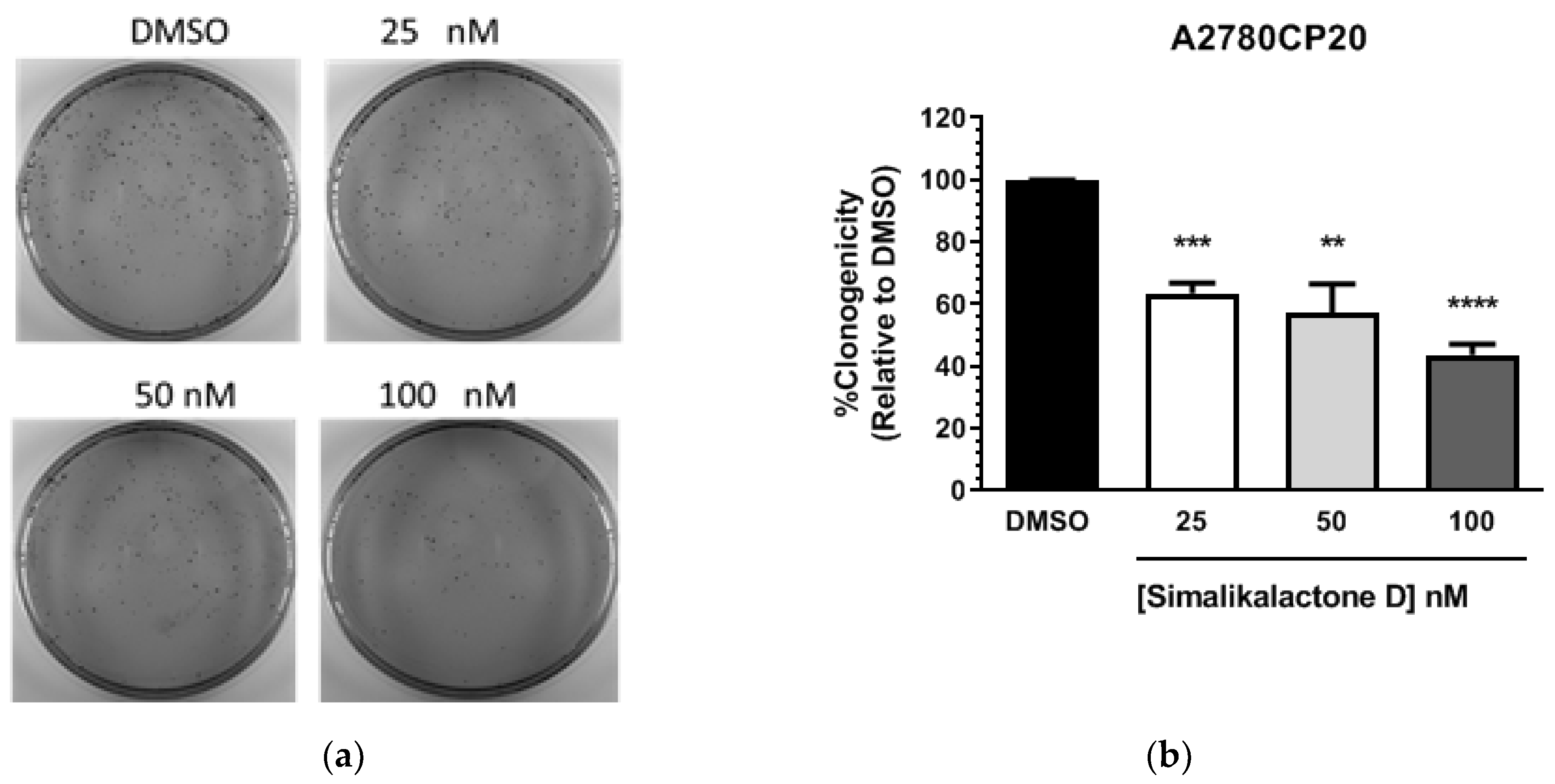
2.3. Potential Antiproliferative Activity of Simalikalactone D (SKD)
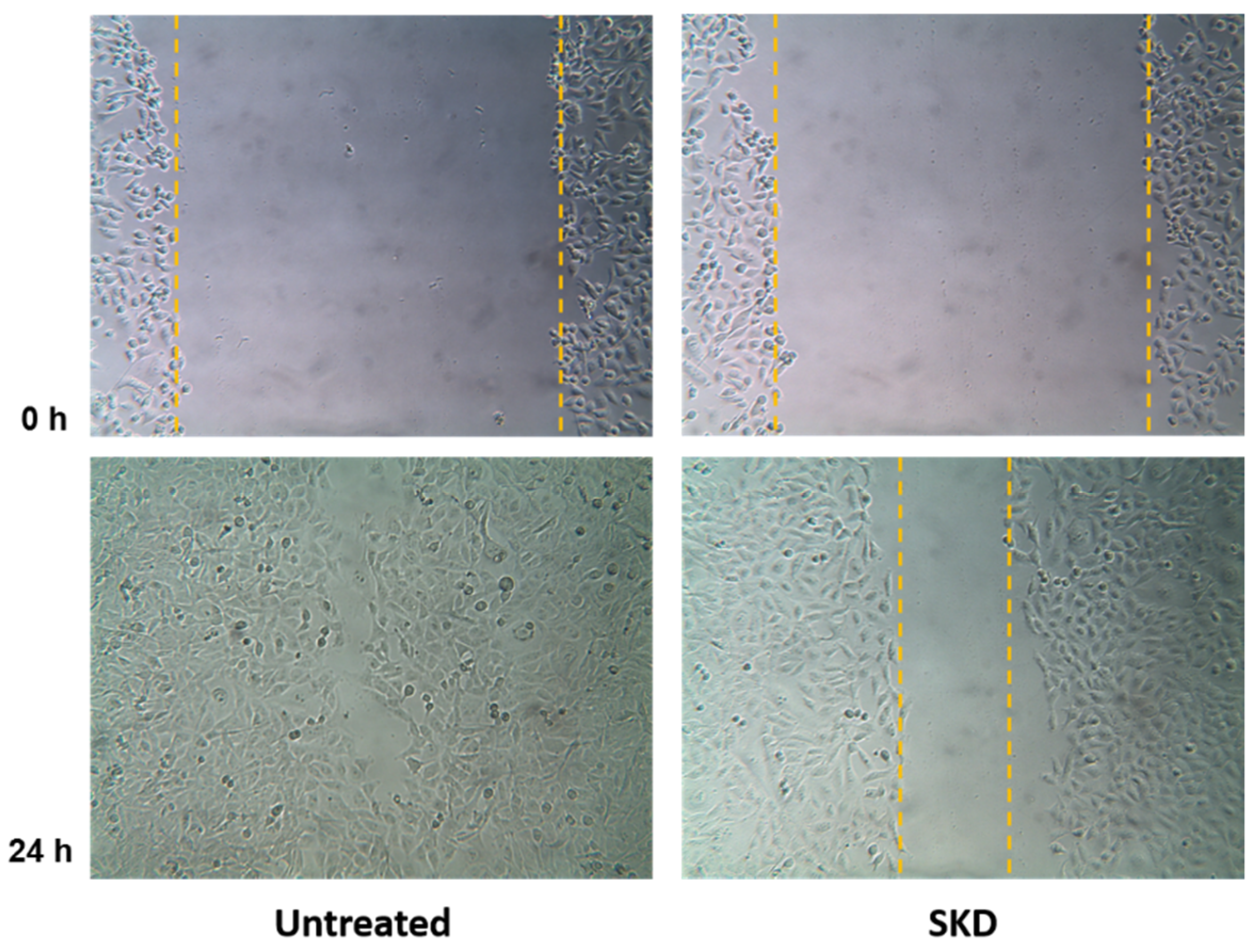
2.4. Anti-Migratory Activity of Simalikalactone D (SKD)
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Spectroscopic Data of Simalikalactone D (SKD)
3.3.1. Simalikalactone D (SKD)
3.3.2. Crystal Data for Simalikalactone D (SKD)
3.4. Biological Evaluation
3.4.1. Cell Lines and Culture Conditions
3.4.2. Cell Viability and Colony Formation Assays
3.4.3. MTT (3-(4,5-Dymethyl thiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide) Assay for Cell Viability
3.4.4. Sulforhodamine B (SRB) Assay
3.4.5. Wound Healing Assay (Scratch Assay) Using MDA-MB-231 Cancer Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Global Cancer Facts & Figures, 4th ed.; American Cancer Society: Atlanta, GA, USA, 2018; pp. 1–76. [Google Scholar]
- Greenwell, M.; Rahman, P.K. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Shah, U.; Shah, R.; Acharya, S.; Acharya, N. Novel Anticancer Agents from Plant Sources. Chin. J. Nat. Med. 2013, 11, 16–23. [Google Scholar] [CrossRef]
- Majolo, F.; Delwing, L.K.D.O.B.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Vangapandu, S.; Sindelar, R.W.; Walker, L.A.; Sindelar, R.D. Biologically Active Quassinoids and Their Chemistry: Potential Leads for Drug Design. Curr. Med. Chem. 2005, 12, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Liou, Y.F.; Okano, M.; Lee, K.H. Antitumor agents XLVI: In vitro effects of esters of brusatol, bisbrusatol, and related compounds on nucleic acid and protein synthesis of P-388 lymphocytic leukemia cells. J. Pharm. Sci. 1982, 71, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.J.; Liu, Y.; Han, S.; Yang, C. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell Biosci. 2019, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef]
- Blicharska, N.M.; Zhixiu, L. Anti-proliferative effect of Brusatol and Brucein D on human breast cancer cells. J. Undergrad. Life Sci. 2016, 10, 45–49. [Google Scholar]
- Alves, I.A.; Miranda, H.M.; Soares, L.A.; Randau, K.P. Simaroubaceae family: Botany, chemical composition and biological activities. Rev. Bras. Farmacogn. 2014, 24, 481–501. [Google Scholar] [CrossRef]
- Little, E.L., Jr.; Woodbury, R.O.; Wadsworth, F.H. Trees of Puerto Rico and the Virgin Islands. In United States Department of Agriculture, Forest Service, Agricultural Handbook No. 449; United States Government Printing Office: Washington, DC, USA, 1974; Volume 2. [Google Scholar]
- Claudio, K.; Hernández, J.; Rivera, J.; Ortiz, I.; Carvajal, A.; Perez, M.; Pagán, M.; Ospina, C.A. Biological Screening of Select Puerto Rican Plants for cytotoxic and antitumor activities. Proc. R. Health Sci. J. 2015, 34, 25–30. [Google Scholar]
- Arisawa, M.; Fujita, A.; Morita, N.; Kinghorn, A.D.; Cordell, G.A.; Farnsworth, N.R. Plant Anticancer Agents XXXV. Further Constituents of Simaba multiflora. Planta Med. 1985, 51, 348–349. [Google Scholar] [CrossRef]
- Cabral, J.A.; McChesney, J.D.; Milhous, W.K. A new antimalarial quassinoid from Simaba guianensis. J. Nat. Prod. 1993, 56, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Cachet, N.; Hoakwie, F.; Bertani, S.; Bourdy, G.; Deharo, E.; Stien, D.; Houel, E.; Gornitzka, H.; Fillaux, J.; Chevalley, S.; et al. Antimalarial activity of simalikalactone E, a new quassinoid from Quassia amara L. (Simaroubaceae). Antimicrob. Agents Chemother. 2009, 53, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Bedir, E.; Khan, S.I.; Tekwani, B.L.; Khan, I.A.; Takamatsu, S.; Pelletier, J.; Walker, L.A. A New Antimalarial Quassinoid from Simaba orinocensis. J. Nat. Prod. 2004, 67, 772–777. [Google Scholar] [CrossRef]
- Samaa, W.; Ajaiyeobab, E.O.; Choudhary, M.I. Larvicidal properties of simalikalactone D from Quassia africana (Simaroubaceae) baill and baill, on the malaria vector Anopheles gambiae. Afr. J. Tradit. Complement. Alter. Med. 2014, 11, 84–88. [Google Scholar] [CrossRef][Green Version]
- O’Neill, M.J.; Bray, D.H.; Boardman, P.; Phillipson, J.D.; Warhurst, D.C.; Peters, W.; Suffness, M. Plants as sources of antimalarial drugs: In vitro antimalarial activities of some quassinoids. Antimicrob. Agents Chemother. 1986, 30, 101–104. [Google Scholar] [CrossRef]
- Bertani, S.; Houel, E.; Bourdy, G.; Stien, D.; Jullian, V.; Landau, I.; Deharo, E. Quassia amara L. (Simaroubaceae) leaf tea: Effect of the growing stage and desiccation status on the antimalarial activity of a traditional preparation. J. Ethnopharmacol. 2007, 111, 40–42. [Google Scholar] [CrossRef]
- Houël, E.; Bertani, S.; Bourdy, G.; Deharo, E.; Jullian, V.; Valentin, A.; Chevalley, S.; Stien, D. Quassinoid constituents of Quassia amara L. leaf herbal tea. Impact on its antimalarial activity and cytotoxicity. J. Ethnopharmacol. 2009, 126, 114–118. [Google Scholar] [CrossRef]
- Ozeki, A.; Hitotsuyanagi, Y.; Hashimoto, E.; Itokawa, H.; Takeya, K.; de Mello Alves, S. Cytotoxic quassinoids from Simaba cedron. J. Nat. Prod. 1998, 61, 776–780. [Google Scholar] [CrossRef]
- Hunter, K.W.; Crawford, N.P.; Alsarraj, J. Mechanisms of metastasis. Breast Cancer Res. 2008, 10, S2. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Liu, Z.P. Natural products as anti-invasive and anti-metastatic agents. Curr. Med. Chem. 2011, 18, 808–829. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Bracke, M.E.; Vanhoecke, B.W.A.; Derycke, L.; Bolca, S.; Possemiers, S.; Heyerick, A.; Stevens, C.V.; Keukeleire, D.D.; Depypere, H.T.; et al. Plant polyphenolics as anti-invasive cancer agents. Anticancer Agents Med. Chem. 2008, 8, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio. Protoc. 2016, 6, e1984. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Silva, T.; Meerow, A.W.; Goenaga, R.; Irish, B. Ornamental plant germplasm exploration in tropical forests of Puerto Rico. Proc. Fla. State Hort. Soc. 2007, 120, 4–7. [Google Scholar]
- Ewel, J.J.; Whitmore, J.L. The ecological life zones of Puerto Rico and the US Virgin Islands. In USDA Forest Service Institute of Tropical Forestry Research Paper ITF-18; Institute of Tropical Forestry: Rio Piedras, Puerto Rico, 1973. [Google Scholar]
- Phadke, P.A.; Mercer, R.R.; Harms, J.F.; Jia, Y.; Frost, A.R.; Jewell, J.L.; Bussard, K.M.; Nelson, S.; Moore, C.; Kappes, J.C.; et al. Kinetics of metastatic breast cancer cell trafficking in bone. Clin. Cancer Res. 2006, 12, 1431–1440. [Google Scholar] [CrossRef]
| Extract | Dry Weight (g) |
|---|---|
| Crude 1 | 113 |
| Hexane | 31 |
| Chloroform | 40 |
| Ethyl acetate | 6 |
| Butanol | 15 |
| Extract/Fraction | A2780CP20 IC50 (µg/mL) a | MDA-MB-231 IC50 (µg/mL) a |
|---|---|---|
| Crude extract | 0.75 | 2.41 |
| Hexane extract | 3.67 | 0.0024 |
| Chloroform extract | 0.14 | 0.0022 |
| Ethyl acetate extract | 36.0 | N.T. |
| Butanol extract | 0.58 | N.T. |
| SH2C3 fraction b | 0.044 | N.T. |
| Extract/Fraction | SH-SY5Y %GI a |
|---|---|
| Crude extract | 75 |
| Hexane extract | 55 |
| Chloroform extract | 83 |
| Ethyl acetate extract | 76 |
| Butanol extract | 16 |
| SH2C2 fraction b | 80 |
| SH2C3 fraction b | 88 |
| SH2C4 fraction b | 76 |
| SH2C5 fraction b | 64 |
| Atom a | Reported SKD b δH, δC | Revised SKD c δH, δC | SKE d δH, δC | Orinocinolide d δH, δC |
|---|---|---|---|---|
| 8 e | 47.7 | 45.9 | 46.1 | 44.1 |
| 10 e | 45.9 | 47.7 | 50.4 | 46.5 |
| 11 | 3.77, 79.4 | 4.64, 74.4 | 4.75, 74.2 | 4.70, 74.8 |
| 12 | 4.63, 74.4 | 3.79, 79.4 | 3.83, 79.8 | 3.66, 78.9 |
| 19 | 1.18, 22.9 | 1.19, 11.4 | 1.35, 12.5 | 1.20, 12.0 |
| 21 | 1.43, 16.6 | 1.44, 22.9 | 1.45, 22.8 | 1.39, 23.4 |
| 24 | 1.22, 11.4 | 1.21,16.6 | 1.21, 16.7 | 1.18, 16.7 |
| Cell Line | IC50 (nM) a |
|---|---|
| A2780CP20 (Ovarian cancer) c | 55 a |
| MDA-MB-231 (Breast cancer) d,g | 65 a, 63 b |
| MDA-MB-435 (Breast cancer) d | 58 b |
| 4T1 (Breast cancer) e | >100 b |
| MCF10A (Breast epithelial cells) | 67 f |
| PC3 (Prostate cancer) | >100 a |
| HCT-116 (Colon cancer) | >100 a |
| SH-SY5Y (Neuroblastoma) | >100 (39.8 µM) b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez, B.; Reyes, J.; Conde, I.; Ramos, Z.; Lozada, E.; Cruz, A.M.; Asencio, G.; Carvajal, A.; Dharmawardhane, S.; Piñero-Cruz, D.M.; et al. Simalikalactone D, a Potential Anticancer Compound from Simarouba tulae, an Endemic Plant of Puerto Rico. Plants 2020, 9, 93. https://doi.org/10.3390/plants9010093
Mendez B, Reyes J, Conde I, Ramos Z, Lozada E, Cruz AM, Asencio G, Carvajal A, Dharmawardhane S, Piñero-Cruz DM, et al. Simalikalactone D, a Potential Anticancer Compound from Simarouba tulae, an Endemic Plant of Puerto Rico. Plants. 2020; 9(1):93. https://doi.org/10.3390/plants9010093
Chicago/Turabian StyleMendez, Belmari, Jeyshka Reyes, Isabel Conde, Zulma Ramos, Eunice Lozada, Ailed M. Cruz, Gabriela Asencio, Augusto Carvajal, Suranganie Dharmawardhane, Dalice M. Piñero-Cruz, and et al. 2020. "Simalikalactone D, a Potential Anticancer Compound from Simarouba tulae, an Endemic Plant of Puerto Rico" Plants 9, no. 1: 93. https://doi.org/10.3390/plants9010093
APA StyleMendez, B., Reyes, J., Conde, I., Ramos, Z., Lozada, E., Cruz, A. M., Asencio, G., Carvajal, A., Dharmawardhane, S., Piñero-Cruz, D. M., Hernández, E., Vivas, P., & Ospina, C. A. (2020). Simalikalactone D, a Potential Anticancer Compound from Simarouba tulae, an Endemic Plant of Puerto Rico. Plants, 9(1), 93. https://doi.org/10.3390/plants9010093








