Antioxidant and Enzyme Inhibitory Properties of the Polyphenolic-Rich Extract from an Ancient Apple Variety of Central Italy (Mela Rosa dei Monti Sibillini)
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition Profile
2.2. In Vitro Antioxidant Capacity Assays
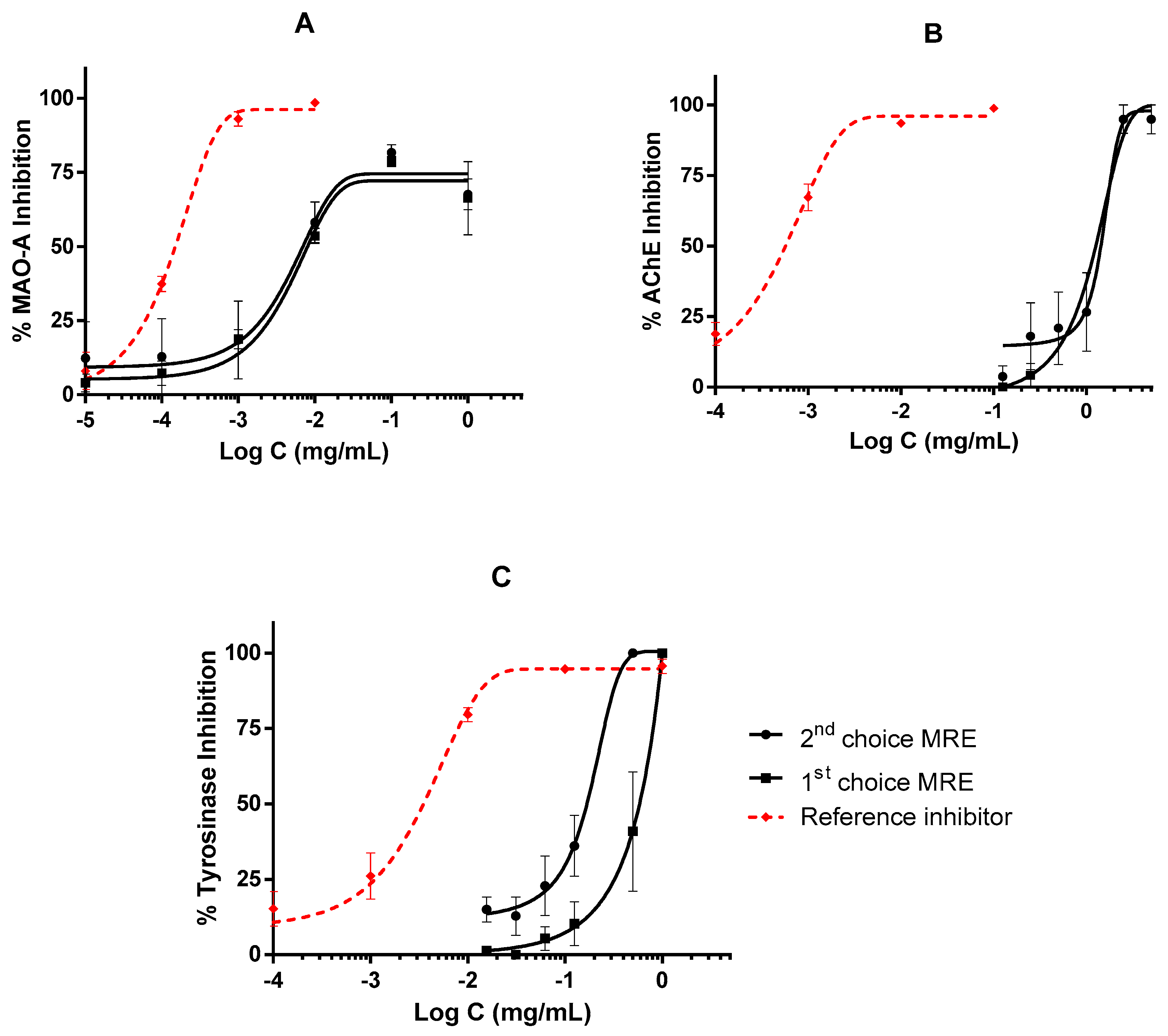
2.3. Inhibitory Activities on Biological Enzymes
3. Discussion
4. Materials and Methods
4.1. Preparation and Purification of Apple Extracts
4.2. HPLC Analysis of Polar Constituents
4.2.1. Reagents and Standards
4.2.2. Method Validation
4.3. In vitro Antioxidant Capacity Assays
4.3.1. Quantification of Total Phenolic Content
4.3.2. Free Radical Scavenging Activity (DPPH Assay)
4.3.3. Radical Cation Decolorization ABTS Assay
4.4. Biological Enzymes Inhibitory Activities
4.4.1. Reagents and Chemicals
4.4.2. Bioassays Regarding Metabolic Enzymes
4.4.3. Bioassays Regarding CNS Enzymes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bignami, C.; Scossa, A.; Vagnoni, G. Evaluation of old Italian apple cultivars by means of sensory analysis. Acta Hortic. 2003, 598, 85–90. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.K.; Canterino, S.; Bounous, G. Application of sensory, nutraceutical and genetic techniques to create a quality profile of ancient apple cultivars. J. Food Qual. 2012, 35, 169–181. [Google Scholar] [CrossRef]
- McCann, M.J.; Gill, C.I.R.; O’Brien, G.; Rao, J.R.; McRoberts, W.C.; Hughes, P.; McEntee, R.; Rowland, I.R. Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem. Toxicol. 2007, 45, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Petriccione, M.; Rega, P.; Scortichini, M.; Napolitano, A. A reappraisal of traditional apple cultivars from Southern Italy as a rich source of phenols with superior antioxidant activity. Food Chem. 2013, 140, 672–679. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Hudault, S.; Lunken, R.C.; Christeller, J.T. Apple peels, from seven cultivars, have lipase-inhibitory activity and contain numerous ursenoic acids as identified by LC-ESI-QTOF-HRMS. J. Agric. Food Chem. 2011, 60, 482–491. [Google Scholar] [CrossRef]
- Nkuimi Wandjou, J.G.; Sut, S.; Giuliani, C.; Fico, G.; Papa, F.; Ferraro, S.; Giovanni, C.; Maggi, F.; Dall’Acqua, S. Characterization of nutrients, polyphenols and volatile components of the ancient apple cultivar ‘Mela Rosa Dei Monti Sibillini’from Marche region, central Italy. Int. J. Food Sci. Nutr. 2019, 70, 796–812. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Pacifico, S.; Cefarelli, G.; Mastellone, C.; Fiorentino, A. ‘Limoncella’apple, an Italian apple cultivar: Phenolic and flavonoid contents and antioxidant activity. Food Chem. 2007, 104, 1333–1337. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Sun, L.; Liu, D.; Sun, J.; Yang, X.; Fu, M.; Guo, Y. Simultaneous separation and purification of chlorogenic acid, epicatechin, hyperoside and phlorizin from thinned young Qinguan apples by successive use of polyethylene and polyamide resins. Food Chem. 2017, 230, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Thu, N.N.; Tien, P.G.; Van Chuyen, N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J. Nutr. Sci. Vitaminol. 2007, 53, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou, K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr. Metab. 2010, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Guo, Y.; Fu, C.; Li, J.; Li, Z. Simultaneous separation and purification of total polyphenols, chlorogenic acid and phlorizin from thinned young apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Tuszyński, T. Antioxidant activity of apples–an impact of maturity stage and fruit part. Acta Sci. Pol. Technol. Aliment. 2011, 10, 443–454. [Google Scholar] [PubMed]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Monsanto, M.; Hooshyar, N.; Meuldijk, J.; Zondervan, E. Modeling and optimization of green tea precipitation for the recovery of catechins. Sep. Purif. Technol. 2014, 129, 129–136. [Google Scholar] [CrossRef]
- Huber, G.M.; Rupasinghe, H.P.V. Phenolic profiles and antioxidant properties of apple skin extracts. J. Food Sci. 2009, 74, C693–C700. [Google Scholar] [CrossRef]
- Karaman, Ş.; Tütem, E.; Başkan, K.S.; Apak, R. Comparison of antioxidant capacity and phenolic composition of peel and flesh of some apple varieties. J. Sci. Food Agric. 2013, 93, 867–875. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Fiorentino, A.; Monaco, P.; Oriano, P.; Pacifico, S. Annurcoic acid: A new antioxidant ursane triterpene from fruits of cv. Annurca apple. Food Chem. 2006, 98, 285–290. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Hemmati, S.; Shirooie, S.; Nabavi, S.M.; Bonakdar, A.T.; Fayaznia, R.; Asgardoon, M.H.; Dehnavi, A.Z.; Ghafouri, M.; Nkuimi Wandjou, J.G.; et al. Protective effects of hydroalcoholic extracts from an ancient apple variety ‘Mela Rosa dei Monti Sibillini’against renal ischemia/reperfusion injury in rats. Food Funct. 2019, 10, 7544–7552. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar]
- Schmidt, J.S.; Lauridsen, M.B.; Dragsted, L.O.; Nielsen, J.; Staerk, D. Development of a bioassay-coupled HPLC-SPE-ttNMR platform for identification of α-glucosidase inhibitors in apple peel (Malus× domestica Borkh.). Food Chem. 2012, 135, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Bangert, A.; Kottra, G.; Geillinger, K.E.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Inhibition of the intestinal sodium--Coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol. Nutr. Food Res. 2014, 58, 1795–1808. [Google Scholar] [CrossRef]
- Saxena, S.; Verma, J.; Gautam, S. Potential prophylactic properties of apple and characterization of potent bioactive from cv.“Granny Smith” displaying strong antimutagenicity in models including human lymphoblast TK6+/− cell line. J. Food Sci. 2016, 81, H508–H518. [Google Scholar] [CrossRef]
- de Oliveira Raphaelli, C.; dos Santos Pereira, E.; Camargo, T.M.; Vinholes, J.; Rombaldi, C.V.; Vizzotto, M.; Nora, L. Apple Phenolic Extracts Strongly Inhibit α-Glucosidase Activity. Plant. Foods Hum. Nutr. 2019, 74, 430–435. [Google Scholar] [CrossRef]
- Gaudout, D.; Megard, D.; Inisan, C.; Esteve, C.; Lejard, F. Phloridzin-Rich Phenolic Fraction and Use Thereof as a Cosmetic, Dietary or Nutraceutical Agent. U.S. Patent No. 7,041,322, 9 May 2006. [Google Scholar]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Ikarashi, N.; Takeda, R.; Ito, K.; Ochiai, W.; Sugiyama, K. The inhibition of lipase and glucosidase activities by acacia polyphenol. Evid. Based Complement Alternat. Med. 2011, 2011, 272075. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, H.; Akazome, Y.; Shoji, T.; Yamaguchi, A.; Yasue, M.; Kanda, T.; Ohtake, Y. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J. Agric. Food Chem. 2007, 55, 4604–4609. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Rupasinghe, H.P. Polyphenols: Multipotent therapeutic agents in neurodegenerative diseases. Oxid. Med. Cell Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouayed, J. Polyphenols: A potential new strategy for the prevention and treatment of anxiety and depression. Curr. Nutr. Food Sci. 2010, 6, 13–18. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H.; Eberhardt, M.V.; Lee, C.Y. Antioxidant and antiproliferative activities of selected New York apple cultivars. N. Y. Fruit Q. 2001, 9, 15. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Beghelli, D.; Isani, G.; Roncada, P.; Andreani, G.; Bistoni, O.; Bertocchi, M.; Lupidi, G.; Alunno, A. Antioxidant and ex vivo immune system regulatory properties of Boswellia serrata extracts. Oxid. Med. Cell. Longev. 2017, 2017, 7468064. [Google Scholar] [CrossRef] [Green Version]
- Censi, R.; Vargas Peregrina, D.; Lacava, G.; Agas, D.; Lupidi, G.; Sabbieti, M.; Di Martino, P. Cosmetic Formulation Based on an Açai Extract. Cosmetics 2018, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Carpéné, C.; Les, F.; Cásedas, G.; Peiro, C.; Fontaine, J.; Chaplin, A.; Mercader, J.; López, V. Resveratrol anti-obesity effects: Rapid inhibition of adipocyte glucose utilization. Antioxidants 2019, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Olsen, H.T.; Stafford, G.I.; Van Staden, J.; Christensen, S.B.; Jäger, A.K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L. J. Ethnopharmacol. 2008, 117, 500–502. [Google Scholar] [CrossRef]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Senol, F.S.; Orhan, I.E.; Ozgen, U.; Renda, G.; Bulut, G.; Guven, L.; Karaoglan, S.E.; Sevindik, H.G.; Skalicka-Wozniak, K.; Caliskan, U.K.; et al. Memory-vitalizing effect of twenty-five medicinal and edible plants and their isolated compounds. S. Afr. J. Bot. 2016, 102, 102–109. [Google Scholar] [CrossRef]


| MRE | ANE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First-Choice | Second-Choice | ||||||||||
| RT | λ | ||||||||||
| Compounds (mg/kg) | Mean | SD | RSD% | Mean | SD | RSD% | Mean | SD | RSD% | ||
| Hydroxybenzoic acids | |||||||||||
| Gallic acid | 5.9 | 272 | 64.5 | 1.9 | 3.0 | 40.6 | 1.4 | 3.4 | 70.8 | 7.6 | 10.7 |
| Flavan-3-ols | |||||||||||
| Catechin | 17.6 | 280 | 20,914.5 | 353.9 | 1.7 | 13,397.9 | 357.8 | 2.7 | 4667.7 | 675.9 | 14.5 |
| Epicatechin | 23.9 | 280 | 38,754.3 | 7686.1 | 19.8 | 42,925.6 | 73.0 | 0.2 | 12,303.2 | 387.3 | 3.1 |
| Procianidin B2 | 24.3 | 230 | 21,692.8 | 251.3 | 1.2 | 30,747.9 | 171.8 | 0.6 | 13,123.0 | 207.4 | 1.6 |
| Procianidin A2 | 29.9 | 230 | 5948.8 | 59.7 | 1.0 | 1456.5 | 37.0 | 2.5 | 1709.9 | 97.0 | 5.7 |
| Anthocyanins | |||||||||||
| Cyanidin 3-glucoside | 25.8 | 520 | 44.9 | 2.4 | 5.4 | 85.1 | 4.4 | 5.1 | 798.6 | 8.1 | 1.0 |
| Flavonols | |||||||||||
| Rutin | 31.47 | 265 | 14,172.9 | 378.1 | 2.7 | 17,390.6 | 215.5 | 1.2 | 4596.1 | 248.7 | 5.4 |
| Quercetin 3-D-galactoside | 32 | 265 | 4760.3 | 26.2 | 0.6 | 4601.6 | 120.3 | 2.6 | 4284.2 | 158.3 | 3.7 |
| Kampferol-3-glucoside | 33.6 | 265 | 3518.8 | 35.2 | 1.0 | 2531.4 | 141.2 | 5.6 | 3582.7 | 85.6 | 2.4 |
| Quercetin | 35.8 | 365 | 18.7 | 0.7 | 3.9 | 220.7 | 3.9 | 1.8 | 582.0 | 35.6 | 6.1 |
| Kampferol | 37.8 | 365 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Hydrocinnamic acids | |||||||||||
| Neochlorogenic acid | 10.58 | 325 | 325.9 | 1.0 | 0.3 | 166.5 | 8.7 | 5.2 | 637.8 | 52.3 | 8.2 |
| Chlorogenic acid | 22.3 | 325 | 31,786.1 | 660.6 | 2.1 | 34,787.4 | 358.2 | 1.0 | 65,753.3 | 1125.5 | 1.7 |
| Caffeic acid | 22.9 | 325 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| p-Coumaric acid | 28.9 | 325 | 0.0 | 0.0 | 0.0 | 70.7 | 9.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| trans-Ferulic acid | 30.5 | 325 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Dihydrochalcones | |||||||||||
| Phloridzin | 32.7 | 280 | 18,714.3 | 552.1 | 3.0 | 22,066.9 | 8.2 | 0.0 | 6011.9 | 357.0 | 5.9 |
| Phloretin | 36.3 | 280 | 37.4 | 1.0 | 2.6 | 89.1 | 8.6 | 9.6 | 38.1 | 2.9 | 7.7 |
| Total Polyphenols | 160,754.1 | 170,578.5 | 118,159.3 | ||||||||
| Triterpenes | |||||||||||
| Annurcoic acid | 43.1 | 210 | 17,262.3 | 610.8 | 3.5 | 23,293.5 | 111.1 | 0.5 | 15,382.9 | 1273.2 | 8.3 |
| Oleanolic acid | 45.8 | 210 | 7611.4 | 76.0 | 1.0 | 8449.3 | 574.6 | 6.8 | 8727.6 | 32.6 | 0.4 |
| Ursolic acid | 45.9 | 210 | 18,097.4 | 428.8 | 2.4 | 20,571.3 | 193.0 | 0.9 | 18,864.6 | 804.8 | 4.3 |
| Total triterpenes | 42,971.0 | 52,314.1 | 42,975.1 | ||||||||
| Total mg/kg | 203,725.1 | 222,892.6 | 161,134.4 | ||||||||
| Total % | 20.4 | 22.3 | 16.1 | ||||||||
| FOLIN | DPPH | ABTS | |||||
|---|---|---|---|---|---|---|---|
| TEAC | IC50 | TEAC | IC50 | ||||
| Samples | mgGAE/g | mgTE/g | mmol TE/g | µg/ML | mgTE/g | mmol TE/g | µg/mL |
| First-choice MRE | 740.0 ± 38.7 | 611.4 | 2.4 | 9.9 ± 0.6 | 682.3 | 2.7 | 6.6 ± 0.3 |
| Second-choice MRE | 547.1 ± 45.0 | 505.8 | 2.0 | 12.0 ± 0.5 | 643.3 | 2.6 | 7.0 ± 0.0 |
| ANE | 517.0 ± 23.1 | 172.6 | 0.7 | 26.4 ± 0.6 | 402.3 | 1.6 | 12.0 ± 0.9 |
| Positive control Trolox | 5.3 ± 1.1 | 4.7 ± 0.2 | |||||
| Enzymatic Inhibition Assay | IC50 (µg/mL) | ||
|---|---|---|---|
| First-Choice MRE | Second-Choice MRE | Reference Inhibitor | |
| α-glucosidase | 907.2 | 823.9 ** | 378.9 (Acarbose) |
| Lipase | 1584.9 *** | 844.7 * | 0.8 (Orlistat) |
| MAO-A | 8.03 * | 6.98 * | 0.15 (Clorgyline) |
| AChE | 1259.0 **** | 1430.6 **** | 0.6 (Galantamine) |
| TYR | 586.1 ** | 168.4 # | 3.5 (Kojic acid) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkuimi Wandjou, J.G.; Mevi, S.; Sagratini, G.; Vittori, S.; Dall’Acqua, S.; Caprioli, G.; Lupidi, G.; Mombelli, G.; Arpini, S.; Allegrini, P.; et al. Antioxidant and Enzyme Inhibitory Properties of the Polyphenolic-Rich Extract from an Ancient Apple Variety of Central Italy (Mela Rosa dei Monti Sibillini). Plants 2020, 9, 9. https://doi.org/10.3390/plants9010009
Nkuimi Wandjou JG, Mevi S, Sagratini G, Vittori S, Dall’Acqua S, Caprioli G, Lupidi G, Mombelli G, Arpini S, Allegrini P, et al. Antioxidant and Enzyme Inhibitory Properties of the Polyphenolic-Rich Extract from an Ancient Apple Variety of Central Italy (Mela Rosa dei Monti Sibillini). Plants. 2020; 9(1):9. https://doi.org/10.3390/plants9010009
Chicago/Turabian StyleNkuimi Wandjou, Joice Guileine, Serena Mevi, Gianni Sagratini, Sauro Vittori, Stefano Dall’Acqua, Giovanni Caprioli, Giulio Lupidi, Giacomo Mombelli, Sabrina Arpini, Pietro Allegrini, and et al. 2020. "Antioxidant and Enzyme Inhibitory Properties of the Polyphenolic-Rich Extract from an Ancient Apple Variety of Central Italy (Mela Rosa dei Monti Sibillini)" Plants 9, no. 1: 9. https://doi.org/10.3390/plants9010009
APA StyleNkuimi Wandjou, J. G., Mevi, S., Sagratini, G., Vittori, S., Dall’Acqua, S., Caprioli, G., Lupidi, G., Mombelli, G., Arpini, S., Allegrini, P., Les, F., López, V., & Maggi, F. (2020). Antioxidant and Enzyme Inhibitory Properties of the Polyphenolic-Rich Extract from an Ancient Apple Variety of Central Italy (Mela Rosa dei Monti Sibillini). Plants, 9(1), 9. https://doi.org/10.3390/plants9010009










