Using Leaf Temperature to Improve Simulation of Heat and Drought Stresses in a Biophysical Model
Abstract
1. Introduction
2. Results
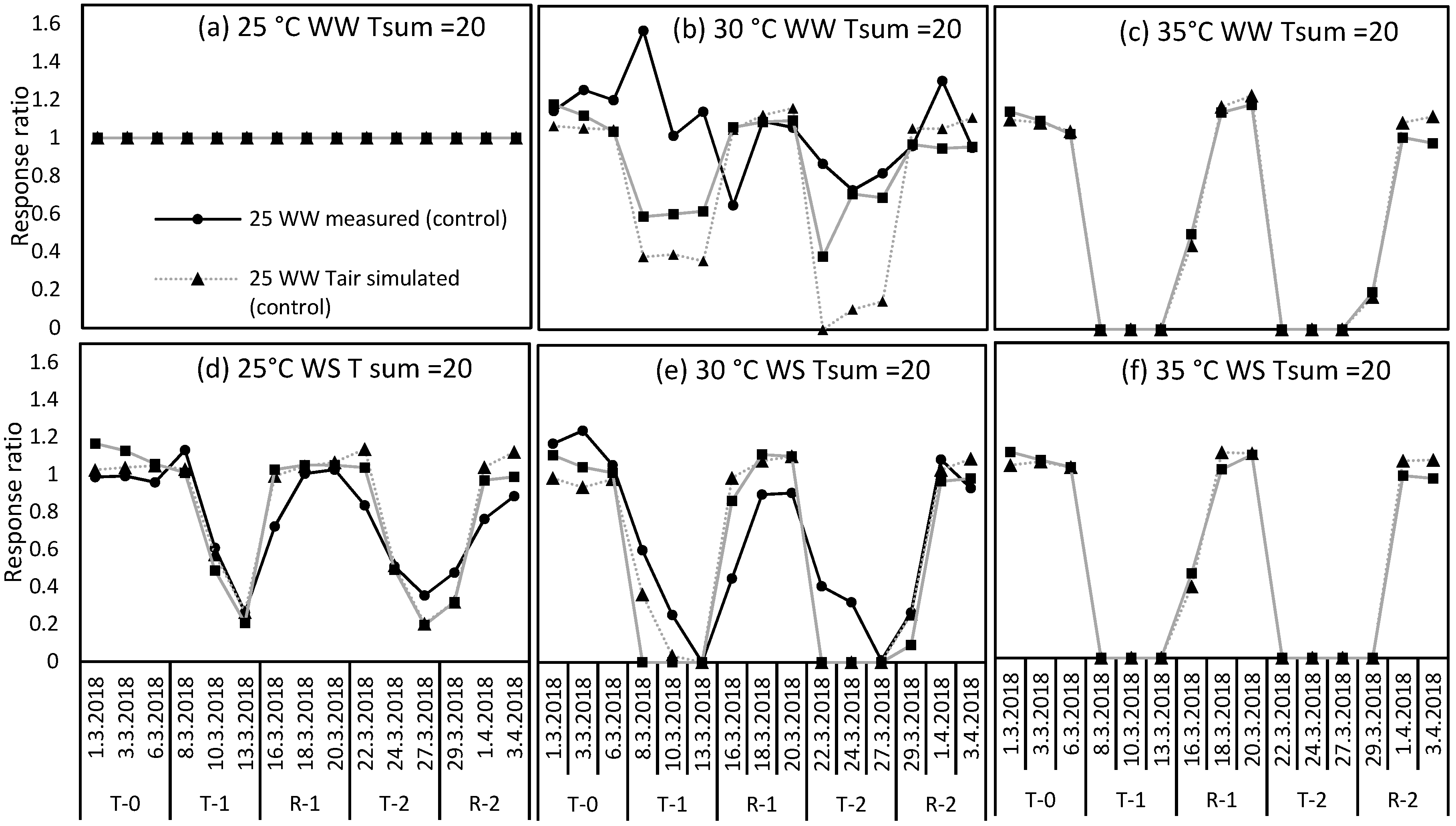
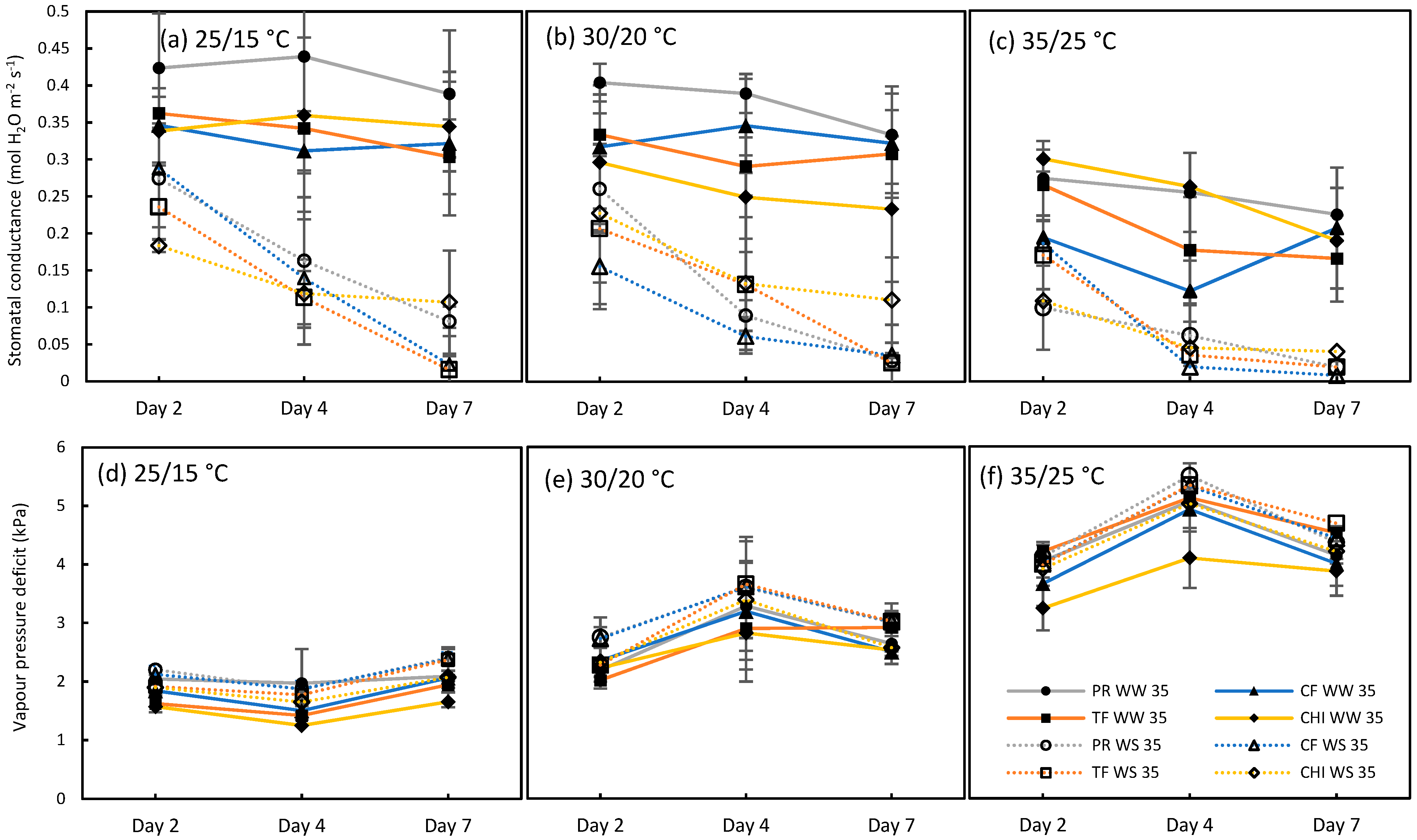
2.1. Test for the Limited Homeothermy of Pastures
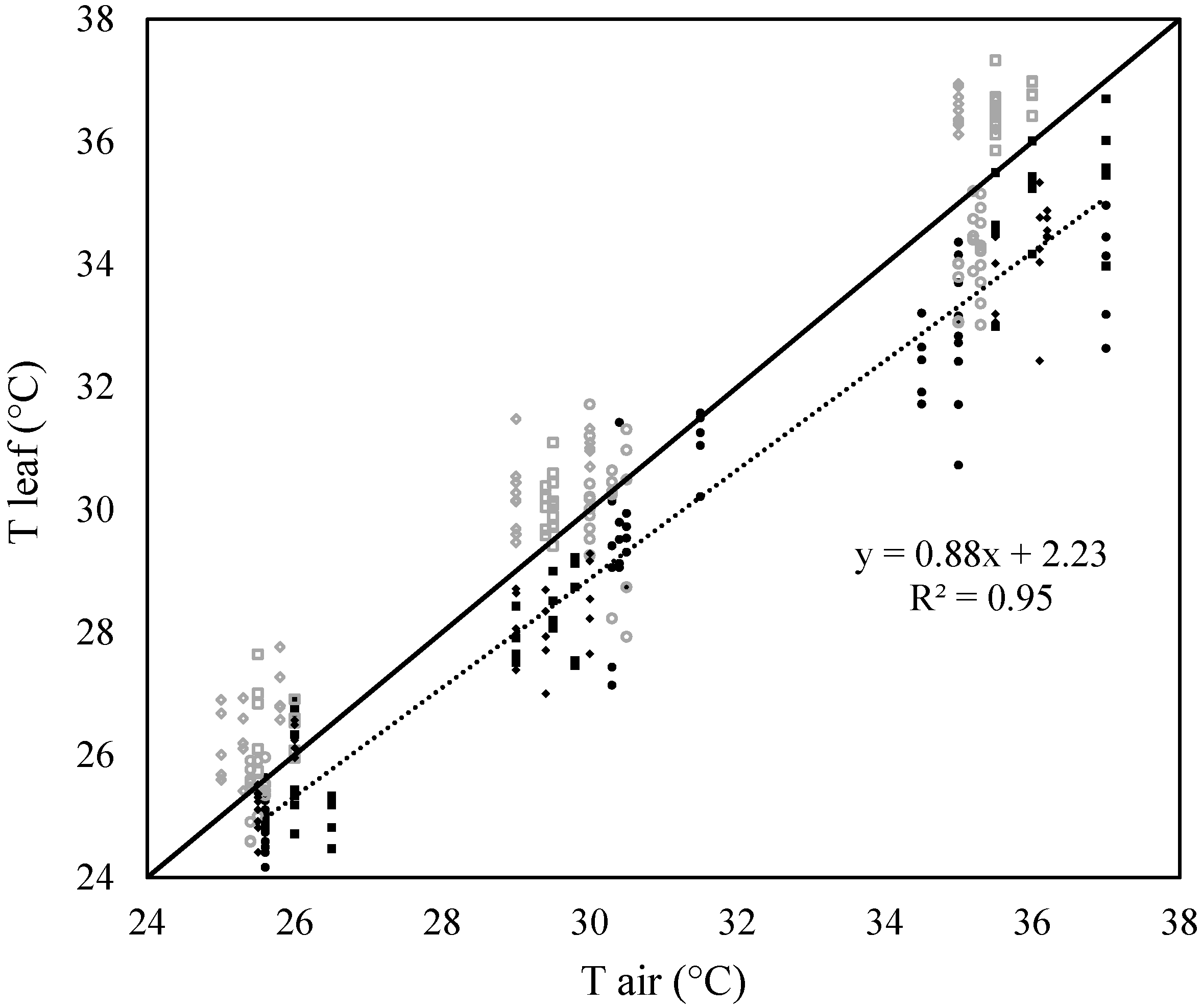
2.2. Ability of the Leaf Energy Budget Equation to Model Leaf Temperature
2.3. Use of T Leaf and T Air to Simulate Photosynthesis
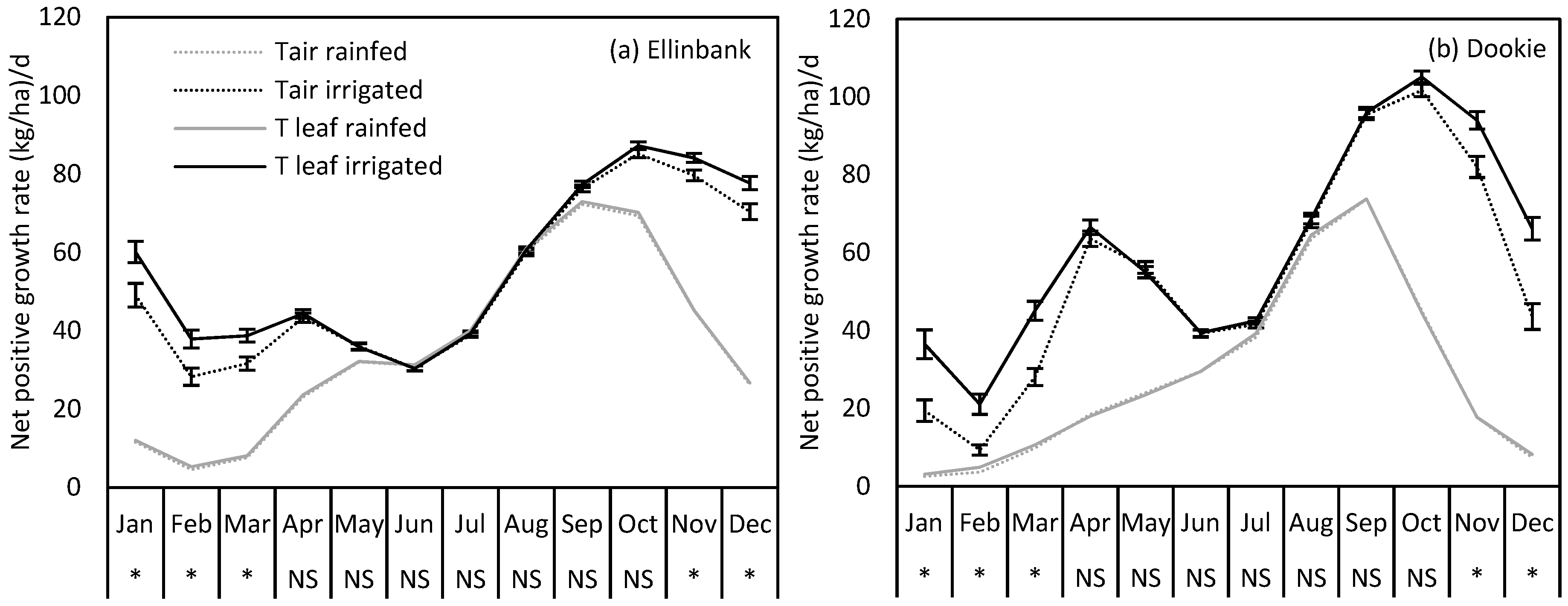
2.4. Uncertainty in Perennial Ryegrass Growth at Ellinbank and Dookie when Using Air Temperature in the Simulations
3. Discussion
4. Materials and Methods
4.1. Validation of Leaf Energy Budget Equation
4.1.1. Experimental Description
4.1.2. Measurements
4.2. Leaf Temperature Calculation Using Energy Budget Equation
4.3. Simulation of Photosynthesis Pattern; Comparison Between Tair and Tleaf
4.4. Parameterization of High Temperature Stress Recovery (T-sum) Function
4.5. Evaluating Effects of Using Leaf Temperature Compared to Air Temperature on Pasture Growth Rate
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CSIRO; BOM. Climate Change in Australia-Projections for Australia’s s Natural Resource Management Regions: Technical Report; CSIRO and Bureau of Meteorology: Canberra, Australia, 2015. [Google Scholar]
- Harrison, M.; Cullen, B.; Rawnsley, R. Modelling the sensitivity of agricultural systems to climate change and extreme climatic events. Agric. Syst. 2016, 148, 135–148. [Google Scholar] [CrossRef]
- Cullen, B.R.; Johnson, I.R.; Eckard, R.J.; Lodge, G.M.; Walker, R.G.; Rawnsley, R.P.; McCaskill, M.R. Climate change effects on pasture systems in south-eastern Australia. Crop Pasture Sci. 2009, 60, 933. [Google Scholar] [CrossRef]
- Moore, A.; Ghahramani, A. Climate change and broadacre livestock production across southern Australia. 1. Impacts of climate change on pasture and livestock productivity, and on sustainable levels of profitability. Glob. Chang. Biol. 2013, 19, 1440–1455. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.M.R.S.; Cullen, B.; Eckard, R. Changing patterns of pasture production in south eastern Australia from 1960 to 2015. Crop Pasture Sci. 2020, 71, in press. [Google Scholar] [CrossRef]
- Paulsen, G.M. High temperature responses of crop plants. In Physiology and Determination of Crop Yield; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1994; pp. 365–389. [Google Scholar]
- Beard, J. Turfgrass Science and Culture; Engle Wood Cliffs, N, J., Printice Hall: London, UK, 1973. [Google Scholar]
- Woledge, J.; Dennis, W.D. The Effect of Temperature on Photosynthesis of Ryegrass and White Clover Leaves. Ann. Bot. 1982, 50, 25–35. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Huang, B. Elevated CO2-mitigation of high temperature stress associated with maintenance of positive carbon balance and carbohydrate accumulation in Kentucky bluegrass. PLoS ONE 2014, 9, e89725. [Google Scholar] [CrossRef] [PubMed]
- Peri, P.L.; Moot, D.J.; McNeil, D.L. A canopy photosynthesis model to predict the dry matter production of cocksfoot pastures under varying temperature, nitrogen and water regimes. Grass Forage Sci. 2003, 58, 416–430. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; Coleman, J.S.; Hallberg, R.L. Recovery of net CO2 assimilation after heat stress is correlated with recovery of oxygen-evolving-complex proteins in Zea mays L. Photosynthetica 1997, 34, 13–20. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Physiological responses to heat stress alone or in combination with drought: A comparison between tall fescue and perennial ryegrass. HortScience 2001, 36, 682–686. [Google Scholar] [CrossRef]
- Perera, R.S.; Cullen, B.R.; Eckard, R.J. Growth and Physiological Responses of Temperate Pasture Species to Consecutive Heat and Drought Stresses. Plants 2019, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Chovancek, E.; Zivcak, M.; Botyanszka, L.; Hauptvogel, P.; Yang, X.; Misheva, S.; Hussain, S.; Brestic, M. Transient Heat Waves May Affect the Photosynthetic Capacity of Susceptible Wheat Genotypes Due to Insufficient Photosystem I Photoprotection. Plants 2019, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Sun, X.; Amombo, E.; Zhu, Q.; Zhao, Z.; Chen, L.; Xu, Q.; Fu, J. High correlation between thermotolerance and photosystem II activity in tall fescue. Photosynth Res 2014, 122, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Thornton, P.; Ericksen, P.; Herrero, M.; Challinor, A. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef]
- Asseng, S.; Foster, I.A.N.; Turner, N.C. The impact of temperature variability on wheat yields. Glob. Chang. Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Keating, B.A.; Carberry, P.S.; Hammer, G.L.; Probert, M.E.; Robertson, M.J.; Holzworth, D.; Huth, N.I.; Hargreaves, J.N.G.; Meinke, H.; Hochman, Z.; et al. An overview of APSIM, a model designed for farming systems simulation. Eur. J. Agron. 2003, 18, 267–288. [Google Scholar] [CrossRef]
- Grant, R.F.; Kimball, B.A.; Conley, M.M.; White, J.W.; Wall, G.W.; Ottman, M.J. Controlled Warming Effects on Wheat Growth and Yield: Field Measurements and Modeling. Agron. J. 2011, 103, 1742. [Google Scholar] [CrossRef]
- Brisson, N.; Gary, C.; Justes, E.; Roche, R.; Mary, B.; Ripoche, D.; Zimmer, D.; Sierra, J.; Bertuzzi, P.; Burger, P.; et al. An overview of the crop model stics. Eur. J. Agron. 2003, 18, 309–332. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Michaletz, S.; Weiser, M.; Zhou, J.; Kaspari, M.; Helliker, B.; Enquist, B. Plant Thermoregulation: Energetics, Trait–Environment Interactions, and Carbon Economics. Trends Ecol. Evol. 2015, 30, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Gates, D.M. Transpiration and Leaf Temperature. Annu. Rev. Plant Physiol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Linacre, E.T. Further studies of the heat transfer from a leaf. Plant Physiol. 1967, 42, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Siebert, S.; Ewert, F.; Eyshi Rezaei, E.; Kage, H.; Graß, R. Impact of heat stress on crop yield—on the importance of considering canopy temperature. Environ. Res. Lett. 2014, 9, 044012. [Google Scholar] [CrossRef]
- Webber, H.; Martre, P.; Asseng, S.; Kimball, B.; White, J.; Ottman, M.; Wall, G.; De Sanctis, G.; Doltra, J.; Grant, R.; et al. Canopy temperature for simulation of heat stress in irrigated wheat in a semi-arid environment: A multi-model comparison. Field Crop. Res. 2017, 202, 21–35. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Blumenthal, C.S.; Bekes, F.; Batey, I.L.; Wrigley, C.W.; Moss, H.J.; Mares, D.J.; Barlow, E.W.R. Interpretation of grain quality results from wheat variety trials with reference to high temperature stress. Aust. J. Agric. Res. 1991, 42, 325. [Google Scholar] [CrossRef]
- Penman, H.L. Natural evaporation from open water, bare soil and grass. Proc. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1948, 193, 120–145. [Google Scholar] [CrossRef]
- Johnson, I. DairyMod and the SGS Pasture Model: A Mathematical Description of the Biophysical Model Structure; IMJ Consultants: Dorrigo, NSW, Australia, 2016. [Google Scholar]
- Johnson, I.R. Biophysical Pasture Model Documentation: Model Documentation for DairyMod. EcoMod and the SGS Pasture Model; IMJ Consultants: Dorrigo, NSW, Australia, 2008. [Google Scholar]
- Mitchell, K. Growth of pasture species under controlled environment. 1. Growth at various levels of constant temperature. N. Z. J. Sci. Technol. 1956, 38, 203–215. [Google Scholar] [CrossRef][Green Version]
- Langworthy, A.; Rawnsley, R.; Freeman, M.; Corkrey, R.; Harrison, M.; Pembleton, K.; Lane, P.; Henry, D.A. Effect of stubble-height management on crown temperature of perennial ryegrass, tall fescue and chicory. Crop Pasture Sci. 2019, 70, 183. [Google Scholar] [CrossRef]
- Rumball, W. Grasslands Puna’ chicory (Cichorium intybusL.). N. Z. J. Exp. Agric. 1986, 14, 105–107. [Google Scholar] [CrossRef]
- Korte, C.; Watkin, B.; Harris, W. Tillering in ‘Grasslands Nui’perennial ryegrass swards 3. Aerial tillering in swards grazed by sheep. N. Z. J. Agric. Res. 1987, 30, 9–14. [Google Scholar] [CrossRef][Green Version]
- Yang, J.Z.; Matthew, C.; Rowland, R. Tiller axis observations for perennial ryegrass (Lolium perenne) and tall fescue (Festuca arundinacea): Number of active phytomers, probability of tiller appearance, and frequency of root appearance per phytomer for three cutting heights. N. Z. J. Agric. Res. 1998, 41, 11–17. [Google Scholar] [CrossRef]
- Briske, D. Developmental morphology and physiology of grasses. In Grazing Management: An Ecological Perspective; Heitschmidt, R.K., Stuth, J.W., Eds.; Timber Press: Portland, OR, USA, 1991; pp. 85–108. [Google Scholar]
- Mitchell, K.J.; Lucanus, R. Growth of pasture species under controlled environment III. Growth at various levels of constant temperature with 8 and 16 hours of uniform light per day. N. Z. J. Agric. Res. 1962, 5, 135–144. [Google Scholar] [CrossRef][Green Version]
- Neukam, D.; Ahrends, H.; Luig, A.; Manderscheid, R.; Kage, H. Integrating Wheat Canopy Temperatures in Crop System Models. Agronomy 2016, 6, 7. [Google Scholar] [CrossRef]
- Adriana, F.; Jossana, S.; Cerci, M.; Simone, M.S.B. The first anatomical and histochemical study of tough lovegrass (Eragrostis plana Nees, Poaceae). Afr. J. Agric. Res. 2015, 10, 2940–2947. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Burke, J.J. Energy exchange and leaf temperature behavior of 3 plant-species. Environ. Exp. Bot. 1991, 31, 295–302. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Hong, T.D.; Ellis, R.H.; Batts, G.R.; Morison, J.I.L.; Hadley, P. The duration and rate of grain growth, and harvest index, of wheat (Triticum aestivum L.) in response to temperature and CO2. J. Exp. Bot. 1996, 47, 623–630. [Google Scholar] [CrossRef]
- Porter, J.; Gawith, M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Pinto, R.S.; Reynolds, M.; Mathews, K.; McIntyre, C.L.; Olivares Villegas, J.-J.; Chapman, S.C. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor. Appl. Genet. 2010, 121, 1001–1021. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.S.; Reynolds, M.P. Common genetic basis for canopy temperature depression under heat and drought stress associated with optimized root distribution in bread wheat. Theor. Appl. Genet. 2015, 128, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Reginato, R.J.; Hatfield, J.L. Normalizing the stress-degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Volaire, F.; Norton, M. Summer dormancy in perennial temperate grasses. Ann. Bot. 2006, 98, 927–933. [Google Scholar] [CrossRef]
- Parker, T.; Berry, G.; Reeder, M. The Structure and Evolution of Heat Waves in Southeastern Australia. J. Clim. 2014, 27, 5768–5785. [Google Scholar] [CrossRef]
- Langworthy, A.; Rawnsley, R.; Freeman, M.; Pembleton, K.; Corkrey, R.; Harrison, M.; Lane, P.; Henry, D. Potential of summer-active temperate (C3) perennial forages to mitigate the detrimental effects of supraoptimal temperatures on summer home-grown feed production in south-eastern Australian dairying regions. Crop Pasture Sci. 2018, 69, 808. [Google Scholar] [CrossRef]
- van Oort, P.A.J.; Saito, K.; Zwart, S.J.; Shrestha, S. A simple model for simulating heat induced sterility in rice as a function of flowering time and transpirational cooling. Field Crop. Res. 2014, 156, 303–312. [Google Scholar] [CrossRef]
- Kerkhoff, A.; Enquist, B.; Elser, J.; Fagan, W.F. Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Glob. Ecol. Biogeogr. 2005, 14, 585–598. [Google Scholar] [CrossRef]
- Michaletz, S.; Cheng, D.; Kerkhoff, A.; Enquist, B.J. Convergence of terrestrial plant production across global climate gradients. Nature 2014, 512, 39–43. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Fuentes, S.; De Bei, R.; Pech, J.; Tyerman, S. Computational water stress indices obtained from thermal image analysis of grapevine canopies. Irrig. Sci. 2012, 30, 523–536. [Google Scholar] [CrossRef]
- Taylor, S.E. Optimal leaf form. In Perspectives of Biophysical Ecology; Springer: Berlin, Germany, 1975; pp. 73–86. [Google Scholar]
- Tedeschi, L. Assessment of the adequacy of mathematical models. Agric. Syst. 2006, 89, 225–247. [Google Scholar] [CrossRef]
- Bibby, J.; Toutenburg, H. Prediction and Improved Estimation in Linear Models; Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- Jeffrey, S.; Carter, J.; Moodie, K.; Beswick, A. Using spatial interpolation to construct a comprehensive archive of Australian climate data. Environ. Model. Softw. 2001, 16, 309–330. [Google Scholar] [CrossRef]
- Holloway Phillips, M.-M.; Brodribb, T. Minimum hydraulic safety leads to maximum water-use efficiency in a forage grass. Plant Cell Environ. 2011, 34, 302–313. [Google Scholar] [CrossRef] [PubMed]
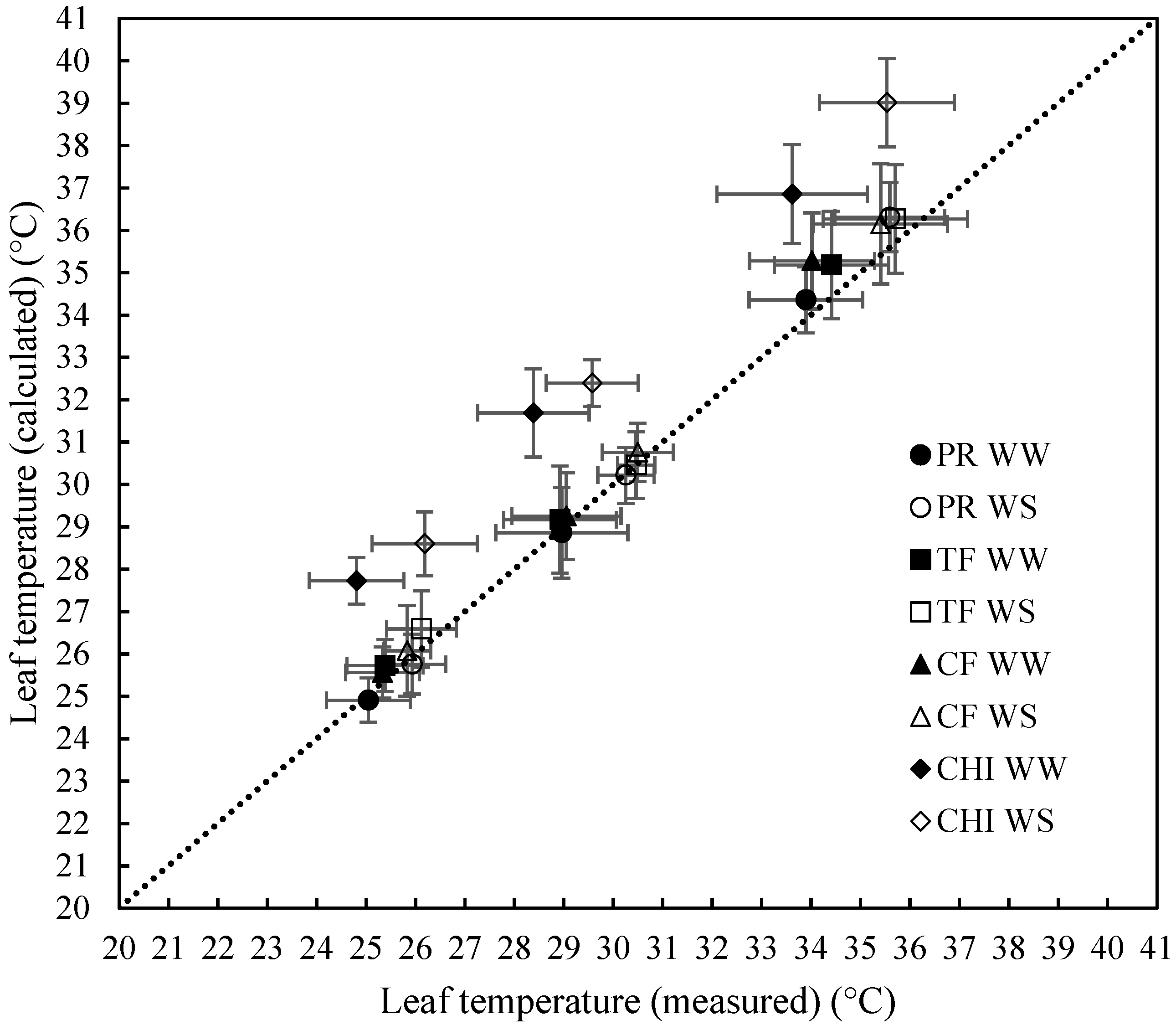



| Model Statistics | All Data | Perennial Ryegrass | Cocksfoot | Tall Fescue | Chicory | Well-Watered | Water-Stressed |
|---|---|---|---|---|---|---|---|
| Mean (measured) | 30.02 | 30.05 | 29.83 | 30.23 | 29.93 | 29.32 | 30.74 |
| Mean (calculated) | 31.00 | 30.18 | 30.31 | 30.61 | 32.99 | 30.37 | 31.65 |
| Mean bias | −0.98 | −0.13 | −0.48 | −0.39 | −3.06 | −1.05 | −0.91 |
| R2 (Coeff. of determination) | 0.89 | 0.95 | 0.97 | 0.96 | 0.94 | 0.86 | 0.91 |
| r (Pearson’s correlation coeff.) | 0.94 | 0.98 | 0.99 | 0.98 | 0.97 | 0.93 | 0.95 |
| Mean Prediction Error (MPE) | 5.88% | 3.18% | 3.03% | 2.86% | 10.79% | 6.38% | 5.36% |
| Modelling Efficiency (MEF) | 0.80 | 0.94 | 0.95 | 0.95 | 0.37 | 0.76 | 0.83 |
| Variance Ratio (V) | 0.92 | 0.94 | 0.93 | 0.97 | 0.96 | 0.91 | 0.91 |
| Cb | 1.00 | 0.97 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CCC | 0.94 | 0.95 | 0.98 | 0.98 | 0.97 | 0.92 | 0.95 |
| n | 294 | 76 | 69 | 79 | 70 | 150 | 144 |
| Tsum R = 50 | Tsum R = 20 | ||||
|---|---|---|---|---|---|
| T Air | T Leaf | T Air | T Leaf | ||
| 25 °C | WW | −0.39 | −0.22 | −0.39 | −0.14 |
| WS | 0.87 | 0.86 | 0.86 | 0.86 | |
| 30 °C | WW | 0.18 | −0.07 | 0.09 | −0.09 |
| WS | 0.88 | 0.93 | 0.86 | 0.87 | |
| (RMSE) | (MAE) | |||
|---|---|---|---|---|
| Tair | Tleaf | Tair | Tleaf | |
| 25 °C WS | 0.16 | 0.15 | 0.12 | 0.13 |
| 30 °C WW | 0.54 | 0.36 | 0.42 | 0.25 |
| 30 °C WS | 0.24 | 0.26 | 0.19 | 0.20 |
| GLF Water Range | Stomatal Conductance mol m−2 s−1 |
|---|---|
| 0.91–1 | 0.4 |
| 0.81–0.9 | 0.225 |
| 0.71–0.8 | 0.18 |
| 0.61–0.7 | 0.11 |
| 0.51–0.6 | 0.05 |
| 0.41–0.5 | 0.035 |
| 0.31–0.4 | 0.025 |
| 0.21–0.3 | 0.01 |
| 0.11–0.2 | 0.008 |
| 0–0.1 | 0.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, R.S.; Cullen, B.R.; Eckard, R.J. Using Leaf Temperature to Improve Simulation of Heat and Drought Stresses in a Biophysical Model. Plants 2020, 9, 8. https://doi.org/10.3390/plants9010008
Perera RS, Cullen BR, Eckard RJ. Using Leaf Temperature to Improve Simulation of Heat and Drought Stresses in a Biophysical Model. Plants. 2020; 9(1):8. https://doi.org/10.3390/plants9010008
Chicago/Turabian StylePerera, Ruchika S., Brendan R. Cullen, and Richard J. Eckard. 2020. "Using Leaf Temperature to Improve Simulation of Heat and Drought Stresses in a Biophysical Model" Plants 9, no. 1: 8. https://doi.org/10.3390/plants9010008
APA StylePerera, R. S., Cullen, B. R., & Eckard, R. J. (2020). Using Leaf Temperature to Improve Simulation of Heat and Drought Stresses in a Biophysical Model. Plants, 9(1), 8. https://doi.org/10.3390/plants9010008






