Mineral Element Composition in Grain of Awned and Awnletted Wheat (Triticum aestivum L.) Cultivars: Tissue-Specific Iron Speciation and Phytate and Non-Phytate Ligand Ratio
Abstract
1. Introduction
2. Results
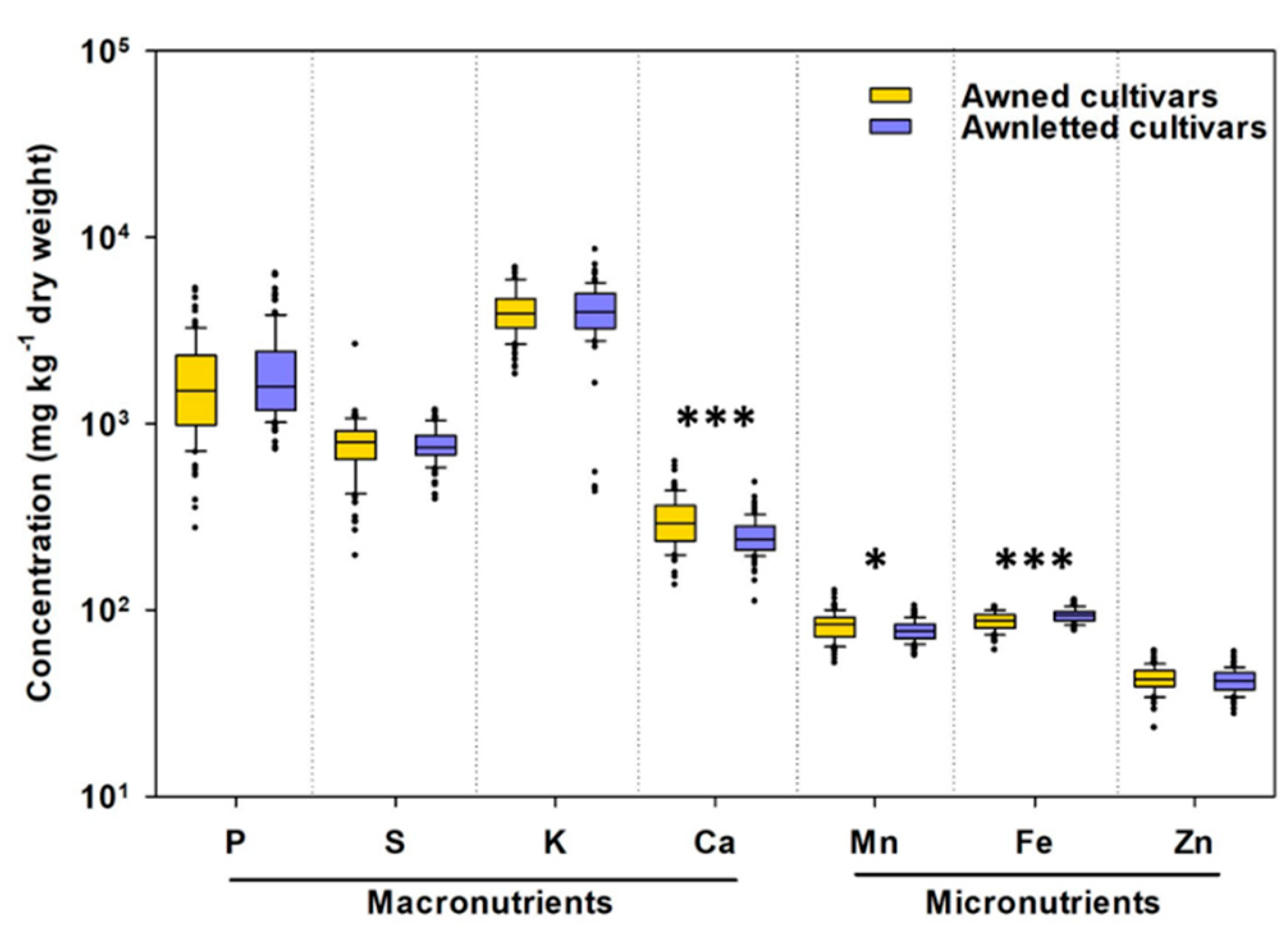
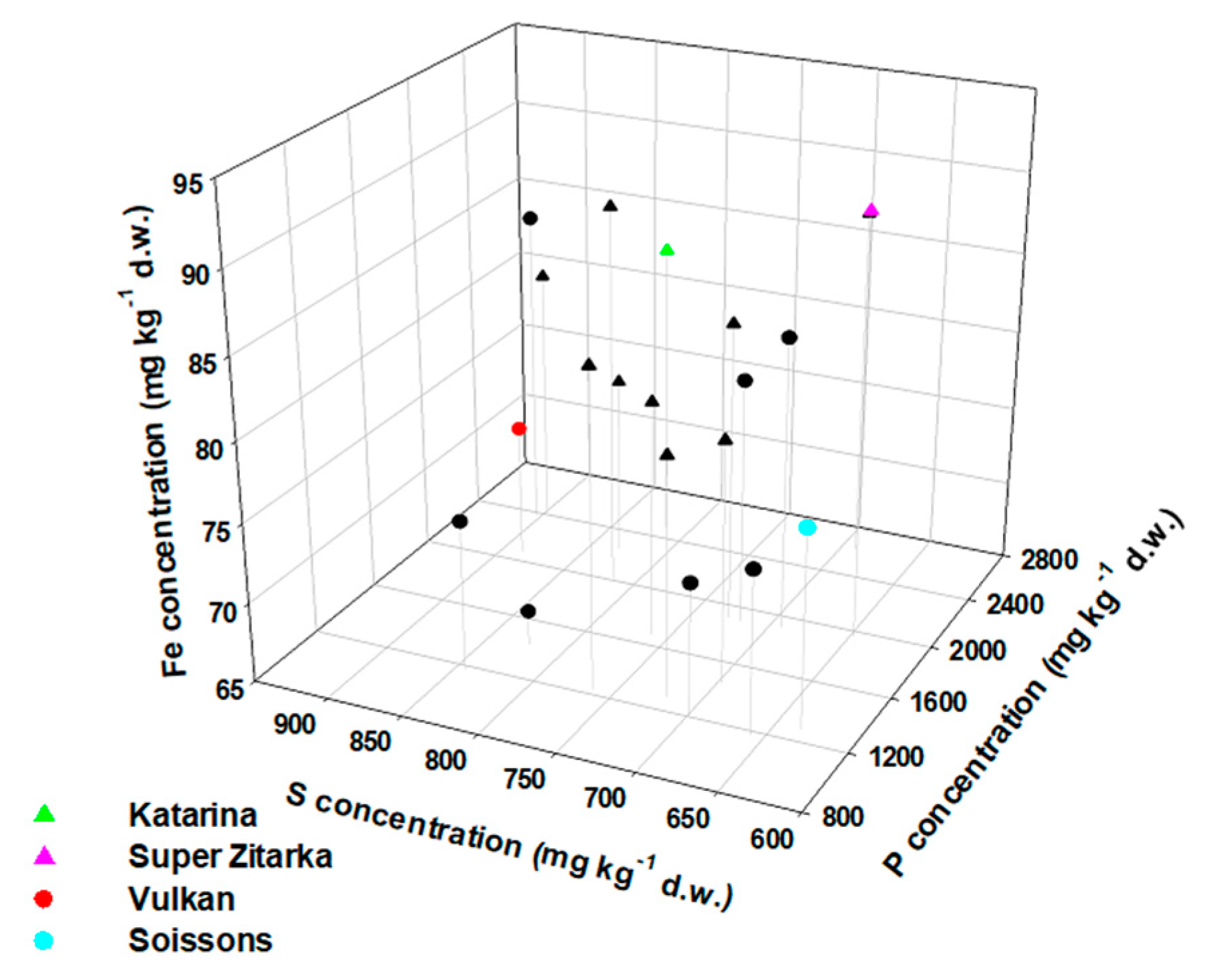
2.1. Total Concentrations of Mineral Elements in Whole Wheat Grain
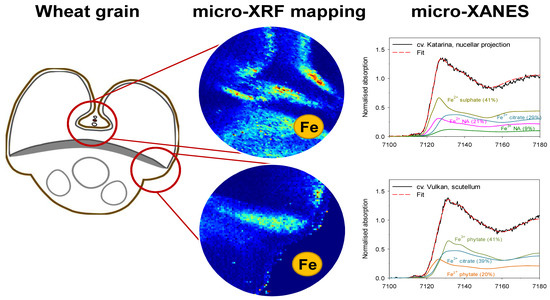
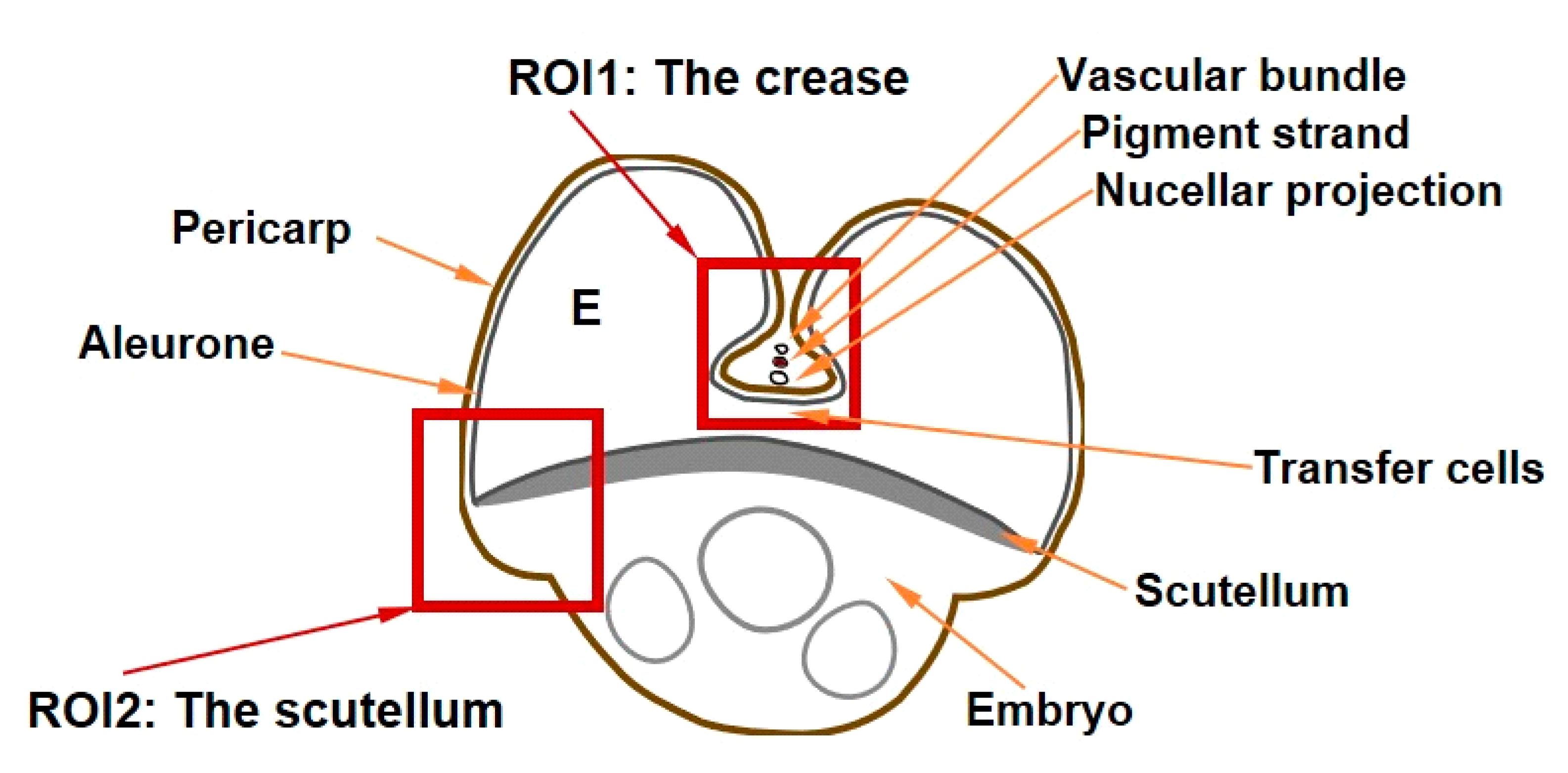
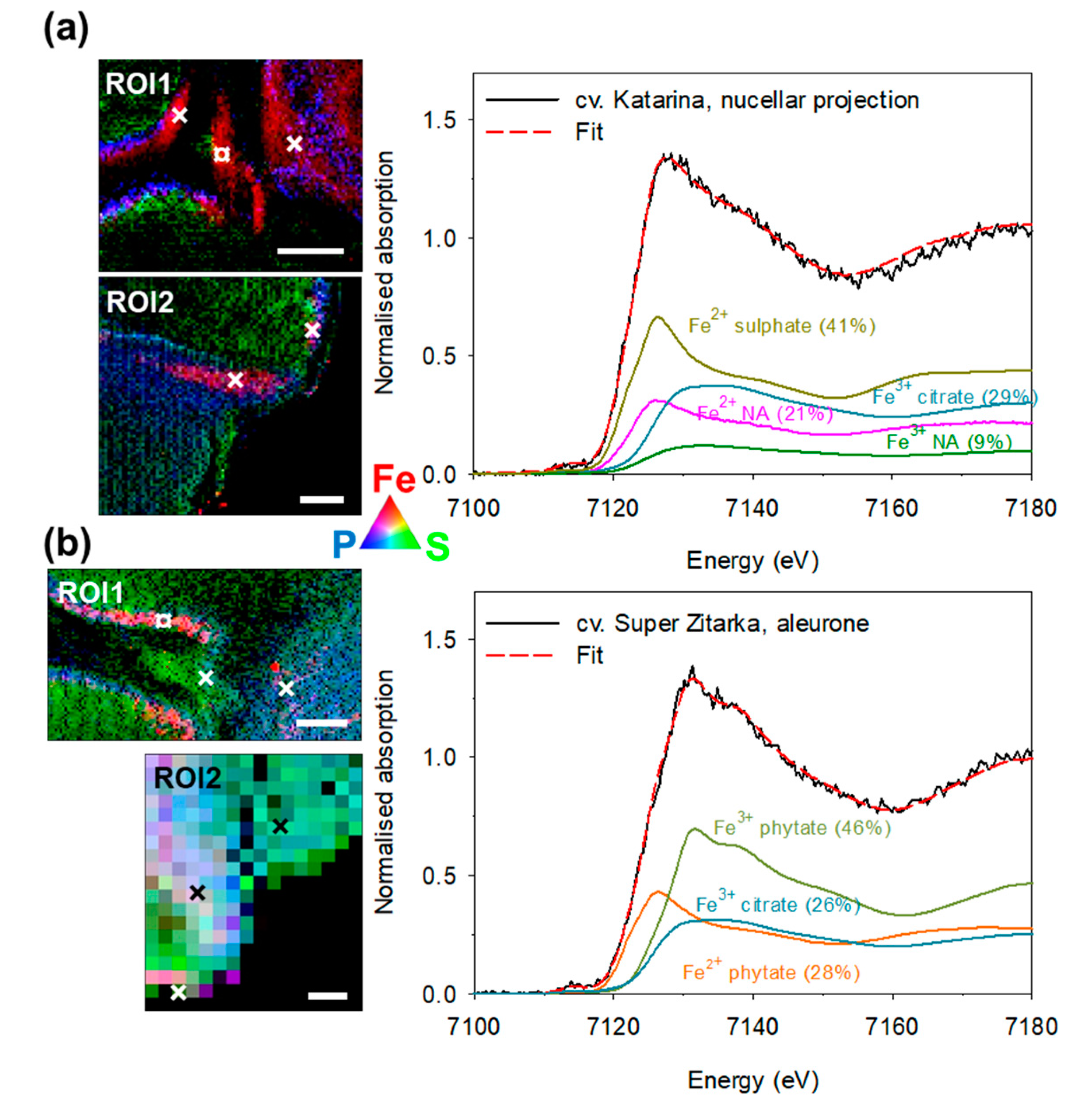
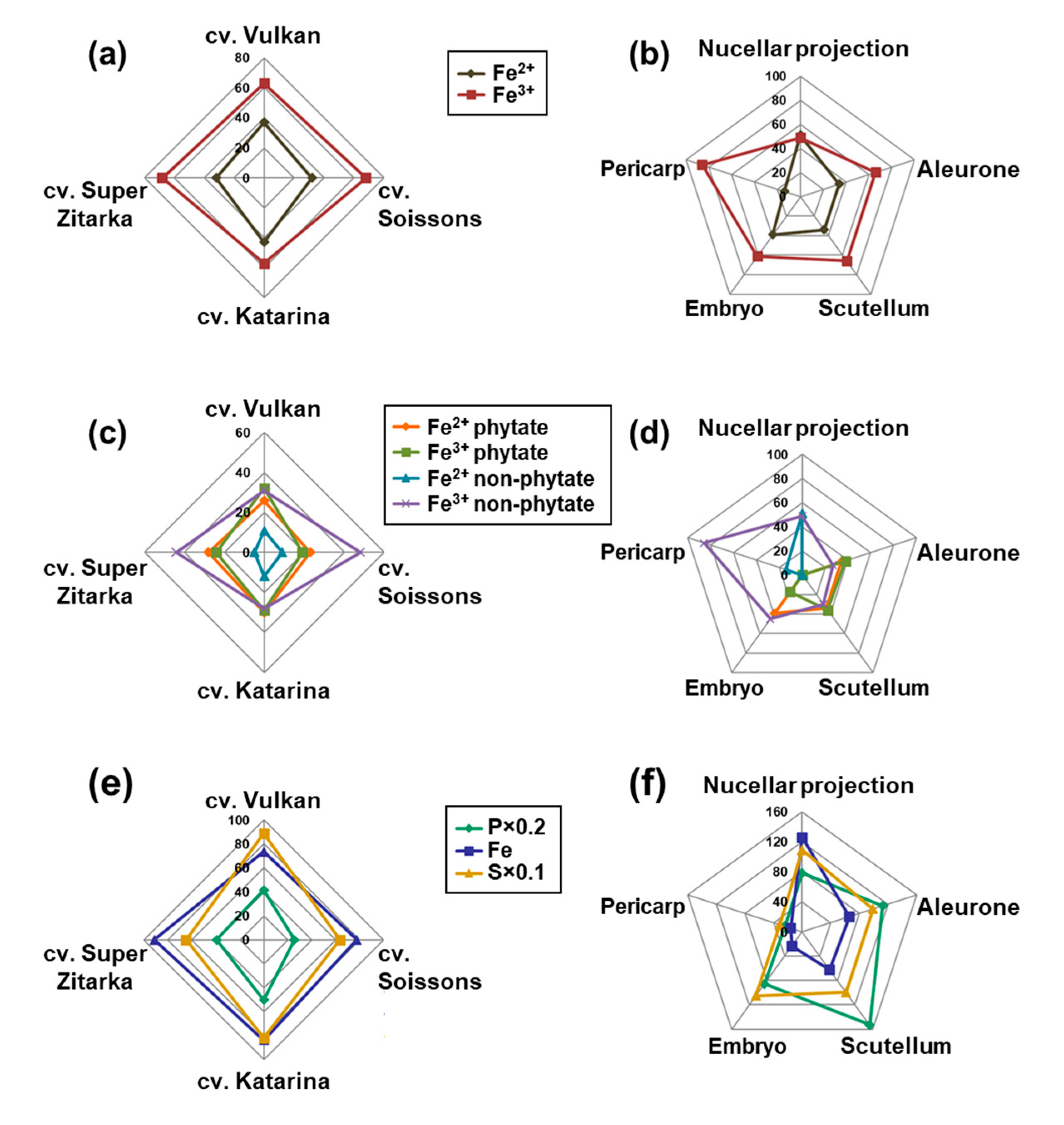
2.2. Tissue-Specific Iron, Phosphorus and Sulphur Concentrations, Iron Speciation and Iron Ligands
3. Discussion
3.1. Awned Cultivars Accumulate More Ca and Mn But Less Fe in Grain than Awnletted Wheat Cultivars
3.2. Iron Speciation and Iron Ligands in Wheat Grain Are Stable across Cultivars Differing in Total Iron Concentration
3.3. Distinct Tissue Specificity in Iron Speciation, Iron Ligands and Iron Concentration in Wheat Grain
4. Conclusions
5. Materials and Methods
5.1. Plant Material and Total Element Concentration in Grain
5.2. Sample Preparation and Micro-XRF Mapping and Micro-XANES Analysis
5.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef]
- DellaPenna, D. Nutritional genomics: Manipulating plant micronutrients to improve human health. Science 1999, 285, 375–379. [Google Scholar] [CrossRef]
- White, P.J.; George, T.S.; Gregory, P.J.; Bengough, A.G.; Hallett, P.D.; McKenzie, B.M. Matching roots to their environment. Ann. Bot. 2013, 112, 207–222. [Google Scholar] [CrossRef]
- Clemens, S. Zn and Fe biofortification: The right chemical environment for human bioavailability. Plant Sci. 2014, 225, 52–57. [Google Scholar] [CrossRef]
- Fan, M.-S.; Zhao, F.-J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of decreasing mineral density in wheat grain over the last 160 years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef]
- Sands, D.C.; Morris, C.E.; Dratz, E.A.; Pilgeram, A.L. Elevating optimal human nutrition to a central goal of plant breeding and production of plant-based foods. Plant Sci. 2009, 177, 377–389. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J. Iron biofortification of staple crops: Lessons and challenges in plant genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.-Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Pongrac, P.; Kreft, I.; Vogel-Mikuš, K.; Regvar, M.; Germ, M.; Vavpetič, P.; Grlj, N.; Jeromel, L.; Eichert, D.; Budič, B.; et al. Relevance for food sciences of quantitative spatially resolved element profile investigations in wheat (Triticum aestivum) grain. J. R. Soc. Interface 2013, 10. [Google Scholar] [CrossRef]
- Collings, R.; Harvey, L.J.; Hooper, L.; Hurst, R.; Brown, T.J.; Ansett, J.; King, M.; Fairweather-Tait, S.J. The absorption of iron from whole diets: A systematic review. Am. J. Clin. Nutr. 2013, 98, 65–81. [Google Scholar] [CrossRef]
- Zhao, F.J.; Su, Y.H.; Dunham, S.J.; Rakszegi, M.; Bedo, Z.; McGrath, S.P.; Shewry, P.R. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- Detterbeck, A.; Pongrac, P.; Rensch, S.; Reuscher, S.; Pečovnik, M.; Vavpetič, P.; Pelicon, P.; Holzheu, S.; Krämer, U.; Clemens, S. Spatially resolved analysis of variation in barley (Hordeum vulgare) grain micronutrient accumulation. New Phytol. 2016, 211, 1241–1254. [Google Scholar] [CrossRef]
- Pinson, S.R.M.; Tarpley, L.; Yan, W.; Yeater, K.; Lahner, B.; Yakubova, E.; Huang, X.-Y.; Zhang, M.; Guerinot, M.L.; Salt, D.E. Worldwide genetic diversity for mineral element concentrations in rice grain. Crop Sci. 2015, 55, 294. [Google Scholar] [CrossRef]
- Bashir, E.M.A.; Ali, A.M.; Ali, A.M.; Melchinger, A.E.; Parzies, H.K.; Haussmann, B.I.G. Characterization of Sudanese pearl millet germplasm for agro-morphological traits and grain nutritional values. Plant Genet. Resour. Character. Util. 2014, 12, 35–47. [Google Scholar] [CrossRef]
- Available online: https://npgsweb.ars-grin.gov/gringlobal/descriptors.aspx? (accessed on 8 January 2020).
- Grillet, L.; Mari, S.; Schmidt, W. Iron in seeds—Loading pathways and subcellular localization. Front. Plant Sci. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Li, X.-F.; Bin, D.; Hong-Gang, W. Awn anatomy of common wheat (Triticum aestivum L.) and its relatives. Caryologia 2010, 63, 391–397. [Google Scholar] [CrossRef][Green Version]
- Pongrac, P.; Vogel-Mikuš, K.; Jeromel, L.; Vavpetič, P.; Pelicon, P.; Kaulich, B.; Gianoncelli, A.; Eichert, D.; Regvar, M.; Kreft, I. Spatially resolved distributions of the mineral elements in the grain of tartary buckwheat (Fagopyrum tataricum). Food Res. Int. 2013, 54, 125–131. [Google Scholar] [CrossRef]
- Vogel-Mikuš, K.; Pelicon, P.; Vavpetič, P.; Kreft, I.; Regvar, M. Elemental analysis of edible grains by micro-PIXE: Common buckwheat case study. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2009, 267, 2884–2889. [Google Scholar] [CrossRef]
- Garnett, T.P.; Graham, R.D. Distribution and remobilization of iron and copper in wheat. Ann. Bot. 2005, 95, 817–826. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Long-distance Transport in the Xylem and Phloem. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; Chapter 3; pp. 49–70. ISBN 978-0-12-384905-2. [Google Scholar]
- Singh, S.P.; Vogel-Mikuš, K.; Arčon, I.; Vavpetič, P.; Jeromel, L.; Pelicon, P.; Kumar, J.; Tuli, R. Pattern of iron distribution in maternal and filial tissues in wheat grains with contrasting levels of iron. J. Exp. Bot. 2013, 64, 3249–3260. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, S.; Karaca, N.; Vogel-Mikuš, K.; Kavčič, A.; Filiz, E.; Tanyolac, B. The conservation of VIT1-dependent iron distribution in seeds. Front. Plant Sci. 2019, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Ibeas, M.A.; Grant-Grant, S.; Coronas, M.F.; Vargas-Pérez, J.I.; Navarro, N.; Abreu, I.; Castillo-Michel, H.; Avalos-Cembrano, N.; Paez Valencia, J.; Perez, F.; et al. The diverse iron distribution in eudicotyledoneae seeds: From arabidopsis to quinoa. Front. Plant Sci. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Brier, N.; Gomand, S.V.; Donner, E.; Paterson, D.; Smolders, E.; Delcour, J.A.; Lombi, E. Element distribution and iron speciation in mature wheat grains (Triticum aestivum L.) using synchrotron X-ray fluorescence microscopy mapping and X-ray absorption near-edge structure (XANES) imaging. Plant. Cell Environ. 2016, 39, 1835–1847. [Google Scholar] [CrossRef]
- Regvar, M.; Eichert, D.; Kaulich, B.; Gianoncelli, A.; Pongrac, P.; Vogel-Mikuš, K.; Kreft, I. New insights into globoids of protein storage vacuoles in wheat aleurone using synchrotron soft X-ray microscopy. J. Exp. Bot. 2011, 62, 3929–3939. [Google Scholar] [CrossRef]
- Kyriacou, B.; Moore, K.L.; Paterson, D.; de Jonge, M.D.; Howard, D.L.; Stangoulis, J.; Tester, M.; Lombi, E.; Johnson, A.A.T. Localization of iron in rice grain using synchrotron X-ray fluorescence microscopy and high resolution secondary ion mass spectrometry. J. Cereal Sci. 2014, 59, 173–180. [Google Scholar] [CrossRef]
- O’Dell, B.L.; De Boland, A.R.; Koirtyohann, S.R. Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J. Agric. Food Chem. 1972, 20, 718–723. [Google Scholar] [CrossRef]
- Singh, S.P.; Vogel-Mikuš, K.; Vavpetič, P.; Jeromel, L.; Pelicon, P.; Kumar, J.; Tuli, R. Spatial X-ray fluorescence micro-imaging of minerals in grain tissues of wheat and related genotypes. Planta 2014, 240, 277–289. [Google Scholar] [CrossRef]
- Pongrac, P.; Scheers, N.; Sandberg, A.S.; Potisek, M.; Arčon, I.; Kreft, I.; Kump, P.; Vogel-Mikuš, K. The effects of hydrothermal processing and germination on Fe speciation and Fe bioaccessibility to human intestinal Caco-2 cells in Tartary buckwheat. Food Chem. 2016, 199, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Rebetzke, G.J.; Bonnett, D.G.; Reynolds, M.P. Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. J. Exp. Bot. 2016, 67, 2573–2586. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Becerra, H.F.; Erdem, H.; Yazici, A.; Tutus, Y.; Torun, B.; Ozturk, L.; Cakmak, I. Grain concentrations of protein and mineral nutrients in a large collection of spelt wheat grown under different environments. J. Cereal Sci. 2010, 52, 342–349. [Google Scholar] [CrossRef]
- Detterbeck, A.; Nagel, M.; Rensch, S.; Weber, M.; Börner, A.; Persson, D.P.; Schjoerring, J.K.; Christov, V.; Clemens, S. The search for candidate genes associated with natural variation of grain Zn accumulation in barley. Biochem. J. 2019, 476, 1889–1909. [Google Scholar] [CrossRef]
- Morgounov, A.; Gómez-Becerra, H.F.; Abugalieva, A.; Dzhunusova, M.; Yessimbekova, M.; Muminjanov, H.; Zelenskiy, Y.; Ozturk, L.; Cakmak, I. Iron and zinc grain density in common wheat grown in Central Asia. Euphytica 2007, 155, 193–203. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Riefolo, C.; Nicastro, G.; De Simone, V.; Di Gesù, A.M.; Beleggia, R.; Platani, C.; Cattivelli, L.; De Vita, P. Phytate and mineral elements concentration in a collection of Italian durum wheat cultivars. Field Crop. Res. 2009, 111, 235–242. [Google Scholar] [CrossRef]
- Murphy, K.M.; Hoagland, L.A.; Yan, L.; Colley, M.; Jones, S.S. Genotype × environment interactions for mineral concentration in grain of organically grown spring wheat. Agron. J. 2011, 103, 1734. [Google Scholar] [CrossRef]
- Joshi, A.K.; Crossa, J.; Arun, B.; Chand, R.; Trethowan, R.; Vargas, M.; Ortiz-Monasterio, I. Genotype × environment interaction for zinc and iron concentration of wheat grain in eastern Gangetic plains of India. Field. Crop. Res. 2010, 116, 268–277. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Huang, Y.; Zhu, L.; Zhang, S.; Zhao, Y.; Guo, J.; Chen, J.; Chen, R. Identification and characterization of the zinc-regulated transporters, iron-regulated transporter-like protein (ZIP) gene family in maize. BMC Plant Biol. 2013, 13, 114. [Google Scholar] [CrossRef]
- Moore, K.L.; Rodríguez-Ramiro, I.; Jones, E.R.; Jones, E.J.; Rodríguez-Celma, J.; Halsey, K.; Domoney, C.; Shewry, P.R.; Fairweather-Tait, S.; Balk, J. The stage of seed development influences iron bioavailability in pea (Pisum sativum L.). Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Morris, E.R.; Ellis, R. Isolation of Monoferric Phytate from Wheat Bran and its Biological Value as an Iron Source to the Rat. J. Nutr. 1976, 106, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Thiel, J. Development of endosperm transfer cells in barley. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Borisjuk, L.; Junker, B.H.; Mock, H.-P.; Rolletschek, H.; Seiffert, U.; Weschke, W.; Wobus, U. Barley Grain Development: Toward an Integrative View. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Amsterdam, The Netherlands, 2010; Volume 281, Chapter 2; pp. 49–89. [Google Scholar]
- Grillet, L.; Ouerdane, L.; Flis, P.; Hoang, M.T.T.; Isaure, M.-P.; Lobinski, R.; Curie, C.; Mari, S. Ascorbate efflux as a new strategy for iron reduction and transport in plants. J. Biol. Chem. 2014, 289, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Nečemer, M.; Kump, P.; Ščančar, J.; Jaćimović, R.; Simčič, J.; Pelicon, P.; Budnar, M.; Jeran, Z.; Pongrac, P.; Regvar, M.; et al. Application of X-ray fluorescence analytical techniques in phytoremediation and plant biology studies. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1240–1247. [Google Scholar] [CrossRef]
- Vogel-Mikuš, K.; Pongrac, P.; Pelicon, P. Micro-PIXE elemental mapping for ionome studies of crop plants. Int. J. PIXE 2014, 24, 217–233. [Google Scholar] [CrossRef]
- Wang, P.; McKenna, B.A.; Menzies, N.W.; Li, C.; Glover, C.J.; Zhao, F.J.; Kopittke, P.M. Minimizing experimental artefacts in synchrotron-based X-ray analyses of Fe speciation in tissues of rice plants. J. Synchrotron Radiat. 2019, 26, 1272–1279. [Google Scholar] [CrossRef]
- Koren, Š.; Arčon, I.; Kump, P.; Nečemer, M.; Vogel-Mikuš, K. Influence of CdCl2 and CdSO4 supplementation on Cd distribution and ligand environment in leaves of the Cd hyperaccumulator Noccaea (Thlaspi) praecox. Plant Soil 2013, 370, 125–148. [Google Scholar] [CrossRef][Green Version]
- Kump, P.; Vogel-Mikuš, K. Quantification of 2D elemental distribution maps of intermediate-thick biological sections by low energy synchrotron μ-X-ray fluorescence spectrometry. J. Instrum. 2018, 13. [Google Scholar] [CrossRef]
- Solé, V.A.; Papillon, E.; Cotte, M.; Walter, P.; Susini, J. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 63–68. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M.A. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongrac, P.; Arčon, I.; Castillo-Michel, H.; Vogel-Mikuš, K. Mineral Element Composition in Grain of Awned and Awnletted Wheat (Triticum aestivum L.) Cultivars: Tissue-Specific Iron Speciation and Phytate and Non-Phytate Ligand Ratio. Plants 2020, 9, 79. https://doi.org/10.3390/plants9010079
Pongrac P, Arčon I, Castillo-Michel H, Vogel-Mikuš K. Mineral Element Composition in Grain of Awned and Awnletted Wheat (Triticum aestivum L.) Cultivars: Tissue-Specific Iron Speciation and Phytate and Non-Phytate Ligand Ratio. Plants. 2020; 9(1):79. https://doi.org/10.3390/plants9010079
Chicago/Turabian StylePongrac, Paula, Iztok Arčon, Hiram Castillo-Michel, and Katarina Vogel-Mikuš. 2020. "Mineral Element Composition in Grain of Awned and Awnletted Wheat (Triticum aestivum L.) Cultivars: Tissue-Specific Iron Speciation and Phytate and Non-Phytate Ligand Ratio" Plants 9, no. 1: 79. https://doi.org/10.3390/plants9010079
APA StylePongrac, P., Arčon, I., Castillo-Michel, H., & Vogel-Mikuš, K. (2020). Mineral Element Composition in Grain of Awned and Awnletted Wheat (Triticum aestivum L.) Cultivars: Tissue-Specific Iron Speciation and Phytate and Non-Phytate Ligand Ratio. Plants, 9(1), 79. https://doi.org/10.3390/plants9010079