Effect of Arbuscular Mycorrhizal Colonization on Cadmium-Mediated Oxidative Stress in Glycine max (L.) Merr.
Abstract
1. Introduction
- (a)
- Cd decreases AMF colonization of soybean roots;
- (b)
- The two components of this experimental system (soybean and the R. intraradices isolate) behave as efficient accumulators of Cd;
- (c)
- The mycorrhization with R. intraradices improves the soybean growth under Cd stress; and
- (d)
- The symbiotic association with the AMF avoids the increase of lipid peroxidation in soybean caused by Cd oxidative stress.
2. Results
2.1. Effect of Cd on Soybean Mycorrhizal Colonization
2.2. Soil and Tissues Cd, P, and Fe Contents in Presence and Absence of AMF after Exposure to Cd
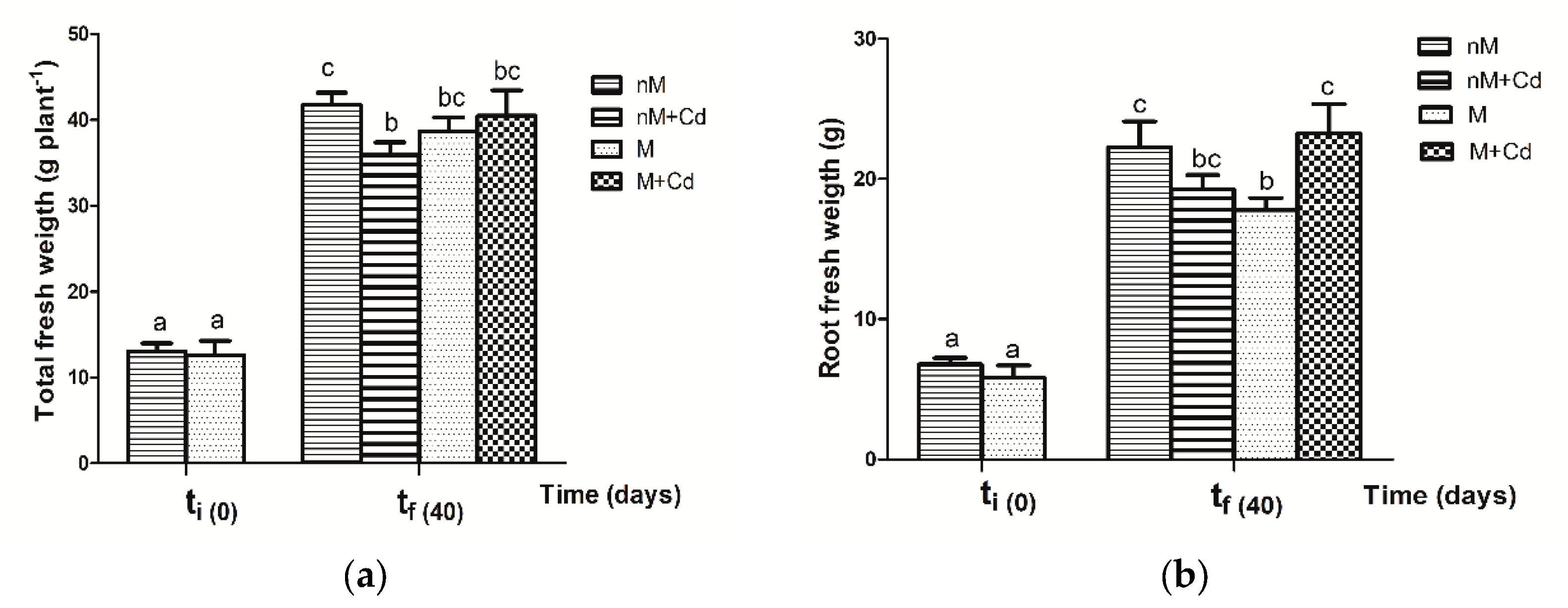
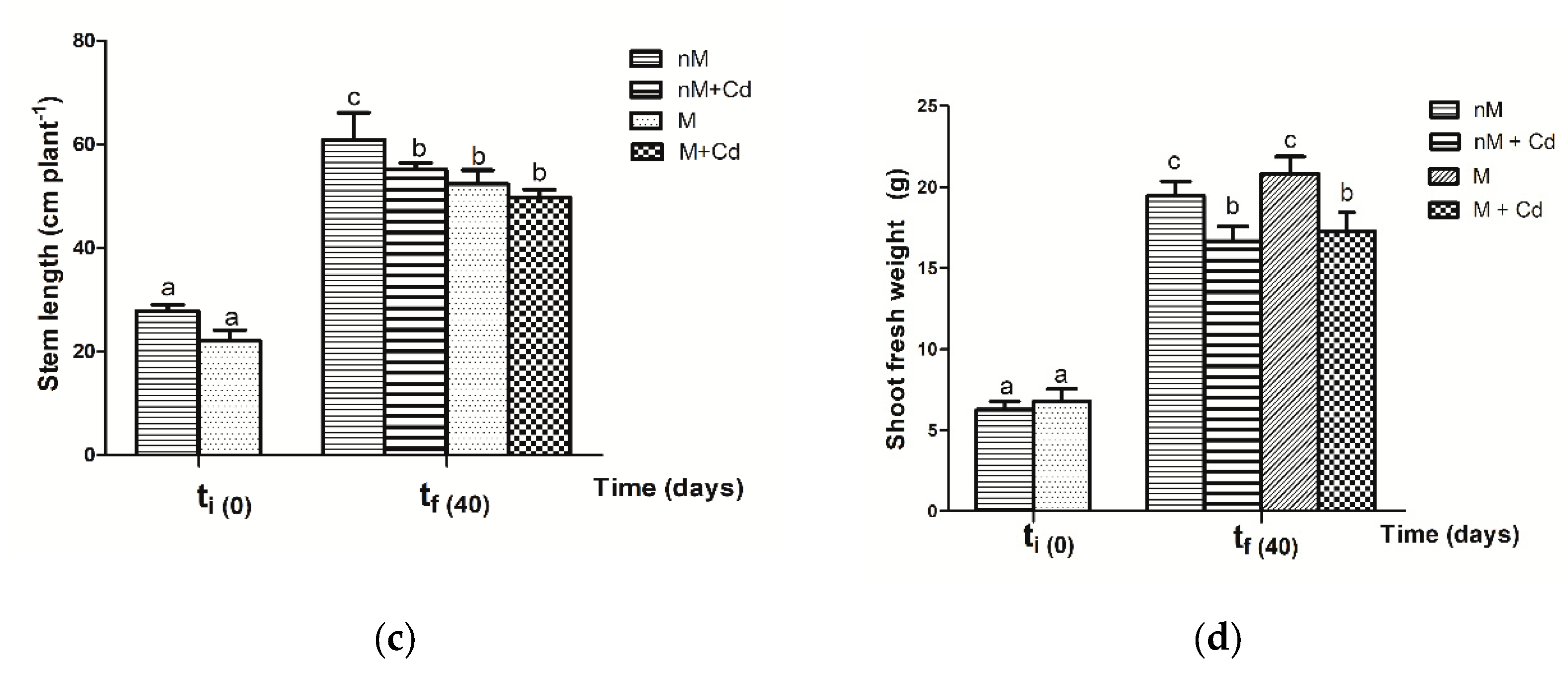
2.3. Growth Parameters of Soybean Plants after Cd Exposure
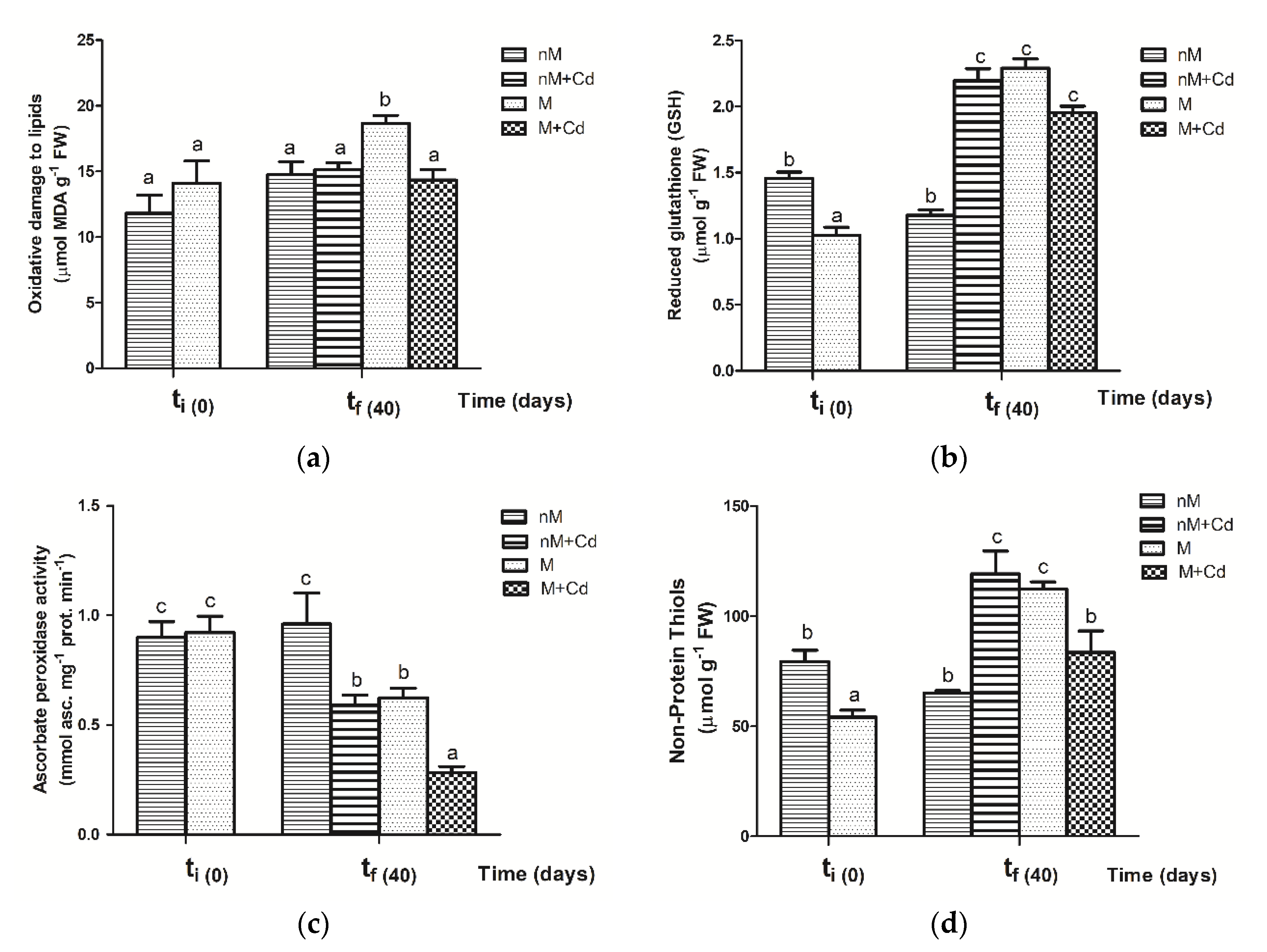
2.4. Oxidative Damage Response of Soybean Plants to Cd Exposure
3. Discussion
3.1. Colonization of Soybean Roots by Rhizophagus Intraradices
3.2. Soil and Plant Cd, P, and Fe Contents
3.3. Response of Soybean Growth Parameters to Cd Exposure
3.4. Oxidative Damage Response to R. intraradices Colonization of Soybean Not Exposed to Cd
3.5. Oxidative Damage Response to R. intraradices Colonization of Soybean with Cd Exposure
4. Materials and Methods
4.1. Biological Material and Experimental Design
4.2. Evaluation of AMF Colonization
4.3. Determination of Soil and Biomass Element Content
4.4. Growth Parameters
4.5. Measurements of Oxidative Damage and Antioxidant Defenses
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aloui, A.; Recorbet, G.; Robert, F.; Schoefs, B.; Bertrand, M.; Henry, C.; Gianinazzi-Pearson, V.; Dumas-Gaudot, E.; Aschi-Smiti, S. Arbuscular mycorrhizal symbiosis elicits shoot proteome changes that are modified during cadmium stress alleviation in Medicago truncatula. BMC Plant Biol. 2011, 11, 75. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Dalurzo, H.C.; Gomez, M.; Romero-Puertas, M.C.; del Río, L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Bhadury, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Bio/Technol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Corpas, F.J.; Rodríguez-Serrano, M.; Gomez, M.; del Río, L.A.; Sandalio, L.M. Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J. Plant Physiol. 2007, 164, 1346–1357. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.S.; Nievas, C.; Pérez Chaca, M.V.; Garibotto, F.; Gonzalez, U.; Luna, C.; Giménez, M.S.; Zirulnik, F. Cadmium-induced oxidative damage and antioxidative defense mechanisms in roots and leaves of Vigna mungo. Plant Growth Regul. 2008, 56, 285–295. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Marchand, C.; Collin, V.; Decottignies, P.; Tsan, P.; Lancelin, J.-M.; Trost, P.; Miginiac-Maslow, M.; Noctor, G.; et al. Glutathionylation of chloroplast thioredoxin f is a redox signaling mechanism in plants. Proc. Natl. Acad. Sci. USA 2005, 102, 16478–16483. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Balestrasse, B.K.; Gallego, S.M.; Tomaro, M.L. Oxidation of the enzymes involved in nitrogen assimilation plays an important role in the cadmium-induced toxicity in soybean plants. Plant Soil 2006, 284, 187–194. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; p. 750. [Google Scholar]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef]
- Kaldorf, M.; Kuhn, A.J.; Schörder, W.H.; Hildebrandt, U.; Bothe, H. Selective element deposits in maize colonized by a heavy metal tolerance conferring arbuscular mycorrhizal fungus. J. Plant Physiol. 1999, 154, 718–728. [Google Scholar] [CrossRef]
- Meharg, A.A.; Cairney, J.W.C. Co-evolution of mycorrhizal symbionts and their hosts to metal-contaminated environments. Adv. Ecol. Res. 2000, 30, 69–112. [Google Scholar] [CrossRef]
- González-Chávez, M.C.; Carrillo-Gónzalez, R.; Wright, S.F.; Nichols, K. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef]
- Joner, E.J.; Briones, R.; Leyval, C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 2000, 226, 227–234. [Google Scholar] [CrossRef]
- Leyval, C.; Turnau, K.; Haseldwandter, K. Effect of heavy metal pollution on mycorrhizal colonization and function: Physiological, ecological and applied aspects. Mycorrhiza 1997, 7, 139–153. [Google Scholar] [CrossRef]
- Joner, E.J.; Leyval, C. Uptake of 109Cd by roots and hyphae of a Glomus mosseae/Trifolium subterraneum mycorrhiza from soil amended with high and low concentrations of cadmium. New Phytol. 1997, 135, 353–360. [Google Scholar] [CrossRef]
- Azcón, R.; Perálvarez, M.C.; Biró, B.; Roldán, A.; Ruíz-Lozano, J.M. Antioxidant activities and metal acquisition in mycorrhizal plants growing in a heavy-metal multi contaminated soil amended with treated lignocellulosica growaste. Appl. Soil. Ecol. 2009, 41, 168–177. [Google Scholar] [CrossRef]
- Garg, N.; Kaur, H. Response of antioxidant enzymes, phytochelatins and glutathione production towards Cd and Zn stresses in Cajanus cajan (L.) Millisp. genotypes colonized by arbuscular mycorrhizal fungi. J. Agron. Crop Sci. 2013, 199, 118–133. [Google Scholar] [CrossRef]
- Rivera-Becerril, F.; Calantzis, C.; Turnau, K.; Caussanel, J.-P.; Belimov, A.; Gianizzani, S.; Strasser, R.J.; Gianizzani-Pearson, V. Cadmium accumulation and buffering of cadmium-induced stress by arbuscular mycorrhiza in three Pisum sativum L. genotypes. J. Exp. Bot. 2002, 53, 1177–1185. [Google Scholar] [CrossRef]
- Rivera-Becerril, F.; van Tuinen, D.; Martin-Laurent, F.; Metwally, A.; Dietz, K.-J.; Gianizzani, S.; Gianizzani-Pearson, V. Molecular changes in Pisum sativum L. roots during arbuscular mycorrhiza buffering of cadmium stress. Mycorrhiza 2005, 16, 51–60. [Google Scholar] [CrossRef]
- Leung, H.M.; Wang, Z.W.; Ye, Z.H.; Yung, K.L.; Peng, X.L.; Cheung, K.C. Interactions between arbuscular mycorrhizae and plants in phytoremediation of metal-contaminated soils: A review. Pedosphere 2013, 23, 549–563. [Google Scholar] [CrossRef]
- Chen, B.D.; Liu, Y.; Shen, H.; Li, X.L.; Christie, P. Uptake of cadmium from an experimentally contaminated calcareous soil by arbuscular mycorrhizal maize (Zea mays L.). Mycorrhiza 2004, 14, 347–354. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of arbuscular mycorrhizal (AM) fungi on growth, cadmium uptake, osmolyte, and phytochelatin synthesis in Cajanus cajan (L.) Millisp. under NaCl and Cd stresses. J. Plant Growth Regul. 2012, 31, 292–308. [Google Scholar] [CrossRef]
- Bano, S.A.; Ashfaq, D. Role of mycorrhiza to reduce heavy metal stress. Nat. Sci. 2013, 5, 16–20. [Google Scholar] [CrossRef]
- Jiang, Q.Y.; Zhuo, F.; Long, S.H.; Zhao, H.D.; Yang, D.J.; Ye, Z.H.; Li, S.S.; Jinga, Y.X. Can arbuscular mycorrhizal fungi reduce Cd uptake and alleviate Cd toxicity of Lonicera japonica grown in Cd-added soils? Sci. Rep. 2016, 6, 21805. [Google Scholar] [CrossRef]
- Pawlowska, T.E.; Charvat, I. Heavy-metal stress and development patterns on arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 2004, 70, 6643–6649. [Google Scholar] [CrossRef]
- Shute, T.; Macfie, S.M. Cadmium and zinc accumulation in soybean: A threat to food safety? Sci. Total Environ. 2006, 371, 63–73. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Ferrol, N.; Azcón-Aguilar, C.; Pérez-Tienda, J. Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: An overview on the mechanisms involved. Plant Sci. 2019, 280, 441–447. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Benabdellah, K.; Ferrol, N.; Azcón-Aguilar, C. Mechanisms underlying heavy metals tolerance in arbuscular mycorrhizas. In Mycorrhizas-Functional Processes and Ecological Impact; Azcón-Aguilar, C., Barea, J., Gianinazzi, S., Gianinazzi-Pearson, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 107–122. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Giacomino, A.; Aceto, M.; Mentasti, E. Adsorption of heavy metals on vermiculite: Influence of pH and organic ligands. J. Colloids Interface Sci. 2006, 299, 537–546. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; García-Sánchez, A. Removal of heavy metals from waste waters by vermiculites. Environ. Technol. 2003, 24, 615–625. [Google Scholar] [CrossRef]
- Najafi, P.; Asgari, K.; Samadi, N. Heavy metal elimination from industrial wastewater using natural substrate on pitcher irrigation. Int. J. Recycl. Org. Waste Agric. 2016, 5, 333–337. [Google Scholar] [CrossRef]
- Dunbar, K.R.; McLaughlin, M.J.; Reid, R. The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L.). J. Exp. Bot. 2003, 381, 349–354. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Andrade, S.A.L.; Gratão, P.L.; Schiavinato, M.A.; Silveira, A.P.D.; Azevedo, R.A.; Mazzafera, P. Zn uptake, physiological response and stress attenuation in mycorrhizal jack bean growing in soil with increasing Zn concentrations. Chemosphere 2009, 75, 1363–1370. [Google Scholar] [CrossRef]
- García-Garrido, J.M.; Ocampo, J.A. Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J. Exp. Bot. 2002, 53, 1377–1386. [Google Scholar] [CrossRef]
- Pozo, M.J.; Verhage, A.; García-Andrade, J.; García, J.M.; Azcón-Aguilar, C. Priming plant defence against pathogens by arbuscular mycorrhizal fungi. In Mycorrhizas—Functional Processes and Ecological Impact; Azcón-Aguilar, C., Barea, J.M., Gianinazzi, S., Gianinazzi-Pearson, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 123–135. [Google Scholar] [CrossRef]
- Fester, T.; Hause, B. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 2005, 15, 373–379. [Google Scholar] [CrossRef]
- Gutjahr, C.; Parniske, M. Cell and developmental biology of arbuscular mycorrhizal symbiosis. Ann. Rev. Cell Dev. Biol. 2013, 29, 593–617. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.-N.; Abd-Allah, E.F. Chapter 15—Mycorrhizal association and ROS in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 453–475. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Baum, C. The potential of rhizosphere microorganisms to promote the plant growth in disturbed soils. In Environmental Protection Strategies for Sustainable Development. Strategies for Sustainability; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 35–64. [Google Scholar] [CrossRef]
- Bressano, M.; Curetti, M.; Giachero, L.; Vargas Gil, S.; Cabello, M.; March, G.; Ducasse, D.A.; Luna, C.M. Mycorrhizal fungi symbiosis as a strategy against oxidative stress in soybean plants. J. Plant Physiol. 2010, 167, 1622–1626. [Google Scholar] [CrossRef]
- Grϋmberg, B.; Urcelay, C.; Schroeder, M.A.; Vargas-Gil, S.; Luna, C.M. The role of inoculum identity in drought stress mitigation by arbuscular mycorrhizal fungi in soybean. Biol. Fertil. Soils 2014, 51, 1–10. [Google Scholar] [CrossRef]
- Pastori, G.M.; Foyer, C. Common components, networks, and pathways of cross-tolerance to stress. The central role of “redox” and abscisic acid-mediated controls. Plant Physiol. 2002, 129, 460–468. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Daniels, B.A.; Skipper, H.D. Methods for the recovery and quantitative estimation of propagules from the soil. In Methods and Principles of Mycorrhizal Research; Schenck, N.C., Ed.; American Phytopathological Society and Press: St. Paul, MN, USA, 1982; pp. 29–35. [Google Scholar]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Gomes, P.C.; Fontes, M.P.F.; da Silva, A.G.; Mendonça, E.; Netto, A.R. Selectivity sequence and competitive adsorption of heavy metals by brazilian soils. Soil Sci. Soc. Am. J. 2001, 65, 1115–1121. [Google Scholar] [CrossRef]
- Reddy, M.R.; Dunn, S.J. Distribution coefficients for nickel and zinc in soils. Environ. Pollut. 1986, 11, 303–313. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedure of clearing roots and staining parasitic and vesicular-arbusuclar mycorrhizal fungi for rapid assessment of infections. Trans. Br. Mycol. Soc. 1970, 55, 159–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, D.L.; Swam, J.A. Anew methods which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for Zinc, Iron, Manganese, and Copper. Am. Soc. Agron. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Bray, R.; Kurtz, L.T. Determination of total, organic and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Akerboom, T.; Sies, H. Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248. [Google Scholar] [CrossRef]
- Gallego, S.; Benavides, M.; Tomaro, M. Effect of cadmium ions on antioxidant defense system in sunflower cotyledons. Biol. Plant. 1999, 42, 49–55. [Google Scholar] [CrossRef]
- R Project for Statistical Computing 3.6.1 for Windows. Free Language; GNU General Public License. 2019. Available online: https://www.r-project.org/ (accessed on 10 July 2019).
| Treatment | Harvest Time | Root Colonization (%) | Arbuscules (%) | Vesicles (%) |
|---|---|---|---|---|
| Without Cd | t0 | 66.76 ± 6.21a | 36.75 ± 6.21a | 1.750 ± 1.10a |
| tf | 85.66 ± 3.09b | 70.83 ± 5.02b | 35.67 ± 0.80b | |
| With Cd | tf | 91.00 ± 3.59b | 72.00 ± 5.1b | 39.33±3.76b 1 |
| Soil Elements Concentration (ppm) | Non-Mycorrhizal Soybean + Cd | Mycorrhizal Soybean+ Cd |
|---|---|---|
| Cd | 10.97 ± 5.06 | 17.72 ± 8.44 |
| P | 529.68 ± 46.3 | 490.53 ± 49.84 |
| Fe | 10375 ± 1742 | 13582 ± 1441 1 |
| Tissues | P (ppm) | Fe (ppm) | Cd (ppm) | |||
|---|---|---|---|---|---|---|
| nM | M | nM | M | nM | M | |
| Roots | 2176.66 ± 140.18ac | 2583.33 ± 59.33be | 3884.20 ± 46.83ac | 5181.20 ± 160.57be | 65.52 ± 0.76ac | 30.27 ± 0.50be |
| Old leaves | 1187.16 ± 54.87adg | 1980.22 ± 204.9bfi | 158.56 ± 11.88adg | 130.34 ± 29.26afi | 0.726 ± 0.020adg | 1.056 ± 0.076bfi |
| New leaves | 2073.35 ± 15.43ach | 2257.14 ± 173.7aei | 123.85 ± 10.11adg | 79.16 ± 1.15afi | 1.600 ± 0.005adh | 0.740 ± 0.046bfj |
| Leaves * | 1630.25 ± 199.7ad | 2118.68 ± 119.95bf | 141.20 ± 10.43ad | 104.75 ± 17.38af | 1.163 ± 0.195ad | 0.898 ± 0.081af 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, A.S.; Lugo, M.A.; Pérez Chaca, M.V.; Vargas-Gil, S.; Zirulnik, F.; Leporati, J.; Ferrol, N.; Azcón-Aguilar, C. Effect of Arbuscular Mycorrhizal Colonization on Cadmium-Mediated Oxidative Stress in Glycine max (L.) Merr. Plants 2020, 9, 108. https://doi.org/10.3390/plants9010108
Molina AS, Lugo MA, Pérez Chaca MV, Vargas-Gil S, Zirulnik F, Leporati J, Ferrol N, Azcón-Aguilar C. Effect of Arbuscular Mycorrhizal Colonization on Cadmium-Mediated Oxidative Stress in Glycine max (L.) Merr. Plants. 2020; 9(1):108. https://doi.org/10.3390/plants9010108
Chicago/Turabian StyleMolina, Alicia S., Mónica A. Lugo, María V. Pérez Chaca, Silvina Vargas-Gil, Fanny Zirulnik, Jorge Leporati, Nuria Ferrol, and Concepción Azcón-Aguilar. 2020. "Effect of Arbuscular Mycorrhizal Colonization on Cadmium-Mediated Oxidative Stress in Glycine max (L.) Merr." Plants 9, no. 1: 108. https://doi.org/10.3390/plants9010108
APA StyleMolina, A. S., Lugo, M. A., Pérez Chaca, M. V., Vargas-Gil, S., Zirulnik, F., Leporati, J., Ferrol, N., & Azcón-Aguilar, C. (2020). Effect of Arbuscular Mycorrhizal Colonization on Cadmium-Mediated Oxidative Stress in Glycine max (L.) Merr. Plants, 9(1), 108. https://doi.org/10.3390/plants9010108







