UHPLC-Q-TOF/MS-Based Metabolomics Approach Reveals the Antifungal Potential of Pinocembroside against Citrus Green Mold Phytopathogen
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of PiCB on Mycelial Growth of P. digitatum
2.2. Effects of PiCB on Mycelial Weights and Water-Retention Rate of P. digitatum
2.3. Effect of PiCB on Hyphal Morphology of P. digitatum Mycelia
2.4. Effect of PiCB on Ultrastructure of P. digitatum Cells
2.5. Effects of PiCB on the Metabolic Profiles of P. digitatum Mycelia
3. Materials and Methods
3.1. Isolation and Purification of Pinocembroside (PiCB)
3.2. Fungal Pathogen and Culture Conditions
3.3. In Vitro Antifungal Activity Assay
3.3.1. Mycelial Growth Assay
3.3.2. Measurement of Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
3.3.3. Determination of Mycelial Weights and Water-Retention Rate
3.4. Microscopic Observations
3.5. Preparation of Metabolite Samples
3.6. UHPLC-Q-TOF/MS Assays
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Otmani, M.; Ait-Oubahou, A.; Zacarías, L. Citrus spp.: Orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 437–516. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Zhang, Y.-F.; Lin, Y.-J.; Chen, Y.; Lu, X.-K. Illumina® Sequencing Reveals Candidate Genes of Carotenoid Metabolism in Three Pummelo Cultivars (Citrus Maxima) with Different Pulp Color. Int. J. Mol. Sci. 2019, 20, 2246. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qi, W.; Peng, X.; Chen, J.; Wan, C. Inhibitory Effect of 7-Demethoxytylophorine on Penicillium italicum and its Possible Mechanism. Microorganisms 2019, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Shen, Y.; Nisar, M.F.; Qi, W.; Chen, C.; Chen, J. The Antifungal Potential of Carvacrol against Penicillium digitatum through 1H-NMR Based Metabolomics Approach. Appl. Sci. 2019, 9, 2240. [Google Scholar] [CrossRef]
- Moraes Bazioli, J.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.M.; Kupper, K.C.; Augusto, F.; de Carvalho, J.E.; Fill, T.P. Biological Control of Citrus Postharvest Phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Galsurker, O.; Diskin, S.; Maurer, D.; Feygenberg, O.; Alkan, N. Fruit Stem-End Rot. Horticulturae 2018, 4, 50. [Google Scholar] [CrossRef]
- Xin, Z.; OuYang, Q.; Wan, C.; Che, J.; Li, L.; Chen, J.; Tao, N. Isolation of antofine from Cynanchum atratum BUNGE (Asclepiadaceae) and its antifungal activity against Penicillium digitatum. Postharvest Biol. Technol. 2019, 157, 110961. [Google Scholar] [CrossRef]
- Qian, X.; Yang, Q.; Zhang, Q.; Abdelhai, M.H.; Dhanasekaran, S.; Serwah, B.N.A.; Gu, N.; Zhang, H. Elucidation of the Initial Growth Process and the Infection Mechanism of Penicillium digitatum on Postharvest Citrus (Citrus reticulata Blanco). Microorganisms 2019, 7, 485. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Cerioni, L.; Sepulveda, M.; Ramallo, J.; Rapisarda, V.A.; Volentini, S.I. Polyhexamethylene guanidine as a fungicide, disinfectant and wound protector in lemons challenged with Penicillium digitatum. Food Microbiol. 2018, 76, 128–134. [Google Scholar] [CrossRef]
- Kellerman, M.; Erasmus, A.; Cronjé, P.J.R.; Fourie, P.H. Thiabendazole residue loading in dip, drench and wax coating applications to control green mould and chilling injury on citrus fruit. Postharvest Biol. Technol. 2014, 96, 78–87. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Yang, Q.; Apaliya, M.T. Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trend. Food Sci. Tech. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, plant- and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Y.; Chen, C.; Wan, C. Inhibition of Key Citrus Postharvest Fungal Strains by Plant Extracts In Vitro and In Vivo: A Review. Plants 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Chen, C.; Li, M.; Yang, Y.; Chen, M.; Chen, J. Chemical Constituents and Antifungal Activity of Ficus hirta Vahl. Fruits. Plants 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, X.; Chen, X.; Ba, Y.; Zhang, N.; Xu, R.; Zhao, W.; Wu, X. Flavonones from Ameliorate Hepatic Steatosis by Activating the SIRT1/AMPK Pathway in HepG2 Cells. Int. J. Mol. Sci. 2018, 19, 2555. [Google Scholar] [CrossRef]
- Li, H.; Hou, Z.; Li, C.; Zhang, Y.; Shen, T.; Hu, Q.; Ren, D. Three pairs of diastereoisomeric flavanone glycosides from Viscum articulatum. Fitoterapia 2015, 102, 156–162. [Google Scholar] [CrossRef]
- Cheng, J.; Yi, X.; Wang, Y.; Huang, X.; He, X. Phenolics from the roots of hairy fig (Ficus hirta Vahl.) exert prominent anti-inflammatory activity. J. Funct. Foods 2017, 31, 79–88. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Liu, X.Z.; Lv, Z.C.; Peng, Y.H. Effects of Ficus hirta Vahl. (Wuzhimaotao) extracts on growth inhibition of HeLa cells. Exp. Toxicol. Pathol. 2012, 64, 743–749. [Google Scholar] [CrossRef]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef]
- Tao, N.; Chen, Y.; Wu, Y.; Wang, X.; Li, L.; Zhu, A. The terpene limonene induced the green mold of citrus fruit through regulation of reactive oxygen species (ROS) homeostasis in Penicillium digitatum spores. Food Chem. 2019, 277, 414–422. [Google Scholar] [CrossRef]
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Rapisarda, V.A.; Volentini, S.I. Antifungal activity of β-carbolines on Penicillium digitatum and Botrytis cinerea. Food Microbiol. 2017, 62, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; OuYang, Q.; Tao, N. Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 2016, 53, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhao, J.; Zhang, B.; Shen, Q.; Shang, Q.; Li, P. Effect of a novel antifungal peptide P852 on cell morphology and membrane permeability of Fusarium oxysporum. BBA Biomembr. 2019, 1861, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Zhang, Y.; Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Quek, S.Y.; Yao, W. Antifungal effects of thymol and salicylic acid on cell membrane and mitochondria of Rhizopus stolonifer and their application in postharvest preservation of tomatoes. Food Chem. 2019, 285, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Gou, J.; Han, X.; Wu, Q.; Zhang, C. In Vitro and In Vivo activities of eugenol against tobacco black shank caused by Phytophthora nicotianae. Pestic. Biochem. Phys. 2017, 142, 148–154. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Luo, X.; Zeng, H.; Ban, X.; He, J.; Wang, Y. The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 2012, 130, 520–527. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral inhibits mycelial growth of Penicillium italicum by a membrane damage mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Li, Q.; Xu, S.; Zhao, X.; Xue, P.; Feng, X. Antifungal activity of zedoary turmeric oil against Phytophthora capsici through damaging cell membrane. Pestic. Biochem. Phys. 2019, 159, 59–67. [Google Scholar] [CrossRef]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Lu, Z.; Sun, C.; Zhang, M.; Zhu, A.; Peng, X. Perillaldehyde, a Promising Antifungal Agent Used in Food Preservation, Triggers Apoptosis through a Metacaspase-Dependent Pathway in Aspergillus flavus. J. Agric. Food Chem. 2016, 64, 7404–7413. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.W.; Walsh, T.J. Amino Acid Metabolism and Transport Mechanisms as Potential Antifungal Targets. Int. J. Mol. Sci. 2018, 19, 909. [Google Scholar] [CrossRef] [PubMed]
- Sant, D.G.; Tupe, S.G.; Ramana, C.V.; Deshpande, M.V. Fungal cell membrane-promising drug target for antifungal therapy. J. Appl. Microbiol. 2016, 121, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Sarkisian, M.R.; Mehal, W.Z.; Pasko, R.; Flavell, R.A. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science 2003, 302, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit–Pathogen Interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Kohyama-Koganeya, A.; Hirabayashi, Y. New insights on glucosylated lipids: Metabolism and functions. BBA Lipids 2013, 1831, 1475–1485. [Google Scholar] [CrossRef]
- Xu, J.; Shao, X.; Li, Y.; Wei, Y.; Xu, F.; Wang, H. Metabolomic Analysis and Mode of Action of Metabolites of Tea Tree Oil Involved in the Suppression of Botrytis cinerea. Front. Microbiol. 2017, 8, 1017–1027. [Google Scholar] [CrossRef]
- Chang, L.; Tang, X.; Lu, H.; Zhang, H.; Chen, Y.Q.; Chen, H.; Chen, W. Role of Adenosine Monophosphate Deaminase during Fatty Acid Accumulation in Oleaginous Fungus Mortierella alpina. J. Agric. Food Chem. 2019, 67, 9551–9559. [Google Scholar] [CrossRef]
- Márquez, S.; Fernández, J.J.; Mancebo, C.; Herrero-Sánchez, C.; Alonso, S.; Sandoval, T.A.; Rodríguez Prados, M.; Cubillos-Ruiz, J.R.; Montero, O.; Fernández, N.; et al. Tricarboxylic Acid Cycle Activity and Remodeling of Glycerophosphocholine Lipids Support Cytokine Induction in Response to Fungal Patterns. Cell Rep. 2019, 27, 525–536. [Google Scholar] [CrossRef]
- Butters, J.A.; Burrell, M.M.; Hollomon, D.W. Purine metabolism in barley powdery mildew and its host. Phys. Plant Pathol. 1985, 27, 65–74. [Google Scholar] [CrossRef]
- Anwar, S.; Bhandari, U.; Panda, B.P.; Dubey, K.; Khan, W.; Ahmad, S. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. J. Pharm. Biomed. Anal. 2018, 159, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent of postharvest citrus sour rot. Crop Prot. 2012, 35, 41–46. [Google Scholar] [CrossRef]
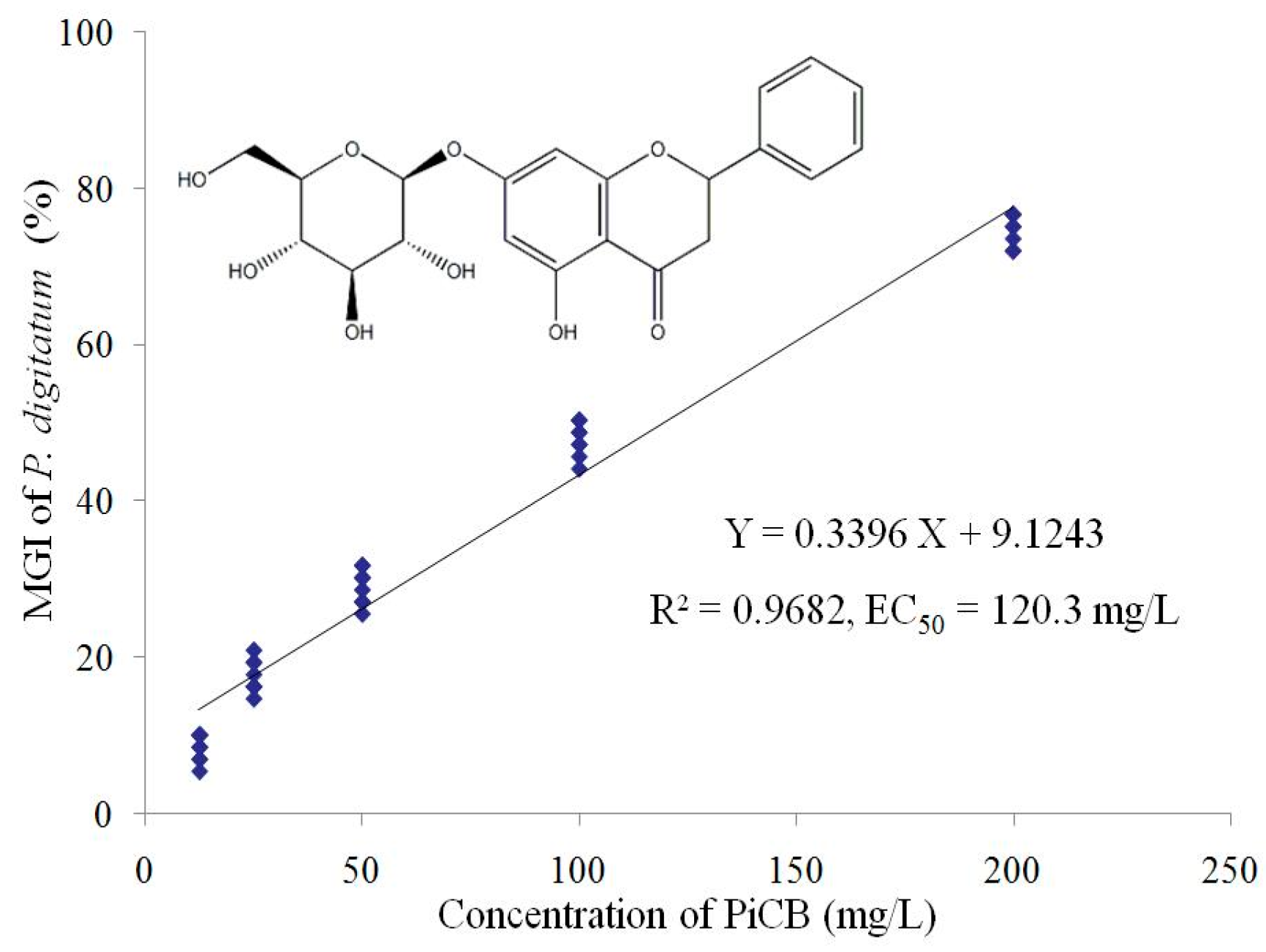
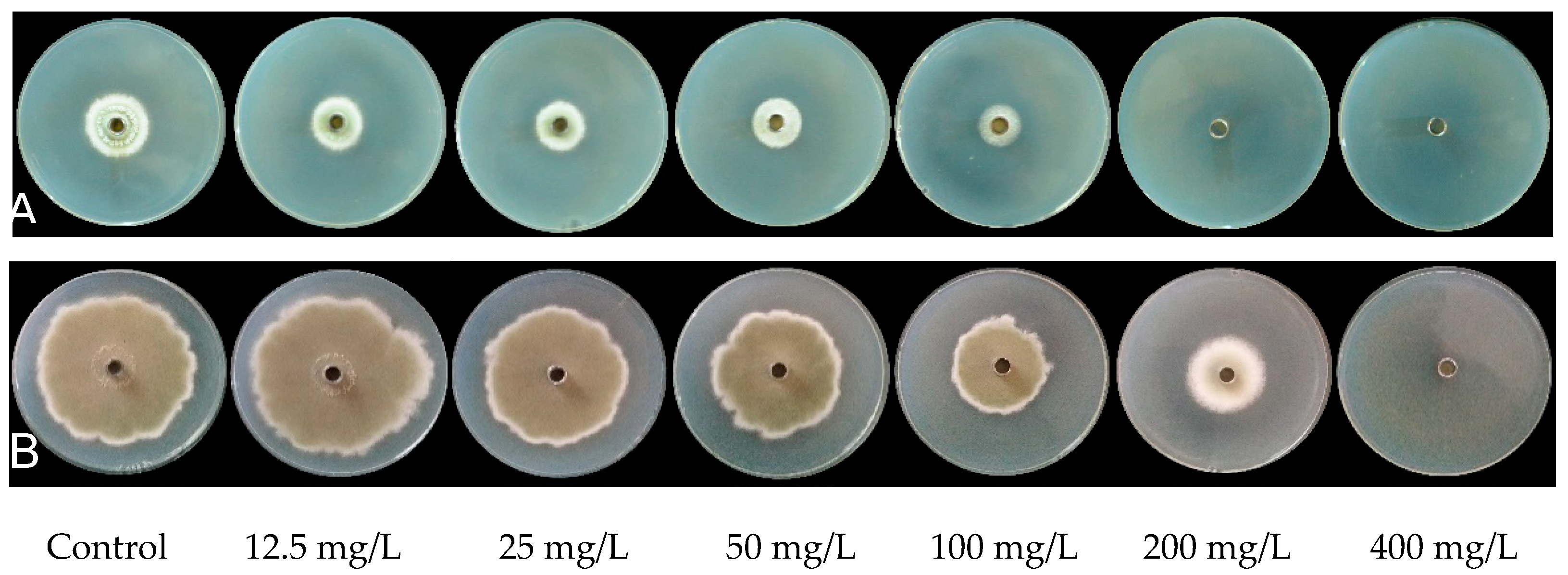
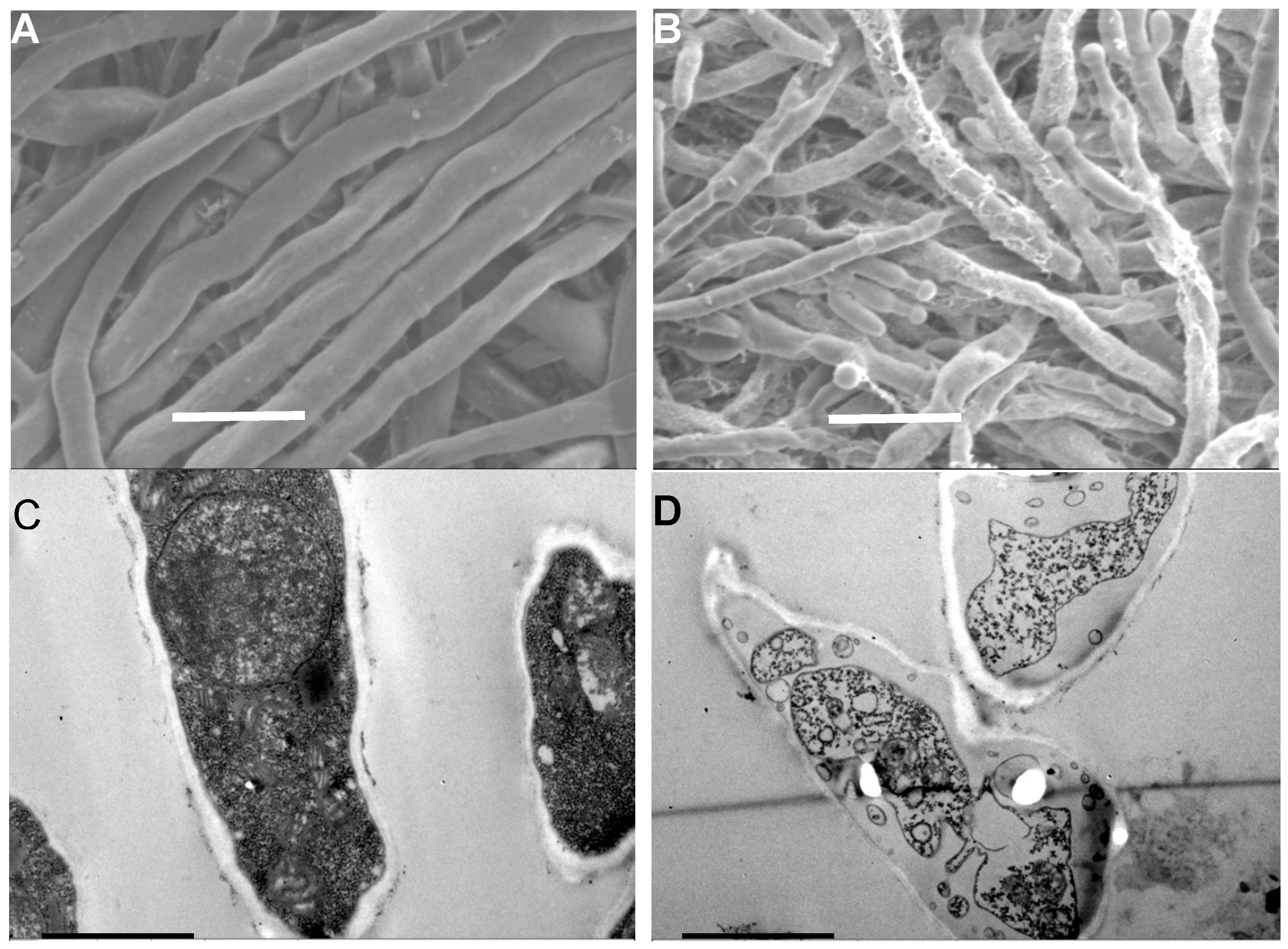
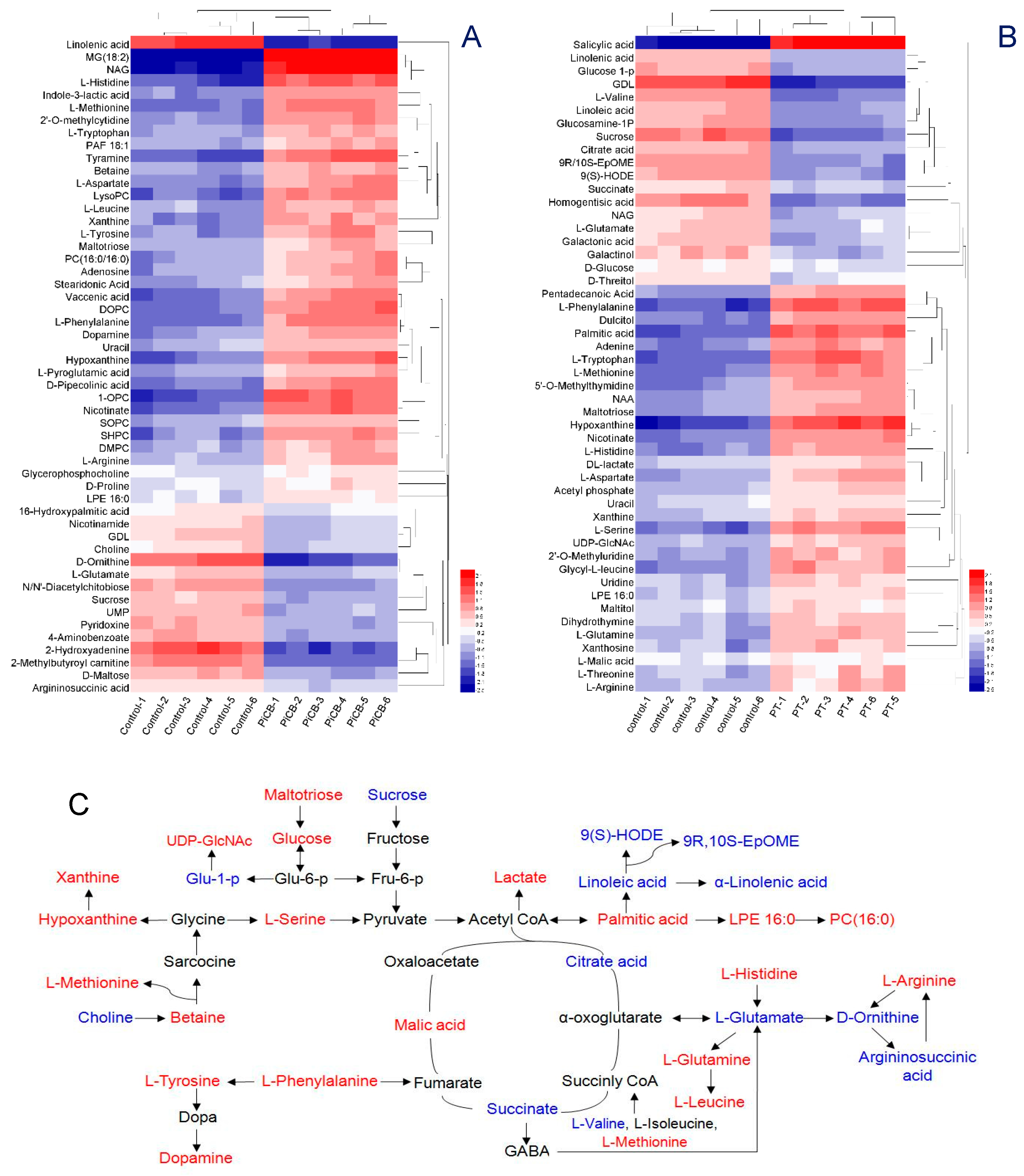
| Concentrations (mg/L) | Mycelial Weight (g/L) | Water-Retention Rate (%) | |
|---|---|---|---|
| Wet Weight | Dry Weight | ||
| 0 | 34.32 ± 1.588 a | 3.82 ± 0.224 a | 88.9 ± 0.14 a |
| 12.5 | 32.73 ± 2.223 a | 3.68 ± 0.127 a | 88.7 ± 0.38 a |
| 25 | 27.13 ± 1.446 b | 3.40 ± 0.042 b | 87.3 ± 0.43 b |
| 50 | 22.37 ± 1.595 c | 3.32 ± 0.076 bc | 85.4 ± 0.69 c |
| 100 | 20.14 ± 0.884 d | 3.14 ± 0.058 cd | 84.5 ± 0.40 c |
| 200 | 18.88 ± 0.527 d | 3.07 ± 0.066 d | 83.4 ± 0.21 d |
| 400 | 18.23 ± 0.388 d | 3.03 ± 0.069 d | 83.3 ± 0.13 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Cai, N.; Chen, J.; Wan, C. UHPLC-Q-TOF/MS-Based Metabolomics Approach Reveals the Antifungal Potential of Pinocembroside against Citrus Green Mold Phytopathogen. Plants 2020, 9, 17. https://doi.org/10.3390/plants9010017
Chen C, Cai N, Chen J, Wan C. UHPLC-Q-TOF/MS-Based Metabolomics Approach Reveals the Antifungal Potential of Pinocembroside against Citrus Green Mold Phytopathogen. Plants. 2020; 9(1):17. https://doi.org/10.3390/plants9010017
Chicago/Turabian StyleChen, Chuying, Nan Cai, Jinyin Chen, and Chunpeng Wan. 2020. "UHPLC-Q-TOF/MS-Based Metabolomics Approach Reveals the Antifungal Potential of Pinocembroside against Citrus Green Mold Phytopathogen" Plants 9, no. 1: 17. https://doi.org/10.3390/plants9010017
APA StyleChen, C., Cai, N., Chen, J., & Wan, C. (2020). UHPLC-Q-TOF/MS-Based Metabolomics Approach Reveals the Antifungal Potential of Pinocembroside against Citrus Green Mold Phytopathogen. Plants, 9(1), 17. https://doi.org/10.3390/plants9010017






