Manganese Homeostasis in Cyanobacteria
Abstract
1. Introduction—The Essential Function of Manganese in Cyanobacteria
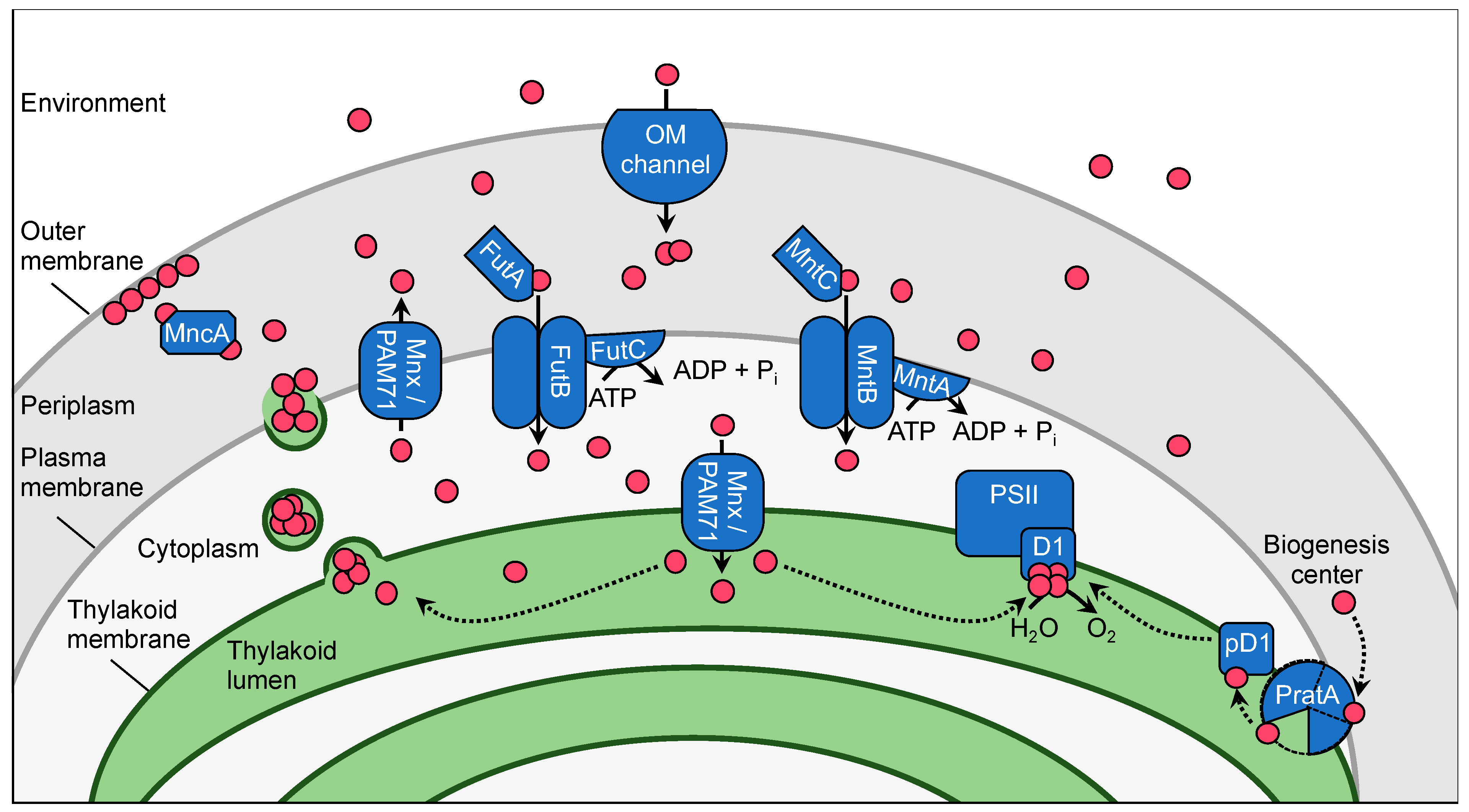
2. The Manganese Homeostasis Network in Synechocystis
2.1. Subcellular Manganese Allocation
2.2. Manganese Transporter
2.2.1. Uptake at the Outer Membrane
2.2.2. Uptake at the Plasma Membrane
2.2.3. Uptake at the Thylakoid Membrane
2.3. Manganese Delivery to Mn Cluster in PSII
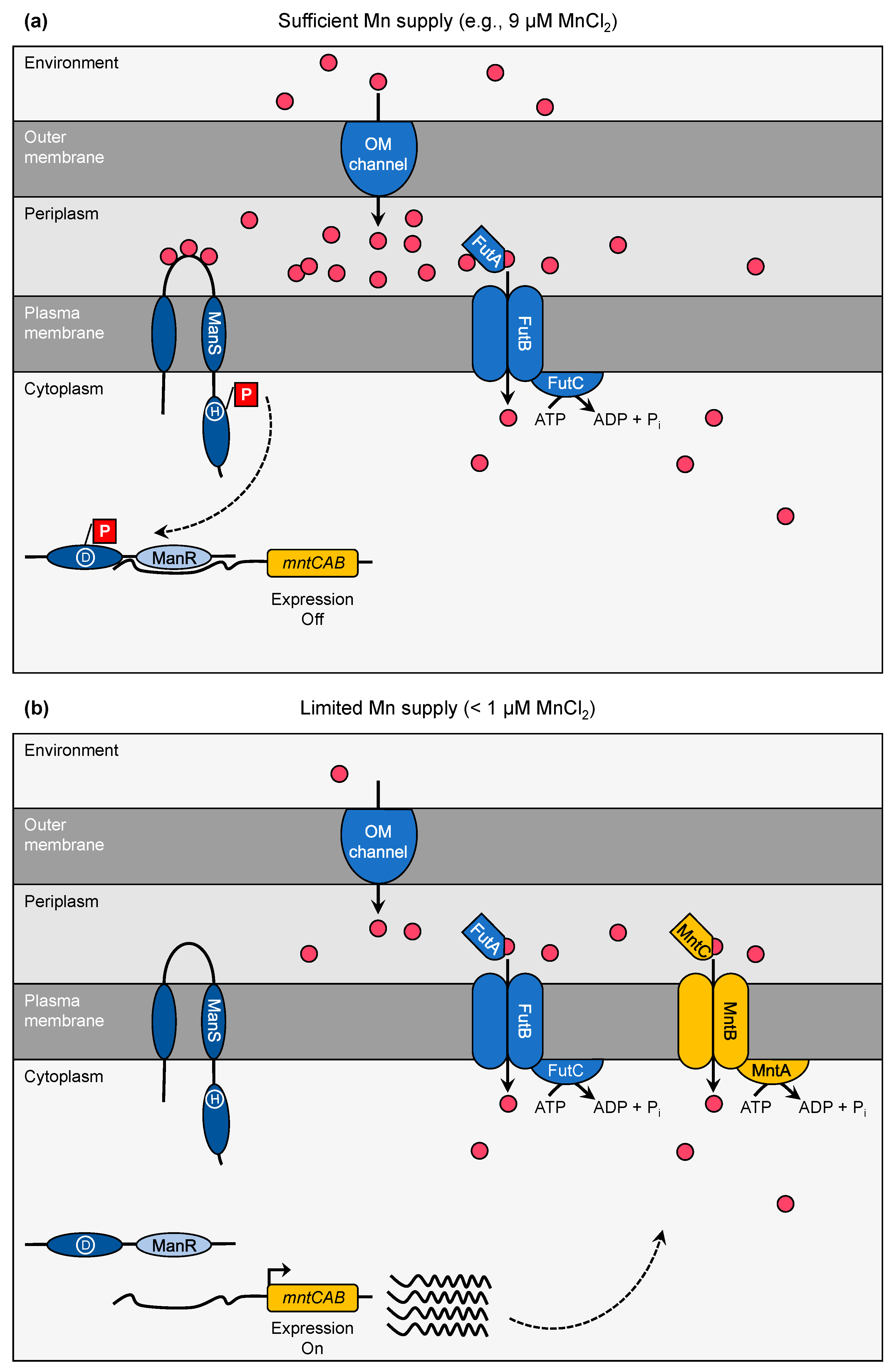
3. Manganese Limitation—Perception and Response
4. Manganese Excess—Risks and Avoidance
Funding
Conflicts of Interest
References
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Klinkhammer, G.P.; Bender, M.L. The distribution of manganese in the Pacific Ocean. Earth Planet. Sci. Lett. 1980, 46, 361–384. [Google Scholar] [CrossRef]
- Middag, R.; de Baar, H.J.W.; Laan, P.; Cai, P.H.; van Ooijen, J.C. Dissolved manganese in the Atlantic sector of the Southern Ocean. Deep. Res. Part II Top. Stud. Oceanogr. 2011, 58, 2661–2677. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Effect of sunlight on redox cycles of manganese in the southwestern Sargasso Sea. Deep Sea Res. Part A Oceanogr. Res. Pap. 1988, 35, 1297–1317. [Google Scholar] [CrossRef]
- Fraústo da Silva, J.J.R.; Williams, R.J.P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Schmidt, S.B. Husted The Biochemical Properties of Manganese in Plants. Plants 2019, 8, 381. [Google Scholar] [CrossRef]
- Allen, J.F.; Martin, W. Out of thin air. Nature 2007, 445, 2–4. [Google Scholar] [CrossRef]
- Daye, M.; Klepac-Ceraj, V.; Pajusalu, M.; Rowland, S.; Farrell-Sherman, A.; Beukes, N.; Tamura, N.; Fournier, G.; Bosak, T. Light-driven anaerobic microbial oxidation of manganese. Nature 2019, 576, 311–314. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Evolution of Oxygenic Photosynthesis. Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the Photosynthetic Oxygen-Evolving Center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Shevela, D.; Messinger, J. Studying the oxidation of water to molecular oxygen in photosynthetic and artificial systems by time-resolved membrane-inlet mass spectrometry. Front. Plant Sci. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Spector, M.; Winget, G.D. Purification of a manganese-containing protein involved in photosynthetic oxygen evolution and its use in reconstituting an active membrane. Proc. Natl. Acad. Sci. USA 1980, 77, 957–959. [Google Scholar] [CrossRef]
- Salomon, E.; Keren, N. Manganese Limitation Induces Changes in the Activity and in the Organization of Photosynthetic Complexes in the Cyanobacterium Synechocystis sp. Strain PCC 6803. Plant Physiol. 2011, 155, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese Deficiency in Plants: The Impact on Photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Socha, A.L.; Guerinot, M. Lou Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Keren, N.; Kidd, M.J.; Penner-Hahn, J.E.; Pakrasi, H.B. A light-dependent mechanism for massive accumulation of manganese in the photosynthetic bacterium Synechocystis sp. PCC 6803. Biochemistry 2002, 41, 15085–15092. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Alberdi, M.; Ivanov, A.G.; Krol, M.; Hüner, N.P.A. Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. J. Exp. Bot. 2013, 64, 343–354. [Google Scholar] [CrossRef]
- Foster, A.W.; Osman, D.; Robinson, N.J. Metal preferences and metallation. J. Biol. Chem. 2014, 289, 28095–28103. [Google Scholar] [CrossRef]
- Tottey, S.; Waldron, K.J.; Firbank, S.J.; Reale, B.; Bessant, C.; Sato, K.; Cheek, T.R.; Gray, J.; Banfield, M.J.; Dennison, C.; et al. Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 2008, 455, 1138–1142. [Google Scholar] [CrossRef]
- Nickelsen, J.; Rengstl, B. Photosystem II Assembly: From Cyanobacteria to Plants. Annu. Rev. Plant Biol. 2013, 64, 609–635. [Google Scholar] [CrossRef]
- Brandenburg, F.; Schoffman, H.; Kurz, S.; Krämer, U.; Keren, N.; Weber, A.P.M.; Eisenhut, M. The Synechocystis manganese exporter mnx is essential for manganese homeostasis in cyanobacteria. Plant Physiol. 2017, 173, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Gandini, C.; Schmidt, S.B.; Husted, S.; Schneider, A.; Leister, D. The transporter SynPAM71 is located in the plasma membrane and thylakoids, and mediates manganese tolerance in Synechocystis PCC6803. New Phytol. 2017, 215, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Rosch, J.W.; Gao, G.; Ridout, G.; Wang, Y.D.; Tuomanen, E.I. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol. Microbiol. 2009, 72, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Klinkert, B.; Ossenbühl, F.; Sikorski, M.; Berry, S.; Eichacker, L.; Nickelsen, J. PratA, a periplasmic tetratricopeptide repeat protein involved in biogenesis of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 2004, 279, 44639–44644. [Google Scholar] [PubMed]
- Schottkowski, M.; Gkalympoudis, S.; Tzekova, N.; Stelljes, C.; Schünemann, D.; Ankele, E.; Nickelsen, J. Interaction of the periplasmic pratA factor and the PsbA (D1) protein during biogenesis of photosystem II in Synechocystis sp. PCC 6803. J. Biol. Chem. 2009, 284, 1813–1819. [Google Scholar] [CrossRef]
- Stengel, A.; Gügel, I.L.; Hilger, D.; Rengstl, B.; Jung, H.; Nickelsen, J. Initial steps of photosystem II de novo assembly and preloading with manganese take place in biogenesis centers in Synechocystis. Plant Cell 2012, 24, 660–675. [Google Scholar] [CrossRef]
- Bartsevich, V.V.; Pakrasi, H.B. Molecular identification of an ABC transporter complex for manganese: Analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J. 1995, 14, 1845–1853. [Google Scholar] [CrossRef]
- Bartsevich, V.Y.; Pakrasi, H.B. Manganese transport in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 1996, 271, 26057–26061. [Google Scholar] [CrossRef]
- Sharon, S.; Salomon, E.; Kranzler, C.; Lis, H.; Lehmann, R.; Georg, J.; Zer, H.; Hess, W.R.; Keren, N. The hierarchy of transition metal homeostasis: Iron controls manganese accumulation in a unicellular cyanobacterium. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 1990–1997. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Suzuki, I.; Yamamoto, H.; Lyukevich, A.; Bodrova, I.; Los, D.A.; Piven, I.; Zinchenko, V.; Kanehisa, M.; Murata, N. A two-component Mn2+-sensing system negatively regulates expression of the mntCAB operon in Synechocystis. Plant Cell 2002, 14, 2901–2913. [Google Scholar] [CrossRef]
- Ogawa, T.; Bao, D.H.; Katoh, H.; Shibata, M.; Pakrasi, H.B.; Bhattacharyya-Pakrasi, M. A two-component signal transduction pathway regulates manganese homeostasis in Synechocystis 6803, a photosynthetic organism. J. Biol. Chem. 2002, 277, 28981–28986. [Google Scholar] [CrossRef] [PubMed]
- Chandler, L.E.; Bartsevich, V.V.; Pakrasi, H.B. Regulation of manganese uptake in Synechocystis 6803 by rfrA, a member of a novel family of proteins containing a repeated five-residues domain. Biochemistry 2003, 42, 5508–5514. [Google Scholar] [CrossRef] [PubMed]
- Cyanobase. Available online: http://genome.microbedb.jp/mnt.html (accessed on 29 November 2019).
- Duy, D.; Soll, J.; Philippar, K. Solute channels of the outer membrane: From bacteria to chloroplasts. Biol. Chem. 2007, 388, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Lea-Smith, D.J.; Bombelli, P.; Vasudevan, R.; Howe, C.J. Photosynthetic, respiratory and extracellular electron transport pathways in cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Celia, H.; Noinaj, N.; Zakharov, S.D.; Bordignon, E.; Botos, I.; Santamaria, M.; Barnard, T.J.; Cramer, W.A.; Lloubes, R.; Buchanan, S.K. Structural insight into the role of the Ton complex in energy transduction. Nature 2016, 538, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.B.; Lou, W.J.; Ke, W.T.; Song, W.Y.; Price, N.M.; Qiu, B.S. New insights into iron acquisition by cyanobacteria: An essential role for ExbB-ExbD complex in inorganic iron uptake. ISME J. 2015, 9, 297–309. [Google Scholar] [CrossRef]
- Zorina, A.; Sinetova, M.A.; Kupriyanova, E.V.; Mironov, K.S.; Molkova, I.; Nazarenko, L.V.; Zinchenko, V.V.; Los, D.A. Synechocystis mutants defective in manganese uptake regulatory system, ManSR, are hypersensitive to strong light. Photosynth. Res. 2016, 130, 11–17. [Google Scholar] [CrossRef]
- Katoh, H.; Hagino, N.; Grossman, A.R.; Ogawa, T. Genes essential to iron transport in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2001, 183, 2779–2784. [Google Scholar] [CrossRef]
- Eisenhut, M.; Hoecker, N.; Schmidt, S.B.; Basgaran, R.M.; Flachbart, S.; Jahns, P.; Eser, T.; Geimer, S.; Husted, S.; Weber, A.P.M.; et al. The Plastid Envelope CHLOROPLAST MANGANESE TRANSPORTER1 Is Essential for Manganese Homeostasis in Arabidopsis. Mol. Plant 2018, 11, 955–969. [Google Scholar] [CrossRef]
- Schneider, A.; Steinberger, I.; Herdean, A.; Gandini, C.; Eisenhut, M.; Kurz, S.; Morper, A.; Hoecker, N.; Rühle, T.; Labs, M.; et al. The evolutionarily conserved protein PHOTOSYNTHESIS AFFECTED MUTANT71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis. Plant Cell 2016, 28, 892–910. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Thomine, S. Importing Manganese into the Chloroplast: Many Membranes to Cross. Mol. Plant 2018, 11, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Järvi, S.; Suorsa, M.; Aro, E.M. Photosystem II repair in plant chloroplasts-Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Photoreduction of manganese oxides in seawater. Mar. Chem. 1994, 46, 133–152. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. Order of stability of metal complexes. Nature 1948, 162, 746–747. [Google Scholar] [CrossRef]
- Csatorday, K.; Gombos, Z.; Szalontai, B. Mn2+ and Co2+ toxicity in chlorophyll biosynthesis. Proc. Natl. Acad. Sci. USA 1984, 81, 476–478. [Google Scholar] [CrossRef]
- Bollivar, D.W.; Beale, S. The Chlorophyll Biosynthetic Enzyme Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase. Plant Physiol. 1996, 112, 105–114. [Google Scholar] [CrossRef]
- Tottey, S.; Block, M.A.; Allen, M.; Westergren, T.; Albrieux, C.; Scheller, H.V.; Merchant, S.; Jensen, P.E. Arabidopsis CHL27, located in both envelope and thylakoid membranes, is required for the synthesis of protochlorophyllide. Proc. Natl. Acad. Sci. USA 2003, 100, 16119–16124. [Google Scholar] [CrossRef]
| Protein | Gene ID 1 | Assigned Function | Reference |
|---|---|---|---|
| MncA | sll1358 | Periplasmic Mn metalloprotein | [20] |
| PratA | slr2048 | Delivery of Mn to pD1 protein during PSII biosynthesis | [25,26,27] |
| MntC | sll1598 | High-affinity Mn uptake at the plasma membrane under Mn-limiting conditions | [28,29] |
| MntA | sll1599 | ||
| MntB | sll1600 | ||
| FutA1 | slr1295 | Candidate for constitutive Mn uptake at the plasma membrane | [30] |
| FutA2 | slr0513 | ||
| FutB | slr0327 | ||
| FutC | sll1878 | ||
| Mnx/PAM71 | sll0615 | Mn export from cytoplasm into thylakoid lumen and periplasm, respectively | [22,23] |
| ManS/Hik27 | slr0640 | Mn-sensing two-component system to control expression of the mntCAB operon | [31,32] |
| ManR/Rre16 | slr1837 | ||
| RfrA | sll1350 | Expression regulator of unknown high-affinity Mn importer at the plasma membrane | [33] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenhut, M. Manganese Homeostasis in Cyanobacteria. Plants 2020, 9, 18. https://doi.org/10.3390/plants9010018
Eisenhut M. Manganese Homeostasis in Cyanobacteria. Plants. 2020; 9(1):18. https://doi.org/10.3390/plants9010018
Chicago/Turabian StyleEisenhut, Marion. 2020. "Manganese Homeostasis in Cyanobacteria" Plants 9, no. 1: 18. https://doi.org/10.3390/plants9010018
APA StyleEisenhut, M. (2020). Manganese Homeostasis in Cyanobacteria. Plants, 9(1), 18. https://doi.org/10.3390/plants9010018





