1. Introduction
This study characterizes the chemical form of Selenium (Se) and its localization in the Brazil nut (
Bertholletia excelsa H.B.K., Lecythidaceae), as well as the variation in Se concentration within and among different commercially available batches.
Bertholletia is a monotypic tree genus in the Lecythidaceae family, and its only species,
B. excelsa, produces large, oil-rich seeds. These, known as Brazil nuts, are of biological and nutritional interest, because they accumulate extraordinarily high Se levels. Selenium (Se) is an essential micronutrient for humans and other mammals. This element plays an important role in the organism, and its inadequate nutritional supplementation can cause a number of health disorders [
1]. The main forms of Se found in humans are organic, in the form of the amino acids selenocysteine (SeCys), analog to cysteine (Cys), and selenomethionine (SeMet), analog to methionine (Met). SeCys is a structural part of the active site of twenty-five different selenoproteins [
2], which play roles in the maintenance of physiological homeostasis, including the cellular redox state regulation and hormonal biosynthesis.
Adequate Se intake varies and depends on personal physiological and biological parameters such as body weight, age and sex [
3,
4]. Therefore, the Recommended Dietary Allowance (RDA) of Se in the United States and Canada ranges from 15 μg Se/day (infants from 0 to 6 months old) to 70 μg Se/day (women from 14 to 50 years old during lactation), while the recommendation for male and female adults, between 18 and 71 years old, corresponds to 55 μg Se/day [
4]. The RDA varies in different countries [
5]. The Austrian, German and Swiss nutrition societies recommend higher Se intake for adult women, 60 μg Se/day, and adult men, 70 μg Se/day [
6], while in Japan the recommended Se intake is 25 μg Se/day for adult women and 35 μg Se/day for adult men [
5].
Despite its importance to human metabolism, Se can become toxic above a certain threshold, due to its interference with sulfur (S) metabolism [
7]. There is a narrow window between Se deficiency, adequacy and toxicity. The tolerable Se intake limit is considered 400 μg Se/day [
4,
8], while the intake of Se associated with toxicity (selenosis) was estimated to be around 1200 μg Se/day (in people exposed to large amounts of organic Se in China) [
8]. Long-term exposure to moderate Se levels can results in chronic Se toxicity, and exposure to high Se levels can in some cases cause death due to acute toxicity. Chronic selenosis symptoms range from fragile or depigmented hair and nails to loss of these parts [
9], and characteristic acute selenosis symptoms include diarrhea, nausea, skin rash, disorders to the nervous system, fatigue and irritability [
4]. There are also possible risks of supra-nutritional Se levels, as suggested by several recent papers on the complex U-shaped relationship between Se dose and diseases such as type 2 diabetes or cancers [
10,
11,
12].
While Se toxicity is a great concern, deficiency is an even bigger problem worldwide. Low dietary Se intake, less than 40 μg/day [
13], is estimated to negatively affect more than one billion people worldwide [
14] including areas in China, Eastern Europe, Brazil and Sub-Saharan Africa, Australia and New Zealand [
3]. In low Se areas in China, two specific diseases related to Se deficiency occur, i.e., Keshan disease [
13,
15] and Kaschin-Beck disease [
16,
17]. The general symptoms of Se deficiency are related to impaired cellular redox capacity, thyroid function and immune defense [
7,
18,
19]. Not surprisingly, several studies have found a positive correlation between Se deficiency and incidence of different types of cancer [
20,
21,
22] as well as decreased survival in HIV-positive patients [
7]. Deficiency symptoms may also include muscle weakness, muscle pain (myalgia) and heart dysfunction [
23], irreversible brain injury and impaired fertility [
7].
Selenium enters the food chain via plants, so an important source of Se to the human diet is plant-based food; this is particularly important for populations in low Se areas that rely on a vegetarian diet [
5]. The Se concentration in crops varies greatly, not only due to species differences, but also due to variation in soil Se concentration worldwide, which is determined by geological processes [
3]. In addition, local Se speciation and bioavailability are influenced by physico-chemical aspects of the soil [
13]. The most common bioavailable Se forms are inorganic selenate (SeO
42−), found in well aerated, alkaline and oxidized soils, or selenite (SeO
32−), present in more acidic and reducing environments like wetlands [
24]. Organic forms of Se, such as SeCys and selenomethionine (SeMet), analog to methionine (Met), can also be present in soil, through plant decomposition and microbiome activity; selenides and elemental Se can also be present, but are not very bioavailable [
25,
26].
To better provide Se to populations in low Se areas, different strategies are used to augment Se levels in crops, practices called biofortification [
27,
28,
29,
30]. An important factor in biofortification is the plant physiological capacity to take up, metabolize, translocate and accumulate Se. Plant species differ in their capacity to take up and assimilate Se. Selenium is not considered a nutrient for plants, but it is a beneficial element in low concentrations (~10 mg kg
−1 dry weight, DW), owing to increased antioxidant capacity, which may lead to increased photosynthesis, stress resistance and ultimately growth [
31]. Selenium can become toxic to plants at tissue levels above 100 mg kg
−1 DW, because they non-specifically take up selenate (SeO
42−) via sulfate (SO
42−) transporters and assimilate it into the Se analogs of the amino acids Cys (SeCys) and Met (SeMet) [
32]. The misincorporation of SeCys and SeMet results in protein malfunction and systemic oxidative stress [
33].
While biofortification can overcome the physiological limitations of crop species, it usually requires costly fertilizer supplementation in agricultural areas with low soil Se concentration [
3]. Naturally high-Se food sources can be a simple solution to the challenge of providing sufficient Se to populations in low-Se areas. Selenium accumulation capacity varies dramatically among plant species; in natural Se-containing areas the Se levels in vegetation can differ 100-fold [
34]. Plants can be generally divided into three large groups based on Se content found in all their organs in natural environments: hyperaccumulators are plants that can exceed the threshold of 1000 mg Se kg
−1 (DW), while secondary accumulators can accumulate from 100–1000 mg kg
−1 DW and non-accumulators do not exceed 100 mg kg
−1 DW [
24]. Non-accumulators, i.e., most species, including crops, and secondary accumulators, e.g.,
Brassica crop species and several wild Brassicaceae and Asteraceae, tend to contain more inorganic Se, while hyperaccumulators such as
Stanleya pinnata (Brassicacea) and
Astragalus bisulcatus (Fabaceae) and
Lecythis ollaria (Lecythidaceae, the monkey pot tree), typically sequester organic forms of Se that do not interfere with S metabolism and therefore are less toxic [
35]. Selenium hyperaccumulation likely evolved independently in different plant families [
36], and it is hypothesized to function in herbivory and pathogen protection as well as allelopathic interaction [
37].
Among different plant-derived food Se sources, the Brazil nut (
B. excelsa H.B.K), endemic to different countries in South America and a relative of the monkey pot tree, contains the highest reported concentration of Se among other nuts/seeds; a few Brazil nuts are sufficient to provide the Se RDA listed for North America, Asia and Europe [
38]. Nuts are included in the healthy diet recommendations of several countries, due to their high nutritional value, fiber content, unsaturated fatty acids and minerals [
39], however moderation consumption of nuts is advised due to high caloric values. Therefore, the regular intake of Brazil nuts could be recommended not only as a suitable strategy to prevent Se deficiency, but for several other health benefits such as anti-inflammatory properties, improvement of the cellular redox homeostasis and the reduced risk of different chronic diseases [
39]. However, the Se content in these seeds can vary greatly according to soil properties with respect to Se concentration and bioavailability [
38,
40]. Since there is a very narrow window between adequate and toxic Se intake for humans, it is crucial to determine the variation in Se concentration within commercially available Brazil nut batches and also among batches from different companies, which could directly affect consumers. In addition, it is important to analyze the chemical forms of Se present in the seed, which could affect its nutritional value and potential toxicity to consumers. Last, it is also interesting to investigate any possible correlations between Se and other nutrients in the seed.
In light of these considerations, this study characterized the chemical form of Se and its localization in the nut, as well as the variation in Se concentration within and among two different commercially available batches (hence referred to as A and B). Furthermore, levels of macronutrients and micronutrients in these nuts were characterized, and their interactions with Se investigated. These studies have significance for Brazil nut consumers, sellers and producers. This study also has intrinsic value; because this species has such unique properties with respect to Se, it is interesting to study its Se metabolic properties in detail.
4. Conclusions
This study analyzed the variation in Se concentration, as well as Se tissue localization and chemical speciation, and the relation of Se with other nutrients in 26 seeds in two different commercially available Brazil nut batches, 13 seeds per batch. Several important findings that are of basic biological interest are presented. The Se was found to be present in a tissue layer 1 to 2 mm below the seed surface, along its periphery. Based on µXANES fitting, the forms accumulated were organic C-Se-C compound(s) that may include SeMet, MetSeCys and/or Se-lanthionine. Together, this information provides novel insight into Se physiology and metabolism in this extraordinary Se-accumulating plant species. The findings also have significance for Brazil nut consumers, producers, sellers, and regulatory agencies. Brazil nuts contain the highest Se levels of any plant-based food source [
35,
36,
42], and are therefore a valuable source of this essential micronutrient. However, Se can easily become toxic at elevated levels, and thus it is vital to inform and protect consumers from possible toxic effects of overconsumption of these high-Se seeds.
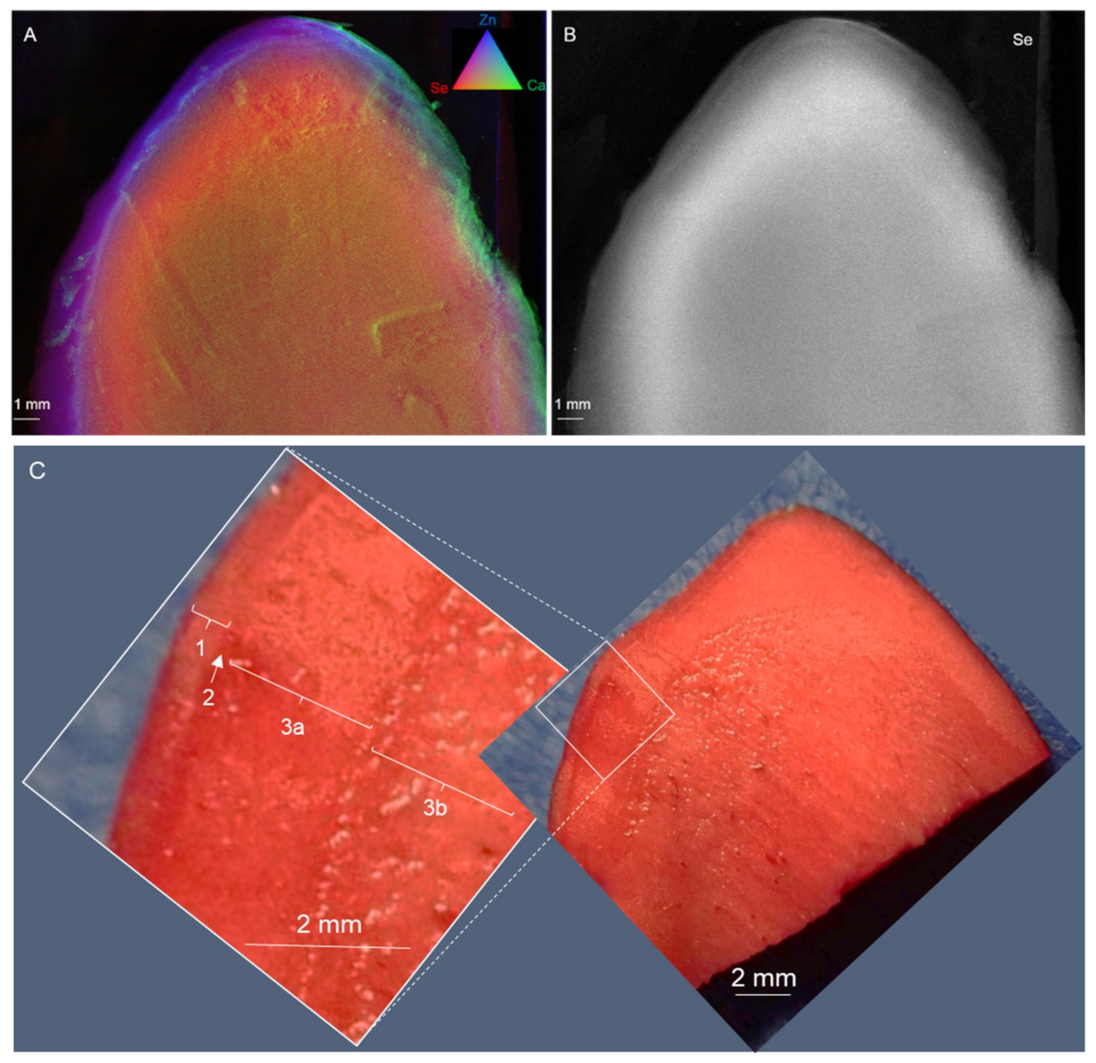
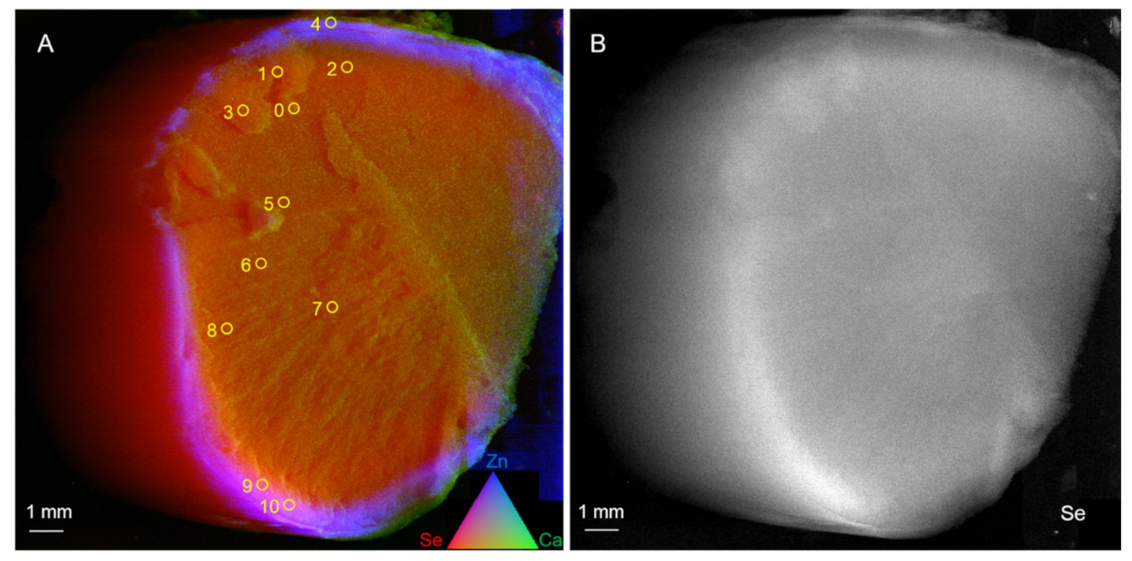
The anatomy of
B. excelsa seeds is unusual: it consists almost entirely of embryo hypocotyl parenchyma, with a thin layer of endosperm around it and a meristematic layer in between (procambium). The area of concentrated Se appears to be below the endosperm along the outside of the embryo hypocotyl, corresponding with the outer parenchyma layer, and possibly the procambium. The Se in the Brazil nuts was mainly organic, reported to be the most effective dietary source of Se [
7]. There was large seed-to-seed variation (up to 8-fold) in Se concentration and the averages between the batches was ~2-fold. The levels of Se found were such that the consumption of one seed (5 g) was enough to meet or even exceed the recommended daily allowance (RDA) for Se.
While healthy, Brazil nuts should be consumed in moderation, it is important to emphasize that the levels of Se found in these two batches were high enough to exceed the maximum allowable daily intake of Se (400 μg) if consumed at the recommended serving size of 30 g (6 seeds). Depending on the batch, the recommended serving size may even exceed the Se intake level reported to cause toxicity symptoms (1200 μg). Therefore, unless low Se levels in batches of seeds can be demonstrated, it would be safer for the recommended serving size for Brazil nuts to be reduced to 15 g (3 seeds) to ensure safe Se intake, and to warn consumers to not exceed this limit. In addition, it would be helpful to include on the package the geographic origin of the Brazil nut and ideally the Se concentration of the specific batch, with an indication of the % of RDA for Se. Furthermore, to avoid Se toxicity due to overconsumption, the package size of Brazil nuts from high-Se geographic areas may be reduced, or these seeds could preferentially be sold as part of mixed nuts packages.