Can Cynodon dactylon Suppress the Growth and Development of the Invasive Weeds Tagetes minuta and Gutenbergia cordifolia?
Abstract
1. Introduction
2. Results
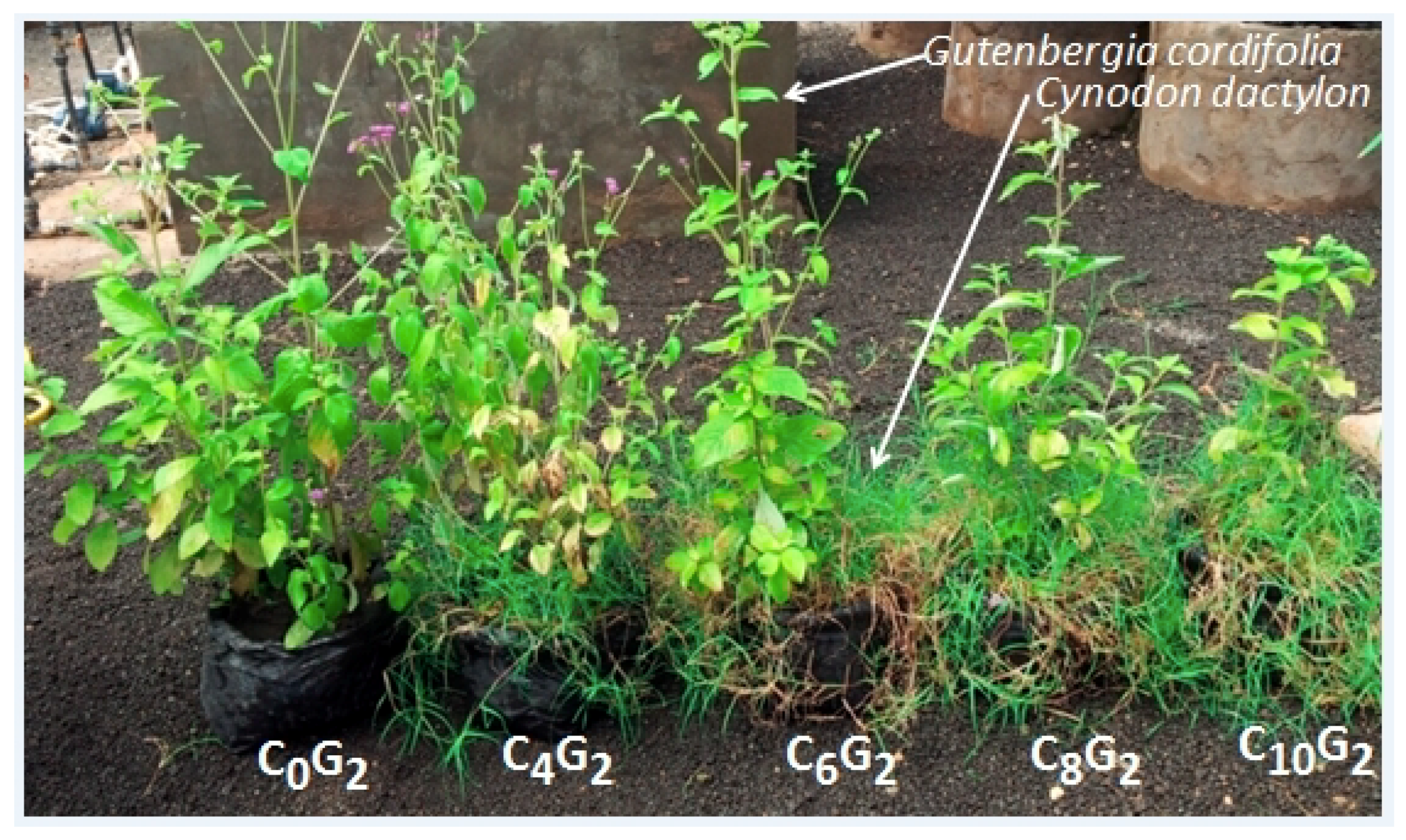
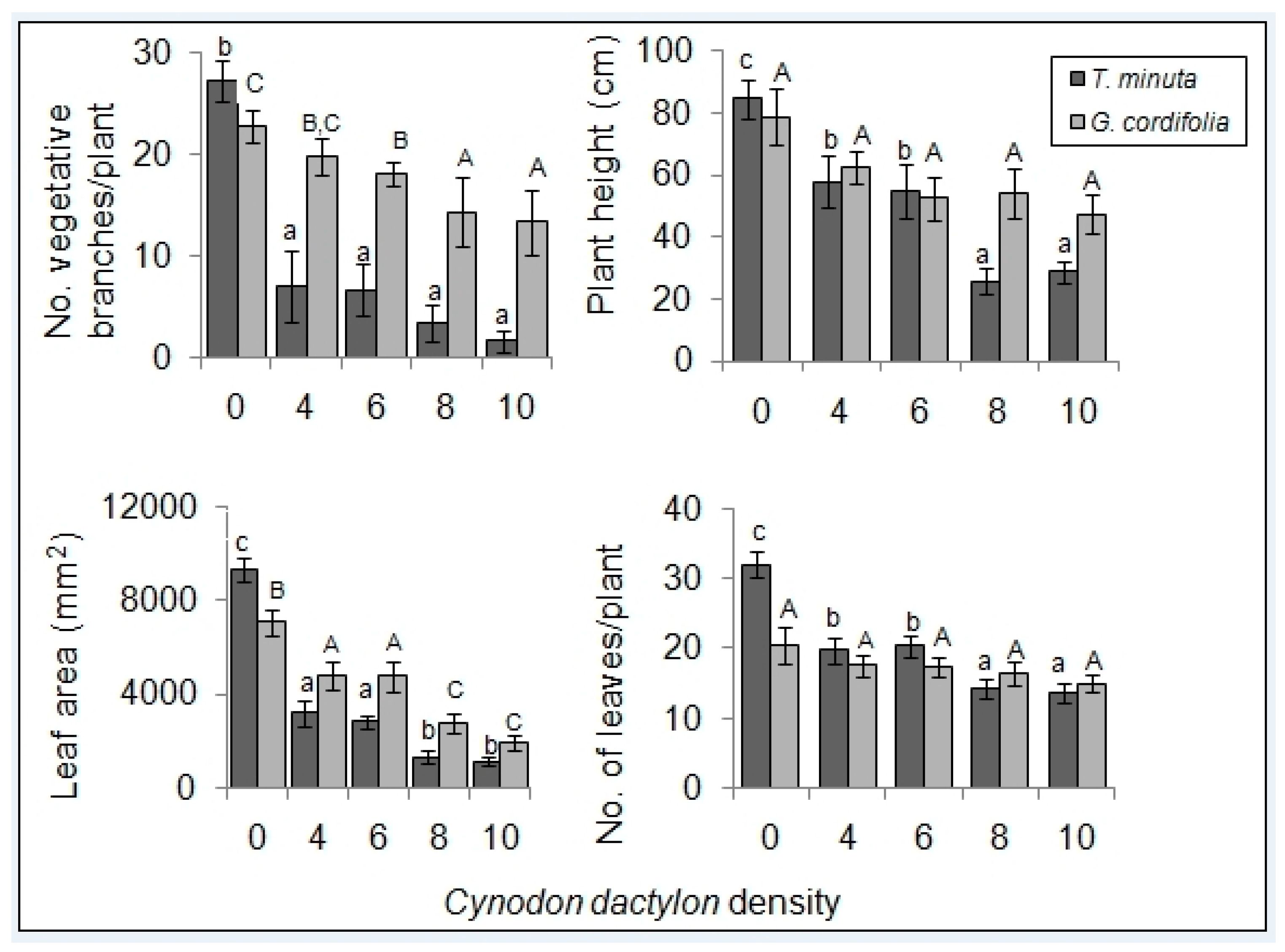
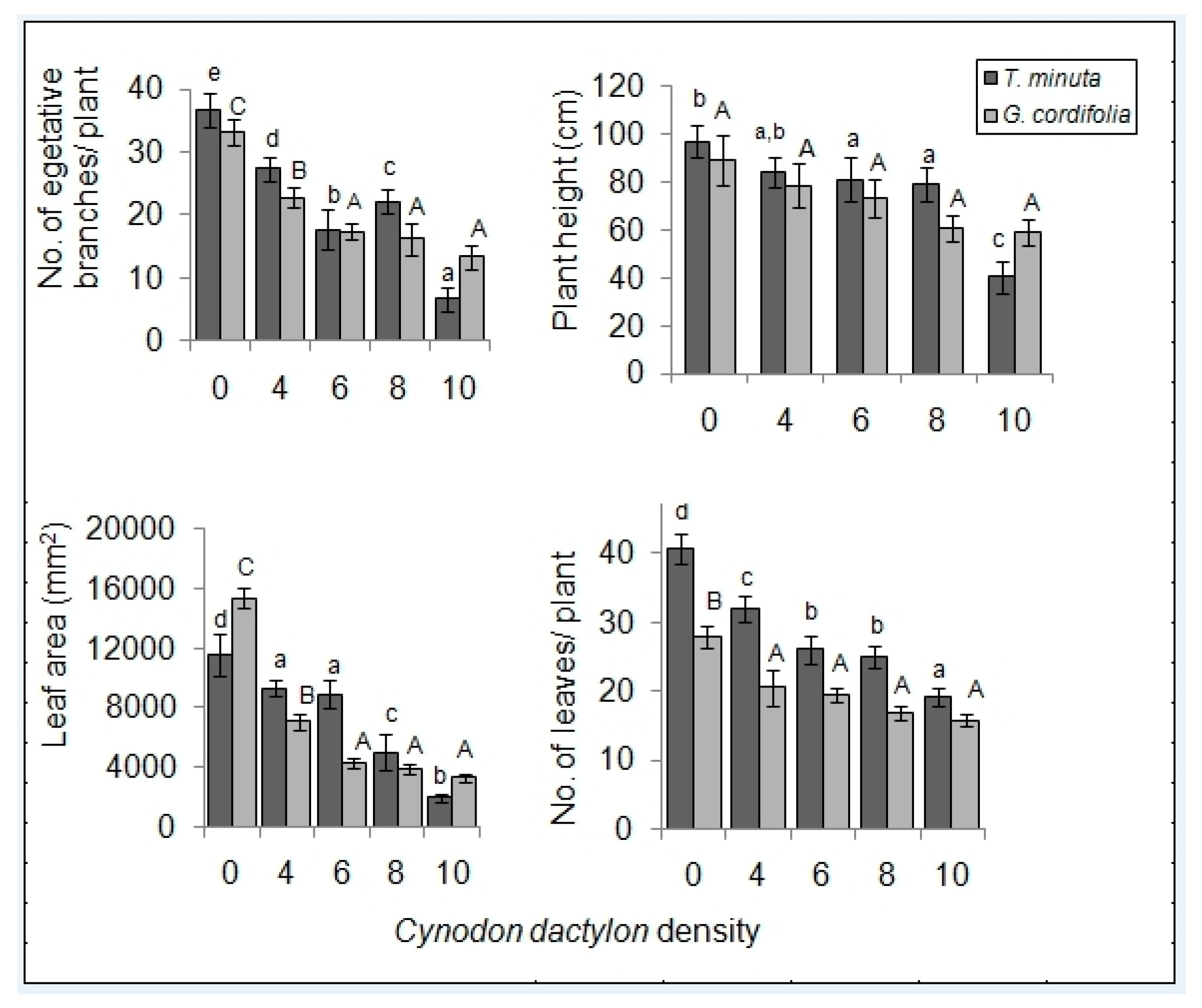
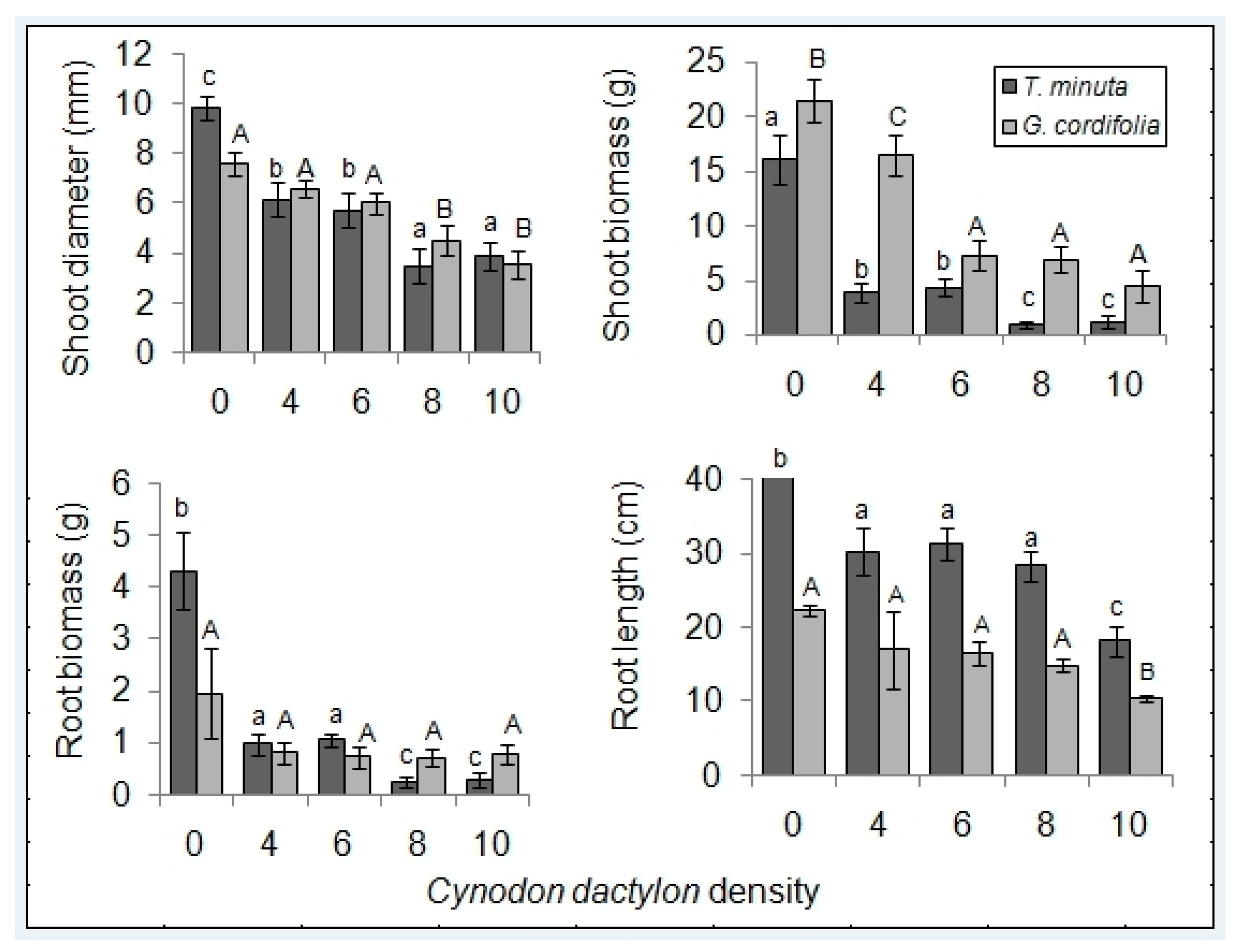
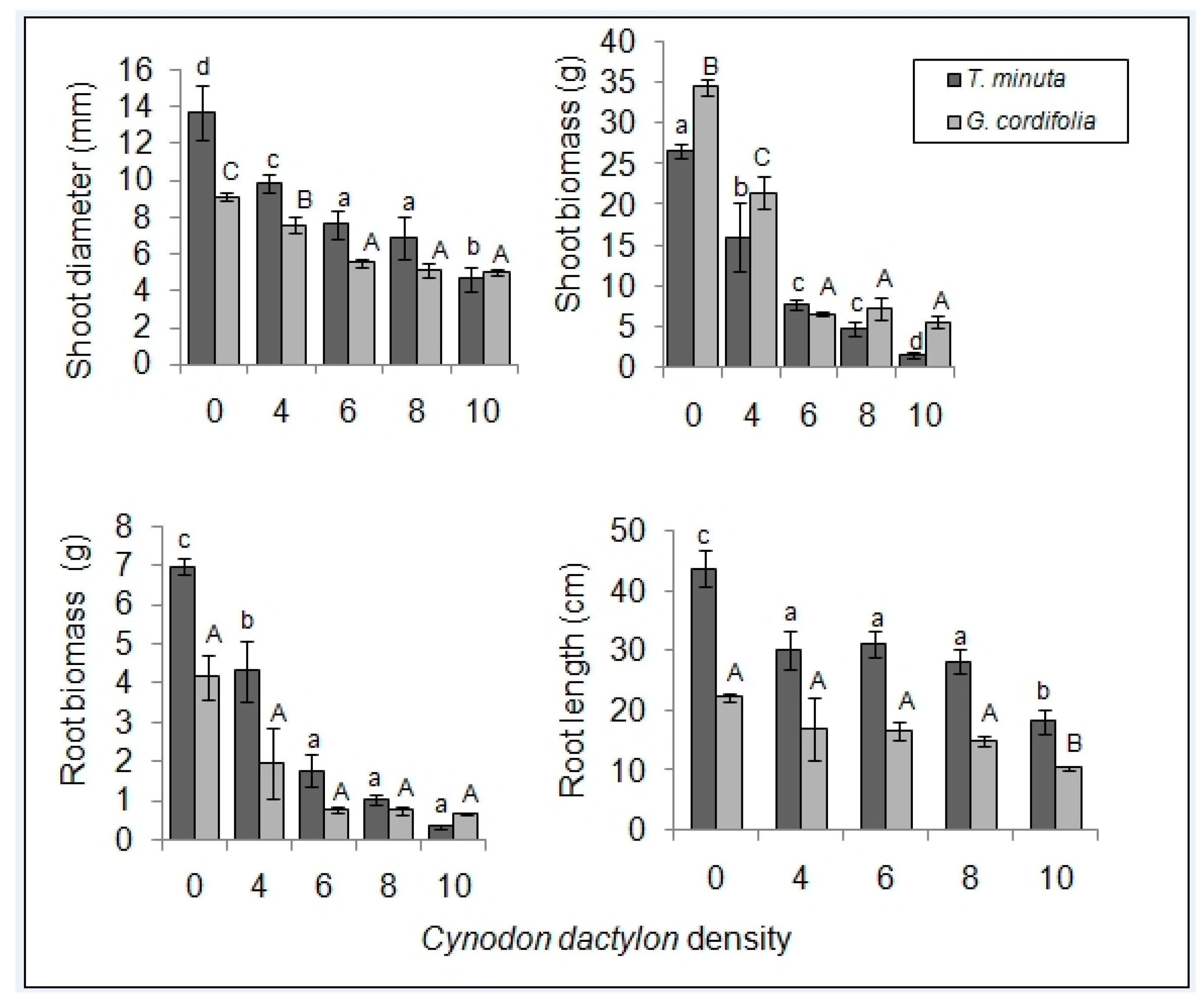
2.1. Invasive Plant Growth Parameters
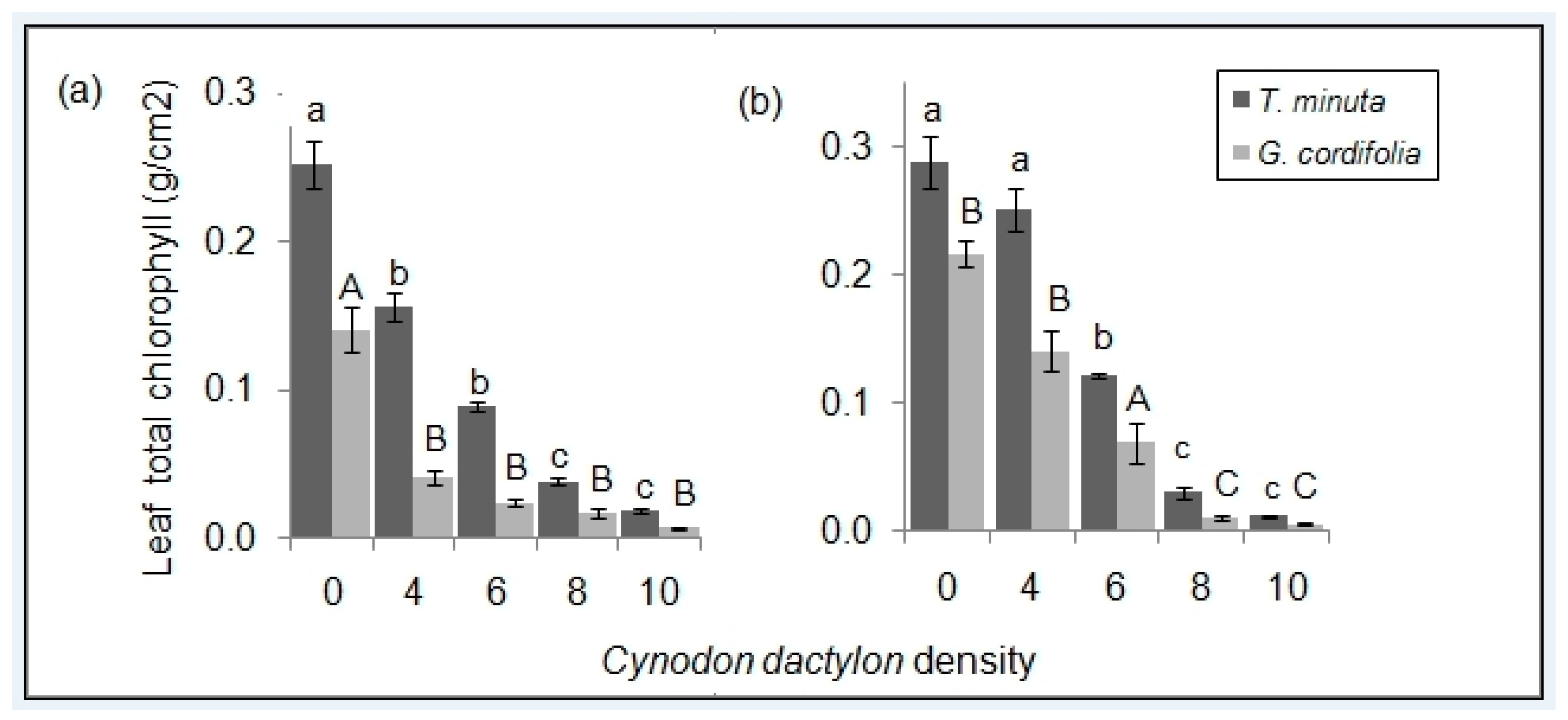
2.2. Leaf Pigmentations
3. Discussion
3.1. C. dactylon Effects on Invasive Plants Growth Parameters
3.2. C. dactylon Effects on Invasive Plants Leaf Pigmentations
4. Materials and Methods
4.1. Study Area Location and Climate
4.2. Study Species
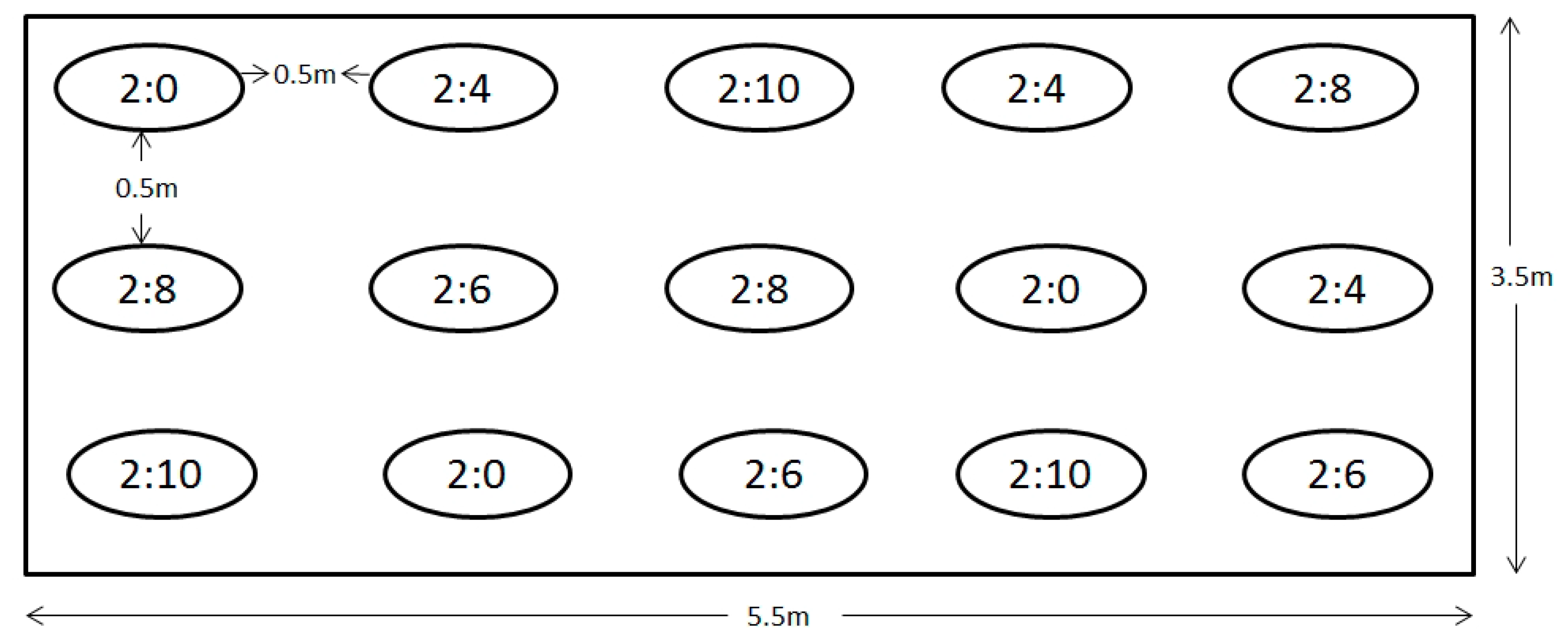
4.3. Experimental Design
4.4. Growth Parameters Measured
4.5. Measurement of Leaf Pigments
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bell, C. A Historical View of Weed Control Technology. Available online: http://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=17593 (accessed on 2 February 2017).
- Holt, J.S.; Lebaron, H.M. Significance and distribution of herbicide resistance. Weed Technol. 1990, 4, 141–149. [Google Scholar] [CrossRef]
- Hiscox, J.; Israelstam, G. A Method for the Extraction of Chlorophyll from Leaf Tissue without Maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Horowitz, M. Competitive Effects of Cynodon dactylon, Sorghum halepense and Cyperus rotundus on Cotton and Mustard. Exp. Agric. 1973, 9, 263–273. [Google Scholar] [CrossRef]
- Altieri, M.A.; Liebman, M. Weed Management in Agro Ecosystems: Ecological Approaches; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- FAO. Improving Weed Management. In Proceedings of the FAO/IWSS Expert Consultation on Improving Weed Management in Developing Countries, Rome, Italy, 6–10 September 1982. [Google Scholar]
- Altieri, M.A. Biodiversity and Pest Management in Agro Ecosystems; Food Products, CRC Press: London, UK, 1994. [Google Scholar]
- Poorter, M.; Pagad, S.; Ullah, M. Invasive Alien Species and Protected Areas: A Scoping Report, Part I; The Global Invasive Species Programme (GISP): Narobi, Kenya, 2007. [Google Scholar]
- Benayas, J.M.; Fernández, A.; Aubenau, A. Clipping Herbaceous Vegetation Improves Early Performance of Planted Seedlings of the Mediterranean Shrub Quercus Coccifera. Web Ecol. 2007, 7, 120–131. [Google Scholar] [CrossRef]
- Burton, G.; Hanna, W. Bermuda Grass. In Forages the Science of Grassland Agriculture; Heath, M., Barnes, R., Metcalfe, D., Eds.; Iowa State University Press: Ames, IA, USA, 1985. [Google Scholar]
- Juraimi, A.; Drennan, D.; Anuar, N. Competitive Effect of Cynodon dactylon (L.) Pers. on Four Crop Species, Soybean [Glycine max (L.) Merr.], Maize (Zea mays), Spring Wheat (Triticum aestivum) and Faba Bean [Vicia faba (L.)]. Asian J. Plant Sci. 2005, 4, 90–94. [Google Scholar]
- Cannell, M.G.R. Environmental Impacts of Forest Monocultures: Water Use, Acidification, Wildlife Conservation, and Carbon Storage. New For. 1999, 17, 239–262. [Google Scholar] [CrossRef]
- Browgram, R. Effects of Intensity of Defoliation on Regrowth of Pasture. Aust. J. Agric. Res. 1956, 7, 377–387. [Google Scholar]
- Falster, D.S.; Westoby, M. Plant Height and Evolutionary Games. Trends Ecol. Evol. 2003, 18, 337–343. [Google Scholar] [CrossRef]
- Xu, L.; Sofia, M.A.; Fei-hai, Y.; Ming Dong, N.; Anten, P.R.; Marinus, J.A. Effects of Trampling on Morphological and Mechanical Traits of Dryland Shrub Species Do Not Depend on Water Availability. PLoS ONE 2013, 8, e53021. [Google Scholar] [CrossRef]
- Dekker, J. Soil Weed Seed Banks and Weed Management. J. Crop Prod. 2011, 2, 139–166. [Google Scholar] [CrossRef]
- Buhler, D.D.; Hartzler, R.G.; Forcella, F. Weed Seed Bank Dynamics: Implications to Weed Management. J. Crop Prod. 1997, 1, 145–168. [Google Scholar] [CrossRef]
- Martina, J.P.; Von Ende, C.N. Correlation of Soil Nutrient Characteristics and Reed Canarygrass (Phalaris Arundinacea: Poaceae) Abundance in Northern Illinois (USA). Am. Midl. Nat. 2008, 160, 430–437. [Google Scholar] [CrossRef]
- Marais, A.; Hardy, M.; Booyse, M.; Botha, A. Effects of Monoculture, Crop Rotation, and Soil Moisture Content on Selected Soil Physicochemical and Microbial Parameters in Wheat Fields. Appl. Environ. Soil Sci. 2012, 2012, 593623. [Google Scholar] [CrossRef]
- Zhang, F.; Li, L. Using Competitive and Facilitative Interactions in Intercropping Systems Enhances Crop Productivity and Nutrient-Use Efficiency Using Competitive and Facilitative Interactions in Intercropping Systems Enhances Crop Productivity and Nutrient-Use Efficiency. Plant Soil 2003, 248, 305–312. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, J.; Xing-Guo, B.; Xiao-Fei, L.; Jian-Hua, Z. Intercropping Enhances Productivity and Maintains the Most Soil Fertility Properties Relative to Sole Cropping. PLoS ONE 2014, 9, e113984. [Google Scholar] [CrossRef]
- Zhao, D.; Raja, R.; Vijaya, G.; Reddy, V.R. Nitrogen Deficiency Effects on Plant Growth, Leaf Photosynthesis, and Hyperspectral Reflectance Properties of Sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Boussadia, O.; Steppe, K.; Zgallai, H.; Ben El Hadj, S.; Braham, M.; Lemeur, R.; Van Labeke, M.C. Effects of Nitrogen Deficiency on Leaf Photosynthesis, Carbohydrate Status and Biomass Production in Two Olive Cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Pavlovik, D.; Nikolic, B.; Durovic, S.; Waisi, H.; Andelikovic, A.; Marisavljevic, D. Chlorophyll as a Measure of Plant Health: Agro-Ecological Aspects. Pestic. Phytomed. (Belgrade) 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Saifuddin, M.; Hossain, A.; Normaniza, O. Impacts of Shading on Flower Formation and Longevity, Leaf Chlorophyll and Growth of Bougainvillea Glabra. Asian J. Plant Sci. 2010, 9, 20–27. [Google Scholar] [CrossRef][Green Version]
- Neill, S. The Functional Role of Anthocyanins in Leaves. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2002. [Google Scholar]
- Mahmoodzadeh, H.; Mahmoodzadeh, M. Allelopathic Effects of Cynodon dactylon L. on Germination and Growth of Triticum aestivum. Ann. Biol. Res. 2013, 5, 118–123. [Google Scholar]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Emerson, B.R. The Relation between Maximum Rate of Photo- Synthesis and Concentration of Chlorophyll. J. Gen. Phys. 1929, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Climate-data.org. Available online: https://en.climate-data.org/africa/tanzania/arusha/tengeru-205827/ (accessed on 19 October 2019).
- Cohn, E.J.; Van Auken, O.W.; Bush, J.K. University of Notre Dame Competitive Interactions between Cynodon dactylon and Acacia smallii Seedlings at Different Nutrient Levels. Am. Midl. Nat. 1989, 121, 265–272. [Google Scholar] [CrossRef]
- Soule, J. Medicinal and beverage uses of Tagetes (Tageteae: Compositae). Am. J. Bot. 1993, 80, 177. [Google Scholar]
- Hulina, N. Wild Marigold—Tagetes minuta L. New Weed on the Island of Hvar, and New Contribution to the Knowledge of Its Distribution in Dalmatia (Croatia). Agric. Conspec. Sci. (ACS) 2008, 73, 23–26. [Google Scholar]
- Wells, M.; Balsinhas, A.; Joffe, H.; Engelbrecht, M.; Harding, G.; Stirton, H. A Catalogue of Problem Plants in Southern Africa: Incorporating the National Weed List of Southern Africa (Memoirs van die Botaniese Opname van Suid-Afrika); Botanical Research Institute: Fort Worth, TX, USA, 1986. [Google Scholar]
- USAID-Tanzania. A Report on Tanzania Environmental Threats and Opportunities Assessment; US Department of Agriculture Forest Service—International Programs: Washington, WA, USA, 2012. [Google Scholar]
- Martinez-Ghersa, M.A.; Ghersa, C.M.; Benech-Arnold, R.L.; Donough, R.M.; Sanchez, R.A. Adaptive traits regulating dormancy and germination of invasive species. Plant Species Biol. 2000, 15, 127–137. [Google Scholar] [CrossRef]
- Meissner, E.; Ruth, N. Allelopathic influence of Tagetes-and Bidens-infested soils on seedling growth of certain crop species. S. Afr. J. Plant Soil 1986, 3, 176–180. [Google Scholar] [CrossRef]
- Winoto-Suatmadji, R. Studies on the Effect of Tagetes Species on Plant Parasitic Nematodes; H.W. Wageningen: Amsterdam, The Netherlands, 1969. [Google Scholar]
- Amorim, M.; Helena, R.; Rui, M.; Da Costa, G.; Carlos, L.; Margarida, M.; Bastos, S.M. Sesquiterpene Lactones: Adverse Health Effects and Toxicity Mechanisms. Crit. Rev. Toxicol. 2013, 43, 559–579. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F.; Word, K. Germacranolides from Gutenbergia. Phytochemistry 1990, 29, 2706–2708. [Google Scholar] [CrossRef]
- Anderson, P.K.; Morales, F.J. Whitefly and Whitefly-Borne Viruses in the Tropics: Building a Knowledge Base for Global Action; CIAT, Agricultural Pests: Arusha, Tanzania, 2005; p. 351. [Google Scholar]
- Gharabadiyan, F.; Jamali, S.; Yazdi, A.; Hadizadeh, M.; Eskandari, A. Weed hosts of root-knot nematodes in tomato fields. J. Plant Prot. Res. 2012, 52, 230–234. [Google Scholar] [CrossRef]
- UNESCO. Information Presented to the Bureau of the World Heritage Committee; World Heritage Center: Paris, France, 2001; Available online: http://whc.unesco.org/en/soc/2490 (accessed on 22 March 2016).
- Ngondya, I.B. Ecological Effects of Selected Invasive Plants and Their Nature Based Management Approaches. Ph.D. Thesis, Nelson Mandela African Institution of Science and Technology, Arusha, Tanzania, 2017. [Google Scholar]
- Heshmati, G.A.; Pessarakli, M. Threshold Model in Studies of Ecological Recovery in Bermudagrass (Cynodon dactylon L.) Under Nutrient Stress Conditions. J. Plant Nutr. 2011, 34, 2183–2192. [Google Scholar] [CrossRef]
- Kelty, M.; Cameron, I. Plot Design for the Analysis of Species Interactions in Mixed Stands. Common Wealth For. Rev. 1995, 744, 322–332. [Google Scholar]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- SERAS. Standard Operating Procedures No. 2034: Plant Biomass Determination. Available online: https://clu-in.org/download/ert/2034-R00.pdf (accessed on 19 October 2019).
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Makoi, J.H.J.R.; Bambara, S.; Ndakidemi, P.A. Rhizosphere Phosphatase Enzyme Activities and Secondary Metabolites in Plants as Affected by the Supply of ‘Rhizobium’, Lime and Molybdenum in ‘Phaseolus vulgaris’ L. Aust. J. Crop Sci. 2010, 4, 590–597. [Google Scholar]



| Parameters | T. minuta | G. cordifolia | ||||||
|---|---|---|---|---|---|---|---|---|
| MS | F | H | P | MS | F | H | P | |
| Veg. branches per/plant | - | - | 10.0 | 0.03 | 46 | 4.4 | - | 0.02 |
| Plant height (cm) | 1732 | 32.2 | - | <0.01 | 452 | 1.9 | - | 0.18 |
| Leaf area (mm2) | - | - | 12.2 | 0.01 | 1213 | 6.6 | - | 0.01 |
| No. of leaves/plant | 162 | 23.9 | - | <0.01 | 12 | 1.3 | - | 0.33 |
| Shoot diameter (mm) | 19 | 21.6 | - | 0.01 | 8 | 8.2 | - | <0.01 |
| Shoot biomass (g) | - | - | 11.7 | 0.02 | - | - | 11.1 | 0.02 |
| Root biomass (g) | - | - | 12.2 | 0.01 | - | - | 1.3 | 0.86 |
| Root length (cm) | 142 | 5.2 | - | 0.02 | 44 | 1.9 | - | 0.17 |
| No. of panicles/plant | 526 | 68.7 | - | 3.1 × 10−7 | 201 | 37.3 | - | 5.6 × 10−6 |
| Chlorophyll (unit?) | 0.03 | 116.6 | - | <0.01 | - | - | 13.2 | 0.01 |
| Anthocyanins (Abs g.DM−1) | - | - | 11.7 | 0.02 | 0.01 | 2.5 | - | 0.11 |
| Parameters | T. minuta | G. cordifolia | ||||||
|---|---|---|---|---|---|---|---|---|
| MS | F | H | P | MS | F | H | P | |
| Veg. branches per/plant | 379 | 65.1 | - | <0.01 | 184 | 14.3 | - | <0.01 |
| Plant height (cm) | 1414 | 13.3 | - | 0.01 | 480 | 1.7 | - | 0.22 |
| Leaf area (mm2) | 43,487,857 | 31.4 | - | <0.01 | - | - | 12.3 | 0.02 |
| No. of leaves/plant | 206 | 17.4 | - | <0.01 | 67 | 7.9 | - | 0.01 |
| Shoot diameter (mm) | 35 | 38.7 | - | <0.01 | 9.7 | 32.8 | - | <0.01 |
| Shoot biomass (g) | - | - | 13.5 | 0.01 | - | - | 10.5 | 0.03 |
| Root biomass (g) | - | - | 13.2 | 0.01 | - | - | 7.0 | 0.13 |
| Shoot biomass (g) | - | - | 13.5 | 0.01 | - | - | 10.5 | 0.03 |
| Root length (cm) | 250 | 13.8 | - | <0.01 | 54.8 | 2.8 | - | 0.08 |
| No. of panicles/plant | 439 | 41.6 | - | 3.3 × 10−6 | - | - | 12.8 | 0.01 |
| Chlorophyll (..) | - | - | 13.0 | 0.01 | - | - | 13.5 | 0.01 |
| Anthocyanins ( Abs g.DM−1) | 0.0676 | 28.7 | - | <0.01 | 0.024 | 12.5 | - | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngondya, I.B.; Treydte, A.C.; Ndakidemi, P.A.; Munishi, L.K. Can Cynodon dactylon Suppress the Growth and Development of the Invasive Weeds Tagetes minuta and Gutenbergia cordifolia? Plants 2019, 8, 576. https://doi.org/10.3390/plants8120576
Ngondya IB, Treydte AC, Ndakidemi PA, Munishi LK. Can Cynodon dactylon Suppress the Growth and Development of the Invasive Weeds Tagetes minuta and Gutenbergia cordifolia? Plants. 2019; 8(12):576. https://doi.org/10.3390/plants8120576
Chicago/Turabian StyleNgondya, Issakwisa B., Anna C. Treydte, Patrick A. Ndakidemi, and Linus K. Munishi. 2019. "Can Cynodon dactylon Suppress the Growth and Development of the Invasive Weeds Tagetes minuta and Gutenbergia cordifolia?" Plants 8, no. 12: 576. https://doi.org/10.3390/plants8120576
APA StyleNgondya, I. B., Treydte, A. C., Ndakidemi, P. A., & Munishi, L. K. (2019). Can Cynodon dactylon Suppress the Growth and Development of the Invasive Weeds Tagetes minuta and Gutenbergia cordifolia? Plants, 8(12), 576. https://doi.org/10.3390/plants8120576





