Leaf Production and Expansion: A Generalized Response to Drought Stresses from Cells to Whole Leaf Biomass—A Case Study in the Tomato Compound Leaf
Abstract
1. Introduction
2. Results
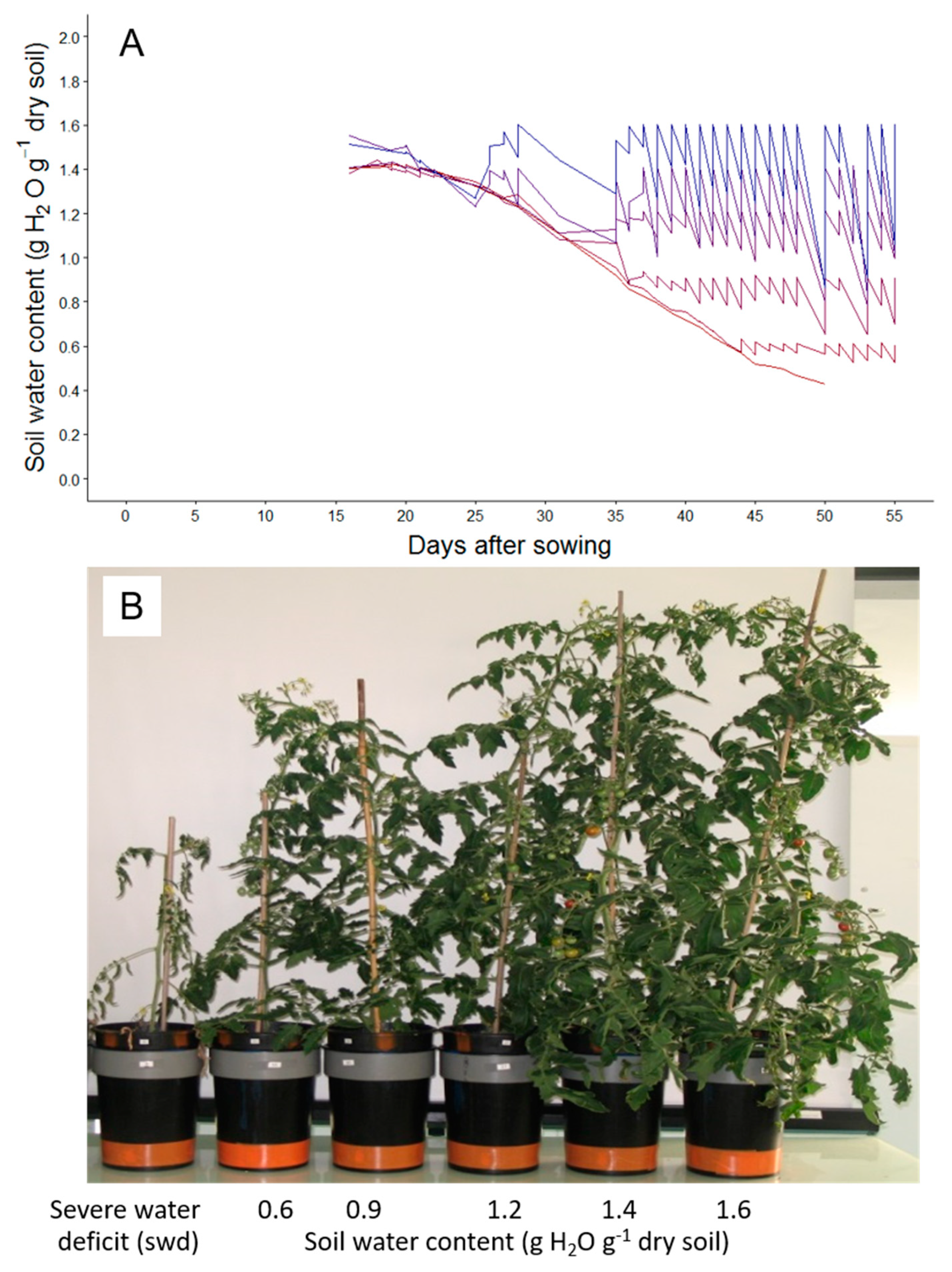
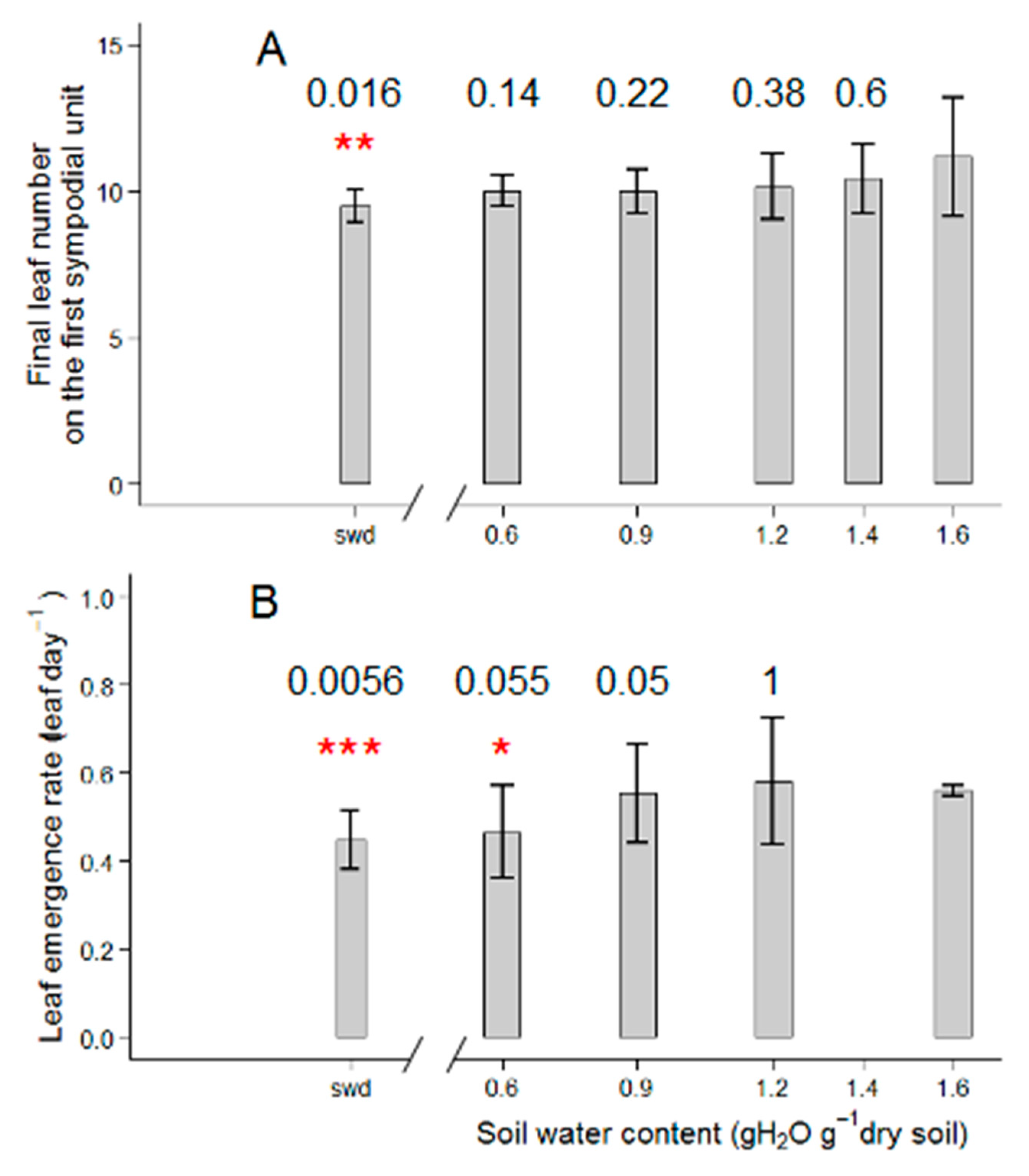
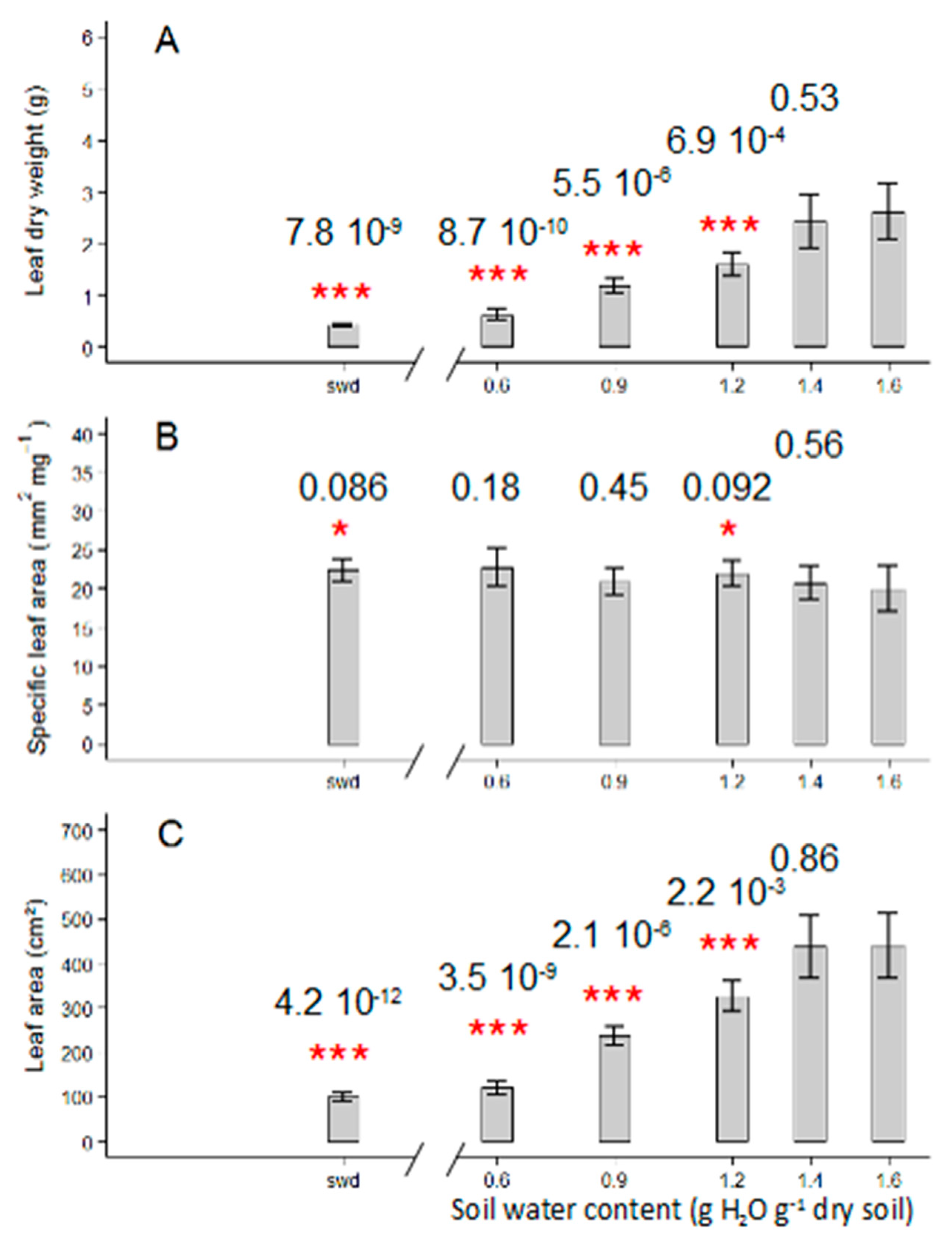
2.1. Tomato Leaf Biomass of the First Sympodial Unit Is Affected by the Different Drought Treatments, Mainly Through a Reduction in Individual Leaf Area and Dry Mass
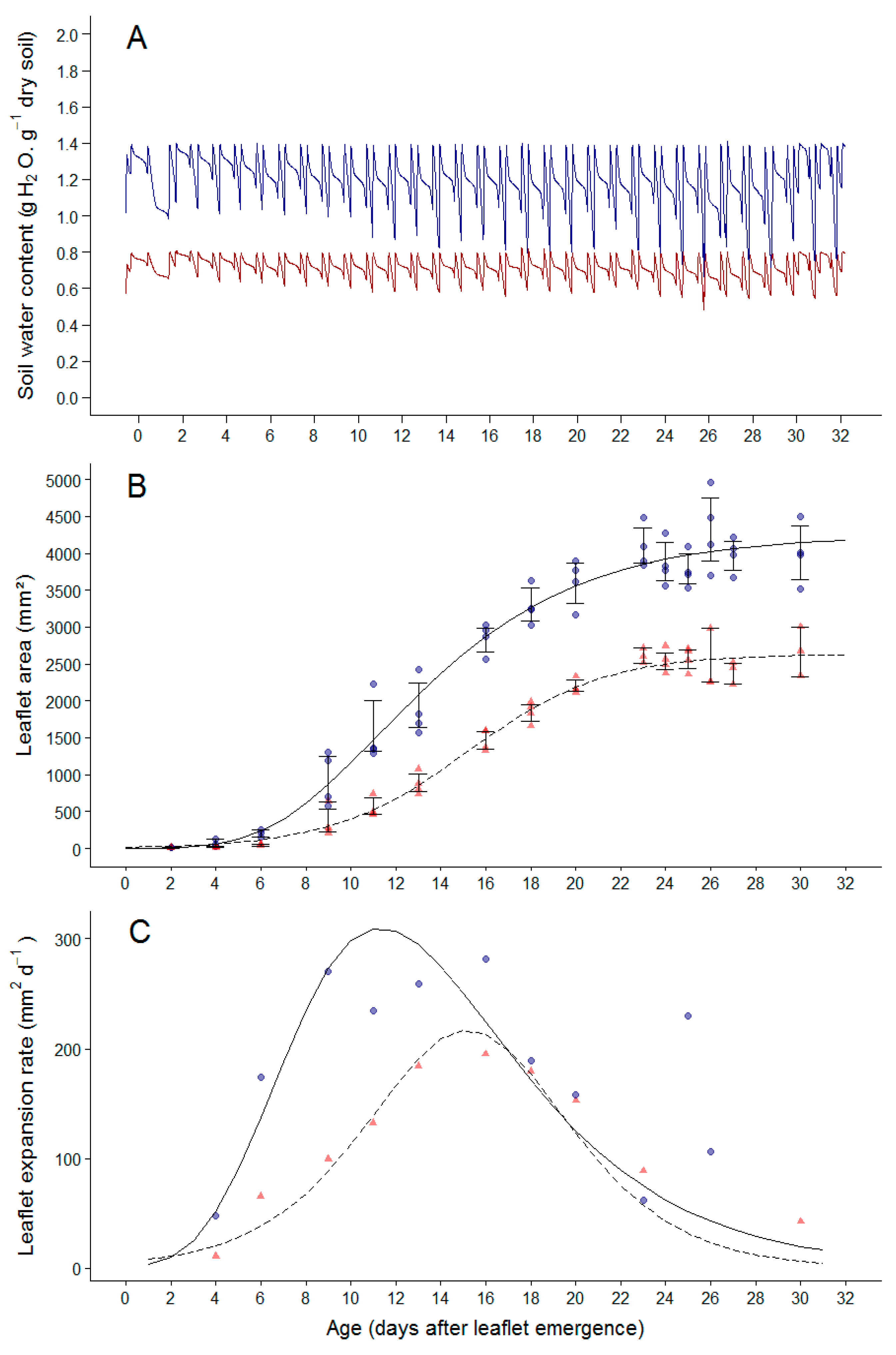
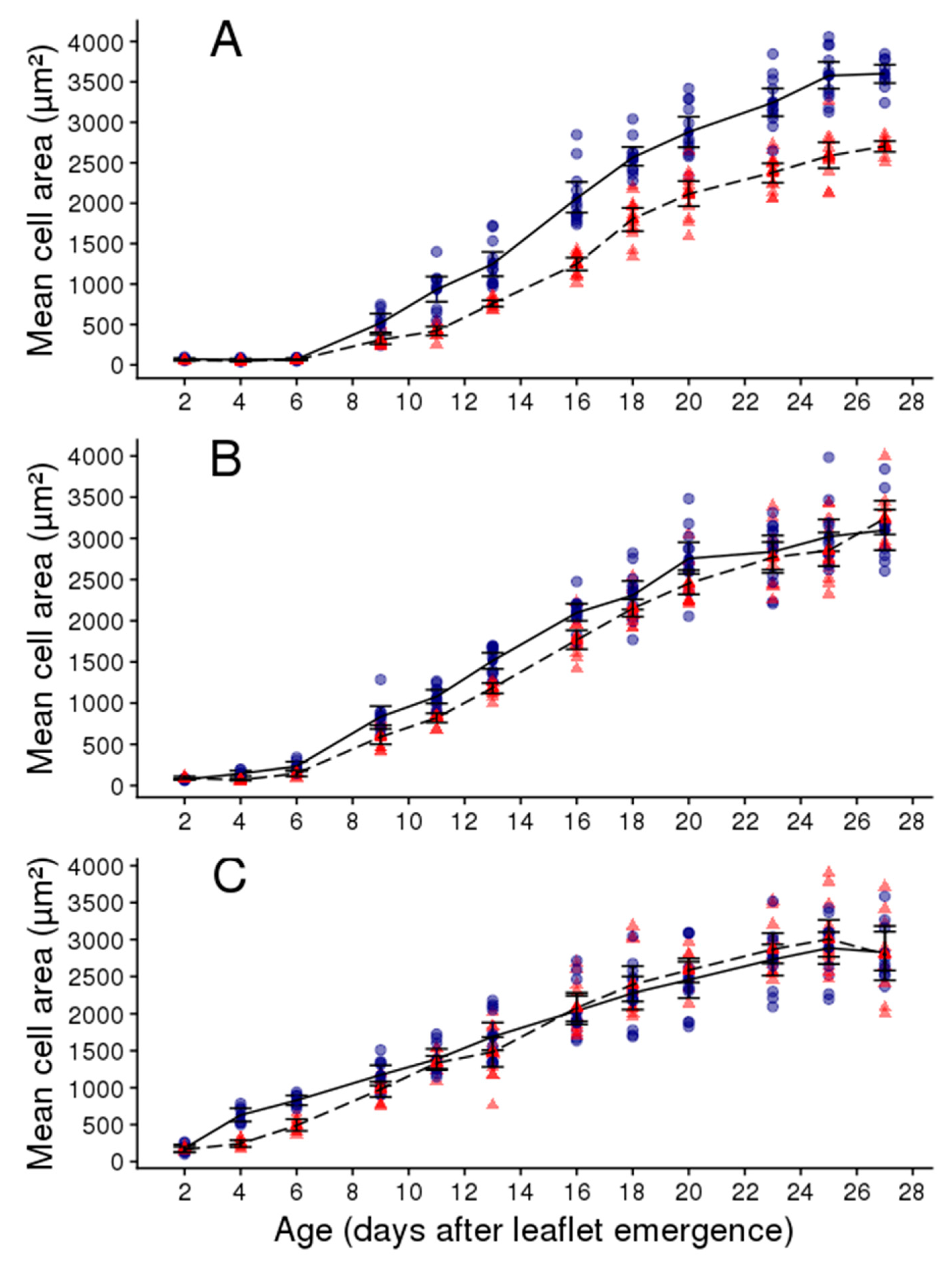
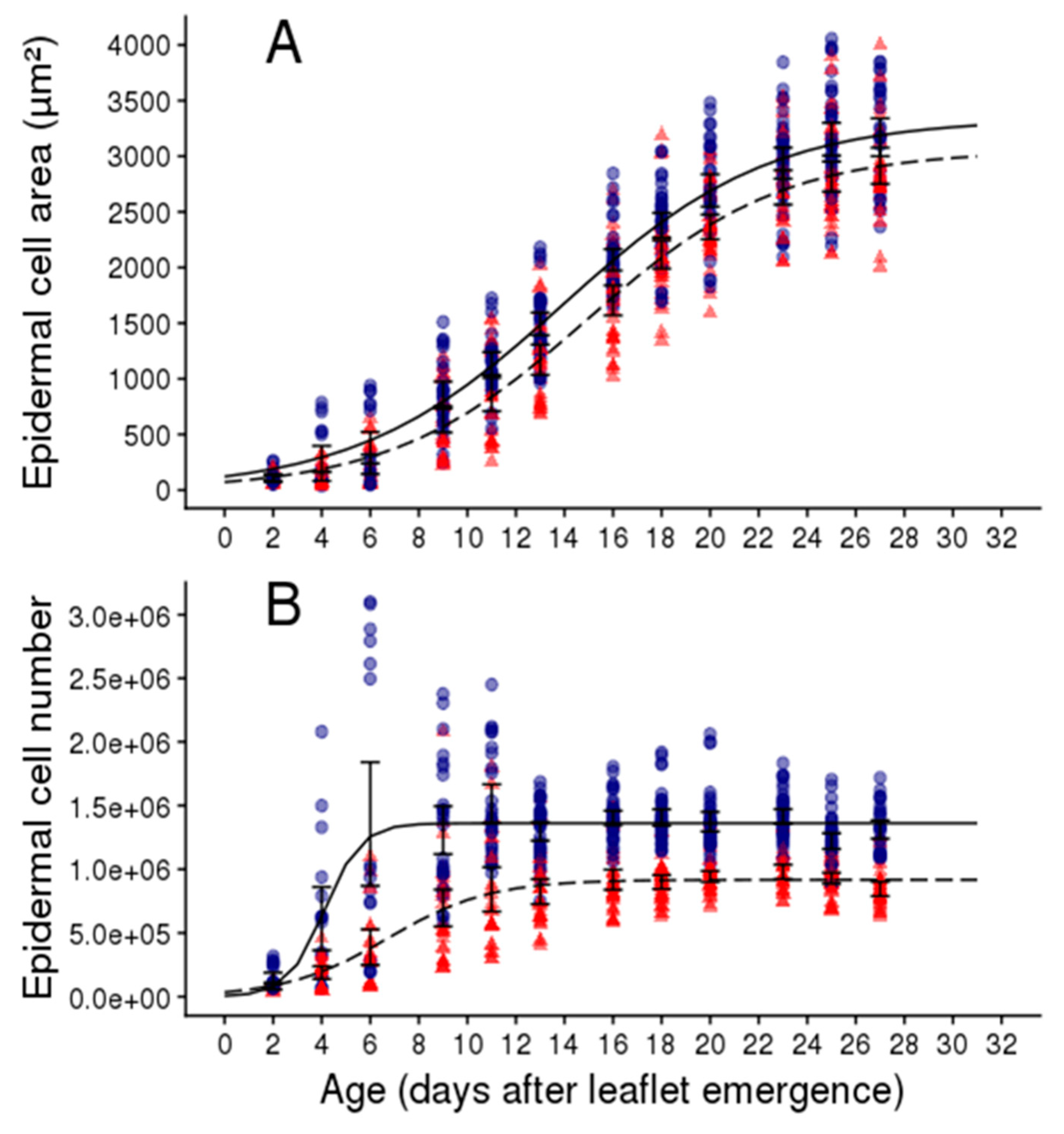
2.2. Soil Water Deficit Affects the Dynamic of Leaflet Expansion, Epidermal Cell Production and Epidermal Cell Expansion
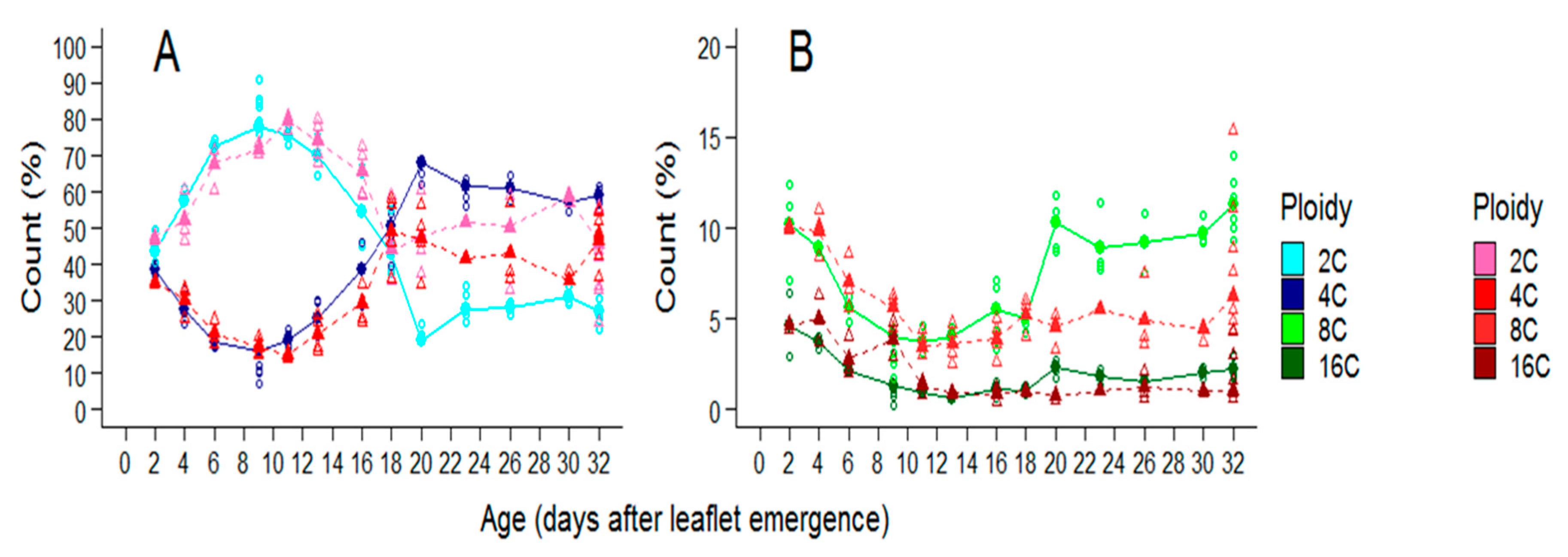
2.3. Soil Water Deficit Affects Both the Dynamic of Ploidy Distribution and the Final Ploidy Distribution in Leaflets
3. Discussion
3.1. Different Drought Dose Responses for Growth and Development-Related Traits
3.2. Drought Induced Plasticity in Cell Division and Cell Expansion in Leaves
3.3. Drought Induced Plasticity in Ploidy in Leaf Cells
4. Materials and Methods
4.1. Plant Material, Sowing and Seedlings Pre-Culture
4.2. Automatic Adjustment of Soil Water Contents
4.3. Measurements of Leaf Growth Related Traits for Dose Response Analyses
4.4. Measurements of Leaf Growth Related Traits for Dynamic Analyses
4.5. Fitted Curves of Leaflet Expansion Related Traits During Experiment B
4.6. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, E.A. Molecular and Physiological Responses to Water-Deficit Stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer International Publishing: Dordrecht, The Netherlands, 2007; pp. 121–140. [Google Scholar]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-Term Climate Change: Projections, Commitments and Irreversibility. In Working Group 1 Contribution to the IPCC Fifth Assessment Report—Climate Change: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: London, UK, 2013; pp. 1029–1136. [Google Scholar]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Aronne, G. Morpho-Anatomical Traits for Plant Adaptation to Drought. In Plant Responses to Drought Stress; Aroca, R., Ed.; From Morphological to Molecular Features; Springer: Berlin, Germany, 2012; pp. 37–61. [Google Scholar]
- Tardieu, F. Any Trait or Trait-Related Allele Can Confer Drought Tolerance: Just Design the Right Drought Scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Claeys, H.; Inze, D. The Agony of Choice: How Plants Balance Growth and Survival under Water-Limiting Conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Varshney, R.K.; Tuberosa, R. Improving Crop Performance under Drought-Cross Fertilization of Disciplines. J. Exp. Bot. 2017, 68, 1393–1398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tardieu, F.; Reymond, M.; Hamard, P.; Granier, C.; Muller, B. Spatial Distributions of Expansion Rate, Cell Division Rate and Cell Size in Maize Leaves: A Synthesis of the Effects of Soil Water Status, Evaporative Demand and Temperature. J. Exp. Bot. 2000, 51, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Reymond, M.; Muller, B.; Leonardi, A.; Charcosset, A.; Tardieu, F. Combining Quantitative Trait Loci Analysis and an Ecophysiological Model to Analyze the Genetic Variability of the Responses of Maize Leaf Growth to Temperature and Water Deficit. Plant Physiol. 2003, 131, 664–675. [Google Scholar] [CrossRef]
- Aguirrezabal, L.; Bouchier-Combaud, S.; Radziejwoski, A.; Dauzat, M.; Cookson, S.J.; Granier, C. Plasticity to Soil Water Deficit in Arabidopsis thaliana: Dissection of Leaf Development into Underlying Growth Dynamic and Cellular Variables Reveals Invisible Phenotypes. Plant Cell Environ. 2006, 29, 2216–2227. [Google Scholar] [CrossRef]
- Lecoeur, J.; Wery, J.; Turc, O.; Tardieu, F. Expansion of Pea Leaves Subjected to Short Water Deficit: Cell Number and Cell Size are Sensitive to Stress at Different Periods of Leaf Development. J. Exp. Bot. 1995, 46, 1093–1101. [Google Scholar] [CrossRef]
- Skirycz, A.; Inze, D. More from Less: Plant Growth under Limited Water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Tardieu, F.; Granier, C.; Muller, B. Water Deficit and Growth. Co-Ordinating Processes without an Orchestrator? Curr. Opin. Plant Biol. 2011, 14, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Avramova, V.; AbdElgawad, H.; Zhang, Z.F.; Fotschki, B.; Casadevall, R.; Vergauwen, L.; Knapen, D.; Taleisnik, E.; Guisez, Y.; Asard, H.; et al. Drought Induces Distinct Growth Response, Protection, and Recovery Mechanisms in the Maize Leaf Growth Zone. Plant Physiol. 2015, 169, 1382–1396. [Google Scholar] [CrossRef] [PubMed]
- Avramova, V.; AbdElgawad, H.; Vasileva, I.; Petrova, A.S.; Holek, A.; Marien, J.; Asard, H.; Beemster, G.T. High Antioxidant Activity Facilitates Maintenance of Cell Division in Leaves of Drought Tolerant Maize Hybrids. Front. Plant Sci. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Granier, C.; Tardieu, F. Water Deficit and Spatial Pattern of Leaf Development. Variability in Responses can be Simulated Using a Simple Model of Leaf Development. Plant Physiol. 1999, 119, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Verelst, W.; Bertolini, E.; De Bodt, S.; Vandepoele, K.; Demeulenaere, M.; Pe, M.E.; Inze, D. Molecular and Physiological Analysis of Growth-Limiting Drought Stress in Brachypodium distachyon Leaves. Mol. Plant. 2013, 6, 311–322. [Google Scholar] [CrossRef]
- Nelissen, H.; Sun, X.H.; Rymen, B.; Jikumaru, Y.; Kojima, M.; Takebayashi, Y.; Abbeloos, R.; Demuynck, K.; Storme, V.; Vuylsteke, M.; et al. The Reduction in Maize Leaf Growth under Mild Drought Affects the Transition between Cell Division and Cell Expansion and cannot be Restored by Elevated Gibberellic Acid Levels. Plant Biotechnol. J. 2018, 16, 615–627. [Google Scholar] [CrossRef]
- Baerenfaller, K.; Massonnet, C.; Walsh, S.; Baginsky, S.; Bühlmann, P.; Hennig, L.; Hirsch-Hoffmann, M.; Howell, K.A.; Kahlau, S.; Radziejwoski, A.; et al. Systems-Based Analysis of Arabidopsis Leaf Growth Reveals Adaptation to Water Deficit. Mol. Syst. Biol. 2012, 8, 606. [Google Scholar] [CrossRef]
- Dambreville, A.; Griolet, M.; Rolland, G.; Dauzat, M.; Bediee, A.; Balsera, C.; Muller, B.; Vile, D.; Granier, C. Phenotyping Oilseed Rape Growth-Related Traits and Their Responses to Water Deficit: The Disturbing Pot Size Effect. Funct. Plant Biol. 2017, 44, 35–45. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-Analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Tisne, S.; Schmalenbach, I.; Reymond, M.; Dauzat, M.; Pervent, M.; Vile, D.; Granier, C. Keep on Growing under Drought: Genetic and Developmental Bases of the Response of Rosette Area using a Recombinant Inbred Line Population. Plant Cell Environ. 2010, 33, 1875–1887. [Google Scholar] [CrossRef]
- Vile, D.; Pervent, M.; Belluau, M.; Vasseur, F.; Bresson, J.; Muller, B.; Granier, C.; Simonneau, T. Arabidopsis Growth under Prolonged High Temperature and Water Deficit: Independent or Interactive Effects? Plant Cell Environ. 2012, 35, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Bouchabke, O.; Chang, F.; Simon, M.; Voisin, R.; Pelletier, G.; Durand-Tardif, M. Natural Variation in Arabidopsis thaliana as a Tool for Highlighting Differential Drought Responses. PLoS ONE 2008, 3, 1705. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Rolland, G.; Dauzat, M.; Bediee, A.; Baldazzi, V.; Bertin, N.; Guedon, Y.; Granier, C. Are Compound Leaves More Complex Than Simple Ones? A Multi-Scale Analysis. Ann. Bot. 2018, 122, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J.B. Soil Conditions and Plant Growth. Plant Cell Environ. 2002, 25, 311–318. [Google Scholar] [CrossRef]
- Marron, N.D.; Delay, J.M.; Petit, E.; Dreyer, G.; Kahlem, F.M. Del-Motte and F. Brignolas. Physiological Traits of two Populus × euramericana Clones, Luisa Avanzo and Dorskamp, during a Water Stress and Re-Watering Cycle. Tree Physiol. 2002, 22, 849–858. [Google Scholar] [CrossRef]
- Marron, N.; Dreyer, E.; Boudouresque, E.; Delay, D.; Petit, J.M.; Delmotte, F.M.; Brignolas, F. Impact of Successive Drought and Re-Watering Cycles on Growth and Specific Leaf Area of two Populus X canadensis (Moench) Clones: ‘Dorskamp’ and ‘Luisa_Avanzo’. Tree Physiol. 2003, 23, 1225–1235. [Google Scholar] [CrossRef]
- Yegappan, T.M.; Paton, D.M.; Gates, C.T.; Muller, W.J. Water Stress in Sunflower (Helianthus Annuus l.). Effects on Leaf Cells and Leaf Area. Ann. Bot. 1982, 49, 63–68. [Google Scholar] [CrossRef]
- Muller, B.; Pantin, F.; Genard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water Deficits Uncouple Growth from Photosynthesis, Increase C Content, and Modify the Relationships between C and Growth in Sink Organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef]
- Wuyts, N.; Massonnet, C.; Dauzat, M.; Granier, C. Structural Assessment of the Impact of Environmental Constraints on Arabidopsis Leaf Growth: A 3D Approach. Plant Cell Environ. 2012, 35, 1631–1646. [Google Scholar] [CrossRef]
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteille, M.; Stitt, M.; et al. Arabidopsis Plants Acclimate to Water Deficit at Low Cost Through Changes of Carbon Usage: An Integrated Perspective Using Growth, Metabolite, Enzyme, and Gene Expression Analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef]
- Nicholson, D.J. Biological Atomism and Cell Theory. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2010, 41, 202–211. [Google Scholar] [CrossRef]
- Massonnet, C.; Tisne, S.; Radziejwoski, A.; Vile, D.; De Veylder, L.; Dauzat, M.; Granier, C. New Insights into the Control of Endoreduplication: Endoreduplication could be Driven by Organ Growth in Arabidopsis Leaves. Plant Physiol. 2011, 157, 2044–2055. [Google Scholar] [CrossRef]
- Heckenberger, U.; Roggatz, U.; Schurr, U. Effect of Drought Stress on the Cytological Status in Ricinus communis. J. Exp. Bot. 1998, 49, 181–189. [Google Scholar] [CrossRef]
- Clauw, P.; Coppens, F.; Korte, A.; Herman, D.; Slabbinck, B.; Dhondt, S.; Van Daele, T.; De Milde, L.; Vermeersch, M.; Maleux, K.; et al. Leaf Growth Response to Mild Drought: Natural Variation in Arabidopsis Sheds Light on Trait Architecture. Plant Cell 2016, 28, 2417–2434. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Vandenbroucke, K.; Clauw, P.; Maleux, K.; De Meyer, B.; Dhondt, S.; Pucci, A.; Gonzalez, N.; Hoeberichts, F.; Tognetti, V.B.; et al. Survival and Growth of Arabidopsis Plants Given Limited Water are not Equal. Nat. Biotechnol. 2011, 29, 212. [Google Scholar] [CrossRef]
- Tisne, S.; Serrand, Y.; Bach, L.; Gilbault, E.; Ben Ameur, R.; Balasse, H.; Voisin, R.; Bouchez, D.; Durand-Tardif, M.; Guerche, P.; et al. Phenoscope: An Automated Large-Scale Phenotyping Platform Offering High Spatial Homogeneity. Plant J. 2013, 74, 534–544. [Google Scholar] [CrossRef]
- Brichet, N.; Fournier, C.; Turc, O.; Strauss, O.; Artzet, S.; Pradal, C.; Welcker, C.; Tardieu, F.; Cabrera-Bosquet, L. A Robot-Assisted Imaging Pipeline for Tracking the Growths of Maize Ear and Silks in a High-Throughput Phenotyping Platform. Plant Methods 2017, 13, 96. [Google Scholar] [CrossRef]
- Nelissen, H.; Rymen, B.; Coppens, F.; Dhondt, S.; Fiorani, F.; Beemster, G.T.S. Kinematic Analysis of Cell Division in Leaves of Mono-And Dicotyledonous Species: A Basis for Understanding Growth and Developing Refined Molecular Sampling Strategies. In Plant Organogenesis. Methods in Molecular Biology (Methods and Protocols); De Smet, I., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 959, pp. 247–264. [Google Scholar]
- Granier, C.; Tardieu, F. Spatial and Temporal Analyses of Expansion and Cell Cyle in Sunflower Leaves. A Common Pattern of Development for All Zones of a Leaf and Different Leaves of a Plant. Plant Physiol. 1998, 116, 991–1001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schuppler, U.; He, P.H.; John, P.C.L.; Munns, R. Effect of Water Stress on Cell Division and Cell-Division-Cycle 2-Like Cell Cycle Kinase Activity in Wheat Leaves. Plant Physiol. 1998, 117, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Reicheld, J.P.; Vernoux, T.; Lardon, F.; Van Montagu, M.; Inze, D. Specific Checkpoints Regulate Plant Cell Cycle Progression in Response to Oxidative Stress. Plant J. 1999, 17, 647–656. [Google Scholar] [CrossRef]
- Nurse, P.A. Long Twentieth Century of the Cell Cycle and Beyond. Cell 2000, 100, 71–78. [Google Scholar] [CrossRef]
- Granier, C.; Aguirrezabal, L.; Chenu, K.; Cookson, S.J.; Dauzat, M.; Hamard, P.; Thioux, J.J.; Rolland, G.; Bouchier-Combaud, S.; Lebaudy, A.; et al. PHENOPSIS, an Automated Platform for Reproducible Phenotyping of Plant Responses to Soil Water Deficit in Arabidopsis thaliana Permitted the Identification of an Accession with Low Sensitivity to Soil Water Deficit. New Phytol. 2006, 169, 623–635. [Google Scholar] [CrossRef]
- Beemster, G.T.S.; De Veylder, L.; Vercruysse, S.; West, G.; Rombaut, D.; Van Hummelen, P.; Galichet, A.; Gruissem, W.; Inze, D.; Vuylsteke, M. Genome-Wide Analysis of Gene Expression Profiles Associated with Cell Cycle Transitions in Growing Organs of Arabidopsis. Plant Physiol. 2005, 138, 734–743. [Google Scholar] [CrossRef]
- Katagiri, Y.; Hasegawa, J.; Fujikura, U.; Hoshino, R.; Matsunaga, S.; Tsukaya, H. The Coordination of Ploidy and Cell Size Differs Between Cell Layers in Leaves. Development. 2016, 143, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Edgar, B.; Zielke, N.; Gutierrez, C. Endocycles: A Recurrent Evolutionary Innovation for Post-Mitotic Cell Growth. Nat. Rev. Mol. Cell Biol. 2014, 15, 197–210. [Google Scholar] [CrossRef]
- Polyn, S.; Willems, A.; De Veylder, L. Cell Cycle Entry, Maintenance, and Exit during Plant Development. Curr. Opin. Plant Biol. 2015, 23, 1–7. [Google Scholar] [CrossRef]
- Kagan, M.L.; Novoplansky, N.; Sachs, T. Variable Cell Lineages form the Functional Pea Epidermis. Ann. Bot. 1992, 69, 303–312. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, G.; Rolland, G.; Dauzat, M.; Bédiée, A.; Baldazzi, V.; Bertin, N.; Guédon, Y.; Granier, C. Leaf Production and Expansion: A Generalized Response to Drought Stresses from Cells to Whole Leaf Biomass—A Case Study in the Tomato Compound Leaf. Plants 2019, 8, 409. https://doi.org/10.3390/plants8100409
Koch G, Rolland G, Dauzat M, Bédiée A, Baldazzi V, Bertin N, Guédon Y, Granier C. Leaf Production and Expansion: A Generalized Response to Drought Stresses from Cells to Whole Leaf Biomass—A Case Study in the Tomato Compound Leaf. Plants. 2019; 8(10):409. https://doi.org/10.3390/plants8100409
Chicago/Turabian StyleKoch, Garance, Gaëlle Rolland, Myriam Dauzat, Alexis Bédiée, Valentina Baldazzi, Nadia Bertin, Yann Guédon, and Christine Granier. 2019. "Leaf Production and Expansion: A Generalized Response to Drought Stresses from Cells to Whole Leaf Biomass—A Case Study in the Tomato Compound Leaf" Plants 8, no. 10: 409. https://doi.org/10.3390/plants8100409
APA StyleKoch, G., Rolland, G., Dauzat, M., Bédiée, A., Baldazzi, V., Bertin, N., Guédon, Y., & Granier, C. (2019). Leaf Production and Expansion: A Generalized Response to Drought Stresses from Cells to Whole Leaf Biomass—A Case Study in the Tomato Compound Leaf. Plants, 8(10), 409. https://doi.org/10.3390/plants8100409




