A Floristic Survey of Wild Edible Plants in Tuscan Maremma, Italy
Abstract
1. Introduction
2. Results
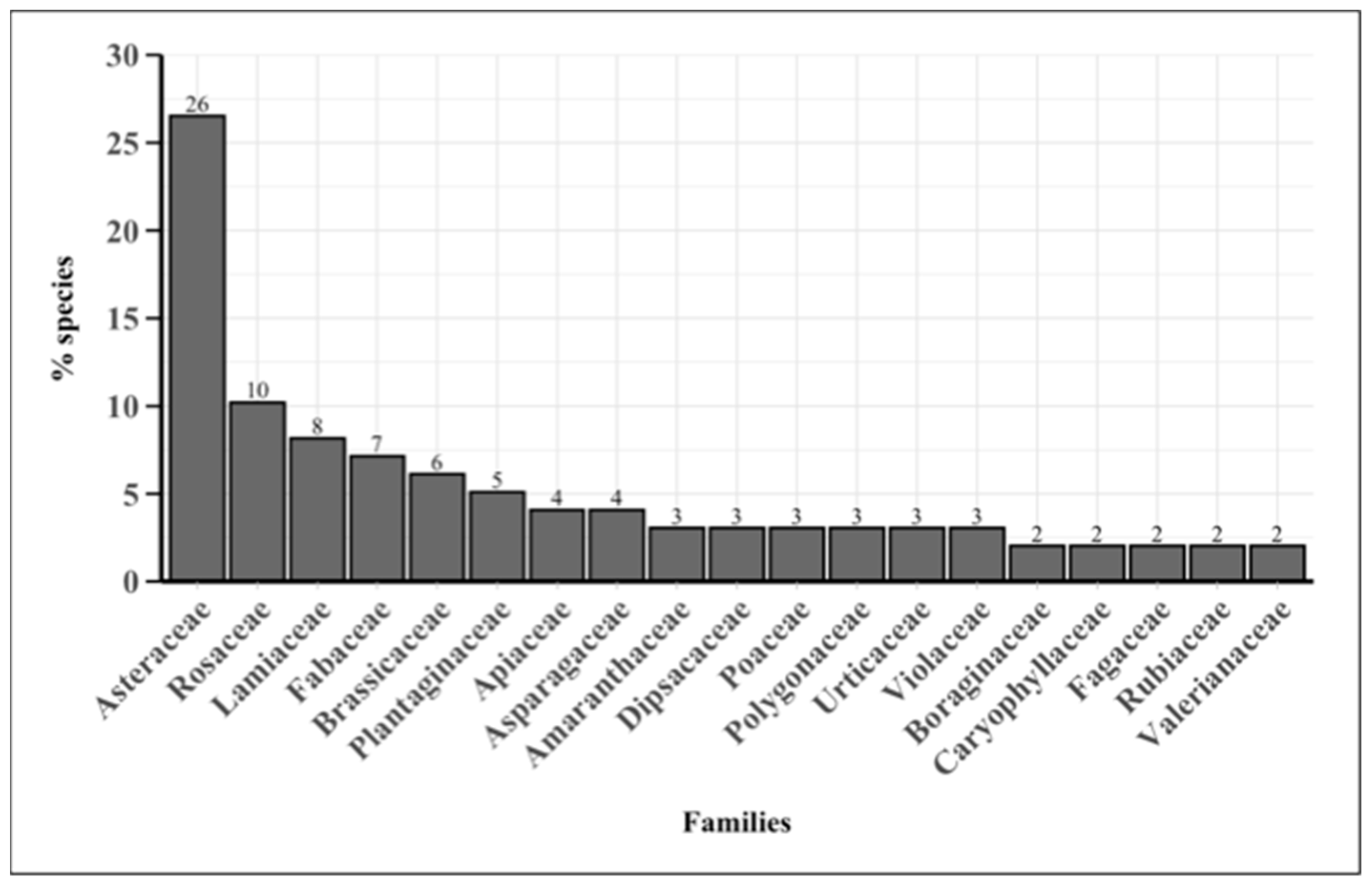
2.1. Floristic Inventory
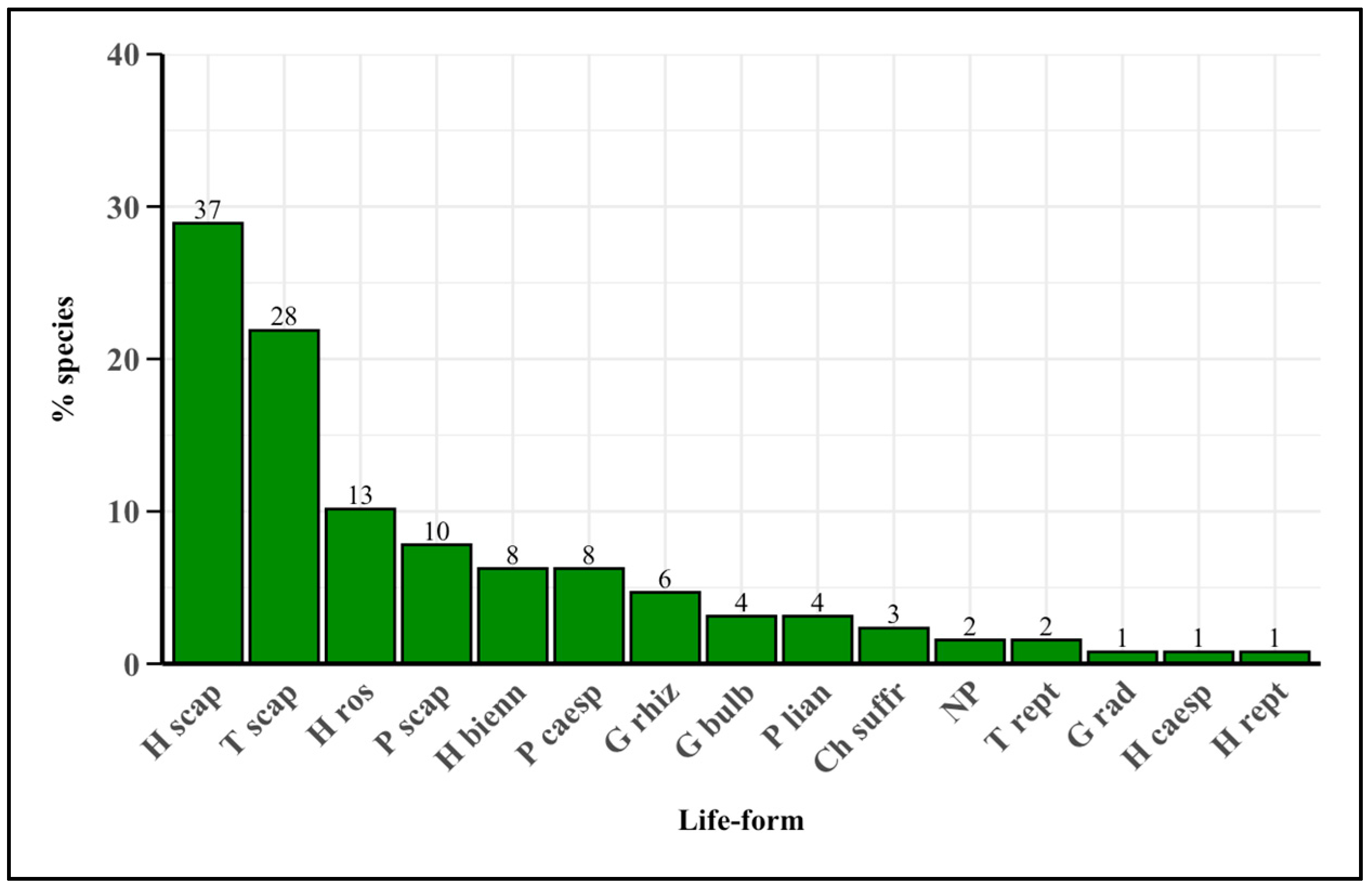
2.2. Life-Form Spectrum
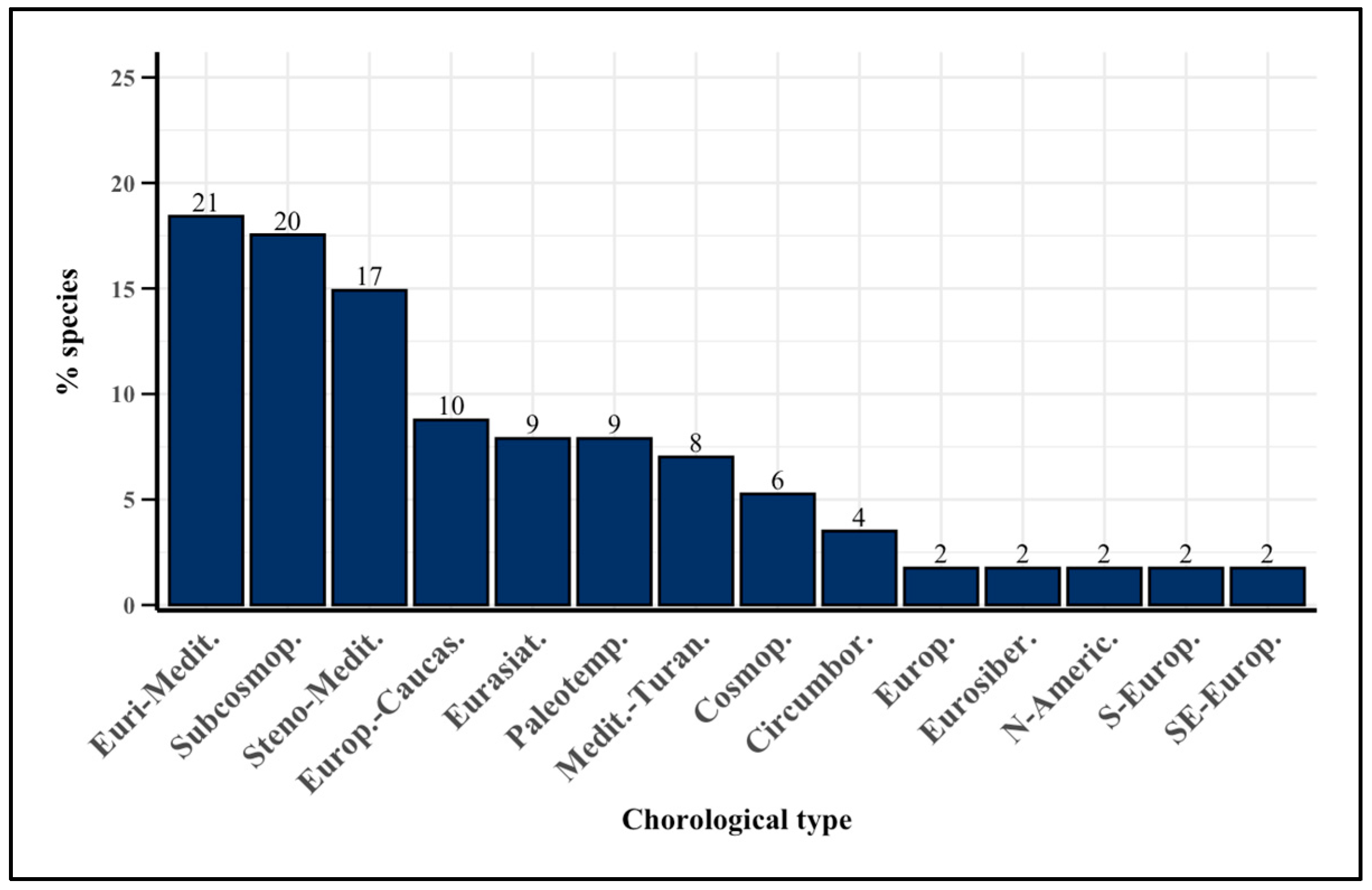
2.3. Chorological Spectrum
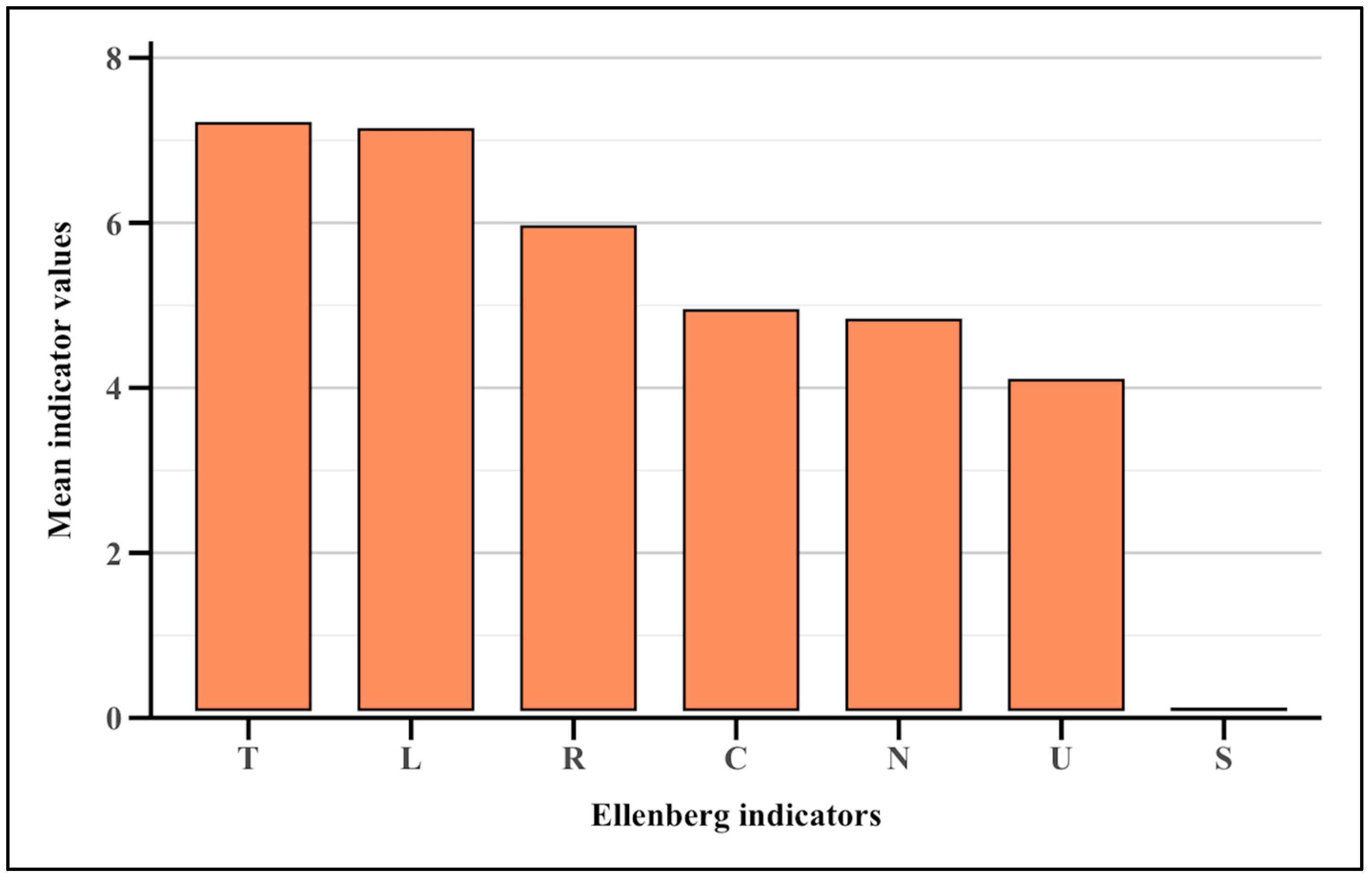
2.4. Ellenberg Indicator Values
2.5. Biochemical Analyses
2.6. Statistical Analyses
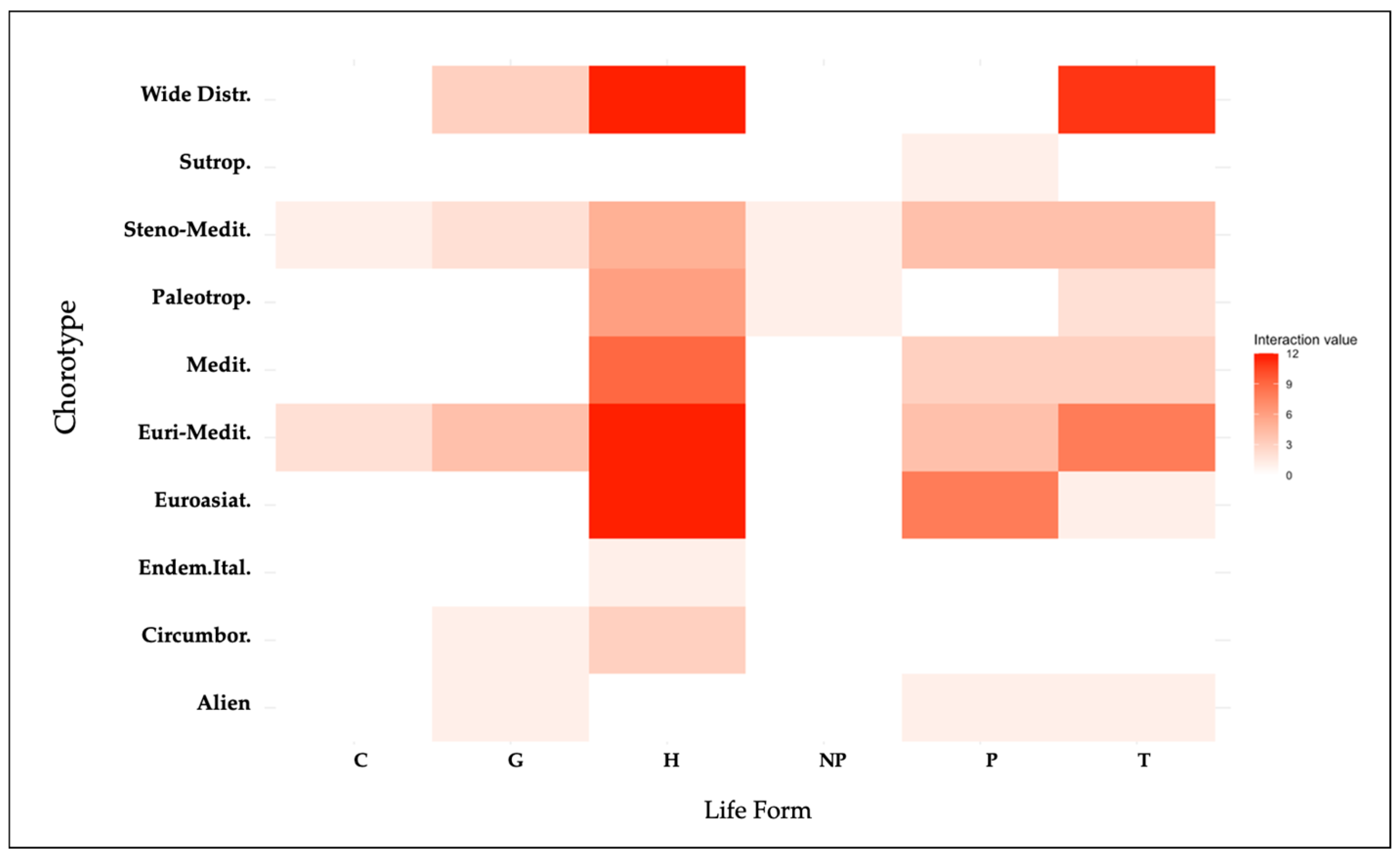
2.6.1. Heatmap Visualization: Insights from MCA Method into Life-Form and Chorological Type Interactions
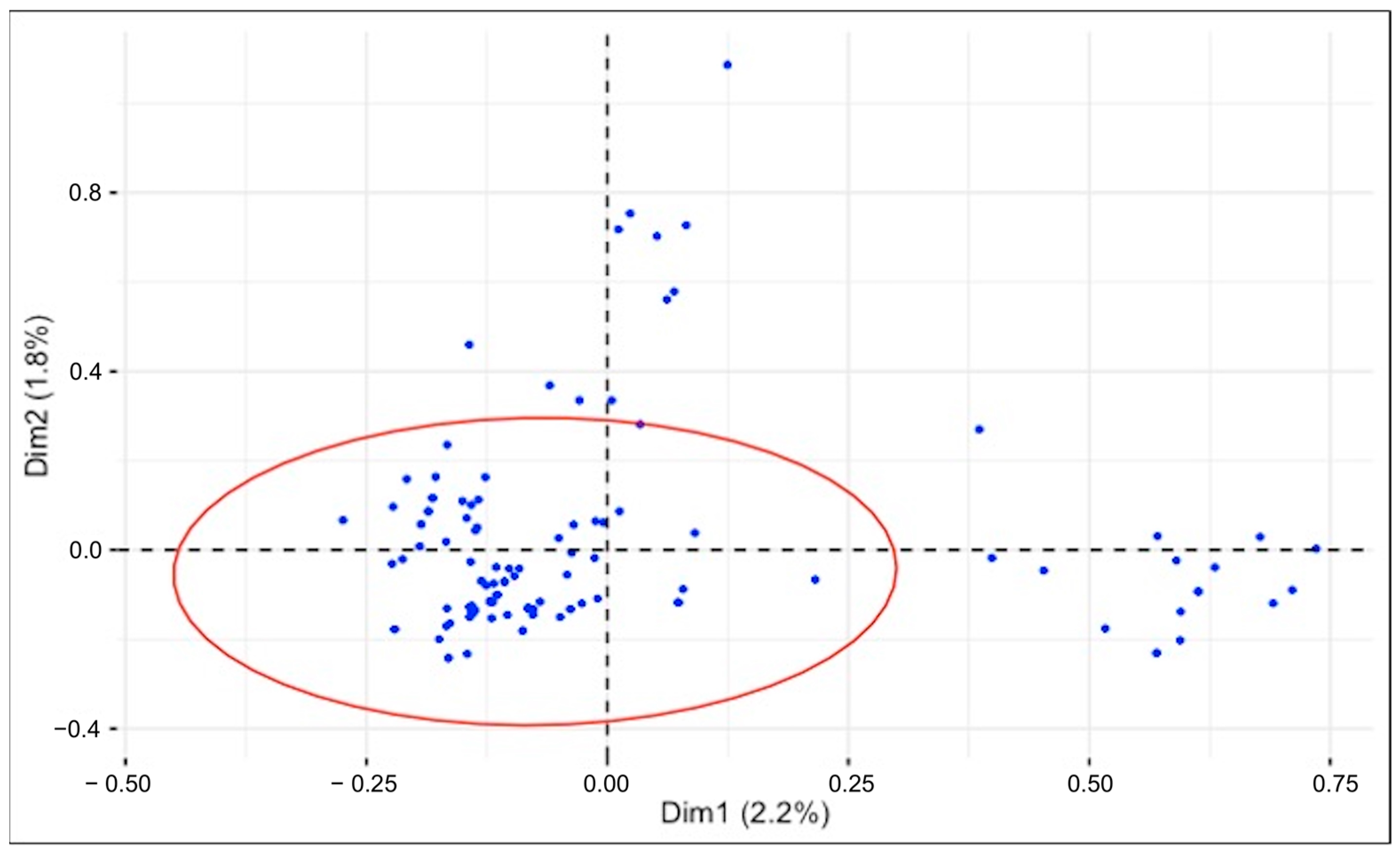
2.6.2. Multiple Correspondence Analysis (MCA) to Identify Interaction Patterns
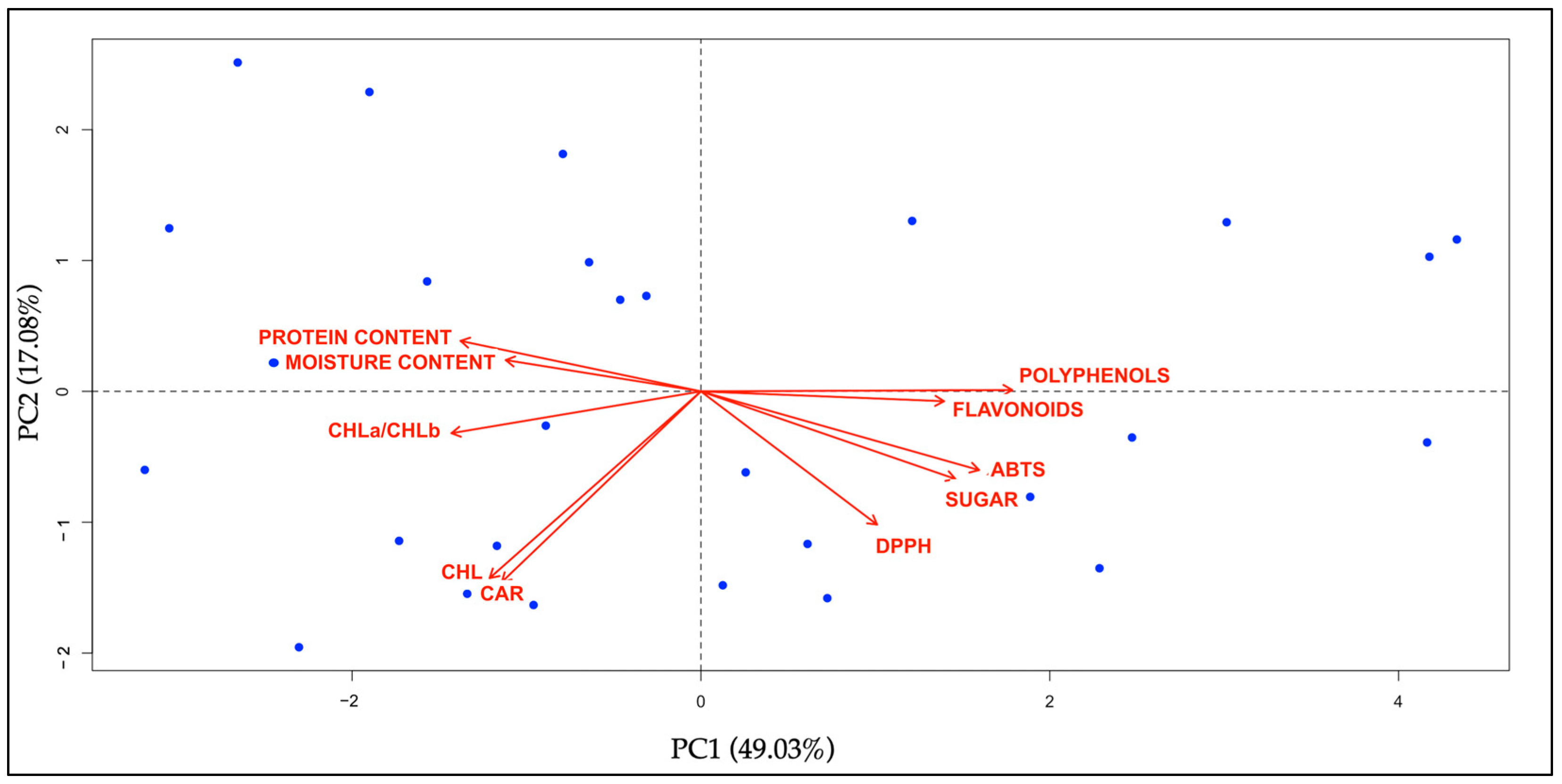
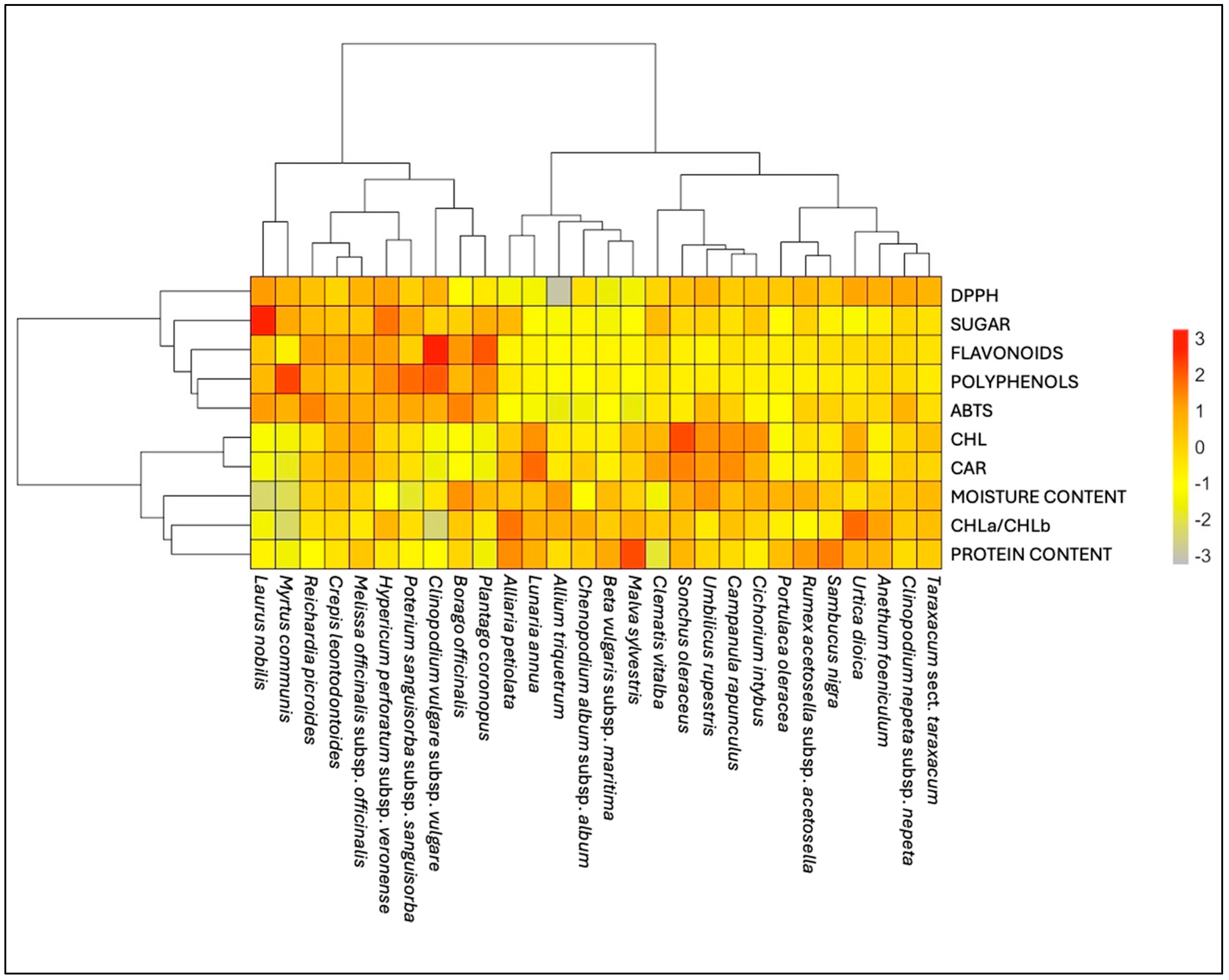
2.6.3. Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) of Phytonutritional Compounds
3. Discussion
3.1. Floristic Inventory
3.2. Life-Form Spectrum
3.3. Chorological Spectrum
3.4. Ellenberg Indicator Values
3.5. Exploring Life-Form and Chorological Type Interactions Through Heatmap Visualization
3.6. Multiple Correspondence Analysis (MCA): From Species Distribution to Key Variable Associations
3.7. Biochemical Analyses
4. Materials and Methods
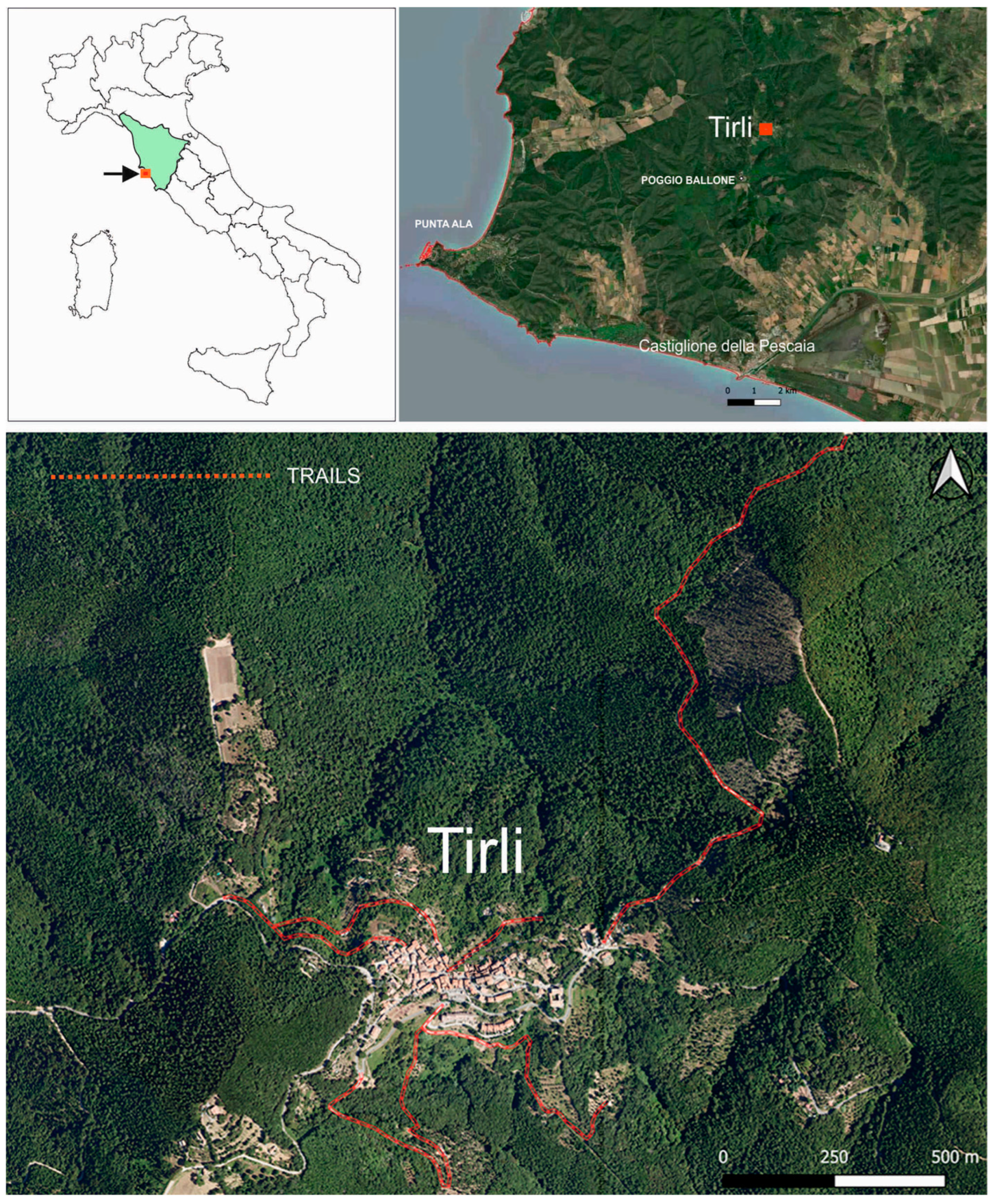
4.1. Study Area
4.2. Data Collection and Analysis
4.3. Biochemical Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) |
| CAR | Carotenoids |
| CE | Catechin equivalent |
| CHLa | Chlorophyll a |
| CHLb | Chlorophyll b |
| DNS | 3,5-dinitrosalicylic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| FW | Fresh weight |
| GAE | Gallic acid equivalent |
| GLU | Glucose |
| MCA | Multiple correspondence analysis |
| HCA | Hierarchical cluster analysis |
| PCA | Principal component analysis |
| TE | Trolox equivalent |
| WEPs | Wild edible plants |
References
- Shirsat, R.; Koche, D. Wild Edible Plants (WEPs): A Review on Their Importance, Possible Threats, and Conservation. In Contemporary Research and Perspectives in Biological Science; Son, P.L., Ed.; BP International: Hong Kong, China, 2024; Volume 2, pp. 97–115. ISBN 978-93-48119-94-0. [Google Scholar]
- Tadesse, D.; Masresha, G.; Lulekal, E.; Wondafrash, M. A Systematic Review Exploring the Diversity and Food Security Potential of Wild Edible Plants in Ethiopia. Sci. Rep. 2024, 14, 17821. [Google Scholar] [CrossRef]
- Ngome, P.I.T.; Shackleton, C.; Degrande, A.; Tieguhong, J.C. Addressing constraints in promoting wild edible plants’ utilization in household nutrition: Case of the Congo Basin forest area. Agric. Food Secur. 2017, 6, 20. [Google Scholar]
- Mahmoud, K.B.; Abdelkefi, F.; Mezzapesa, G.; Perrino, E. Nutritional Value and Functional Properties of an Underexploited Tunisian Wild Beet (Beta macrocarpa Guss.) in Relation to Soil Characteristics. Euro-Mediterr. J. Environ. Integr. 2024, 9, 705–720. [Google Scholar] [CrossRef]
- Keservani, R.K.; Kesharwani, R.K.; Vyas, N.; Jain, S.; Raghuvanshi, R.; Sharma, A.K. Nutraceutical and Functional Food as Future Food: A Review. Pharm. Lett. 2010, 2, 106–116. [Google Scholar]
- Turreira-García, N.; Theilade, I.; Meilby, H.; Sørensen, M. Wild Edible Plant Knowledge, Distribution and Transmission: A Case Study of the Achí Mayans of Guatemala. J. Ethnobiol. Ethnomed. 2015, 11, 1–17. [Google Scholar]
- Singh, V. Biodiversity Conservation. In Textbook of Environment and Ecology; Springer: Berlin/Heidelberg, Germany, 2024; pp. 225–236. [Google Scholar]
- Burlingame, B.; Charrondiere, R.; Mouille, B. Food Composition Is Fundamental to the Cross-Cutting Initiative on Biodiversity for Food and Nutrition. J. Food Compos. Anal. 2009, 22, 361–365. [Google Scholar]
- Cordero, S.; Gálvez, F.; Arenas, J.; Rodríguez-Valenzuela, E. Does Access to Natural Environments Explain Differences in the Use of Wild Plants between Rural and Urban Populations? Bot. Sci. 2021, 99, 104–123. [Google Scholar]
- Borelli, T.; Hunter, D.; Powell, B.; Ulian, T.; Mattana, E.; Termote, C.; Pawera, L.; Beltrame, D.; Penafiel, D.; Tan, A. Born to Eat Wild: An Integrated Conservation Approach to Secure Wild Food Plants for Food Security and Nutrition. Plants 2020, 9, 1299. [Google Scholar] [CrossRef]
- Enri, S.R.; Falla, N.M.; Demasi, S.; Bianchi, D.M.; Squadrone, S.; Lombardi, G.; Scariot, V. Exploring the Potential of Wild Leafy Vegetables Widespread in European Alps as Functional Food. J. Agric. Food Res. 2024, 18, 101368. [Google Scholar]
- Fantasma, F.; Samukha, V.; Saviano, G.; Chini, M.G.; Iorizzi, M.; Caprari, C. Nutraceutical Aspects of Selected Wild Edible Plants of the Italian Central Apennines. Nutraceuticals 2024, 4, 190–231. [Google Scholar] [CrossRef]
- Aberoumand, A.; Deokule, S. Studies on Nutritional Values of Some Wild Edible Plants from Iran and India. Pak. J. Nutr. 2009, 8, 26–31. [Google Scholar] [CrossRef]
- Grace, O.O.; Kayode, J. Ethno-Medicinal Assessment of Wild Edible Plants in Ijesa Region, Osun State, Nigeria. Bull. Pure Appl. Sci. -Bot. 2018, 37, 89–107. [Google Scholar] [CrossRef]
- Lockett, C.T.; Calvert, C.C.; Grivetti, L.E. Energy and Micronutrient Composition of Dietary and Medicinal Wild Plants Consumed during Drought. Study of Rural Fulani, Northeastern Nigeria. Int. J. Food Sci. Nutr. 2000, 51, 195–208. [Google Scholar] [PubMed]
- Ranfa, A.; Orlandi, F.; Maurizi, A.; Bodesmo, M. Ethnobotanical Knowledge and Nutritional Properties of Two Edible Wild Plants from Central Italy: Tordylium apulum L. and Urospermum dalechampii (L.) Scop. ex FW Schmid. J. Appl. Bot. Food Qual. 2015, 88, 249–254. [Google Scholar]
- Bacchetta, L.; Visioli, F.; Cappelli, G.; Caruso, E.; Martin, G.; Nemeth, E.; Bacchetta, G.; Bedini, G.; Wezel, A.; van Asseldonk, T. A Manifesto for the Valorization of Wild Edible Plants. J. Ethnopharmacol. 2016, 191, 180–187. [Google Scholar] [CrossRef]
- Mattirolo, O.; Gallino, B.; Pallavicini, G. Phytoalimurgia pedemontana. Come Alimentarsi con le Piante Selvatiche; Blu Edizioni: Torino, Italy, 2011. [Google Scholar]
- Baldi, A.; Bruschi, P.; Campeggi, S.; Egea, T.; Rivera, D.; Obón, C.; Lenzi, A. The Renaissance of Wild Food Plants: Insights from Tuscany (Italy). Foods 2022, 11, 300. [Google Scholar] [CrossRef]
- Zanin, G.; Zuin, M.; Vigolo, M.; Zanin, G. Il Giardino Fitoalimurgico per La Valorizzazione Delle Specie Spontanee. 2008. Veneto Agricoltura, 7:1–16. Available online: https://hdl.handle.net/11577/2274889 (accessed on 10 January 2025).
- Paura, B.; Di Marzio, P. Making a Virtue of Necessity: The Use of Wild Edible Plant Species (Also Toxic) in Bread Making in Times of Famine According to Giovanni Targioni Tozzetti (1766). Biology 2022, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible halophytes and halo-tolerant species in Apulia region (Southeastern Italy): Biogeography, traditional food use and potential sustainable crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- Lombardi, T.; Ventura, I.; Bertacchi, A. Floristic Inventory of Ethnobotanically Important Halophytes of North-Western Mediterranean Coastal Brackish Areas, Tuscany, Italy. Agronomy 2023, 13, 615. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D. A Second Update to the Checklist of the Vascular Flora Native to Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Paura, B.; Di Marzio, P.; Salerno, G.; Brugiapaglia, E.; Bufano, A. Design a Database of Italian Vascular Alimurgic Flora (AlimurgITA): Preliminary Results. Plants 2021, 10, 743. [Google Scholar] [CrossRef]
- Arrigoni, P.; Mazzanti, A.; Ricceri, C. Contributo Alla Conoscenza Dei Boschi Della Maremma Grossetana. Webbia 1990, 44, 121–149. [Google Scholar]
- Scapini, F.; Nardi, M. Il Parco Regionale della Maremma e il suo territorio. In Una Guida per Conoscere e Capire; Pacini: Pisa, Italy, 2007; Available online: https://hdl.handle.net/2158/262226 (accessed on 10 January 2025).
- Tomei, P.E.; Trimarchi, S. Piante d’uso Etnobotanico in Toscana; Pacini Fazzi: Lucca, Italy, 2017; ISBN 88-6550-587-7. [Google Scholar]
- Ghirardini, M.P.; Carli, M.; Del Vecchio, N.; Rovati, A.; Cova, O.; Valigi, F.; Agnetti, G.; Macconi, M.; Adamo, D.; Traina, M. The Importance of a Taste. A Comparative Study on Wild Food Plant Consumption in Twenty-One Local Communities in Italy. J. Ethnobiol. Ethnomedicine 2007, 3, 1–14. [Google Scholar]
- Guarrera, P.; Savo, V. Wild Food Plants Used in Traditional Vegetable Mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar]
- Raunkiaer, C. Types biologiques pour la géographie botanique. Bull. Acad. R. Sci. 1905, 5, 347–437. [Google Scholar]
- Lichtenthaler, H.; Babani, F. Contents of Photosynthetic Pigments and Ratios of Chlorophyll a/b and Chlorophylls to Carotenoids (A + b)/(X + c) in C4 Plants as Compared to C3 Plants. Photosynthetica 2021, 60, 3. [Google Scholar] [PubMed]
- Abdi, H.; Valentin, D. Multiple Correspondence Analysis. Encycl. Meas. Stat. 2007, 2, 651–657. [Google Scholar]
- Hirzel, A.H.; Le Lay, G. Habitat Suitability Modelling and Niche Theory. J. Appl. Ecol. 2008, 45, 1372–1381. [Google Scholar]
- White, E.P.; Ernest, S.M.; Adler, P.B.; Hurlbert, A.H.; Lyons, S.K. Integrating Spatial and Temporal Approaches to Understanding Species Richness. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3633–3643. [Google Scholar]
- Leonti, M.; Nebel, S.; Rivera, D.; Heinrich, M. Wild Gathered Food Plants in the European Mediterranean: A Comparative Analysis. Econ. Bot. 2006, 60, 130–142. [Google Scholar]
- Töpel, M.; Antonelli, A.; Yesson, C.; Eriksen, B. Past Climate Change and Plant Evolution in Western North America: A Case Study in Rosaceae. PLoS One 2012, 7, e50358. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Romano, A. How Climate Change-Related Abiotic Factors Affect the Production of Industrial Valuable Compounds in Lamiaceae Plant Species: A Review. Front. Plant Sci. 2024, 15, 1370810. [Google Scholar] [CrossRef] [PubMed]
- Waheed, M.; Arshad, F. Adaptive Convergence and Divergence Underpin the Diversity of Asteraceae in a Semi-Arid Lowland Region. Flora 2024, 317, 152554. [Google Scholar] [CrossRef]
- González-Tejero, M.; Casares-Porcel, M.; Sánchez-Rojas, C.; Ramiro-Gutiérrez, J.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.; Censorii, E.; De Pasquale, C.; Della, A. Medicinal Plants in the Mediterranean Area: Synthesis of the Results of the Project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Camangi, F.; Guarrera, P.; Camarda, I.; Tomei, P.; Lentini, F.; Stefani, A.; Leporatti, M.; Pieroni, A. Usi Alimentari. In Etnobotanica: Conservazione di un Patrimonio Culturale Come Risorsa per uno Sviluppo Sostenibile; Edipuglia: Fogliano Redipuglia, Italy, 2013; pp. 113–142. [Google Scholar]
- Guarrera, P.M.; Savo, V. Perceived Health Properties of Wild and Cultivated Food Plants in Local and Popular Traditions of Italy: A Review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The Wild Taxa Utilized as Vegetables in Sicily (Italy): A Traditional Component of the Mediterranean Diet. J. Ethnobiol. Ethnomed. 2018, 14, 1–27. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. The Medicinal Potential of Plants from the Genus Plantago (Plantaginaceae). Ind. Crops Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al-Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive compounds and health benefits of edible Rumex species—A review. Cell. Mol. Biol. 2018, 64, 27–34. [Google Scholar] [CrossRef]
- Badalamenti, N.; Sottile, F.; Bruno, M. Ethnobotany, Phytochemistry, Biological, and Nutritional Properties of Genus Crepis—A Review. Plants 2022, 11, 519. [Google Scholar] [CrossRef]
- Mittu, B.; Chaturvedi, A.; Sharma, R.; Singh, M.; Begum, Z. Viola Tricolor and Viola Odorata. In Edible Flowers; Elsevier: Amsterdam, The Netherlands, 2024; pp. 325–354. [Google Scholar]
- Guarrera, P.M.; Salerno, G.; Caneva, G. Food, Flavouring and Feed Plant Traditions in the Tyrrhenian Sector of Basilicata, Italy. J. Ethnobiol. Ethnomed. 2006, 2, 1–6. [Google Scholar] [CrossRef]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [PubMed]
- Midolo, G.; Axmanová, I.; Divíšek, J.; Dřevojan, P.; Lososová, Z.; Večeřa, M.; Karger, D.N.; Thuiller, W.; Bruelheide, H.; Aćić, S. Diversity and Distribution of Raunkiær’s Life Forms in European Vegetation. J. Veg. Sci. 2024, 35, e13229. [Google Scholar]
- Cain, S.A. Life-Forms and Phytoclimate. Bot. Rev. 1950, 16, 1–32. [Google Scholar]
- Ubaldi, D. Flora, Fitocenosi e Ambiente. Elementi di Geobotanica E Fitosociologia; CLUEB: Bologna, Italy, 2003. [Google Scholar]
- Fiaschi, T.; Bonari, G.; Frignani, F.; Gizzi, G.; Landi, M.; Magrini, S.; Quilghini, G.; Pafumi, E.; Scoppola, A.; Angiolini, C. Vascular Flora of the Isthmus of Feniglia (Southern Tuscany, Italy). Ital. Bot. 2024, 17, 77–101. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volumes 1–3. [Google Scholar]
- Motti, R.; Motti, P. An Ethnobotanical Survey of Useful Plants in the Agro Nocerino Sarnese (Campania, Southern Italy). Hum. Ecol. 2017, 45, 865–878. [Google Scholar]
- Gentile, C.; Spampinato, G.; Patti, M.; Laface, V.L.A.; Musarella, C.M. Contribution to the Ethnobotanical Knowledge of Serre Calabre (Southern Italy). Res. J. Ecol. Environ. Sci. 2022, 2, 35–55. [Google Scholar]
- Ellenberg, H. Zeigerwerte der Gefässpflanzen Mitteleuropas. Scr. Geobot. 1974, 9, 1–166. [Google Scholar]
- Nelson, V. Climate Change and Gender: What Role for Agricultural Research Among Smallholder Farmers in Africa? CIAT Working Document 2010, No. 222. Available online: https://hdl.handle.net/10568/49623 (accessed on 10 January 2025).
- Ali, S. A Study on Different Plant Species of the Rosaceae Family and Their Ethnobotanical Uses among the Local Communities at Swat District, Pakistan. Ethnobot. Res. Appl. 2023, 25, 1–16. [Google Scholar]
- Tardío, J.; Pardo-de-Santayana, M.; Morales, R. Ethnobotanical Review of Wild Edible Plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar]
- Hadjichambis, A.C.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.M. Wild and Semi-Domesticated Food Plant Consumption in Seven Circum-Mediterranean Areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar]
- Nouidha, S.; Selmi, S.; Guigonis, J.; Pourcher, T.; Chekir-Ghedira, L.; Kilani-Jaziri, S. Metabolomics Profiling of Tunisian Sonchus oleraceus L. Extracts and Their Antioxidant Activities. Chem. Biodivers. 2023, 20, e202300290. [Google Scholar] [CrossRef]
- Botella, M.Á.; Hellín, P.; Hernández, V.; Dabauza, M.; Robledo, A.; Sánchez, A.; Fenoll, J.; Flores, P. Chemical Composition of Wild Collected and Cultivated Edible Plants (Sonchus oleraceus L. and Sonchus tenerrimus L.). Plants 2024, 13, 269. [Google Scholar] [CrossRef]
- García-Herrera, P.; Sánchez-Mata, M.; Cámara, M.; Fernández-Ruiz, V.; Díez-Marqués, C.; Molina, M.; Tardío, J. Nutrient Composition of Six Wild Edible Mediterranean Asteraceae Plants of Dietary Interest. J. Food Compos. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- Panfili, G.; Niro, S.; Bufano, A.; D’Agostino, A.; Fratianni, A.; Paura, B.; Falasca, L.; Cinquanta, L. Bioactive Compounds in Wild Asteraceae Edible Plants Consumed in the Mediterranean Diet. Plant Foods Hum. Nutr. 2020, 75, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Gazarini, L.; Rodrigues, M. Acclimation of Myrtus communis to Contrasting Mediterranean Light Environments—Effects on Structure and Chemical Composition of Foliage and Plant Water Relations. Environ. Exp. Bot. 2001, 45, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Khodja, Y.K.; Bachir-Bey, M.; Belmouhoub, M.; Ladjouzi, R.; Dahmoune, F.; Khettal, B. The Botanical Study, Phytochemical Composition, and Biological Activities of Laurus nobilis L. Leaves: A Review. Int. J. Second. Metab. 2023, 10, 269–296. [Google Scholar] [CrossRef]
- Bektašević, M.; Politeo, O.; Roje, M.; Jurin, M. Polyphenol Composition, Anticholinesterase and Antioxidant Potential of the Extracts of Clinopodium vulgare L. Chem. Biodivers. 2022, 19, e202101002. [Google Scholar] [CrossRef]
- Gomaa, N.H.; Hassan, M.O.; Fahmy, G.M.; González, L.; Hammouda, O.; Atteya, A.M. Flavonoid Profiling and Nodulation of Some Legumes in Response to the Allelopathic Stress of Sonchus oleraceus L. Acta Bot. Bras. 2015, 29, 553–560. [Google Scholar] [CrossRef]
- Poonia, A.; Upadhayay, A. Chenopodium album Linn: Review of Nutritive Value and Biological Properties. J. Food Sci. Technol. 2015, 52, 3977–3985. [Google Scholar] [CrossRef]
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant Activity and Phenolic Composition of Wild, Edible, and Medicinal Fennel from Different Mediterranean Countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef]
- Spina, M.; Cuccioloni, M.; Sparapani, L.; Acciarri, S.; Eleuteri, A.M.; Fioretti, E.; Angeletti, M. Comparative Evaluation of Flavonoid Content in Assessing Quality of Wild and Cultivated Vegetables for Human Consumption. J. Sci. Food Agric. 2008, 88, 294–304. [Google Scholar]
- Regione Toscana. GeoScopio–Sistema Informativo Territoriale della Regione Toscana. Available online: https://www.regione.toscana.it/-/geoscopio (accessed on 16 December 2024).
- Gabellini, A.; Viciani, D.; Biagini, P. La Vegetazione Del Complesso Forestale Regionale «Bandite Di Scarlino»(Grosseto, Toscana Meridionale). Atti Della Soc. Toscana Sci. Nat. Resid. Pisa. Memorie. Ser. B 2014, 120, 45–58. [Google Scholar]
- Arrigoni, P.; Raffaelli, M.; Rizzotto, M.; Selvi, F.; Viciani, D.; Lombardi, L.; Foggi, B.; Melillo, C.; Benesperi, R.; Ferretti, G. La Vegetazione Forestale. Serie Boschi e Macchie Di Toscana. 1998. Available online: https://hdl.handle.net/2158/637685 (accessed on 10 January 2025).
- Selvi, F.; Viciani, D. Contributo Alla Conoscenza Vegetazionale Delle Sugherete Toscane. Parlatorea 1999, 3, 45–63. [Google Scholar]
- Biserni, G.; Cornamusini, G. Sistemi Torbiditici Interagenti Nell’avanfossa Oligocenica Nordappenninica: Dati Petrografico-Stratigrafici Del Macigno Della Toscana Meridionale Costiera. Boll. Soc. Geol. Ital. 2002, 121, 253–264. [Google Scholar]
- Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar]
- Regione Toscana, Direzione Difesa del Suolo e Protezione Civile. Sistema Informativo Regionale (SIR) Toscana. Available online: https://www.sir.toscana.it/ (accessed on 16 December 2024).
- Camangi, F.; Stefani, A.; Uncini Manganelli, R.E.; Tomei, P.E.; Trimarchi, S.; Oggiano, N.; Loni, A. L’uso Delle Erbe Nella Tradizione Rurale Della Toscana. Arsia 2007, 3, 23–301. Available online: https://hdl.handle.net/11382/101092 (accessed on 10 January 2025).
- Camangi, F.; Bettini, D.; Stefani, A.; Cecchelli, G.; Santoro, A.M. Piante Spontanee d’Uso Alimentare: Viaggio Alla Scoperta della Cucina Povera a Partire dalla Tradizione Popolare Volterrana; Edizioni ETS: Pisa, Italy, 2010; 223p, Available online: https://hdl.handle.net/11382/307038 (accessed on 10 January 2025).
- Luciano, R.; Gatti, C. Erbe Spontanee Commestibili; Nuova ed. riv. e integrata; Araba Fenice: Boves, Italy, 2014; ISBN 978-88-95853-10-9. [Google Scholar]
- Motti, R. Wild Plants Used as Herbs and Spices in Italy: An Ethnobotanical Review. Plants 2021, 10, 563. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017; Volumes 1–4. [Google Scholar]
- Galasso, G.; Conti, F.; Peruzzi, L.; Alessandrini, A.; Ardenghi, N.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D. A Second Update to the Checklist of the Vascular Flora Alien to Italy. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2024, 158, 297–340. [Google Scholar] [CrossRef]
- Wikiplantbase #Toscana. Available online: https://bot.biologia.unipi.it/wpb/toscana/index (accessed on 16 January 2025).
- Martellos, S.; Bartolucci, F.; Conti, F.; Galasso, G.; Moro, A.; Pennesi, R.; Peruzzi, L.; Pittao, E.; Nimis, P.L. FlorItaly—The Portal to the Flora of Italy. PhytoKeys 2020, 156, 55–71. [Google Scholar]
- Selvi, F. A Critical Checklist of the Vascular Flora of Tuscan Maremma (Grosseto Province, Italy). Flora Mediterr. 2010, 20, 47–139. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.-Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Li, P.; Gao, J.; Hu, H.; Luo, S.; Zhang, L. Postharvest Senescence of Fresh Lotus Pods and Seeds Is Delayed by Treatment with 1-methylcyclopropene. Ann. Appl. Biol. 2016, 169, 440–452. [Google Scholar]
- Teixeira, R.S.S.; da Silva, A.S.; Ferreira-Leitão, V.S.; da Silva Bon, E.P. Amino Acids Interference on the Quantification of Reducing Sugars by the 3, 5-Dinitrosalicylic Acid Assay Mislead Carbohydrase Activity Measurements. Carbohydr. Res. 2012, 363, 33–37. [Google Scholar]
- Mills, H.A.; Jones, J.B., Jr. Plant Analysis Handbook II. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing: Athens, Greece, 1996. [Google Scholar]
- Kamalja, K.K.; Khangar, N.V. Multiple Correspondence Analysis and Its Applications. Electron. J. Appl. Stat. Anal. 2017, 10, 432–462. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pentassuglia, M.; Lombardi, T.; Bambi, G.; Ventura, I.; D’Ambrosio, B.; Bertacchi, A.; Pistelli, L. A Floristic Survey of Wild Edible Plants in Tuscan Maremma, Italy. Plants 2025, 14, 976. https://doi.org/10.3390/plants14060976
Pentassuglia M, Lombardi T, Bambi G, Ventura I, D’Ambrosio B, Bertacchi A, Pistelli L. A Floristic Survey of Wild Edible Plants in Tuscan Maremma, Italy. Plants. 2025; 14(6):976. https://doi.org/10.3390/plants14060976
Chicago/Turabian StylePentassuglia, Mario, Tiziana Lombardi, Giovanni Bambi, Irene Ventura, Benedetta D’Ambrosio, Andrea Bertacchi, and Laura Pistelli. 2025. "A Floristic Survey of Wild Edible Plants in Tuscan Maremma, Italy" Plants 14, no. 6: 976. https://doi.org/10.3390/plants14060976
APA StylePentassuglia, M., Lombardi, T., Bambi, G., Ventura, I., D’Ambrosio, B., Bertacchi, A., & Pistelli, L. (2025). A Floristic Survey of Wild Edible Plants in Tuscan Maremma, Italy. Plants, 14(6), 976. https://doi.org/10.3390/plants14060976









