An Up-to-Date Review Regarding the Biological Activity of Geranium robertianum L.
Abstract
1. Introduction
2. Taxonomy
3. Phytochemical Composition
4. Biological Activity
4.1. Antimicrobial Activity
4.2. Antiviral
4.3. Anti-Inflammatory
4.4. Antioxidant
4.5. Anti-Cancer
4.6. Wound-Healing
4.7. Neuroprotective
4.8. Antiulcerative
4.9. Antidiabetic
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIC | Minimum inhibitory concentration |
| NO synthase | Nitric oxide synthase |
| IC50 | Half maximal inhibitory concentration |
| GI50 | 50% growth inhibition |
| CC50 | Concentration of cytotoxicity 50% |
| LOX | Lipoxygenase |
| DNA | Deoxyribonucleic acid |
| EC50 | Half maximal effective concentration |
| MAPK | Mitogen activated protein kinase |
| NF-κB | Nuclear factor Kappa B |
| PI3K/Akt | Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) |
| TNF-α | Tumor necrosis factor |
| HO-1 | Heme oxygenase |
| Nrf-2 | Nuclear factor erythroid 2-related factor 2 |
References
- Nasim, N.; Sandeep, I.S.; Mohanty, A.S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucl. Int. J. Cytol. Allied Top. 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Czigle, S.; Nagy, M.; Mladěnka, P. Pharmacokinetic and pharmacodynamic herb-drug interactions—Part I. Herbal medicines of the central nervous system. PeerJ 2023, 11, e16149. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology—An Update of Promising Treatment Options. Molecules 2022, 27, 3209. [Google Scholar] [CrossRef] [PubMed]
- Curtasu, M.V.; Nørskov, N.P. Quantitative distribution of flavan-3-ols, procyanidins, flavonols, flavanone and salicylic acid in five varieties of organic winter dormant Salix spp. by LC-MS/MS. Heliyon 2024, 10, e25129. [Google Scholar] [CrossRef]
- Brook, K.; Bennett, J.; Desai, S.P. The Chemical History of Morphine: An 8000-year Journey, from Resin to de-novo Synthesis. J. Anesth. Hist. 2017, 3, 50–55. [Google Scholar] [CrossRef]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Shinkai, R.S.; de Campos, T.T.; Mendes, L.S.; Katekawa, L.; Michel-Crosato, E.; Biazevic, M.G. Phytotherapy: Knowledge, experience and prescription in oral healthcare. Acta Odontol. Latinoam. 2023, 36, 140–149. [Google Scholar] [CrossRef]
- Salm, S.; Rutz, J.; Van Den Akker, M.; Blaheta, R.A.; Bachmeier, B.E. Current state of research on the clinical benefits of herbal medicines for non-life-threatening ailments. Front. Pharmacol. 2023, 14, 1234701. [Google Scholar] [CrossRef]
- Panossian, A. Challenges in phytotherapy research. Front. Pharmacol. 2023, 14, 1199516. [Google Scholar] [CrossRef]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st Century Natural Product Research and Drug Development and Traditional Medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, B. The geranium genus: A comprehensive study on ethnomedicinal uses, phytochemical compounds, and pharmacological importance. Saudi J. Biol. Sci. 2024, 31, 103940. [Google Scholar] [CrossRef]
- Bemowska-Kałabun, O.; Bogucka, A.; Wiłkomirski, B.; Wierzbicka, M. Survival on railway tracks of Geranium robertianum—A glyphosate-tolerant plant. Ecotoxicol. Lond. Engl. 2021, 30, 1186–1202. [Google Scholar] [CrossRef]
- Bawish, B.M.; Rabab, M.A.; Gohari, S.T.; Khattab, M.S.; AbdElkader, N.A.; Elsharkawy, S.H.; Ageez, A.M.; Zaki, M.M.; Kamel, S.; Ismail, E.M. Promising effect of Geranium robertianum L. leaves and Aloe vera gel powder on Aspirin®-induced gastric ulcers in Wistar rats: Anxiolytic behavioural effect, antioxidant activity, and protective pathways. Inflammopharmacology 2023, 31, 3183–3201. [Google Scholar] [CrossRef] [PubMed]
- USDA Plants Database Plant Profile General. Available online: https://plants.sc.egov.usda.gov/plant-profile/GERO (accessed on 3 February 2025).
- Geranium robertianum L.|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:109269-2 (accessed on 1 February 2025).
- Geranium robertianum subsp. Maritimum Bab. ex H.G.Baker. Available online: https://www.gbif.org/species/6285771 (accessed on 1 February 2025).
- Geranium robertianum subsp. Celticum Ostenf. Available online: https://www.gbif.org/fr/species/2890690 (accessed on 1 February 2025).
- Graça, V.C.; Ferreira, I.C.; Santos, P.F. Phytochemical composition and biological activities of Geranium robertianum L.: A review. Ind. Crops Prod. 2016, 87, 363–378. [Google Scholar] [CrossRef]
- Geranium robertianum (Herb-Robert, Mountain Crane’s-Bill): Go Botany. Available online: https://gobotany.nativeplanttrust.org/species/geranium/robertianum/ (accessed on 1 February 2025).
- Graça, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Carvalho, A.M.; Santos-Buelga, C.; Santos, P.F.; Ferreira, I.C. Chemical characterization and bioactive properties of aqueous and organic extracts of Geranium robertianum L. Food Funct. 2016, 7, 3807–3814. [Google Scholar] [CrossRef]
- Paun, G.; Litescu, S.C.; Neagu, E.; Tache, A.; Radu, G.L. Evaluation of Geranium spp., Helleborus spp. and Hyssopus spp. polyphenolic extracts inhibitory activity against urease and α-chymotrypsin. J. Enzym. Inhib. Med. Chem. 2014, 29, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Fodorea, C.S.; Vlase, L.; Suciu, S.; Tămaş, M.; Leucuţa, S.E. Preliminary HPLC study on some polyphenols of Geranium robertianum L. (Geraniaceae). Rev. Med. Chir. Soc. Med. Nat. Iasi 2005, 109, 174–178. [Google Scholar]
- Jemia, M.; Wannes, W.A.; Ouchikh, O.; Bruno, M.; Kchouk, M.E. Antioxidant Activity of Tunisian Geranium Robertianum L. (Geraniaceae). Nat. Prod. Res. 2013, 27, 1–10. [Google Scholar]
- Okuda, T.; Mori, K.; Hatano, T. The distribution of geraniin and mallotusinic acid in the order geraniales. Phytochemistry 1980, 19, 547–551. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Granica, S.; Zwierzyńska, M.; Stefańska, J.; Schopohl, P.; Melzig, M.F.; Kiss, A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 2014, 155, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Graça, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Ferreira, I.C.; Santos, I.C. Bio-guided fractionation of extracts of Geranium robertianum L.: Relationship between phenolic profile and biological activity. Ind. Crops Prod. 2017, 108, 543–552. [Google Scholar] [CrossRef]
- Pedro, L.G.; Pais, M.S.S.; Scheffer, J.J.C. Composition of the essential oil of Geranium robertianum L. Flavour Fragr. J. 1992, 7, 223–226. [Google Scholar] [CrossRef]
- Radulović, N.; Dekić, M.; Stanković, N.; Ghamari, Z.; Baloglu, E.; Sarker, S.; Tapsell, S.; Soares, D.; Raza, M. Geranium robertianum essential oil: Chemical composition, antimicrobial, antioxidant, and anti-inflammatory activities. Food Chem. Toxicol. 2018, 120, 446–453. [Google Scholar]
- Neagu, E.; Roman, G.P.; Radu, G.L.; Nechifor, G. Concentration of the bioactive principles in Geranium robertianum extracts through membrane procedures (ultrafiltration). J. Rom. Biotechnol. Lett. 2010, 15, 5042–5048. [Google Scholar]
- Igwenyi, I.O.; Elekwa, A.E. Phytochemical analysis and determination of vitamin contents of Geranium robertianum. J. Dent. Med. Sci. 2014, 13, 44–47. [Google Scholar]
- Paun, G.; Neagu, E.; Litescu, S.C.; Rotinberg, P.; Radu, G.L. Application of membrane processes for the concentration of Symphytum officinale and Geranium robertianum extracts to obtain compounds with high anti-oxidative activity. J. Serbian Chem. Soc. 2012, 77, 1191–1204. [Google Scholar] [CrossRef]
- Osiane, A. In vitro screening for antioxidant and antimicrobial properties of three Lebanese medicinal plants crude extracts. Pharmacogn. Res. 2019, 11, 127–133. [Google Scholar]
- Neagu, E.; Paun, G.; Moroeanu, V.; Radu, G.L. Evaluation of antioxidant capacity of Geranium robertianum extracts. Rev. Română De Chim. 2010, 55, 321–325. [Google Scholar]
- Catarino, M.D.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Antioxidant and anti-inflammatory activities of Geranium robertianum L. decoctions. Food Funct. 2017, 8, 3355–3365. [Google Scholar] [CrossRef]
- Papović, O.; Pljevljakušić, D.; Marković, M. Ethnopharmacological application of plants from the family Geraniaceae in the Pirot County, Pirot. Zbornik Radova Pirot. 2021, 46, 43–51. [Google Scholar] [CrossRef]
- Petelka, J.; Plagg, B.; Säumel, I.; Zerbe, S. Traditional medicinal plants in South Tyrol (Northern Italy, Southern Alps): Biodiversity and use. J. Ethnobiol. Ethnomed. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Ilić, M.D.; Marčetić, M.D.; Zlatković, B.K.; Lakušić, B.S.; Kovačević, N.N.; Drobac, M.M. Chemical composition of volatiles of eight Geranium L. species from Vlasina Plateau (South Eastern Serbia). Chem. Biodivers. 2020, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, A. Tannins and other polyphenols in Geranium sylvaticum: Identification, intraplant distribution and biological activity. In Turun Yliopiston Julkaisuja—Annales Universitatis Turkuensi; Yliopisto: Turku, Finland, 2017; Volume 569, ISBN 978-951-29-7050-6. ISSN 2343-3175. [Google Scholar]
- Gilca, M.; Tiplica, G.S.; Salavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other Eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Świątek, Ł.; Wasilewska, I.; Boguszewska, A.; Grzegorczyk, A.; Rezmer, J.; Rajtar, B.; Polz-Dacewicz, M.; Sieniawska, E. Herb Robert’s gift against human diseases: Anticancer and antimicrobial activity of Geranium robertianum L. Pharmaceutics 2023, 15, 1561. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Ljubenkov, I.; Katalinić, V.; Šimat, V. Antioxidant and antimicrobial potential of phenolic metabolites from traditionally used Mediterranean herbs and spices. Foods 2019, 8, 579. [Google Scholar] [CrossRef]
- Graça, V.C.; Ferreira, I.C.F.R.; Santos, P.F. Bioactivity of the Geranium genus: A comprehensive review. Curr. Pharm. Des. 2020, 26, 1838–1865. [Google Scholar] [CrossRef]
- Renda, G.; Celik, G.; Korkmaz, B.; Karaoglu, S.A.; Yayli, N. Antimicrobial activity and analyses of six Geranium L. species with headspace SPME and hydrodistillation. J. Essent. Oil-Bear. Plants 2016, 19, 2003–2016. [Google Scholar] [CrossRef]
- Gębarowska, E.; Politowicz, J.; Szumny, A. Chemical composition and antimicrobial activity of Geranium robertianum L. essential oil. Acta Pol. Pharm. —Drug Res. 2017, 74, 699–705. [Google Scholar]
- Bismarck, D.; Dusold, A.; Heusinger, A.; Müller, E. Antifungal in vitro activity of essential oils against clinical isolates of Malassezia pachydermatis from canine ears: A report from a practice laboratory. Complement. Med. Res. 2020, 27, 143–154. [Google Scholar] [CrossRef]
- Panahi, Y.; Akhavan, A.; Sahebkar, A.; Hosseini, S.M.; Taghizadeh, M.; Akbari, H.; Sharif, M.R.; Imani, S. Investigation of the effectiveness of Syzygium aromaticum, Lavandula angustifolia, and Geranium robertianum essential oils in the treatment of acute external otitis: A comparative trial with ciprofloxacin. J. Microbiol. Immunol. Infect. 2014, 47, 211–216. [Google Scholar] [CrossRef]
- Ramesh, H.; Valan, M.F. Rethinking the use of traditional indigenous medicinal plants for the management of COVID-19 in India: A review. Int. J. Ayurveda Pharm. Res. 2021, 9, 53–63. [Google Scholar] [CrossRef]
- Acquadro, S.; Civra, A.; Cagliero, C.; Marengo, A.; Rittà, M.; Francese, R.; Sanna, C.; Bertea, C.; Sgorbini, B.; Lembo, D.; et al. Punica granatum leaf ethanolic extract and ellagic acid as inhibitors of Zika virus infection. Planta Medica 2020, 86, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Promsong, A.; Chuenchitra, T.; Saipin, K.; Tewtrakul, S.; Panichayupakaranant, P.; Satthakarn, S.; Nittayananta, W. Ellagic acid inhibits HIV-1 infection in vitro: Potential role as a novel microbicide. Oral Dis. 2018, 24, 249–252. [Google Scholar] [CrossRef]
- Shin, M.S.; Kang, E.H.; Lee, Y.I. A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antivir. Res. 2005, 67, 163–168. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of ellagic acid from Rhodiola rosea L. as an anti-Ebola virus entry inhibitor. Viruses 2018, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Kiss, A.K.; Kozłowska-Wojciechowska, M. Anti-hyaluronidase and anti-elastase activity screening of tannin-rich plant materials used in traditional Polish medicine for external treatment of diseases with an inflammatory background. J. Ethnopharmacol. 2011, 137, 937–941. [Google Scholar] [CrossRef]
- Ilić, M.; Samardžić, S.; Kotur-Stevuljević, J.; Ušjak, D.; Milenković, M.; Kovačević, N.; Drobac, M. Polyphenol-rich extracts of Geranium L. species as potential natural antioxidant and antimicrobial agents. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6283–6294. [Google Scholar]
- Yonei, Y.; Yagi, M. Verification of improvement of periorbital wrinkles by using Asakado Skin Care Asakado. Glycative Stress Res. 2023, 10, 27–42. [Google Scholar]
- Neagu, E.; Paun, G.; Constantin, D.; Radu, G.L. Cytostatic activity of Geranium robertianum L. extracts processed by membrane procedures. Arab. J. Chem. 2017, 10, S2547–S2553. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, S.; Liu, Y.; Cao, X.; Zhao, M.; Xiao, J.; Feng, C. Geraniin inhibits cell growth and promoted autophagy-mediated cell death in the nasopharyngeal cancer C666-1 cells. Saudi J. Biol. Sci. 2022, 29, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Kostić, O.; Mataruga, Z.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.E.; Yilmaz, A. Neuroprotective effects of Geranium robertianum L. aqueous extract on the cellular Parkinson’s disease model. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 570–579. [Google Scholar]
- Ferreira, F.M.; Peixoto, F.; Nunes, E.; Sena, C.; Seiça, R.; Santos, M.S. “MitoTea”: Geranium robertianum L. decoctions decrease blood glucose levels and improve liver mitochondrial oxidative phosphorylation in diabetic Goto-Kakizaki rats. Acta Biochim. Pol. 2010, 57, 399–402. [Google Scholar] [CrossRef]
- Hamza, N.; Berke, B.; Umar, A.; Cheze, C.; Gin, H.; Moore, N. A review of Algerian medicinal plants used in the treatment of diabetes. J. Ethnopharmacol. 2019, 238, 111841. [Google Scholar] [CrossRef]
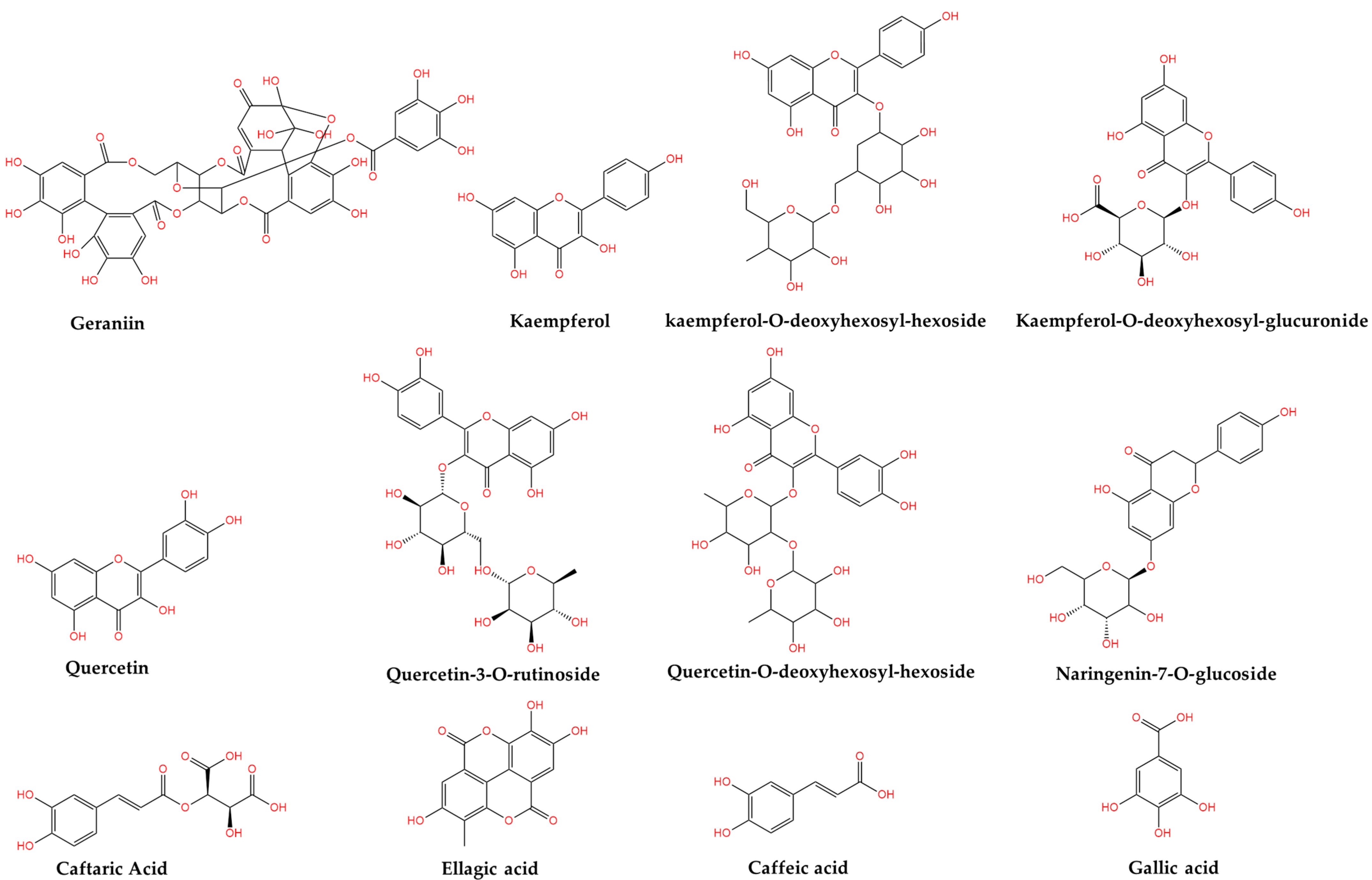
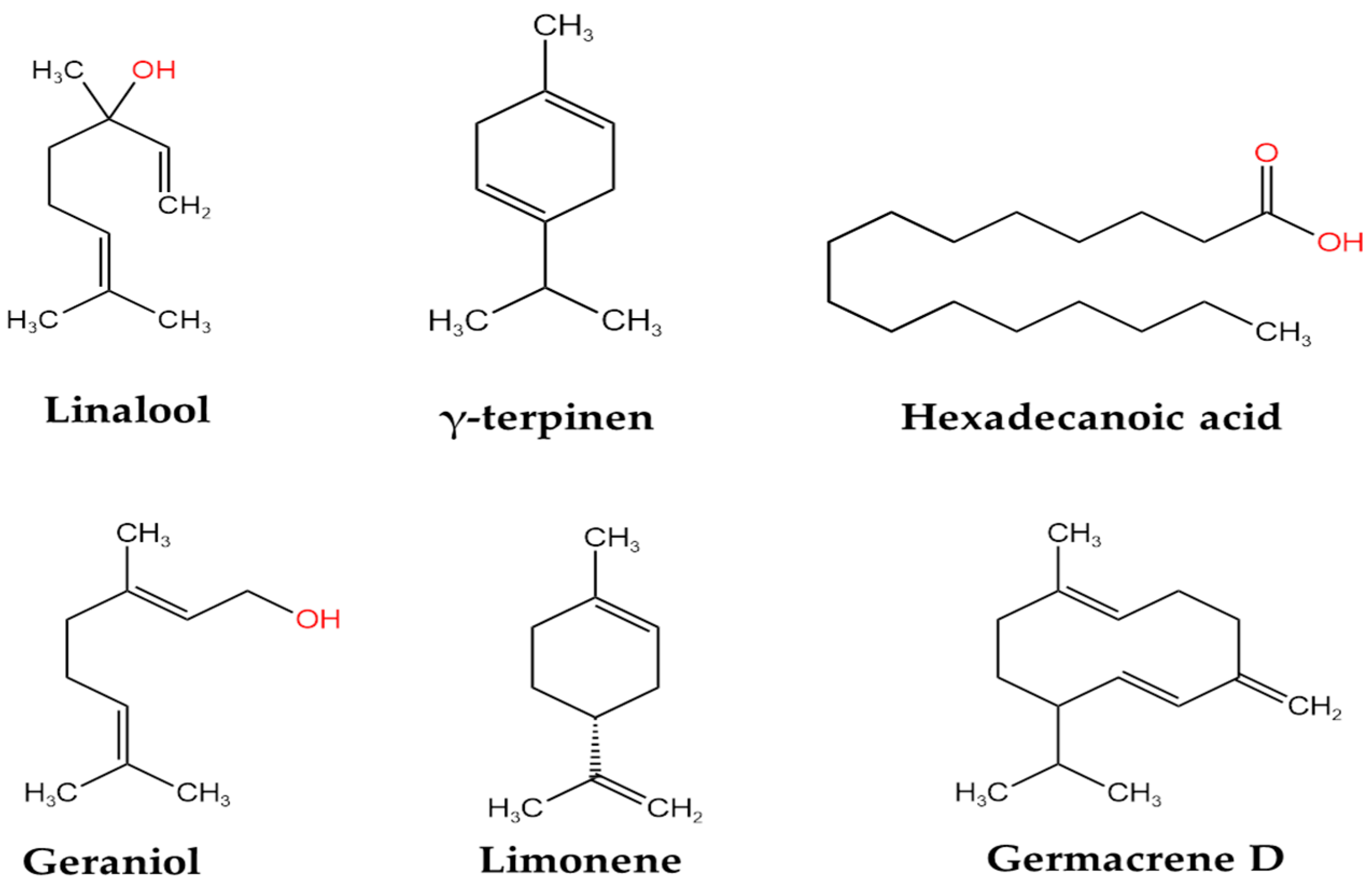

| Study Team and Year | Country | Compound | Concentration | Type of Extract/Plant Material | Method of Analysis |
|---|---|---|---|---|---|
| Okuda et al., 1980 [25] | Japan | Geraniin | 10% of leaves dried weight | acetone extract | HPLC-UV |
| Paun et al., 2012 [22] | Romania | gallic acid ellagic acid | 1070.78 mg/kg 900.13 mg/kg | aqueous extract | HPLC-MS |
| Fodorea et al., 2005 [23] | Romania | ellagic acid | 7599.76 µg/100 mg | alcoholic extract (non-hydrolyzed materials) | HPLC-UV |
| ellagic acid | 10,550.65 µg/100 mg | alcoholic extract (hydrolyzed materials) | |||
| caffeic acid | 6.62 µg/100 mg | ||||
| caftaric acid | 47.41 µg/100 mg | ||||
| Graça et al., 2017 [27] | Portugal | kaempferol-O-deoxyhexosyl-glucuronide | 4.78 ± 0.11 mg/g | methanol extract | HPLC-DAD- ESI/MS |
| kaempferol-O-deoxyhexosyl-hexoside | 2.61 ± 0.07 mg/g | acetone extract | |||
| kaempferol-O-deoxyhexosyl-glucuronid | 2.32 ± 0.05 mg/g | ethyl acetate extract | |||
| quercetin-3-O-rutinoside | 3.39 ± 0.06 mg/g | acetone extract | |||
| quercetin-O-deoxyhexosyl-hexoside | 1.71 ± 0.02 mg/g | methanol extract | |||
| quercetin-O-deoxyhexosyl-glucuronide | 0.9421 ± 0.0004 mg/g | ethyl acetate extract | |||
| Radulovic’ et al., 2012 [29] | Serbia | hexadecanoic acid | 45.3% | diethyl ether extract from the underground part diethyl ether extract from the aerial part | GC-MS |
| Pentacosane | 28.5% | ||||
| hexadecanoic acid | 16.6% | ||||
| hexahydrofarnesyl acetone | 6.5% | ||||
| caryophyllene oxide | 5.4% |
| Extract Type | Mg (mg/L) | Mn (mg/L) | Fe (mg/L) | Ca (mg/L) | Zn (mg/L) |
|---|---|---|---|---|---|
| Aqueous extract | 10.40 ± 0.3 | 0.893 ± 0.07 | 3.2 ± 0.1 | 0.935 ± 0.08 | 0.071 ± 0.006 |
| Hydro-alcoholic (50/50) extract | 9.78 ± 0.7 | 0.819 ± 0.07 | 1.8 ± 0.1 | 0.927 ± 0.08 | 0.069 ± 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haj Ali, D.; Dărăban, A.M.; Ungureanu, D.; Căta, A.; Ienașcu, I.M.C.; Dinu, S.; Dehelean, C.A.; Danciu, C. An Up-to-Date Review Regarding the Biological Activity of Geranium robertianum L. Plants 2025, 14, 918. https://doi.org/10.3390/plants14060918
Haj Ali D, Dărăban AM, Ungureanu D, Căta A, Ienașcu IMC, Dinu S, Dehelean CA, Danciu C. An Up-to-Date Review Regarding the Biological Activity of Geranium robertianum L. Plants. 2025; 14(6):918. https://doi.org/10.3390/plants14060918
Chicago/Turabian StyleHaj Ali, Diana, Adriana Maria Dărăban, Diana Ungureanu, Adina Căta, Ioana Maria Carmen Ienașcu, Stefania Dinu, Cristina Adriana Dehelean, and Corina Danciu. 2025. "An Up-to-Date Review Regarding the Biological Activity of Geranium robertianum L." Plants 14, no. 6: 918. https://doi.org/10.3390/plants14060918
APA StyleHaj Ali, D., Dărăban, A. M., Ungureanu, D., Căta, A., Ienașcu, I. M. C., Dinu, S., Dehelean, C. A., & Danciu, C. (2025). An Up-to-Date Review Regarding the Biological Activity of Geranium robertianum L. Plants, 14(6), 918. https://doi.org/10.3390/plants14060918








