Modification in the Composition of Lactuca sativa L. Plants Exposed to Abiotic Stress Induced by Commonly Used Antibiotics
Abstract
1. Introduction
2. Results and Discussion
2.1. Modification in the Composition of Lactuca sativa L. Plants Exposed to Antibiotic Treatments
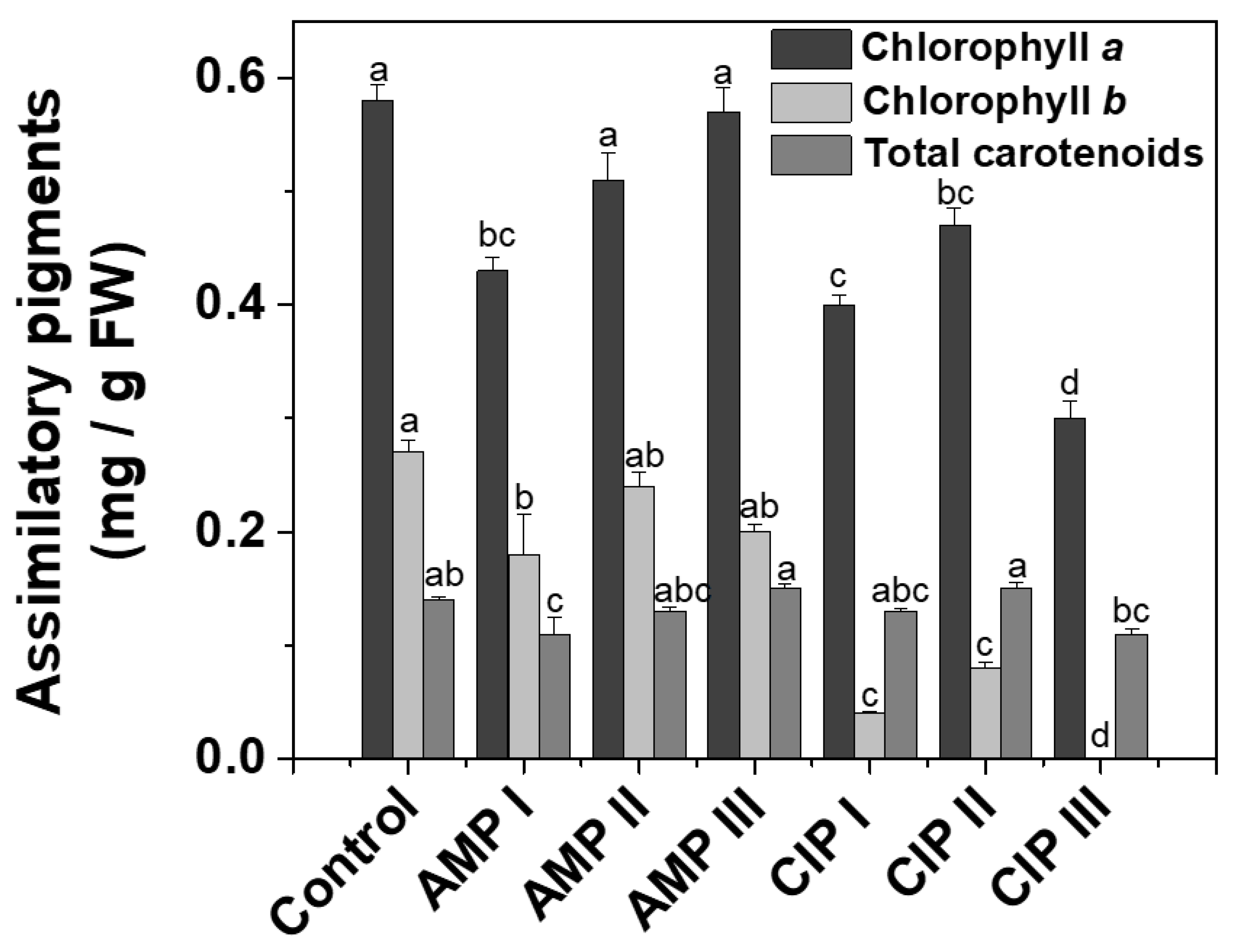
2.1.1. Assimilatory Pigments
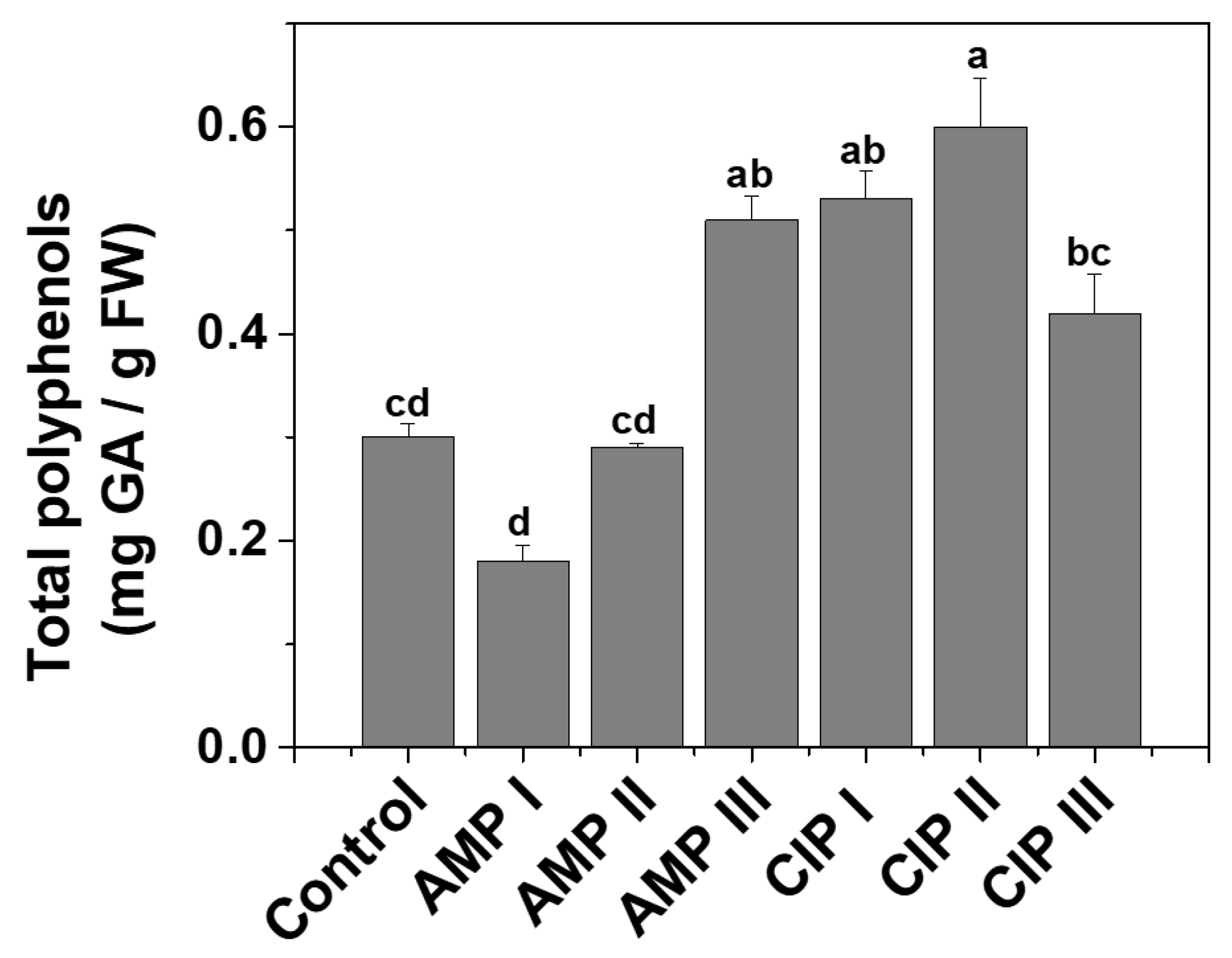
2.1.2. Total Polyphenols
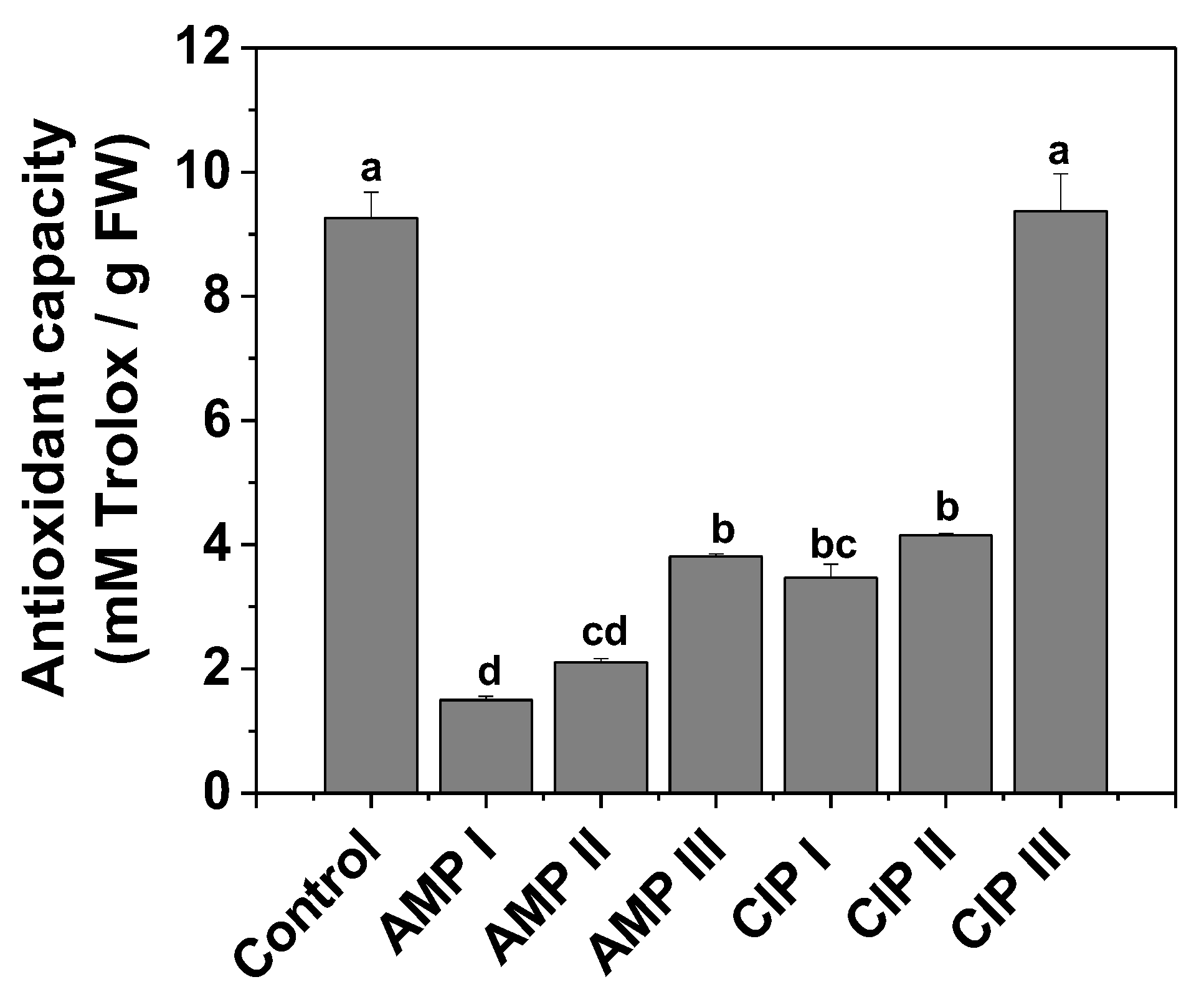
2.1.3. Antioxidant Capacity as DPPH Radical Bleaching
2.2. Variation of the Bioactive Compounds and Antioxidant Capacity in Treated Lactuca sativa L. Plants Relative to Control
2.3. Elemental Composition of Lactuca sativa L. Plants
2.4. Chemometric Evaluation of Antibiotics on the Composition of Lactuca sativa L.
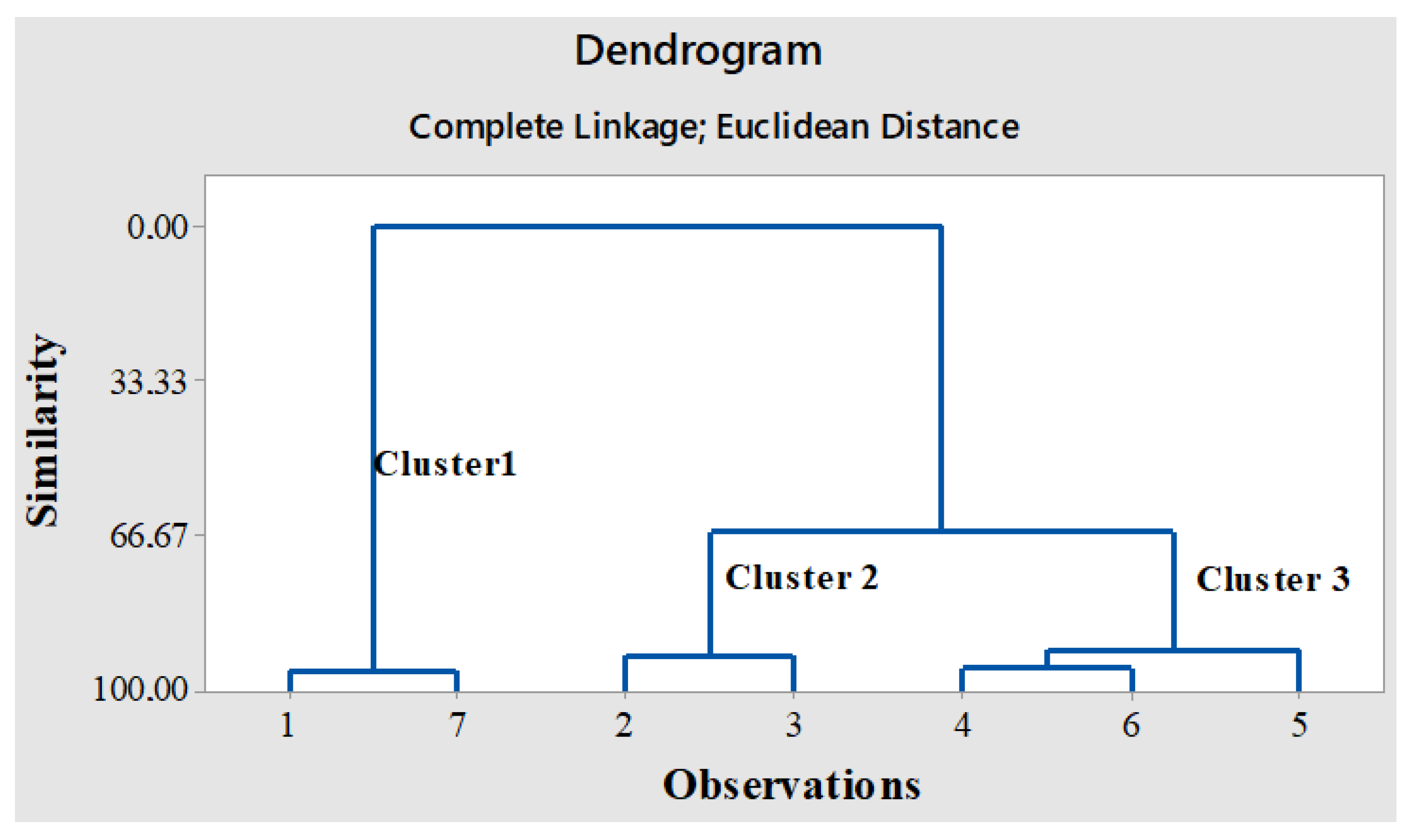
2.4.1. Hierarchical Cluster Analysis (HCA)
2.4.2. Principal Component Analysis
3. Materials and Methods
3.1. Reagents and Materials
3.2. Conditions for Plant Growth
3.3. Determination of the Bioactive Compounds and Antioxidant Capacity
3.3.1. Measurement of Chlorophyll and Carotenoid Content
3.3.2. Obtaining and Evaluating Total Polyphenols
3.3.3. Assessment of Antioxidant Capacity Using the DPPH Method
3.4. Determination of the Elemental Content in Lactuca sativa L. Plants
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Minden, V.; Schnetger, B.; Pufal, G.; Leonhardt, S.D. Antibiotic- induced effects on scaling relationships and on plant element contents in herbs and grasses. Ecol. Evol. 2018, 8, 6699–6713. [Google Scholar] [CrossRef] [PubMed]
- Danilova, N.; Galieva, G.; Kuryntseva, P.; Selivanovskaya, S.; Galitskaya, P. Influence of the Antibiotic Oxytetracycline on the Morphometric Characteristics and Endophytic Bacterial Community of Lettuce (Lactuca sativa L.). Microorganisms 2023, 11, 2828. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Davis, W. Meeting the goal of biological integrity in water-resource programs in the US Environmental Protection Agency. J. N. Am. Benthol. Soc. 1994, 13, 592–597. [Google Scholar] [CrossRef]
- Noumedem, J.A.K.; Djeussi, D.E.; Hritcu, L.; Mihasan, M.; Kuete, V. Lactuca sativa. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 437–449. [Google Scholar]
- Azanu, D.; Mortey, C.; Darko, G.; Weisser, J.J.; Styrishave, B.; Abaidoo, R.C. Uptake of antibiotics from irrigation water by plants. Chemosphere 2016, 157, 107–114. [Google Scholar] [CrossRef]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and accumulative pattern of tetracyclines and sulfonamides in edible vegetables of cucumber, tomato, and lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef]
- Choe, H.; Kantharaj, V.; Lee, K.-A.; Shin, Y.; Chohra, H.; Yoon, Y.-E.; Kim, Y.-N.; Lee, Y.B. Morpho-physiological, biochemical, and molecular responses of lettuce (Lactuca sativa L.) seedlings to chlortetracycline stress. Environ. Exp. Bot. 2024, 219, 105615. [Google Scholar] [CrossRef]
- Wahid, F.; Baig, S.; Bhatti, M.F.; Manzoor, M.; Ahmed, I.; Arshad, M. Growth responses and rubisco activity influenced by antibiotics and organic amendments used for stress alleviation in Lactuca sativa. Chemosphere 2021, 264, 128433. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of ciprofloxacin from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, L.; Monduzzi, M.; Salis, A. Adsorption and release of ampicillin antibiotic from ordered mesoporous silica. J. Colloid Interface Sci. 2017, 497, 217–225. [Google Scholar] [CrossRef]
- Pawłowska, B.; Biczak, R. Drugs in the environment—Impacts on plants: A review. Environ. Toxicol. Pharmacol. 2024, 111, 104557. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity of amoxicillin to the duckweed Spirodela polyrhiza: Growth, oxidative stress, biochemical traits and antibiotic degradation. Chemosphere 2018, 201, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Das, A.; Dave, R.; Bhattacharya, B.K. A non-destructive estimation of chlorophyll-a and -b over different crops using airborne imaging spectroscopy observations. Adv. Space Res. 2024, 73, 1290–1303. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Havaux, M.; Tardy, F.; Lemoine, Y. Photosynthetic light-harvesting function of carotenoids in higher-plant leaves exposed to high light irradiances. Planta 1998, 205, 242–250. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Sharma, K.R.; Agrawal, M.; Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef]
- Silveira, G.L.; Lima, M.G.; Reis, G.B.; Palmieri, M.J.; Andrade-Vieria, L.F. Toxic effects of environmental pollutants: Comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 2017, 178, 359–367. [Google Scholar] [CrossRef]
- Mirza, H.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between macro- and micro-nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Li, F.; Wenzel-Storjohann, A.; Sulieman, S.; Tasdemir, D.; Mühling, K.H. Comparative metabolite profile, biological activity and overall quality of three lettuce (Lactuca sativa L.; Asteraceae) cultivars in response to sulfur nutrition. Pharmaceutics 2021, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Otsuki, H.; Narisawa, T.; Kobayashi, M.; Sawai, S.; Kamide, Y.; Kusano, M.; Aoki, T.; Hirai, M.Y.; Saito, K. A new class of plant lipid is essential for protection against phosphorus depletion. Nat. Commun. 2013, 4, 1510. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.R.M.; Ferreira, I.M.P.L.V.O. Changes in macrominerals, trace elements and pigments content during lettuce (Lactuca sativa L.) growth: Influence of soil composition. Food Chem. 2014, 152, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Sârbu, C.; Nașcu-Briciu, R.D.; Kot-Wasik, A.; Gorinstein, S.; Wasik, A.; Namiesnik, J. Classification and fingerprinting of kiwi and pomelo fruits by multivariate analysis of chromatographic and spectroscopic data. Food Chem. 2012, 130, 94–1002. [Google Scholar] [CrossRef]
- Casoni, D.; Sârbu, C. Comprehensive evaluation of antioxidant activity: A chemometric approach using principal component analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 343–348. [Google Scholar] [CrossRef]
- Jeong, S.W.; Kim, G.S.; Lee, W.S.; Kim, Y.H.; Kang, N.J.; Jin, J.S.; Lee, G.M.; Kim, S.T.; Abd El-Aty, A.M.; Shim, J.H.; et al. The effects of different night-time temperatures and cultivation durations on the polyphenolic contents of lettuce: Application of principal component analysis. J. Adv. Res. 2015, 6, 493–499. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Opriș, O.; Lung, I.; Soran, M.L.; Copolovici, L.; Copolovici, D.M.; Costa, M.C. Impact assessment of paracetamol on Phaseolus vulgaris L. and Triticum aestivum L. plants. Rev. Chim. 2020, 71, 549–557. [Google Scholar] [CrossRef]
- Soran, M.-L.; Sîrb, A.N.; Lung, I.; Opriș, O.; Culicov, O.; Stegarescu, A.; Nekhoroshkov, P.; Gligor, D.-M. A multi-method approach for impact assessment of some heavy metals on Lactuca sativa L. Molecules 2023, 28, 759. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Current Protocols in Food Analytical Chemistry; Units: F4.3.1–F4.3.8; John Wiley & Sons Inc.: New York, NY, USA, 2001. [Google Scholar]
- Ivanova, V.; Stefova, M.; Chinnici, F. Determination of the polyphenol contents in Macedonian grapes and wines by standardized spectrophotometric methods. J. Serbian Chem. Soc. 2010, 75, 45–59. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
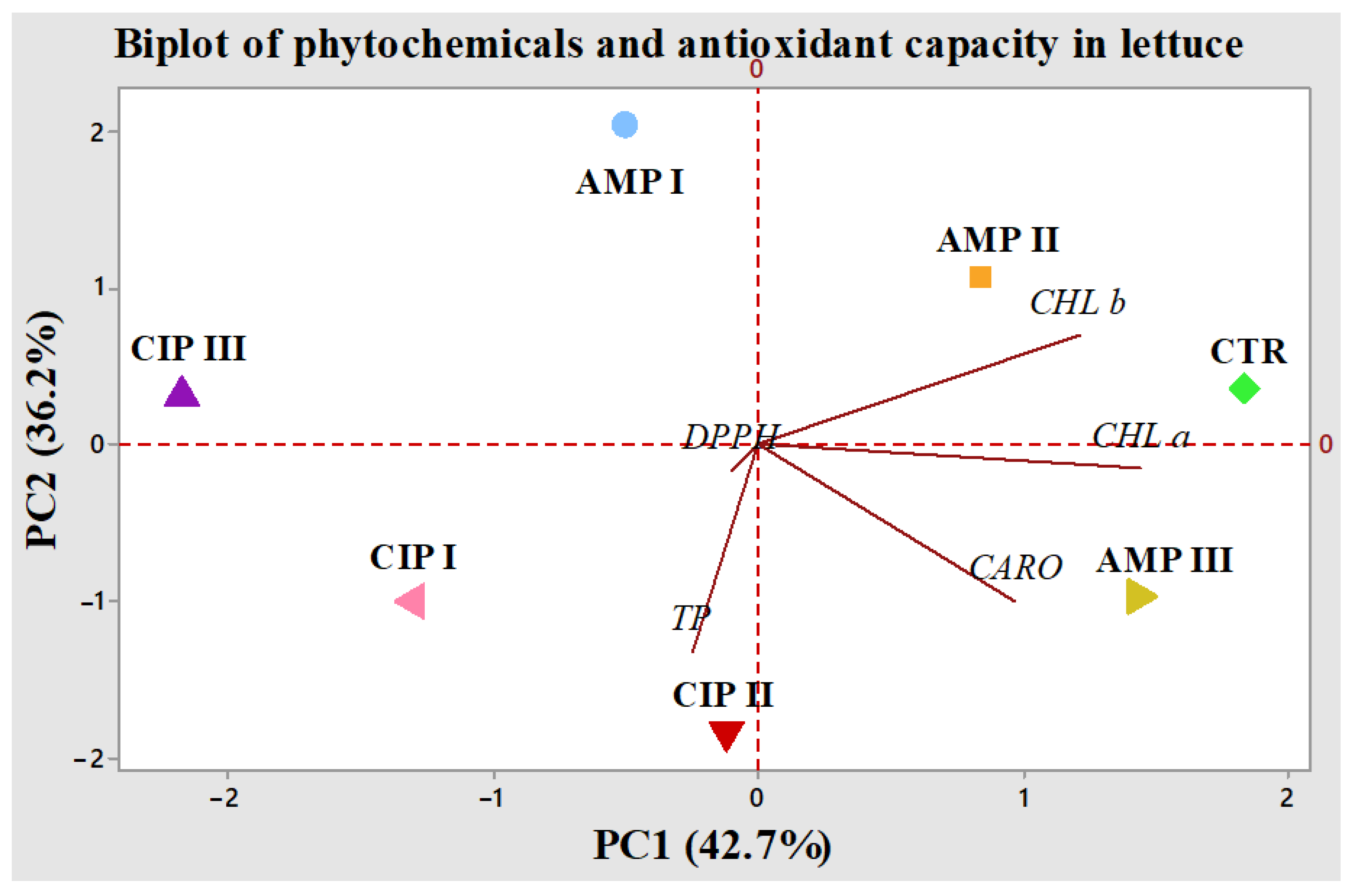
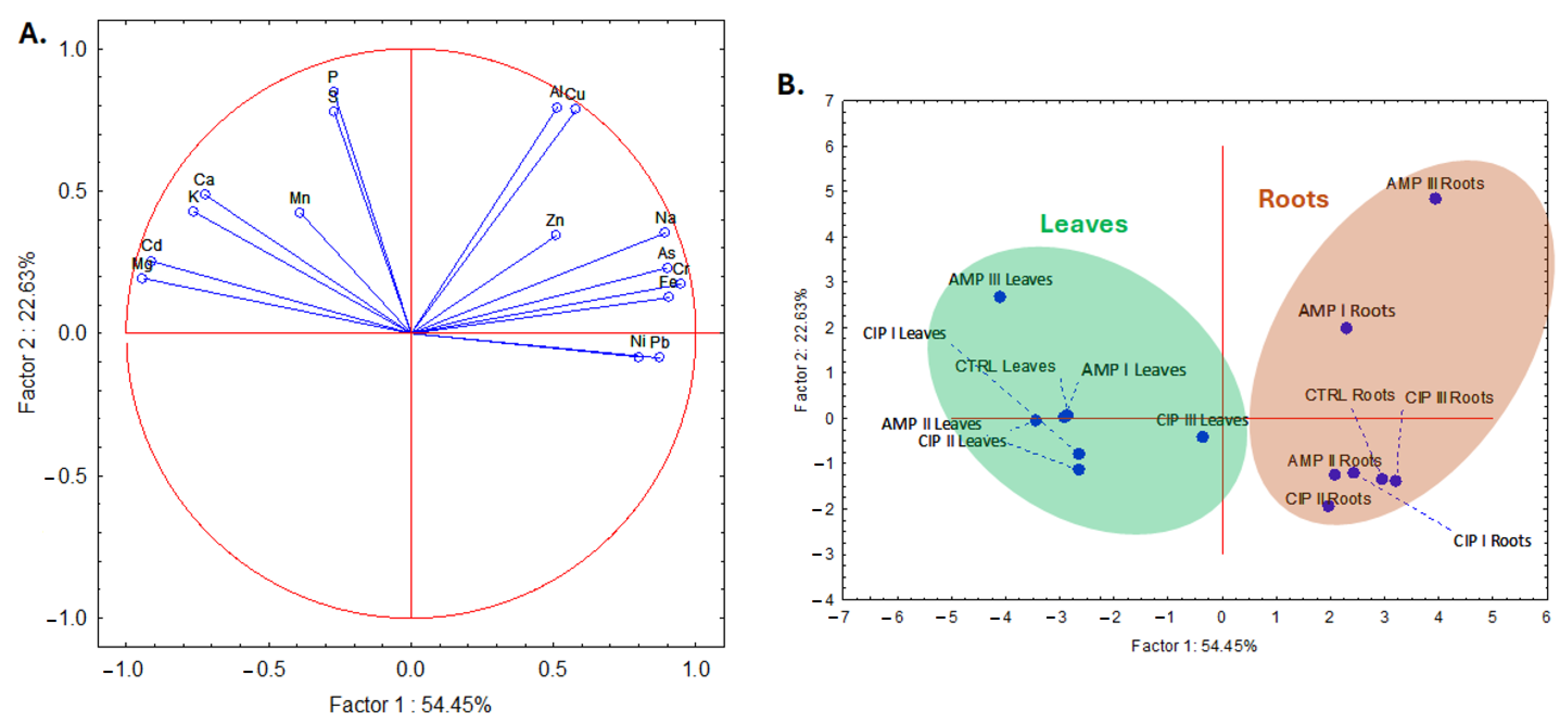
| Treatment | Chlorophyll a | Chlorophyll b | Total Carotenoids | Total Polyphenols | Antioxidant Capacity |
|---|---|---|---|---|---|
| AMP I | −0.25965 | −0.33254 | −0.26195 | −0.41667 | −0.83772 |
| AMP II | −0.12963 | −0.10701 | −0.11536 | −0.05165 | −0.77261 |
| AMP III | −0.02181 | −0.24213 | 0.05312 | 0.67884 | −0.58888 |
| CIP I | −0.30192 | −0.85292 | −0.09598 | 0.74119 | −0.62583 |
| CIP II | −0.18758 | −0.69982 | 0.04893 | 0.96855 | −0.55188 |
| CIP III | −0.47429 | −0.97569 | −0.24118 | 0.37449 | 0.0113 |
| Elemental Content (mg kg−1) ± SE | Treatment Applied | ||||||
|---|---|---|---|---|---|---|---|
| Control | AMP I | AMP II | AMP III | CIP I | CIP II | CIP III | |
| Mn | 140.43 a ± 7.71 | 90.29 ab ± 1.37 | 36.78 c ± 15.73 | 105.79 ab ± 14.65 | 59.28 bc ± 1.58 | 140.24 a ± 15.30 | 59.23 bc ± 5.11 |
| Al | 2107.88 a ± 1163.25 | 318.99 a ± 128.15 | 110.43 a ± 17.34 | 1252.88 a ± 598.30 | 527.26 a ± 191.48 | 109.03 a ± 27.97 | 102.57 a ± 9.36 |
| Cu | 13.38 ab ± 0.39 | 6.01 ab ± 1.34 | 3.26 b ± 0.25 | 21.69 a ± 8.41 | 5.07 b ± 1.52 | 3.89 b ± 0.83 | 2.67 b ± 0.55 |
| Zn | 128.26 a ± 15.48 | 130.41 a ± 7.24 | 102.01 a ± 0.03 | 138.56 a ± 13.87 | 101.10 a ± 10.06 | 101.13 a ± 2.41 | 98.31 a ± 2.46 |
| Fe | 163.01 b ± 22.76 | 110.56 c ± 4.72 | 114.71 bc ± 4.87 | 162.61 bc ± 10.78 | 125.06 bc ± 10.22 | 135.06 bc ± 0.75 | 546.32 a ± 4.12 |
| Pb | 0.54 c ± 0.06 | 0.70 bc ± 0.07 | 1.30 bc ± 0.07 | 0.63 bc ± 0.12 | 2.33 b ± 0.80 | 1.23 bc ± 0.51 | 5.46 a ± 0.07 |
| Cd | 1.01 ab ± 0.05 | 0.93 ab ± 0.03 | 0.812 ab ± 0.02 | 1.20 a ± 0.14 | 0.91 ab ± 0.01 | 0.99 ab ± 0.01 | 0.73 b ± 0.16 |
| As | 0.44 bc ± 0.01 | 0.44 bc ± 0.005 | 0.33 c ± 0.02 | 0.57 b ± 0.07 | 0.38 c ± 0.01 | 0.43 bc ± 0.01 | 1.01 a ± 0.01 |
| Cr | 1.44 b ± 0.11 | 0.88 cd ± 0.03 | 0.99 cd ± 0.09 | 1.28 bc ± 0.14 | 0.84 cd ± 0.03 | 0.83 d ± 0.01 | 5.30 a ± 0.13 |
| Ni | 1.07 b ± 0.01 | 0.66 b ± 0.001 | 1.17 b ± 0.10 | 0.96 b ± 0.13 | 0.60 b ±0.04 | 0.56 b ± 0.01 | 20.82 a ± 0.60 |
| Na | 5597.06 b ± 202.64 | 4779.76 bc ± 186.92 | 3325.60 d ± 225.70 | 5565.07 b ± 327.92 | 3643.17 cd ± 132.44 | 3729.63 cd ± 390.51 | 8841.50 a ± 118.74 |
| K | 142,885.34 b ± 3944.14 | 182,686.34 a ± 5816.71 | 117,905.34 b ± 261.03 | 182,045.24 a ± 14,100.03 | 125,965.59 b ± 8809.50 | 129,186.64 b ± 10365.38 | 125,904.20 b ± 2159.47 |
| Mg | 5720.66 ab ± 138.56 | 5172.65 b ± 268.87 | 4708.73 b ± 72.10 | 7028.67 a ± 899.51 | 4961.66 b ± 28.98 | 5266.66 ab ± 27.69 | 4094.16 b ± 4.91 |
| Ca | 20,958.74 a ± 1719.81 | 18,946.72 a ± 1429.18 | 23,740.70 a ± 2642.03 | 25,886.70 a ± 3334.29 | 21,266.70 a ± 2938.78 | 23,966.70 a ± 2026.29 | 20,556.31 a ± 212.96 |
| P | 6018.00 c ± 28.11287 | 8142.00 ab ± 253.28 | 6159.50 c ± 6.64 | 9055.00 a ± 863.50 | 7009.50 bc ± 34.34 | 6604.00 bc ± 142.97 | 7426.66 abc ± 162.91 |
| S | 2978.3 b ± 100.17 | 2917.8 b ± 61.52 | 3146.30 b ± 27.07 | 3995.30 a ± 336.77 | 3161.30 b ± 21.36 | 3215.30 b ± 79.48 | 3275.30 b ± 50.96 |
| Elemental Content (mg kg−1 FW) ± SE | Treatment Applied | ||||||
|---|---|---|---|---|---|---|---|
| Control | AMP I | AMP II | AMP III | CIP I | CIP II | CIP III | |
| Mn | 78.97 a ± 18.90 | 75.87 a ± 26.72 | 37.52 a ± 6.91 | 101.44 a ± 11.39 | 62.87 a ± 26.10 | 68.04 a ± 17.46 | 79.92 a ± 20.61 |
| Al | 1547.37 a ± 439.68 | 5764.38 a ± 4079.54 | 899.56 a ± 257.93 | 12998.65 a ± 11325.61 | 1605.50 a ± 575.59 | 1244.50 a ± 544.21 | 1402.35 a ± 361.92 |
| Cu | 16.19 a ± 1.10 | 77.89 a ± 47.10 | 20.08 a ± 4.08 | 131.99 a ± 112.71 | 26.68 a ± 7.35 | 18.18 a ± 0.33 | 25.05 a ± 6.76 |
| Zn | 172.14 a ± 14.96 | 160.31 a ± 19.33 | 138.09 a ± 18.59 | 148.73 a ± 49.58 | 139.01 a ± 35.92 | 129.09 a ± 22.72 | 107.13 a ± 9.89 |
| Fe | 1003.93 a ± 251.94 | 893.97 a ± 243.30 | 481.91 a ± 153.54 | 1254.12 a ± 200.17 | 1415.02 a ± 898.05 | 619.92 a ± 197.01 | 1060.94 a ± 262.39 |
| Pb | 5.59 a ± 2.35 | 4.21 a ± 0.66 | 11.06 a ± 7.88 | 9.30 a ± 5.57 | 6.35 a ± 2.58 | 8.70 a ± 6.09 | 7.85 a ± 3.87 |
| Cd | 0.37 a ± 0.04 | 0.67 a ± 0.02 | 0.73 a ± 0.23 | 0.55 a ± 0.17 | 0.62 a ± 0.13 | 0.53 a ± 0.19 | 0.41 a ± 0.04 |
| As | 2.13 a ± 0.46 | 2.50 a ± 0.31 | 1.02 a ± 0.25 | 2.14 a ± 0.23 | 1.57 a ± 0.50 | 1.25 a ± 0.26 | 2.01 a ± 0.46 |
| Cr | 14.35 a ± 1.15 | 10.95 a ± 2.42 | 15.92 a ± 3.57 | 22.08 a ± 7.05 | 10.72 a ± 0.71 | 9.96 a ± 0.79 | 17.52 a ± 4.74 |
| Ni | 10.94 a ± 0.46 | 15.82 a ± 7.32 | 18.11 a ± 3.02 | 14.12 a ± 4.51 | 13.92 a ± 3.24 | 8.47 a ± 1.05 | 28.26 a ± 15.17 |
| Na | 12,912.58 a ± 398.59 | 10,126.62 a ± 1511.03 | 10,568.05 a ± 1207.88 | 20,530.33 a ± 8280.66 | 10,174.92 a ± 555.81 | 10,913.44 a ± 1085.41 | 12,559.47 a ± 3552.92 |
| K | 53,530.93 a ± 9493.49 | 64,769.80 a ± 14727.86 | 79,738.69 a ± 2485.69 | 133,132.41 a ± 29853.89 | 80,383.12 a ± 20190.21 | 81,342.37 a ± 22566.38 | 85,063.88 a ± 21431.83 |
| Mg | 3142.59 a ± 507.63 | 2820.96 a ± 370.20 | 2055.60 a ± 319.18 | 2066.94 a ± 130.65 | 2269.33 a ± 696.52 | 1687.07 a ± 226.85 | 1698.66 a ± 191.82 |
| Ca | 17,184.07 a ± 1634.82 | 17,701.92 a ± 2967.58 | 16,506.80 a ± 3385.47 | 21,579.81 a ± 5342.96 | 14,378.03 a ± 2579.51 | 14,366.89 a ± 863.74 | 17,821.67 a ± 1519.71 |
| P | 4685.18 a ± 574.79 | 7642.63 a ± 398.54 | 6033.33 a ± 163.47 | 9087.04 a ± 2484.91 | 5714.54 a ± 987.05 | 5171.82 a ± 1221.39 | 6203.47 a ± 717.95 |
| S | 2751.48 a ± 19.15 | 3797.57 a ± 309.67 | 3115.33 a ± 316.63 | 3544.99 a ± 956.64 | 2913.82 a ± 613.82 | 2744.33 a ± 594.06 | 2487.65 a ± 194.93 |
| Elemental Content (mg kg−1 Soil) ± SE | Treatment Applied | ||||||
|---|---|---|---|---|---|---|---|
| Control | AMP I | AMP II | AMP III | CIP I | CIP II | CIP III | |
| Mg | 1214.59 a ± 264.55 | 971.29 a ± 203.67 | 846.94 a ± 201.77 | 1455.62 a ± 330.53 | 1643.99 a ± 448.53 | 1185.34 a ± 274.10 | 1178.17 a ± 377.37 |
| Ca | 23,555.90 a ± 6614.20 | 17,047.91 a ± 2587.79 | 18,361.22 a ± 3970.23 | 27,037.06 a ± 6555.50 | 31,227.57 a ± 2624.23 | 22,640.52 a ± 2884.21 | 21,594.44 a ± 6270.07 |
| P | 607.08 a ± 93.20 | 600.67 a ± 140.70 | 421.09 a ± 76.29 | 969.31 a ± 99.83 | 816.68 a ± 68.70 | 705.67 a ± 146.46 | 653.70 a ± 94.95 |
| S | 4083.82 a ± 942.25 | 3263.23 a ± 429.96 | 3563.77 a ± 279.95 | 4481.38 a ± 1026.65 | 3766.59 a ± 482.68 | 3699.08 a ± 321.25 | 3391.38 a ± 215.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lung, I.; Soran, M.-L.; Sârb, A.N.; Stegarescu, A.; Moț, A.C.; Ganea, I.-V.; Gligor, D.-M.; Opriș, O. Modification in the Composition of Lactuca sativa L. Plants Exposed to Abiotic Stress Induced by Commonly Used Antibiotics. Plants 2025, 14, 842. https://doi.org/10.3390/plants14060842
Lung I, Soran M-L, Sârb AN, Stegarescu A, Moț AC, Ganea I-V, Gligor D-M, Opriș O. Modification in the Composition of Lactuca sativa L. Plants Exposed to Abiotic Stress Induced by Commonly Used Antibiotics. Plants. 2025; 14(6):842. https://doi.org/10.3390/plants14060842
Chicago/Turabian StyleLung, Ildiko, Maria-Loredana Soran, Aura Nicoleta Sârb, Adina Stegarescu, Augustin C. Moț, Iolanda-Veronica Ganea, Delia-Maria Gligor, and Ocsana Opriș. 2025. "Modification in the Composition of Lactuca sativa L. Plants Exposed to Abiotic Stress Induced by Commonly Used Antibiotics" Plants 14, no. 6: 842. https://doi.org/10.3390/plants14060842
APA StyleLung, I., Soran, M.-L., Sârb, A. N., Stegarescu, A., Moț, A. C., Ganea, I.-V., Gligor, D.-M., & Opriș, O. (2025). Modification in the Composition of Lactuca sativa L. Plants Exposed to Abiotic Stress Induced by Commonly Used Antibiotics. Plants, 14(6), 842. https://doi.org/10.3390/plants14060842






