Synergistic Antibacterial Interaction of Geraniol and Biogenic Silver Nanoparticles on Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Results and Discussion
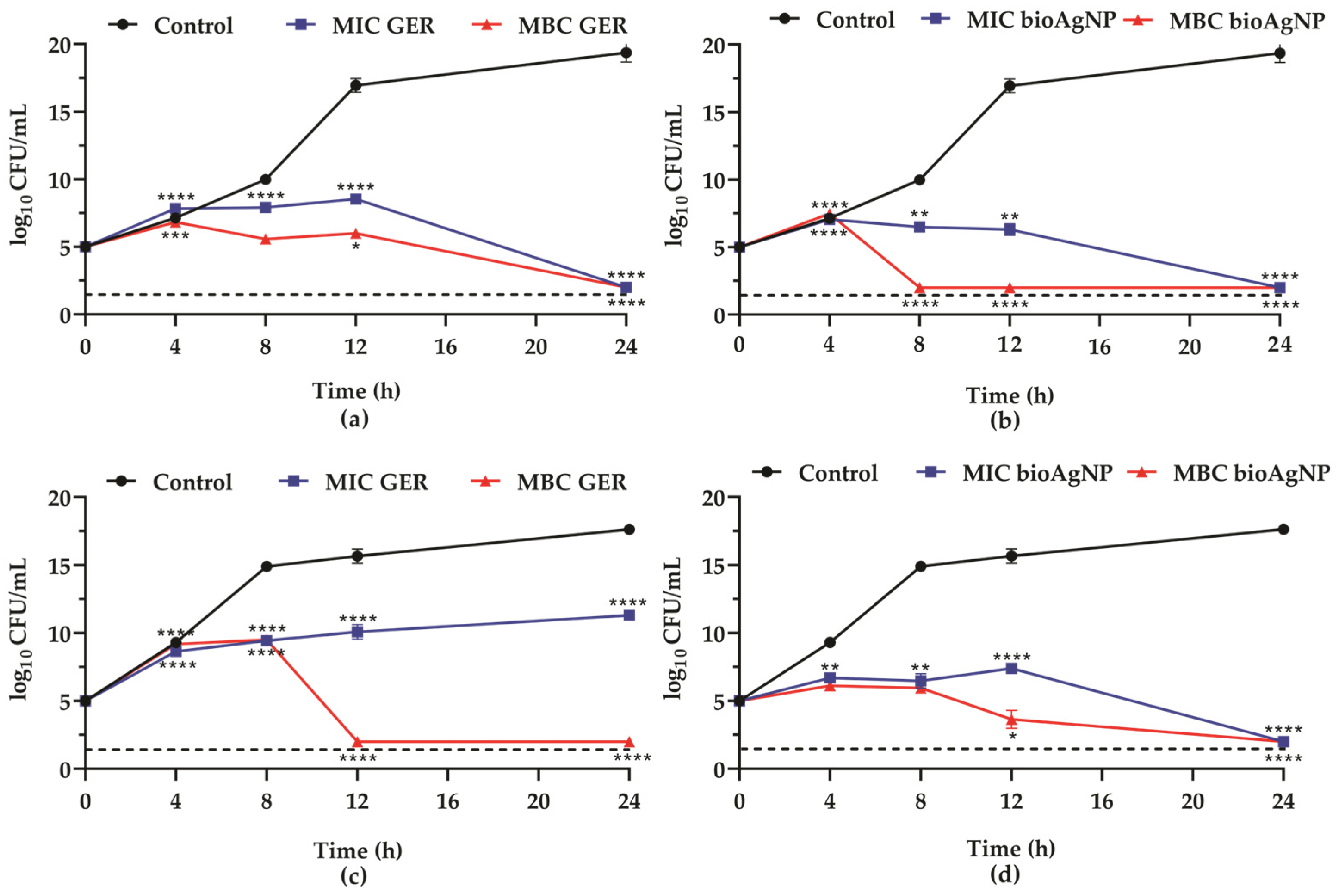
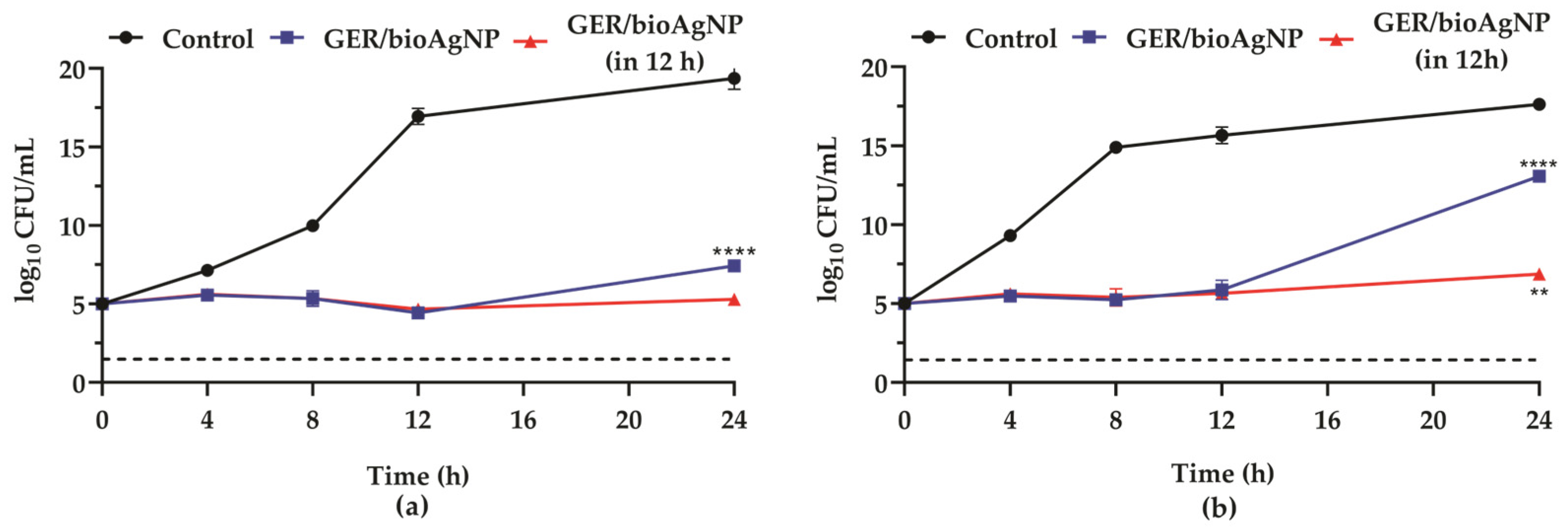
2.1. Geraniol Displays Synergistic Interaction with Biogenic Silver Nanoparticles Against Planktonic Cells of Methicillin-Resistant Staphylococcus aureus
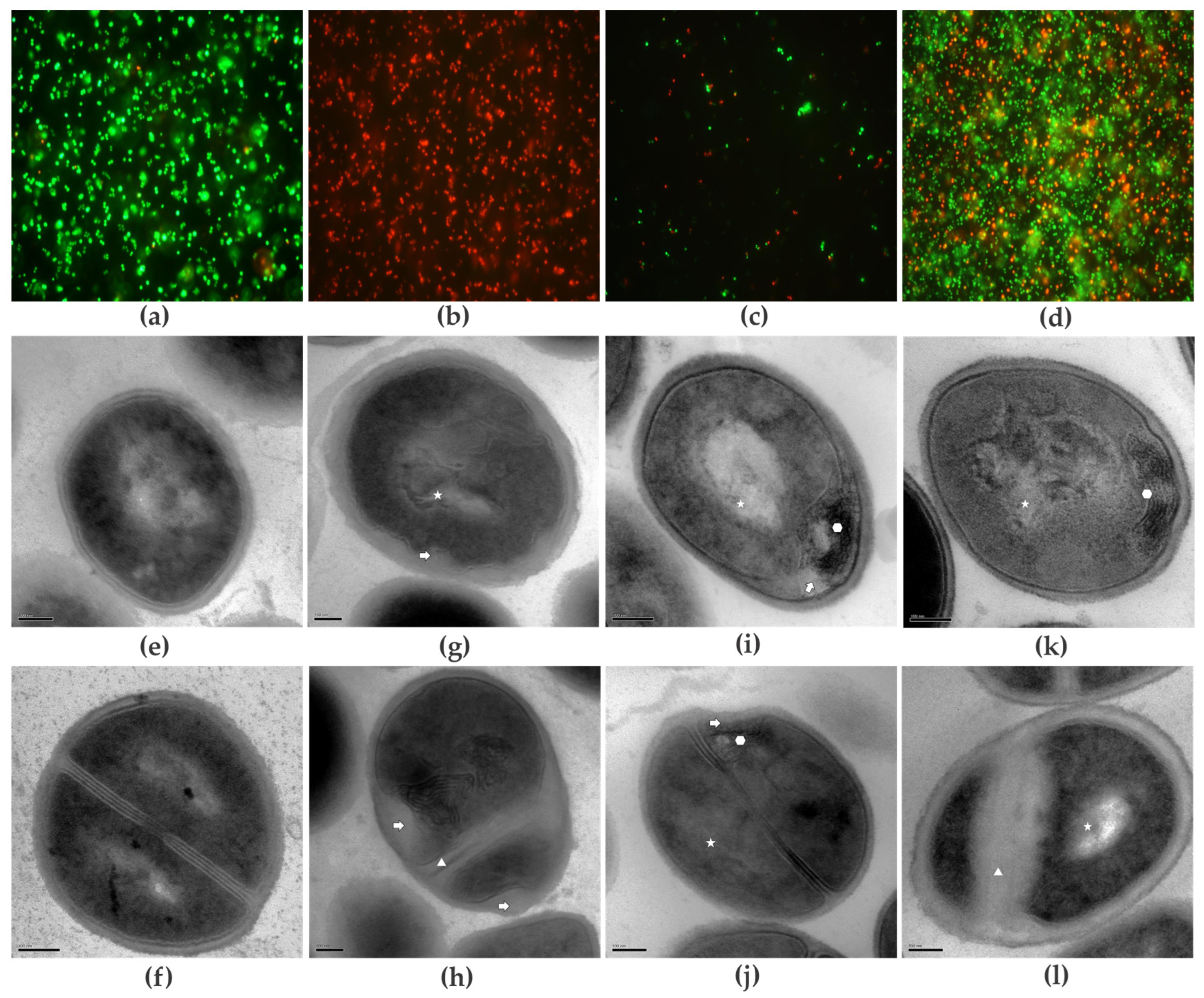
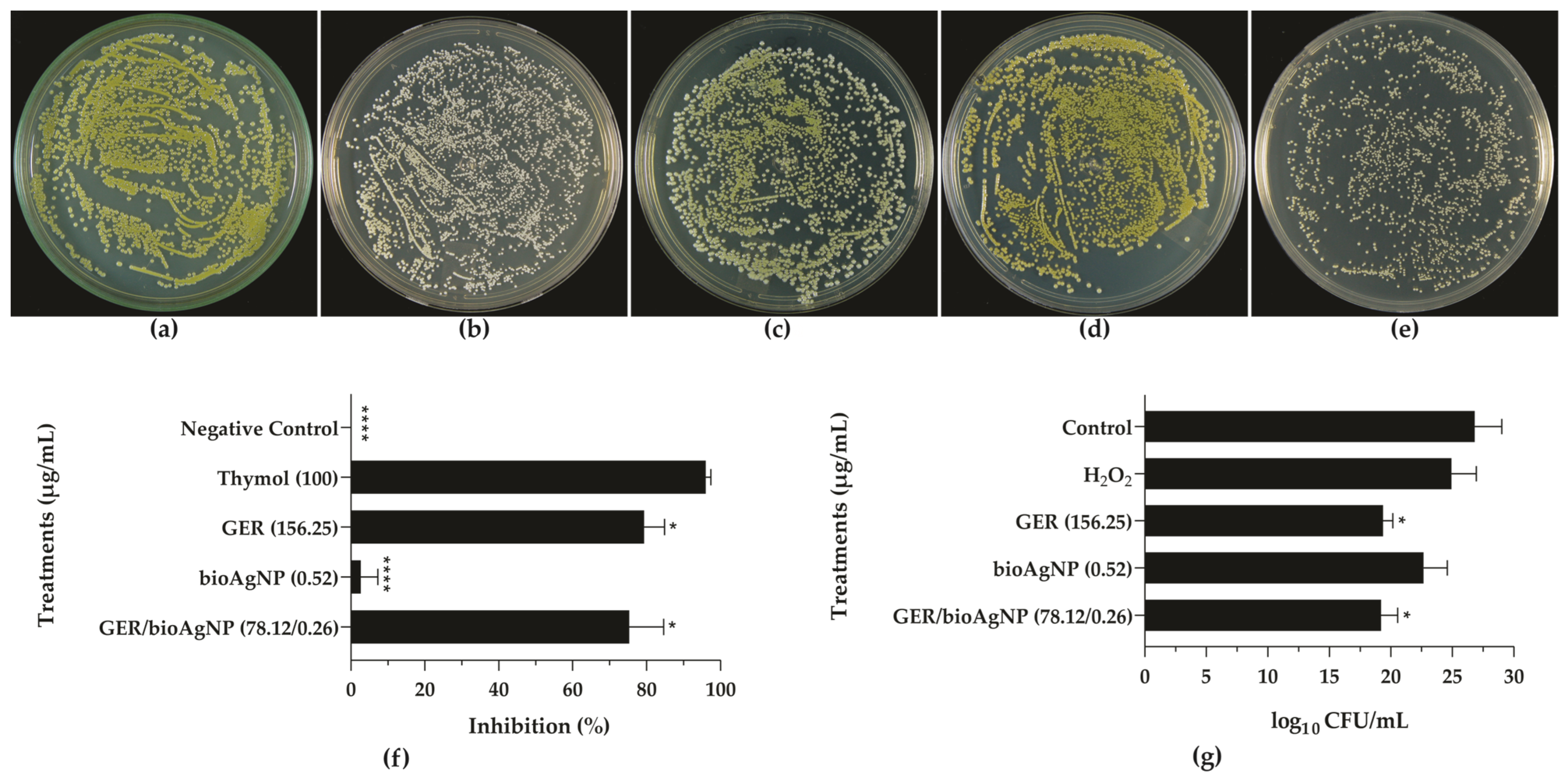
2.2. Geraniol Interferes with the Pigmentation of Methicillin-Resistant Staphylococcus aureus
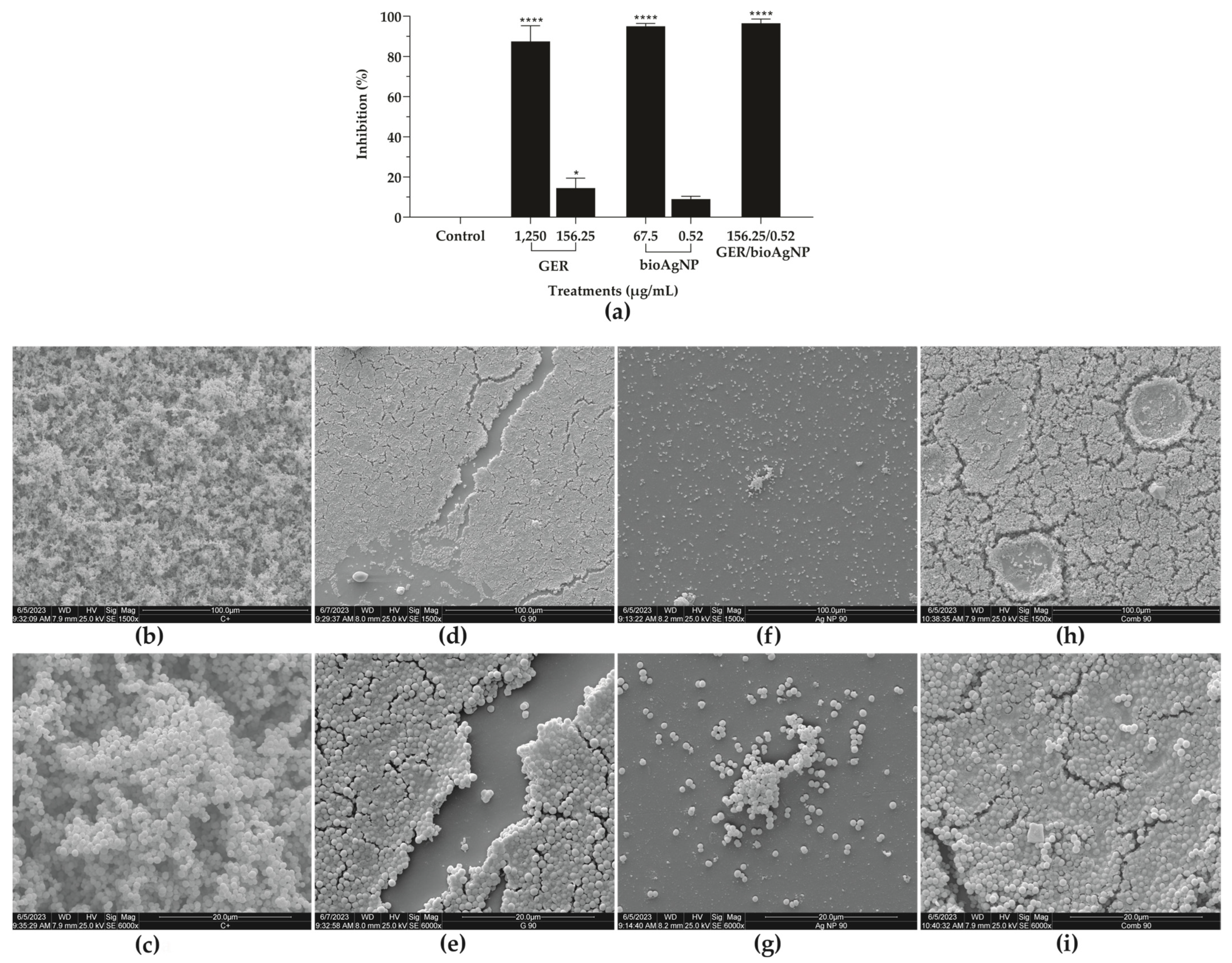
2.3. Geraniol, Alone or Combined with Biogenic Silver Nanoparticles, Inhibits the Adhesion and Biofilm Formation of Methicillin-Resistant Staphylococcus aureus on Abiotic Surfaces
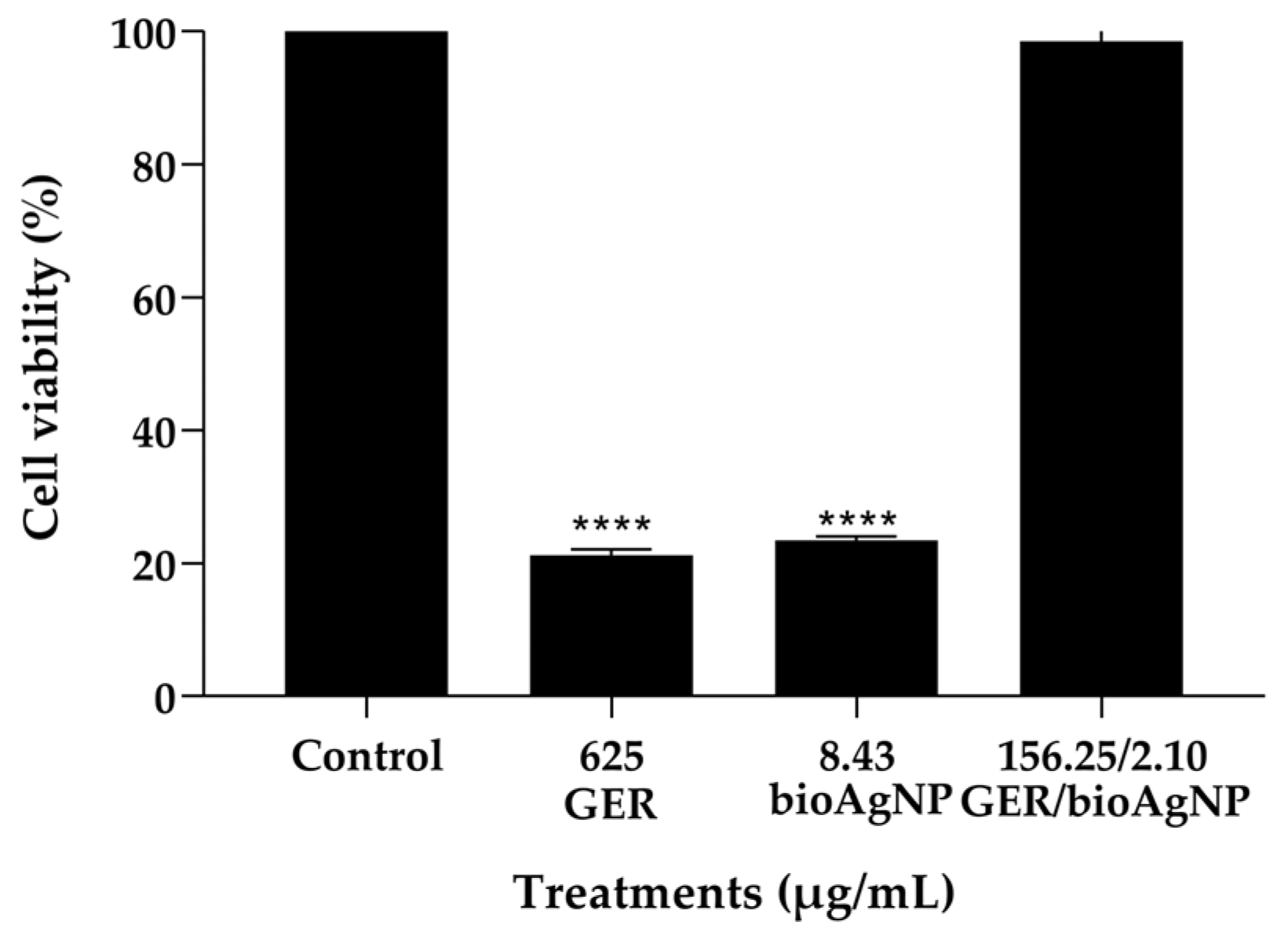
2.4. Geraniol Combined with bioAgNP Does Not Cause Toxicity to HaCat Cells and Galleria mellonella Larvae
3. Materials and Methods
3.1. Chemicals and Culture Media
3.2. Bacterial Strains and Growth Conditions
3.3. Antibacterial Activity Against Planktonic Cells
3.3.1. Minimum Inhibitory (MIC) and Minimum Bactericidal (MBC) Concentrations
3.3.2. Checkerboard Microdilution Assay
3.3.3. Time–Kill Assay
3.4. Mode of Action of Compounds on Planktonic Cells
3.4.1. Cell Viability
3.4.2. Transmission Electron Microscopy (TEM)
3.4.3. Effect on Staphyloxanthin Biosynthesis
3.4.4. Hydrogen Peroxide Killing Assay
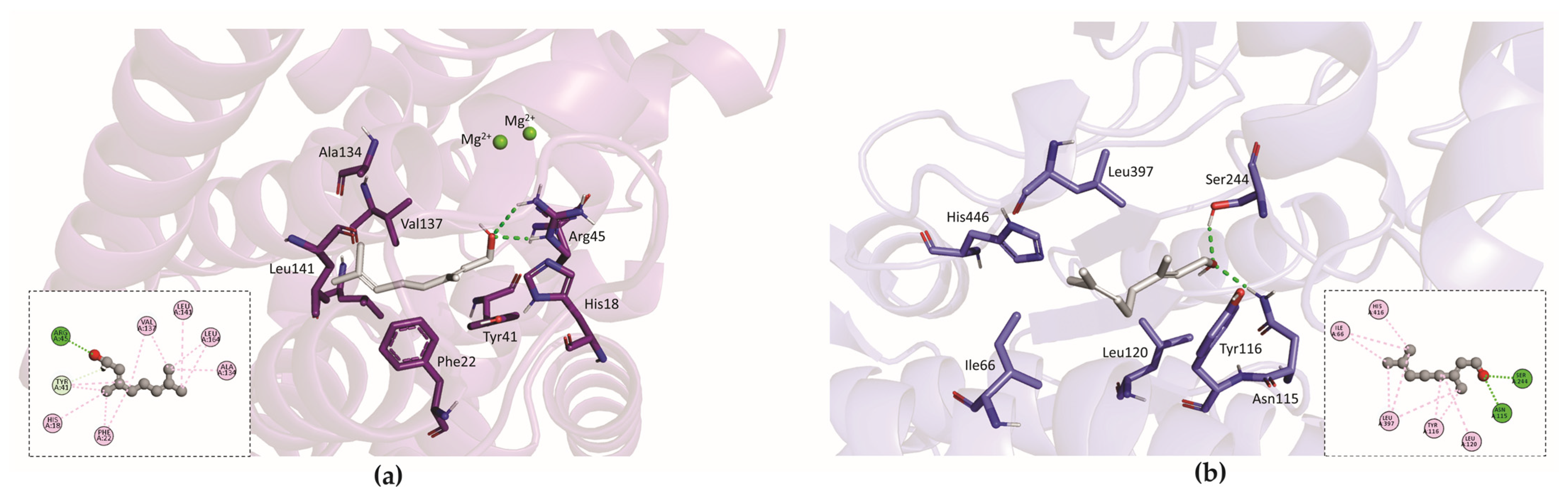
3.5. In Silico Analysis
Molecular Docking
3.6. Antibacterial Effect on Sessile (Biofilms) Cells
3.6.1. Antibiofilm Activity of Geraniol and bioAgNPs, Alone or in Combination, on Pre-Formed Biofilms
3.6.2. Effect of Geraniol and bioAgNPs, Alone or in Combination, on Adhesion and Biofilm Formation
3.6.3. Scanning Electron Microscopy (SEM)
3.7. Toxicity Analyses
3.7.1. Effect of Geraniol and bioAgNPs, Alone or in Combination, on Mammalian Cells
3.7.2. Effect of Geraniol and bioAgNPs, Alone or in Combination, on G. mellonella Larvae
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krismer, B.; Weidenmaier, C.; Zipperer, A.; Peschel, A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 2017, 15, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Morey, A.T.; Santos, J.P.; Gomes, L.V.; Cardoso, J.D.; Pinge-Filho, P.; Perugini, M.R.; Yamauchi, L.M.; Yamada-Ogatta, S.F. Molecular and phenotypic characteristics of methicillin-resistant Staphylococcus aureus isolated from hospitalized patients. J. Infect. Dev. Ctries. 2015, 9, 743–751. [Google Scholar] [CrossRef]
- Duarte, F.C.; Tavares, E.R.; Danelli, T.; Ribeiro, M.A.G.; Yamauchi, L.M.; Yamada-Ogatta, S.F.; Perugini, M.R.E. Disseminated Clonal Complex 5 (CC5) methicillin-resistant Staphylococcus aureus SCCmec type II in a tertiary hospital of Southern Brazil. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e32. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.G.; Smith, C.J.; Diekema, D.J.; Castanheira, M. Bacterial and fungal pathogens isolated from patients with bloodstream infection: Frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (2012–2017). Diagn. Microbiol. Infect. Dis. 2020, 97, 115016. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Mang, N.S.; Loomis, J.; Ortwine, J.K.; Wei, W.; O’Connell, E.J.; Shah, N.J.; Prokesch, B.C. Incidence of drug-resistant pathogens in community-acquired pneumonia at a safety net hospital. Microbiol. Spectr. 2024, 16, e0079224. [Google Scholar] [CrossRef]
- Matuszewska, M.; Murray, G.G.R.; Harrison, E.M.; Holmes, M.A.; Weinert, L.A. The Evolutionary Genomics of Host Specificity in Staphylococcus aureus. Trends Microbiol. 2020, 28, 465–477. [Google Scholar] [CrossRef]
- Douglas, E.J.A.; Laabei, M. Staph wars: The antibiotic pipeline strikes back. Microbiology 2023, 169, 001387. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in: Lancet 2022, 400, 1102. https://doi.org/10.1016/S0140-6736(21)02653-2. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef]
- Kaushik, A.; Kest, H.; Sood, M.; Steussy, B.W.; Thieman, C.; Gupta, S. Biofilm Producing Methicillin-Resistant Staphylococcus aureus (MRSA) Infections in Humans: Clinical Implications and Management. Pathogens 2024, 13, 76. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef] [PubMed]
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 4 September 2024).
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Li, J.; Sun, Z. The Combination of Antibiotic and Non-Antibiotic Compounds Improves Antibiotic Efficacy against Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2023, 24, 15493. [Google Scholar] [CrossRef] [PubMed]
- Bushby, S.R.; Hitchings, G.H. Trimethoprim, a sulphonamide potentiator. Br. J. Pharmacol. Chemother. 1968, 33, 72–90. [Google Scholar] [CrossRef]
- Moellering, R.C., Jr. Rationale for use of antimicrobial combinations. Am. J. Med. 1983, 75, 4–8. [Google Scholar] [CrossRef]
- Ribeiro Neto, J.A.; Pimenta Tarôco, B.R.; Batista Dos Santos, H.; Thomé, R.G.; Wolfram, E.; Maciel de A Ribeiro, R.I. Using the plants of Brazilian Cerrado for wound healing: From traditional use to scientific approach. J. Ethnopharmacol. 2020, 260, 112547. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One Hundred Faces of Geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- Silva-Rodrigues, G.; Castro, I.M.; Borges, P.H.G.; Suzukawa, H.T.; Souza, J.M.; Bartolomeu-Gonçalves, G.; Pelisson, M.; Medeiros, C.I.S.; Bispo, M.L.F.; Almeida, R.S.C.; et al. Geraniol Potentiates the Effect of Fluconazole against Planktonic and Sessile Cells of Azole-Resistant Candida tropicalis: In Vitro and In Vivo Analyses. Pharmaceutics 2024, 16, 1053. [Google Scholar] [CrossRef]
- Velidandi, A.; Dahariya, S.; Pabbathi, N.P.P.; Kalivarathan, D.; Baadhe, R.R. A Review on Synthesis, Applications, Toxicity, Risk Assessment, and Limitations of Plant Extracts Synthesized Silver Nanoparticles. NanoWorld J. 2020, 6, 35–60. [Google Scholar] [CrossRef]
- Swolana, D.; Wojtyczka, R.D. Activity of Silver Nanoparticles against Staphylococcus spp. Int. J. Mol. Sci. 2022, 23, 4298. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.S.; Rahman, M.A.; Ripon, R.K.; Mubarak, M.; Akter, M.; Mahbub, S.; Al Mamun, F.; Sikder, M.T. A systematic review on green synthesis of silver nanoparticles using plants extract and their biomedical applications. Heliyon 2024, 10, e29766. [Google Scholar] [CrossRef]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef]
- Wahab, S.; Salman, A.; Khan, Z.; Khan, S.; Krishnaraj, C.; Yun, S.I. Metallic Nanoparticles: A Promising Arsenal against Antimicrobial Resistance-Unraveling Mechanisms and Enhancing Medication Efficacy. Int. J. Mol. Sci. 2023, 24, 14897. [Google Scholar] [CrossRef]
- Sedighi, O.; Bednarke, B.; Sherriff, H.; Doiron, A.L. Nanoparticle-Based Strategies for Managing Biofilm Infections in Wounds: A Comprehensive Review. ACS Omega 2024, 9, 27853–27871. [Google Scholar] [CrossRef] [PubMed]
- Samuggam, S.; Chinni, S.V.; Mutusamy, P.; Gopinath, S.C.B.; Anbu, P.; Venugopal, V.; Reddy, L.V.; Enugutti, B. Green Synthesis and Characterization of Silver Nanoparticles Using Spondias mombin Extract and Their Antimicrobial Activity against Biofilm-Producing Bacteria. Molecules 2021, 26, 2681. [Google Scholar] [CrossRef]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, Anti-MRSA Activity and Toxicity of Essential Oils from Cymbopogon Species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef]
- Gu, K.; Ouyang, P.; Hong, Y.; Dai, Y.; Tang, T.; He, C.; Shu, G.; Liang, X.; Tang, H.; Zhu, L.; et al. Geraniol inhibits biofilm formation of methicillin-resistant Staphylococcus aureus and increase the therapeutic effect of vancomycin in vivo. Front. Microbiol. 2022, 13, 960728. [Google Scholar] [CrossRef]
- Younis, A.B.; Milosavljevic, V.; Fialova, T.; Smerkova, K.; Michalkova, H.; Svec, P.; Antal, P.; Kopel, P.; Adam, V.; Zurek, L.; et al. Synthesis and characterization of TiO2 nanoparticles combined with geraniol and their synergistic antibacterial activity. BMC Microbiol. 2023, 23, 207. [Google Scholar] [CrossRef]
- Ajlouni, A.W.; Hamdan, E.H.; Alshalawi, R.A.E.; Shaik, M.R.; Khan, M.; Kuniyil, M.; Alwarthan, A.; Ansari, M.A.; Khan, M.; Alkhathlan, H.Z.; et al. Green Synthesis of Silver Nanoparticles Using Aerial Part Extract of the Anthemis pseudocotula Boiss. Plant and Their Biological Activity. Molecules 2022, 28, 246. [Google Scholar] [CrossRef]
- Merghni, A.; Lassoued, M.A.; Noumi, E.; Hadj Lajimi, R.; Adnan, M.; Mastouri, M.; Snoussi, M. Cytotoxic Activity and Antibiofilm Efficacy of Biosynthesized Silver Nanoparticles against Methicillin-Resistant Staphylococcus aureus Strains Colonizing Cell Phones. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 9410024. [Google Scholar] [CrossRef]
- Ankudze, B.; Neglo, D. Green synthesis of silver nanoparticles from peel extract of Chrysophyllum albidum fruit and their antimicrobial synergistic potentials and biofilm inhibition properties. Biometals 2023, 36, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Hairil Anuar, A.H.; Abd Ghafar, S.A.; Hanafiah, R.M.; Lim, V.; Mohd Pazli, N.F.A. Critical Evaluation of Green Synthesized Silver Nanoparticles-Kaempferol for Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus. Int. J. Nanomed. 2024, 19, 1339–1350. [Google Scholar] [CrossRef]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of Essentials oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 20, 791–815. [Google Scholar] [CrossRef]
- Otaguiri, E.S.; Morguette, A.E.B.; Biasi-Garbin, R.P.; Morey, A.T.; Lancheros, C.A.C.; Kian, D.; de Oliveira, A.G.; Kerbauy, G.; Perugini, M.R.E.; Duran, N.; et al. Antibacterial Combination of Oleoresin from Copaifera multijuga Hayne and Biogenic Silver Nanoparticles Towards Streptococcus agalactiae. Curr. Pharm. Biotechnol. 2017, 18, 177–190. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Sistema de Informação Sobre a Biodiversidade Brasileira. Trichilia catigua A. Juss. Available online: https://ala-bie.sibbr.gov.br/ala-bie/species/339244 (accessed on 19 September 2024).
- Nakazato, G.; Celidonio, A.P.S.; Kobayashi, R.K.T.; Panagio, L.A.; Lonni, A.A.G.S.; De Campos, A.C.L.P.; Goncalves, M.C.; Okino, G.A.K. Carta Patente—Processo de Produção de Nanopartículas Biogênicas de Prata, Nanopartículas Biogênicas de Prata e Usos das Nanopartículas Biogênicas de Prata—GRAL Bioativos LTDA. BR Patent Application No. BR1020210163755, 18 September 2021. Available online: http://www.inpi.gov.br (accessed on 30 September 2024).
- Almajid, A.; Almuyidi, S.; Alahmadi, S.; Bohaligah, S.; Alfaqih, L.; Alotaibi, A.; Almarzooq, A.; Alsarihi, A.; Alrawi, Z.; Althaqfan, R.; et al. “Myth Busting in Infectious Diseases”: A Comprehensive Review. Cureus 2024, 16, e57238. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Valliammai, A.; Selvaraj, A.; Muthuramalingam, P.; Priya, A.; Ramesh, M.; Pandian, S.K. Staphyloxanthin inhibitory potential of thymol impairs antioxidant fitness, enhances neutrophil mediated killing and alters membrane fluidity of methicillin resistant Staphylococcus aureus. Biomed. Pharmacother. 2021, 141, 111933. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, A.; Balasubramaniam, B.; Ranjitha, R.; Srinivasan, R.; Packiavathy, I.A.S.V.; Balamurugan, K.; Pandian, S.K.; Ravi, A.V. In vitro and in vivo biofilm inhibitory efficacy of geraniol-cefotaxime combination against Staphylococcus spp. Food Chem. Toxicol. 2019, 125, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Elghali, F.; Ibrahim, I.; Guesmi, M.; Frikha, F.; Mnif, S. Unveiling the impact of selected essential oils on MRSA strain ATCC 33591: Antibacterial efficiency, biofilm disruption, and staphyloxanthin inhibition. Braz. J. Microbiol. 2024, 55, 2057–2069. [Google Scholar] [CrossRef]
- Mishra, N.N.; Liu, G.Y.; Yeaman, M.R.; Nast, C.C.; Proctor, R.A.; McKinnell, J.; Bayer, A.S. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 2011, 55, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.B.; Gatto, C.; Wilkinson, B.J. Interrelationships between Fatty Acid Composition, Staphyloxanthin Content, Fluidity, and Carbon Flow in the Staphylococcus aureus Membrane. Molecules 2018, 23, 1201. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]
- Marshall, J.H.; Wilmoth, G.J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J. Bacteriol. 1981, 147, 900–913. [Google Scholar] [CrossRef]
- Xue, L.; Chen, Y.Y.; Yan, Z.; Lu, W.; Wan, D.; Zhu, H. Staphyloxanthin: A potential target for antivirulence therapy. Infect. Drug Resist. 2019, 12, 2151–2160. [Google Scholar] [CrossRef]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef]
- Beard-Pegler, M.A.; Stubbs, E.; Vickery, A.M. Observations on the resistance to drying of staphylococcal strains. J. Med. Microbiol. 1988, 26, 251–255. [Google Scholar] [CrossRef]
- Pelz, A.; Wieland, K.P.; Putzbach, K.; Hentschel, P.; Albert, K.; Götz, F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J. Biol. Chem. 2005, 280, 32493–32498. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, P.C. Functional expression and extension of staphylococcal staphyloxanthin biosynthetic pathway in Escherichia coli. J. Biol. Chem. 2012, 287, 21575–21583. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, Z.; Zhang, X.; Li, H.; Sun, H.; Zong-Wan, M.; Xia, W. Structural insight into the substrate gating mechanism by Staphylococcus aureus aldehyde dehydrogenase. CCS Chem. 2020, 2, 946–954. [Google Scholar] [CrossRef]
- Lin, F.Y.; Zhang, Y.; Hensler, M.; Liu, Y.L.; Chow, O.A.; Zhu, W.; Wang, K.; Pang, R.; Thienphrapa, W.; Nizet, V.; et al. Dual dehydrosqualene/squalene synthase inhibitors: Leads for innate immune system-based therapeutics. ChemMedChem 2012, 7, 561–564. [Google Scholar] [CrossRef]
- Lin, F.Y.; Liu, C.I.; Liu, Y.L.; Zhang, Y.; Wang, K.; Jeng, W.Y.; Ko, T.P.; Cao, R.; Wang, A.H.; Oldfield, E. Mechanism of action and inhibition of dehydrosqualene synthase. Proc. Natl. Acad. Sci. USA 2010, 107, 21337–21342. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Khan, H.M.; Husain, F.M.; Ahmad, R.; Qais, F.A.; Khan, M.A.; Jalal, M.; Tayyaba, U.; Ali, S.G.; Singh, A.; et al. Antibacterial and antibiofilm activity of Abroma augusta stabilized silver (Ag) nanoparticles against drug-resistant clinical pathogens. Front. Mol. Biosci. 2023, 10, 1292509. [Google Scholar] [CrossRef]
- Yan, Y.; Li, G.; Su, M.; Liang, H. Scutellaria baicalensis Polysaccharide-Mediated Green Synthesis of Smaller Silver Nanoparticles with Enhanced Antimicrobial and Antibiofilm Activity. ACS Appl. Mater. Interfaces 2024, 16, 45289–45306. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef]
- Hall, R.L.; Oser, B.L. Recent Progress in the Consideration of Flavoring Ingredients under the Food Additives Amendment. Ill. GRAS Substances. In Food Technology; The Garrard Press: Oregon, OR, USA, 1965; Volume 19, Part 2. [Google Scholar]
- FDA 2023. Food Additive Status List. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list#ftnG (accessed on 18 November 2024).
- Bitschar, K.; Wolz, C.; Krismer, B.; Peschel, A.; Schittek, B. Keratinocytes as sensors and central players in the immune defense against Staphylococcus aureus in the skin. J. Dermatol. Sci. 2017, 87, 215–220. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Imani, S.; Zhao, Z.; Ullah, S.; Wang, Q. Navigating ESKAPE Pathogens: Considerations and Caveats for Animal Infection Models Development. ACS Infect. Dis. 2024, 10, 2336–2355. [Google Scholar] [CrossRef] [PubMed]
- Nacional Centre for the Replacement Refinement & Reduction of Animals in Research. The 3Rs. Available online: http://www.nc3rs.org.uk/the-3rs (accessed on 26 September 2024).
- Cutuli, M.A.; Petronio Petronio, G.; Vergalito, F.; Magnifico, I.; Pietrangelo, L.; Venditti, N.; Di Marco, R. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 2019, 10, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A.; et al. Geraniol Pharmacokinetics, Bioavailability and Its Multiple Effects on the Liver Antioxidant and Xenobiotic-Metabolizing Enzymes. Front. Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef]
- Teixeira, L.A.; Resende, C.A.; Ormonde, L.R.; Rosenbaum, R.; Figueiredo, A.M.; de Lencastre, H.; Tomasz, A. Geographic spread of epidemic multiresistant Staphylococcus aureus clone in Brazil. J. Clin. Microbiol. 1995, 33, 2400–2404. [Google Scholar] [CrossRef] [PubMed]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: 12th Edition. CLSI: Wayne PA, USA, 2024.
- CLSI Standard M26-A; Methods for Determining Bactericidal Activity of Antimicrobial Agents. Approved Guideline: Volume 19, Number 18; CLSI: Wayne PA, USA, 1999.
- Klepser, M.E.; Ernst, E.J.; Lewis, R.E.; Ernst, M.E.; Pfaller, M.A. Influence of test conditions on antifungal time-kill curve results: Proposal for standardized methods. Antimicrob. Agents Chemother. 1998, 42, 1207–1212. [Google Scholar] [CrossRef]
- Spoladori, L.F.A.; Andriani, G.M.; Castro, I.M.; Suzukawa, H.T.; Gimenes, A.C.R.; Bartolomeu-Gonçalves, G.; Ishida, K.; Nakazato, G.; Pinge-Filho, P.; Machado, R.R.B.; et al. Synergistic Antifungal Interaction between Pseudomonas aeruginosa LV Strain Metabolites and Biogenic Silver Nanoparticles against Candida auris. Antibiotics 2023, 12, 861. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Zhu, X.; Zhao, H.; Lu, Y.; Zhang, C.; Lu, Z. Surfactin effectively inhibits Staphylococcus aureus adhesion and biofilm formation on surfaces. Appl. Microbiol. Biotechnol. 2019, 103, 4565–4574. [Google Scholar] [CrossRef]

| Staphylococcus aureus | GER (µg/mL) | bioAgNPs (µg/mL) | GER/bioAgNPs b (µg/mL) | FICI c | Interaction d | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC a | MIC | MBC | MBC/MIC a | ||||
| BEC 9393 | 625 | 1250 | 2 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 108 | 625 | 1250 | 2 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 149 | 625 | 2500 | 4 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 1 | 625 | 2500 | 4 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 26 | 625 | 2500 | 4 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 37 | 625 | 1250 | 2 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 598 | 625 | 1250 | 2 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 5 | 625 | 2500 | 4 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 39 | 625 | 2500 | 4 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| 518 | 625 | 2500 | 4 | 8.43 | 16.87 | 2 | 156.25/2.10 | 0.5 | Synergy |
| Staphylococcus aureus | SMIC90 | FICI b | Interaction c | ||
|---|---|---|---|---|---|
| GER (µg/mL) | bioAgNPs (µg/mL) | GER/bioAgNPs a (µg/mL) | |||
| BEC 9393 | 1250 | 67.5 | 156.25/0.52 | 0.13 | synergy |
| 108 | 1250 | 67.5 | 156.25/0.26 | 0.13 | synergy |
| 149 | 625 | 16.87 | 156.25/4.21 | 0.49 | synergy |
| 1 | 1250 | 67.5 | 156.25/4.21 | 0.19 | synergy |
| 26 | 625 | 33.75 | 156.25/4.21 | 0.37 | synergy |
| 37 | 1250 | 67.5 | 156.25/0.52 | 0.13 | synergy |
| 598 | 1250 | 67.5 | 156.25/0.26 | 0.12 | synergy |
| 5 | 1250 | 67.5 | 156.25/1.05 | 0.13 | synergy |
| 39 | 625 | 67.5 | 156.25/8.43 | 0.37 | synergy |
| 518 | 1250 | 67.5 | 156.25/0.26 | 0.12 | synergy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, I.M.d.; Antunes, C.; Valentim, C.C.; Spoladori, L.F.d.A.; Suzukawa, H.T.; Correia, G.F.; Silva-Rodrigues, G.; Borges, P.H.G.; Bartolomeu-Gonçalves, G.; Silva, M.L.; et al. Synergistic Antibacterial Interaction of Geraniol and Biogenic Silver Nanoparticles on Methicillin-Resistant Staphylococcus aureus. Plants 2025, 14, 1059. https://doi.org/10.3390/plants14071059
Castro IMd, Antunes C, Valentim CC, Spoladori LFdA, Suzukawa HT, Correia GF, Silva-Rodrigues G, Borges PHG, Bartolomeu-Gonçalves G, Silva ML, et al. Synergistic Antibacterial Interaction of Geraniol and Biogenic Silver Nanoparticles on Methicillin-Resistant Staphylococcus aureus. Plants. 2025; 14(7):1059. https://doi.org/10.3390/plants14071059
Chicago/Turabian StyleCastro, Isabela Madeira de, Camila Antunes, Camila Cristina Valentim, Laís Fernanda de Almeida Spoladori, Helena Tiemi Suzukawa, Guilherme Ferreira Correia, Gislaine Silva-Rodrigues, Paulo Henrique Guilherme Borges, Guilherme Bartolomeu-Gonçalves, Mariana Luiza Silva, and et al. 2025. "Synergistic Antibacterial Interaction of Geraniol and Biogenic Silver Nanoparticles on Methicillin-Resistant Staphylococcus aureus" Plants 14, no. 7: 1059. https://doi.org/10.3390/plants14071059
APA StyleCastro, I. M. d., Antunes, C., Valentim, C. C., Spoladori, L. F. d. A., Suzukawa, H. T., Correia, G. F., Silva-Rodrigues, G., Borges, P. H. G., Bartolomeu-Gonçalves, G., Silva, M. L., Bispo, M. d. L. F., Machado, R. R. B., Nakamura, C. V., Nakazato, G., Pinge-Filho, P., Tavares, E. R., Yamauchi, L. M., & Yamada-Ogatta, S. F. (2025). Synergistic Antibacterial Interaction of Geraniol and Biogenic Silver Nanoparticles on Methicillin-Resistant Staphylococcus aureus. Plants, 14(7), 1059. https://doi.org/10.3390/plants14071059







