Balanced Fertilization Improves Crop Production and Soil Organic Carbon Sequestration in a Wheat–Maize Planting System in the North China Plain
Abstract
1. Introduction
2. Results
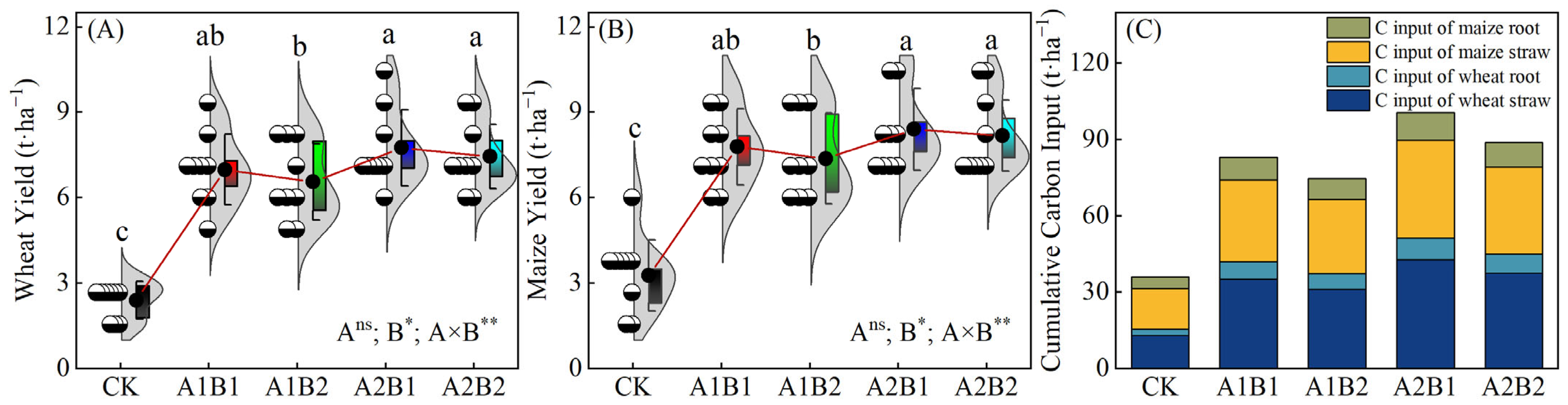
2.1. Crop Yields and Cumulative C Inputs
2.2. SOC Content, Storage, and Sequestration
2.3. Soil Nutrients
2.4. Soil Stoichiometric Characteristics
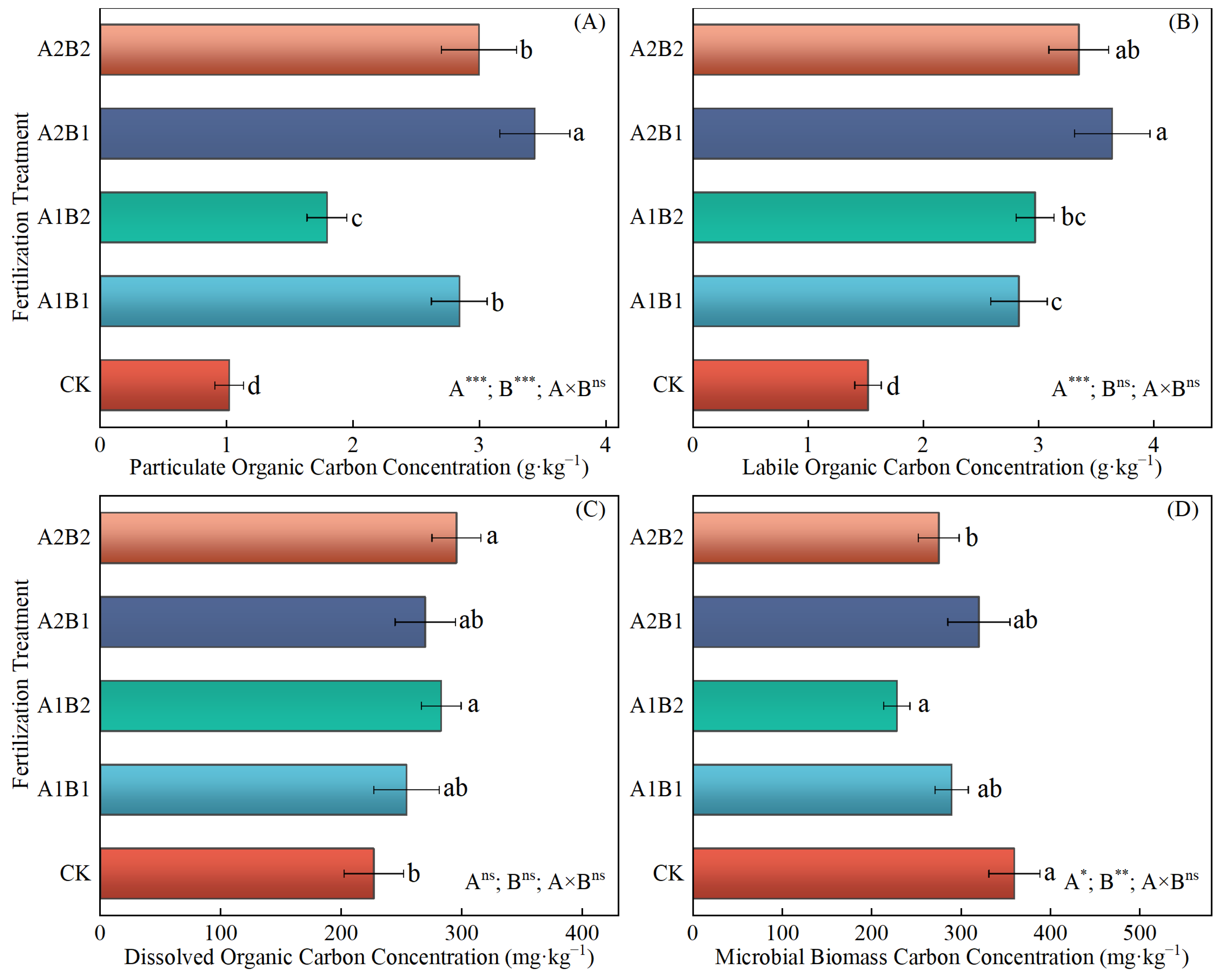
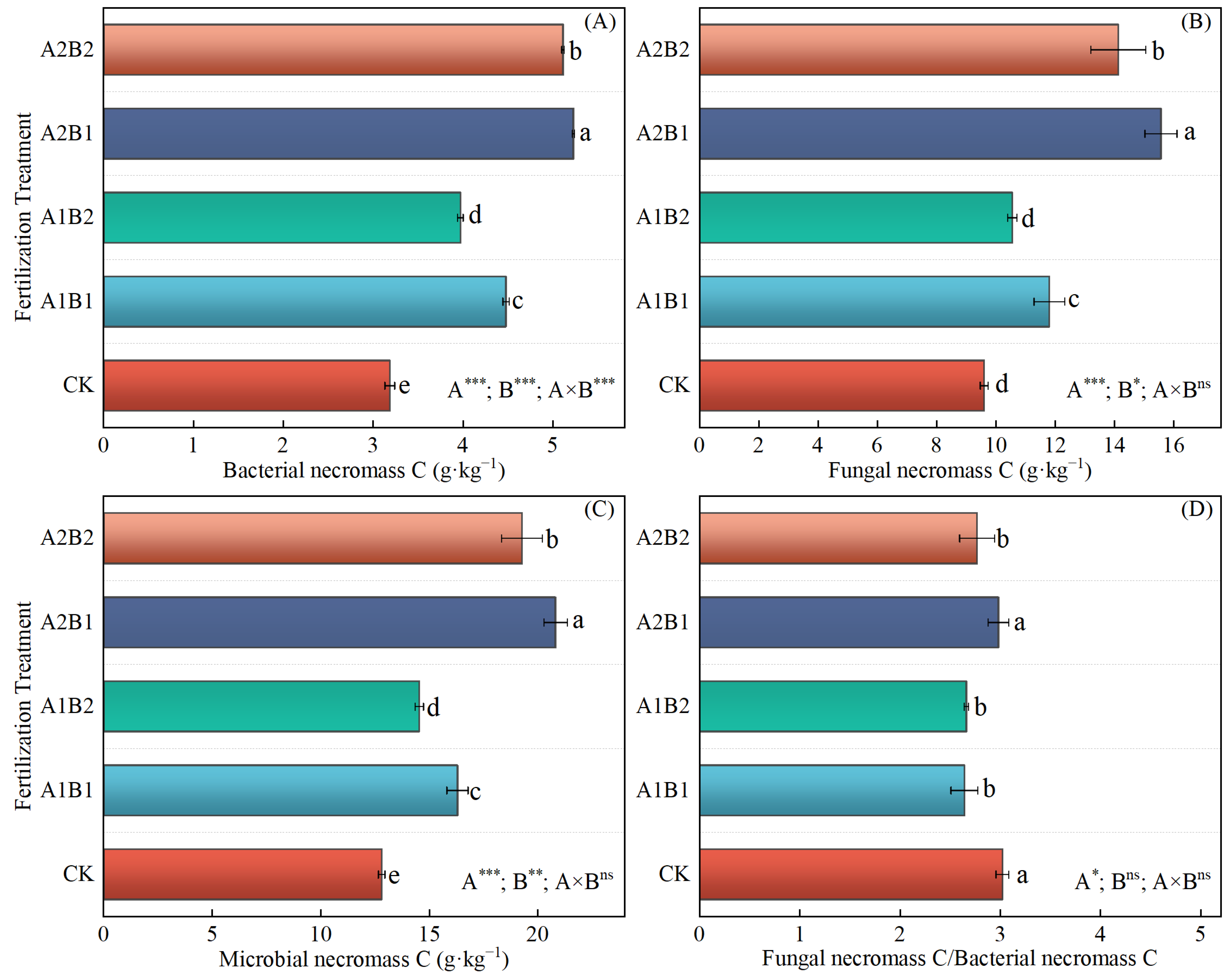
2.5. The Content of Active Components of SOC and Microbial Necromass C
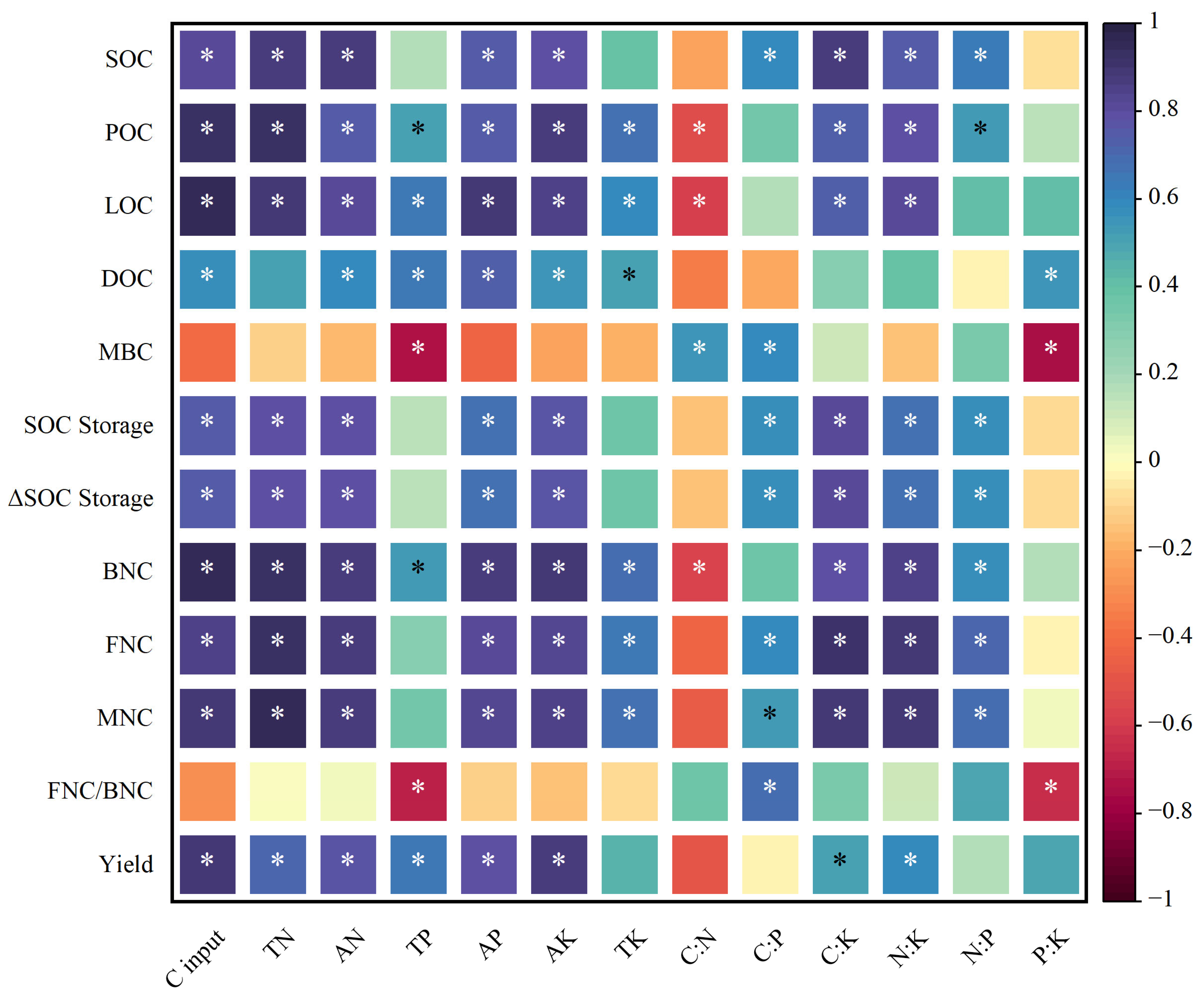
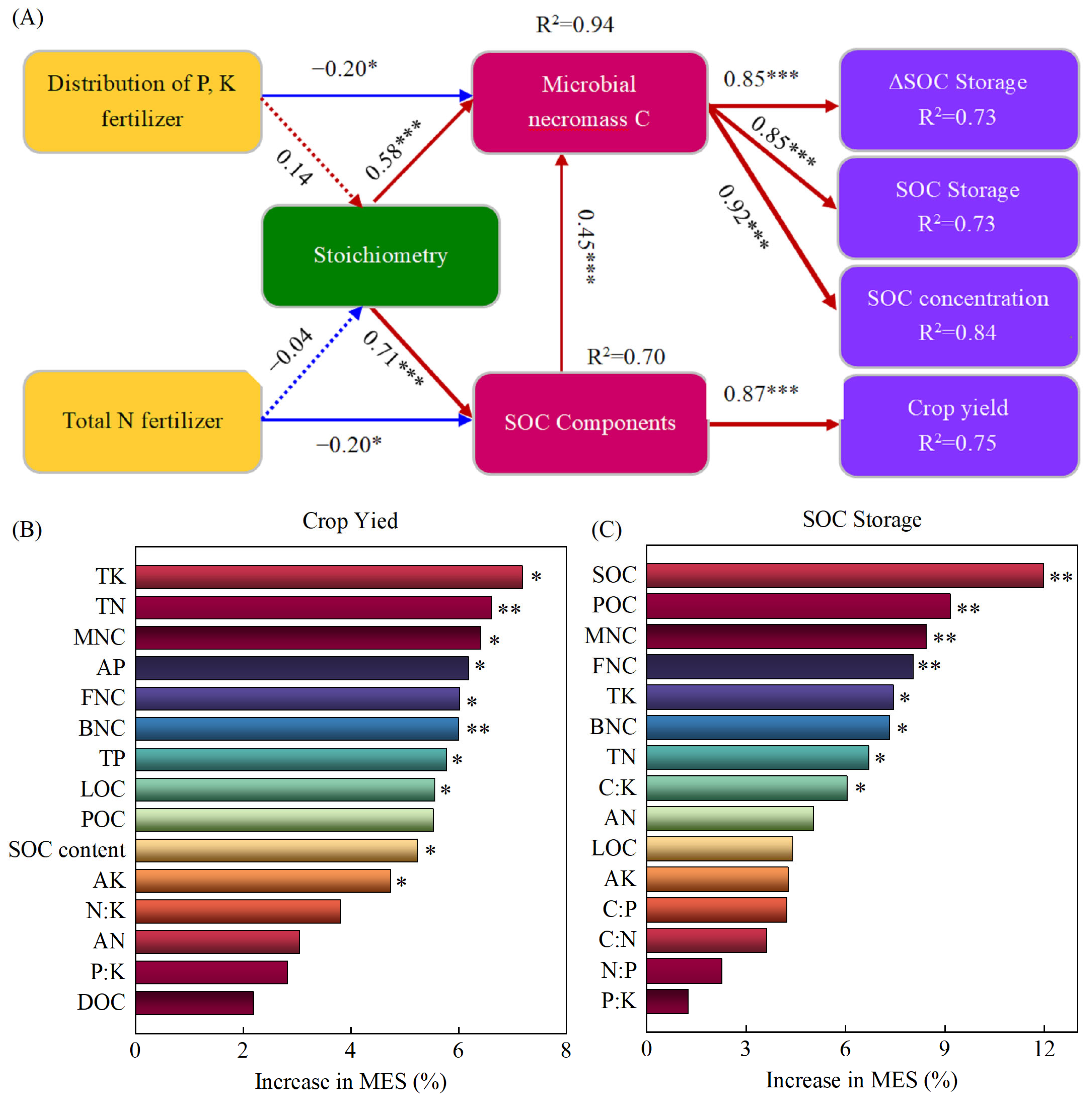
2.6. Relationships Between Soil Chemical Nutrient Indicators, Crop Yield and SOC Indicators
3. Discussion
3.1. The Impacts of Different Fertilization Practices on SOC Storage and Its Regulating Factors
3.2. The Impacts of Different Fertilization Practices on Crop Yield and Its Regulating Factors
4. Materials and Methods
4.1. Description of Experimental Site
4.2. Experimental Design
4.3. Sample Collection and Measurement Methods
4.3.1. Soil Chemical Nutrients
4.3.2. SOC and Its Components Content
4.3.3. Amino Sugar Content
4.4. Calculation of C Input of Crop Residue, SOC Storage, and Sequestration
4.4.1. Carbon (C) Input of Straw Residue
4.4.2. Soil Bulk Density, SOC Storage and Sequestration
4.4.3. Microbial Necromass Carbon (MNC)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Carbon Cycling in Global Drylands. Curr. Clim. Change Rep. 2019, 5, 221–232. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Dougherty, W.J.; Fang, Y.; Badgery, W.; Hoyle, F.C.; Dalal, R.C.; Cowie, A.L. Impact of agricultural management practices on the nutrient supply potential of soil organic matter under long-term farming systems. Soil Tillage Res. 2018, 175, 71–81. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Glidden, S.; Wu, Y.P.; Tang, L.; Liu, Q.Y.; Li, C.S.; Frolking, S. Changes in the soil organic carbon balance on China’s cropland during the last two decades of the 20th century. Sci. Rep. 2017, 7, 7144. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, W.; Li, T.; Cheng, X.; Liu, Q. Balancing straw returning and chemical fertilizers in China: Role of straw nutrient resources. Renew. Sustain. Energy Rev. 2018, 81, 2695–2702. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, P.; Mei, F.; Ling, Y.; Qiao, Y.; Liu, C.; Leghari, S.J.; Guan, X.; Wang, T. Does continuous straw returning keep China farmland soil organic carbon continued increase? A meta-analysis. J. Environ. Manag. 2021, 288, 112391. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Basile-Doelsch, I.; Derrien, D.; Fanin, N.; Fontaine, S.; Guenet, B.; Karimi, B.; Marsden, C.; Maron, P.A. Advancing the mechanistic understanding of the priming effect on soil organic matter mineralisation. Funct. Ecol. 2022, 36, 1355–1377. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, B.; Shen, J.; Zhu, X.; Yi, W.; Li, Y.; Wu, J. Contrasting effects of straw and straw-derived biochar applications on soil carbon accumulation and nitrogen use efficiency in double-rice cropping systems. Agric. Ecosyst. Environ. 2021, 311, 107286. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, T.; Peñuelas, J.; Sardans, J.; Tan, W.; Wei, X.; Cui, Y.; Cui, Q.; Wu, C.; Liu, L.; et al. Crop residue return sustains global soil ecological stoichiometry balance. Glob. Change Biol. 2023, 29, 2203–2226. [Google Scholar] [CrossRef]
- Song, J.; Song, J.; Xu, W.; Gao, G.; Bai, J.; Zhang, Z.; Yu, Q.; Hao, J.; Yang, G.; Ren, G.; et al. Straw return with fertilizer improves soil CO2 emissions by mitigating microbial nitrogen limitation during the winter wheat season. Catena 2024, 241, 108050. [Google Scholar] [CrossRef]
- Guo, Z.; Li, W.; Ul Islam, M.; Wang, Y.; Zhang, Z.; Peng, X. Nitrogen fertilization degrades soil aggregation by increasing ammonium ions and decreasing biological binding agents on a Vertisol after 12 years. Pedosphere 2022, 32, 629–636. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Enguang, N.; Khan, A.; Feng, Y.; Yang, G.; Wang, H. Straw mulching with inorganic nitrogen fertilizer reduces soil CO2 and N2O emissions and improves wheat yield. Sci. Total Environ. 2020, 741, 140488. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Xu, M.; Wang, W.; Sun, X.; Zhao, K. Return rate of straw residue affects soil organic C sequestration by chemical fertilization. Soil Tillage Res. 2011, 113, 70–73. [Google Scholar] [CrossRef]
- Zhao, S.; He, P.; Qiu, S.; Jia, L.; Liu, M.; Jin, J.; Johnston, A.M. Long-term effects of potassium fertilization and straw return on soil potassium levels and crop yields in north-central China. Field Crop. Res. 2014, 169, 116–122. [Google Scholar] [CrossRef]
- Islam, M.U.I.; Jiang, F.; Halder, M.; Liu, S.; Peng, X. Impact of straw return combined with different fertilizations on soil organic carbon stock in upland wheat and maize croplands in China: A meta-analysis. Crop Environ. 2023, 2, 233–241. [Google Scholar] [CrossRef]
- Prasad, J.V.N.S.; Rao, C.S.; Srinivas, K.; Jyothi, C.N.; Venkateswarlu, B.; Ramachandrappa, B.K.; Dhanapal, G.N.; Ravichandra, K.; Mishra, P.K. Effect of ten years of reduced tillage and recycling of organic matter on crop yields, soil organic carbon and its fractions in Alfisols of semi arid tropics of southern India. Soil Tillage Res. 2016, 156, 131–139. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wang, Q.; Liu, J. Impacts of 9 years of a new conservational agricultural management on soil organic carbon fractions. Soil Tillage Res. 2014, 143, 1–6. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, F.; Zou, B.; Chen, Y.; Zhao, J.; Mo, Q.; Li, Y.; Li, X.; Xia, H. Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol. Fertil. Soils 2014, 51, 207–215. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Hu, H.; Qian, C.; Xue, K.; Liang, Y. Reducing the uncertainty in estimating soil microbial-derived carbon storage. Proc. Natl. Acad. Sci. USA 2024, 121, e2401916121. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Gao, H.; Mao, J.; Chu, W.; Thompson, M.L. Biochemical stabilization of soil organic matter in straw-amended, anaerobic and aerobic soils. Sci. Total Environ. 2018, 625, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Yang, D.; Wang, X.; Crowther, T.W.; Vinay, N.; Luo, Z.; Yu, K.; Sun, S.; Zhang, F.; Xiong, Y.; et al. Nutrient limitation of soil organic carbon stocks under straw return. Soil Biol. Biochem. 2024, 192, 109360. [Google Scholar] [CrossRef]
- Khan, S.A.; Mulvaney, R.L.; Ellsworth, T.R.; Boast, C.W. The myth of nitrogen fertilization for soil carbon sequestration. J. Environ. Qual. 2007, 36, 1821–1832. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Hastings, A.; Helmy, M.; Prescher, A.; Osborne, B.; Lanigan, G.; Forristal, D.; Killi, D.; Maratha, P.; Williams, M.; et al. Assessing the combined use of reduced tillage and cover crops for mitigating greenhouse gas emissions from arable ecosystem. Geoderma 2014, 223–225, 9–20. [Google Scholar] [CrossRef]
- Jarecki, M.K.; Lal, R. Crop Management for Soil Carbon Sequestration. Crit. Rev. Plant Sci. 2010, 22, 471–502. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, W.L.; Meng, F.Q.; Smith, P.; Lal, R. Increase in soil organic carbon by agricultural intensification in northern China. Biogeosciences 2015, 12, 1403–1413. [Google Scholar] [CrossRef]
- Ranaivoson, L.; Naudin, K.; Ripoche, A.; Affholder, F.; Rabeharisoa, L.; Corbeels, M. Agro-ecological functions of crop residues under conservation agriculture. A review. Agron. Sustain. Dev. 2017, 37, 26. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Y.; Liu, J.; Yuan, J.; Liang, Y.; Ren, J.; Cai, H. Effects of maize straw and its biochar application on organic and humic carbon in water-stable aggregates of a Mollisol in Northeast China: A five-year field experiment. Soil Tillage Res. 2019, 190, 1–9. [Google Scholar] [CrossRef]
- Jin, V.L.; Schmer, M.R.; Stewart, C.E.; Sindelar, A.J.; Varvel, G.E.; Wienhold, B.J. Long-term no-till and stover retention each decrease the global warming potential of irrigated continuous corn. Glob. Change Biol. 2017, 23, 2848–2862. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Passioura, J.B.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Nutrient availability limits carbon sequestration in arable soils. Soil Biol. Biochem. 2014, 68, 402–409. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Xu, M.; Tong, X.; Sun, F.; Wang, J.; Huang, S.; Zhu, P.; He, X. Long-term combined chemical and manure fertilizations increase soil organic carbon and total nitrogen in aggregate fractions at three typical cropland soils in China. Sci. Total Environ. 2015, 532, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.K.; Wei, L.; Turner, N.C.; Ma, S.C.; Yang, M.D.; Wang, T.C. Improved straw management practices promote in situ straw decomposition and nutrient release, and increase crop production. J. Clean. Prod. 2020, 250, 119514. [Google Scholar] [CrossRef]
- Luo, S.; Gao, Q.; Wang, S.; Tian, L.; Zhou, Q.; Li, X.; Tian, C. Long-term fertilization and residue return affect soil stoichiometry characteristics and labile soil organic matter fractions. Pedosphere 2020, 30, 703–713. [Google Scholar] [CrossRef]
- Li, J.; Wen, Y.; Li, X.; Li, Y.; Yang, X.; Lin, Z.; Song, Z.; Cooper, J.M.; Zhao, B. Soil labile organic carbon fractions and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China Plain. Soil Tillage Res. 2018, 175, 281–290. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J. The effect of chemical fertilizer application on carbon input and export in soil—A pot experiment with wheat using natural 13C abundance method. Geoderma 2012, 189–190, 170–175. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Y.; Wang, L.; Zhu, P. Straw Return with Reduced Nitrogen Fertilizer Maintained Maize High Yield in Northeast China. Agronomy 2019, 9, 229. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Liu, Y.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef]
- Tian, K.; Zhao, Y.; Xu, X.; Hai, N.; Huang, B.; Deng, W. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis. Agric. Ecosyst. Environ. 2015, 204, 40–50. [Google Scholar] [CrossRef]
- Fan, Y.F.; Gao, J.L.; Sun, J.Y.; Liu, J.; Su, Z.J.; Hu, S.P.; Wang, Z.G.; Yu, X.F. Potentials of straw return and potassium supply on maize (Zea mays L.) photosynthesis, dry matter accumulation and yield. Sci. Rep. 2022, 12, 799. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, J.; Sun, J.; Liu, J.; Su, Z.; Wang, Z.; Yu, X.; Hu, S. Effects of straw returning and potassium fertilizer application on root characteristics and yield of spring maize in China inner Mongolia. Agron. J. 2021, 13, 4369–4385. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, H.; Zhu, Q.H.; Wang, X.F.; Zhang, Y.Z.; Yu, X.C.; Peng, X. Carbon sequestration efficiency in paddy soil and upland soil under long-term fertilization in southern China. Soil Tillage Res. 2013, 130, 42–51. [Google Scholar] [CrossRef]
- Ren, Z.; Han, X.; Feng, H.; Wang, L.; Ma, G.; Li, J.; Lv, J.; Tian, W.; He, X.; Zhao, Y.; et al. Long-term conservation tillage improves soil stoichiometry balance and crop productivity based on a 17-year experiment in a semi-arid area of northern China. Sci. Total Environ. 2024, 908, 168283. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Sun, Y.; Liu, P.; Zhang, Q.; Wang, X.; Wang, R.; Li, J. Long-term effects of optimized fertilization, tillage and crop rotation on soil fertility, crop yield and economic profit on the Loess Plateau. Eur. J. Agron. 2023, 143, 126731. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Qi, J.Y.; Jing, Z.H.; He, C.; Liu, Q.Y.; Wang, X.; Kan, Z.R.; Zhao, X.; Xiao, X.P.; Zhang, H.L. Effects of tillage management on soil carbon decomposition and its relationship with soil chemistry properties in rice paddy fields. J. Environ. Manag. 2021, 279, 111595. [Google Scholar] [CrossRef]
- Kan, Z.-R.; Ma, S.-T.; Liu, Q.-Y.; Liu, B.-Y.; Virk, A.L.; Qi, J.-Y.; Zhao, X.; Lal, R.; Zhang, H.-L. Carbon sequestration and mineralization in soil aggregates under long-term conservation tillage in the North China Plain. Catena 2020, 188, 104428. [Google Scholar] [CrossRef]

| Treatment | Bulk Density (g·cm−3) | SOC Content (g·kg−1) | SOC Storage (t·ha−1) | ΔSOC Storage (t·ha−1) | |
|---|---|---|---|---|---|
| Initial | 1.31 | 10.34 | 27.09 | — | |
| CK | 1.29 ± 0.04 a | 9.74 ± 0.80 d | 25.16 ± 2.72 d | −1.93 ± 2.72 d | |
| A1B1 | 1.24 ± 0.03 ab | 11.94 ± 0.53 bc | 29.59 ± 0.77 bc | 2.50 ± 0.77 bc | |
| A1B2 | 1.21 ± 0.04 bc | 10.62 ± 0.37 cd | 25.69 ± 0.92 cd | −1.40 ± 0.92 cd | |
| A2B1 | 1.13 ± 0.03 d | 15.13 ± 1.40 a | 34.20 ± 3.26 a | 7.11 ± 3.26 a | |
| A2B2 | 1.16 ± 0.03 cd | 13.59 ± 1.26 ab | 31.51 ± 2.61 ab | 4.41 ± 2.61 ab | |
| ANOVA | A | 19.69 ** | 28.69 *** | 17.35 ** | 15.41 ** |
| B | 0.01 ns | 6.17 * | 6.94 * | 6.14 * | |
| A × B | 2.77 ns | 0.85 ns | 0.23 ns | 0.22 ns | |
| Treatment | Stoichiometry | ||||||
|---|---|---|---|---|---|---|---|
| C:N | C:P | C:K | N:P | N:K | P:K | ||
| CK | 10.54 ± 0.92 a | 34.16 ± 0.93 b | 1.82 ± 0.04 c | 3.26 ± 0.33 bc | 0.17 ± 0.01 c | 0.05 ± 0.00 d | |
| A1B1 | 9.32 ± 0.26 a | 27.56 ± 1.49 c | 1.91 ± 0.05 c | 2.96 ± 0.08 cd | 0.21 ± 0.00 b | 0.07 ± 0.00 ab | |
| A1B2 | 9.48 ± 0.79 a | 25.28 ± 0.28 c | 1.90 ± 0.02 c | 2.68 ± 0.25 d | 0.20 ± 0.01 b | 0.08 ± 0.00 a | |
| A2B1 | 9.21 ± 0.58 a | 40.96 ± 0.86 a | 2.44 ± 0.11 a | 4.45 ± 0.20 a | 0.26 ± 0.01 a | 0.06 ± 0.00 cd | |
| A2B2 | 9.47 ± 0.64 a | 32.46 ± 2.07 b | 2.10 ± 0.07 b | 3.44 ± 0.31 b | 0.22 ± 0.02 b | 0.06 ± 0.01 bc | |
| ANOVA | A | 0.03 ns | 173.04 *** | 78.22 *** | 75.72 *** | 27.43 *** | 24.50 ** |
| B | 0.38 ns | 47.54 *** | 18.31 ** | 25.15 ** | 9.33 * | 4.50 ns | |
| A × B | 0.02 ns | 15.83 ** | 16.28 ** | 8.12 * | 4.76 ns | 0.50 ns | |
| Fertilization Treatment | Fertilization Rate During Wheat Season (kg·ha−1) | Fertilization Rate During Maize Season (kg·ha−1) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sowing Stage | Jointing Stage | Sowing Stage | Flare Opening Stage | Flowering-Filling Stage | |||||||||||
| N | P2O5 | K2O | N | P2O5 | K2O | N | P2O5 | K2O | N | P2O5 | K2O | N | P2O5 | K2O | |
| CK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A1B1 | 157.5 | 180 | 135 | 67.5 | 0 | 0 | 67.5 | 0 | 0 | 112.5 | 0 | 0 | 45 | 0 | 0 |
| A1B2 | 210 | 180 | 135 | 90 | 0 | 0 | 90 | 0 | 0 | 150 | 0 | 0 | 60 | 0 | 0 |
| A2B1 | 157.5 | 120 | 90 | 67.5 | 0 | 0 | 67.5 | 0 | 0 | 112.5 | 30 | 22.5 | 45 | 30 | 22.5 |
| A2B2 | 210 | 120 | 90 | 90 | 0 | 0 | 90 | 0 | 0 | 150 | 30 | 22.5 | 60 | 30 | 22.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhai, H.; Zan, R.; Tian, Y.; Ma, X.; Ji, H.; Zhang, D. Balanced Fertilization Improves Crop Production and Soil Organic Carbon Sequestration in a Wheat–Maize Planting System in the North China Plain. Plants 2025, 14, 838. https://doi.org/10.3390/plants14060838
Zhang H, Zhai H, Zan R, Tian Y, Ma X, Ji H, Zhang D. Balanced Fertilization Improves Crop Production and Soil Organic Carbon Sequestration in a Wheat–Maize Planting System in the North China Plain. Plants. 2025; 14(6):838. https://doi.org/10.3390/plants14060838
Chicago/Turabian StyleZhang, Huiyu, Hao Zhai, Ruixin Zan, Yuan Tian, Xiaofei Ma, Hutai Ji, and Dingyi Zhang. 2025. "Balanced Fertilization Improves Crop Production and Soil Organic Carbon Sequestration in a Wheat–Maize Planting System in the North China Plain" Plants 14, no. 6: 838. https://doi.org/10.3390/plants14060838
APA StyleZhang, H., Zhai, H., Zan, R., Tian, Y., Ma, X., Ji, H., & Zhang, D. (2025). Balanced Fertilization Improves Crop Production and Soil Organic Carbon Sequestration in a Wheat–Maize Planting System in the North China Plain. Plants, 14(6), 838. https://doi.org/10.3390/plants14060838





