Silicon Deposition and Phytolith Morphological Variation in Culm Sheaths of Dendrocalamus brandisii at Different Growth Stages
Abstract
1. Introduction
2. Results
2.1. Silicon and Phytolith Content in D. brandisii Culm Sheath Blades at Different Growth Stages
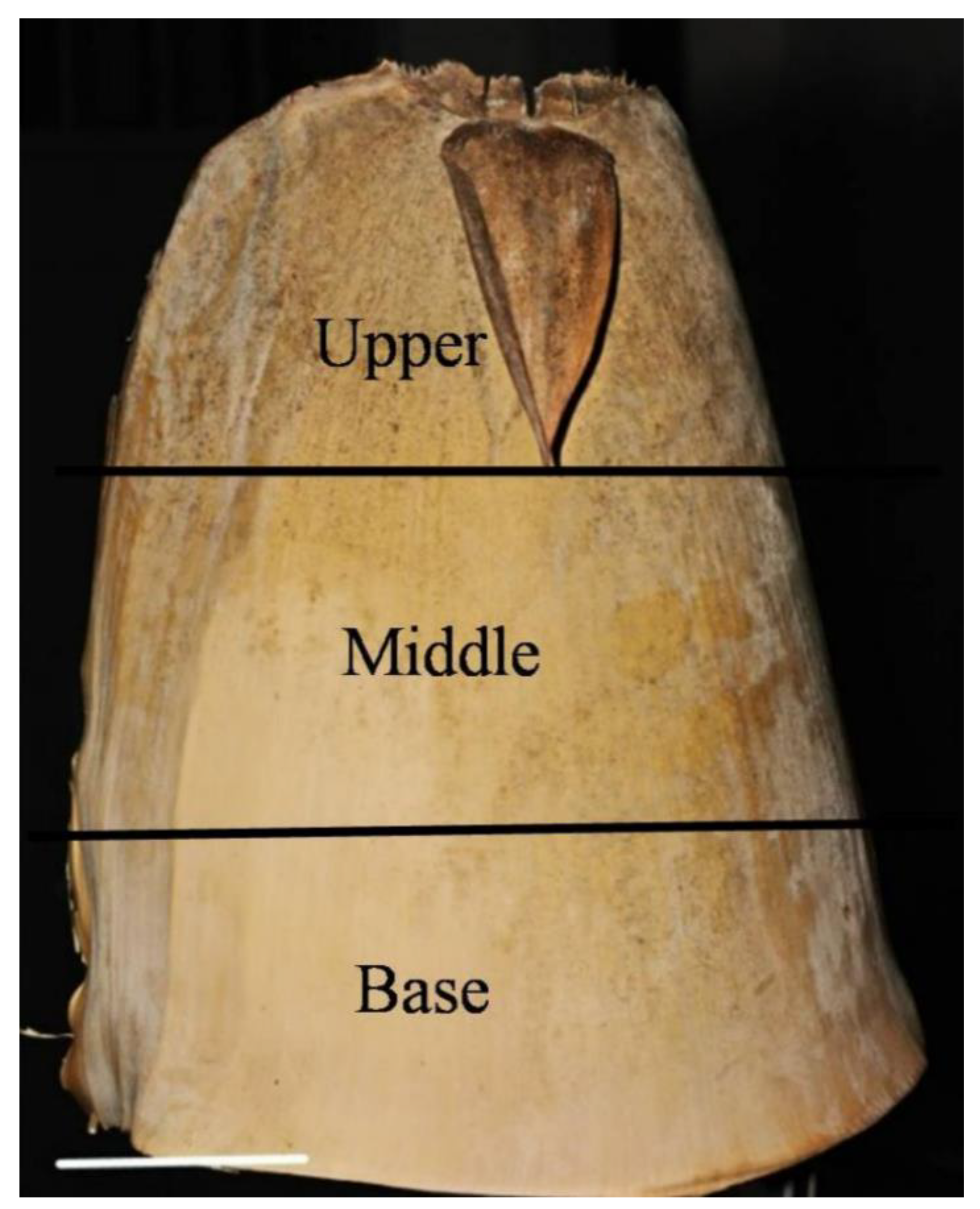
2.2. Silicon and Phytolith Content in the D. brandisii Culm Sheath Bodies at Different Growth Stages
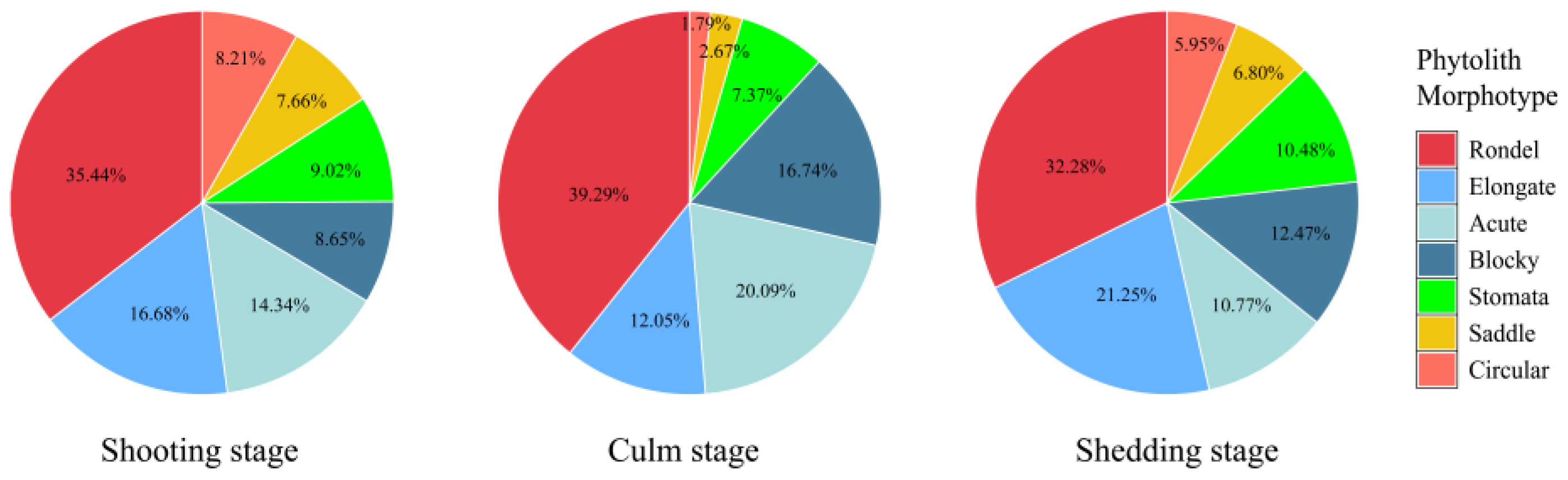
2.3. Phytolith Morphotypes and Their Variation in the D. brandisii Culm Sheath Blades at Different Growth Stages
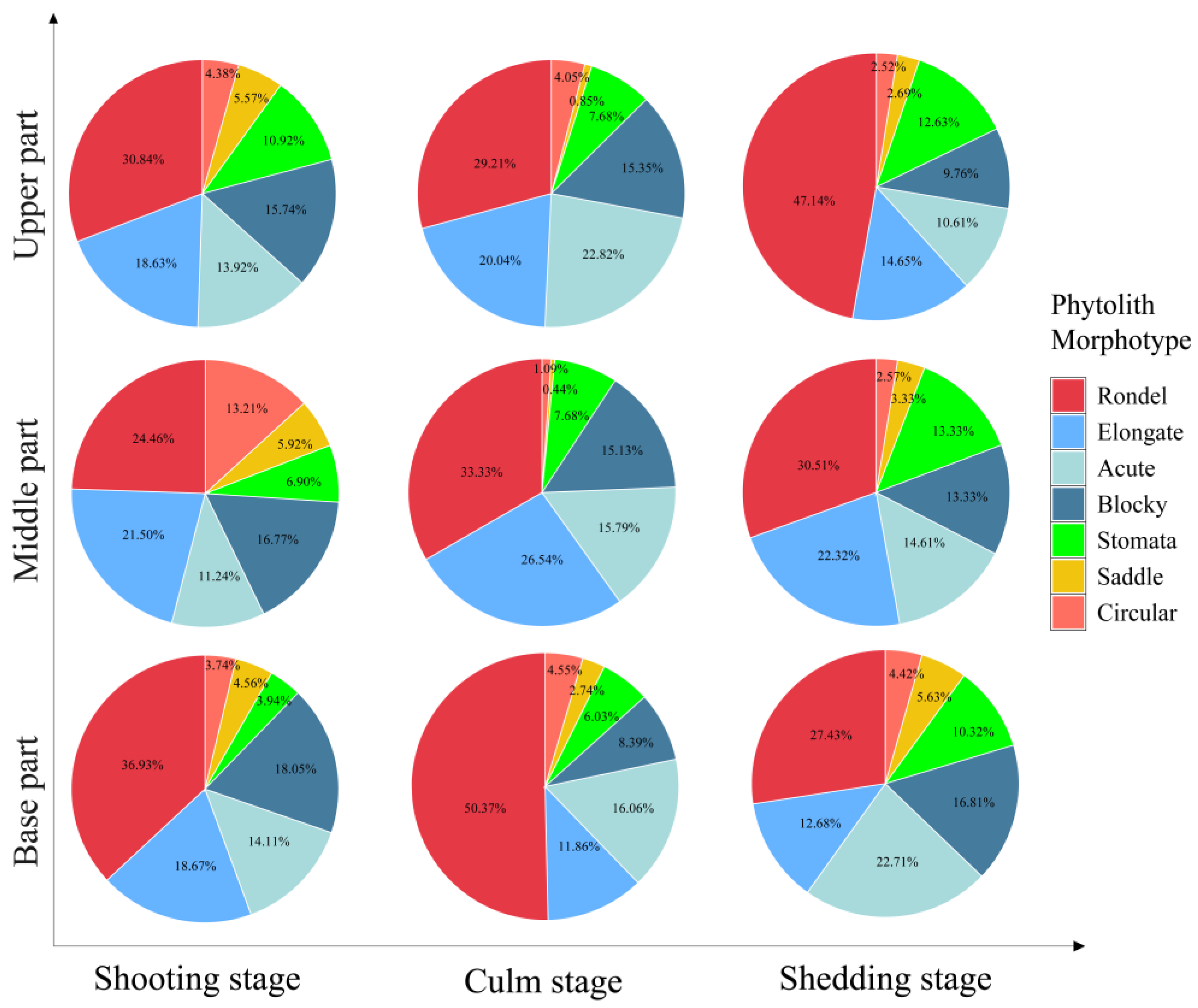
2.4. Phytolith Morphotypes and Their Variation in the D. brandisii Culm Sheath Bodies at Different Growth Stages
2.5. Phytolith Size and Its Variation in the D. brandisii Culm Sheath Blades at Different Growth Stages
2.6. Phytolith Size and Its Variation in D. brandisii Culm Sheath Bodies at Different Growth Stages
3. Discussion
3.1. Variation in Silicon and Phytolith Content in Culm Sheaths
3.2. Variation In Phytolith Morphotypes and Composition in Culm Sheaths
3.3. Evolution of Phytolith Morphotypes with Sheath Growth and Function
4. Materials and Methods
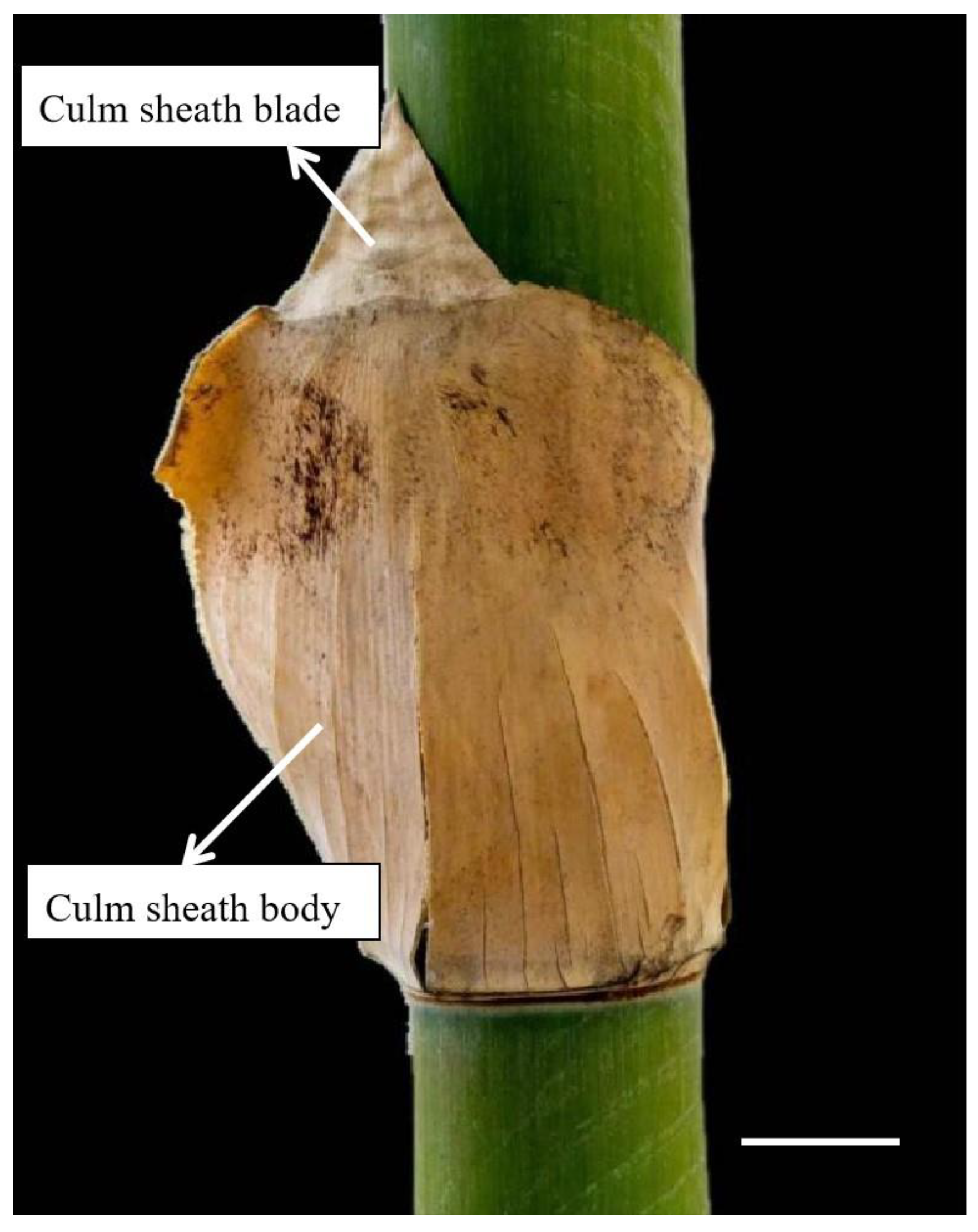
4.1. Materials
4.2. Determination of Silicon and Phytolith Content
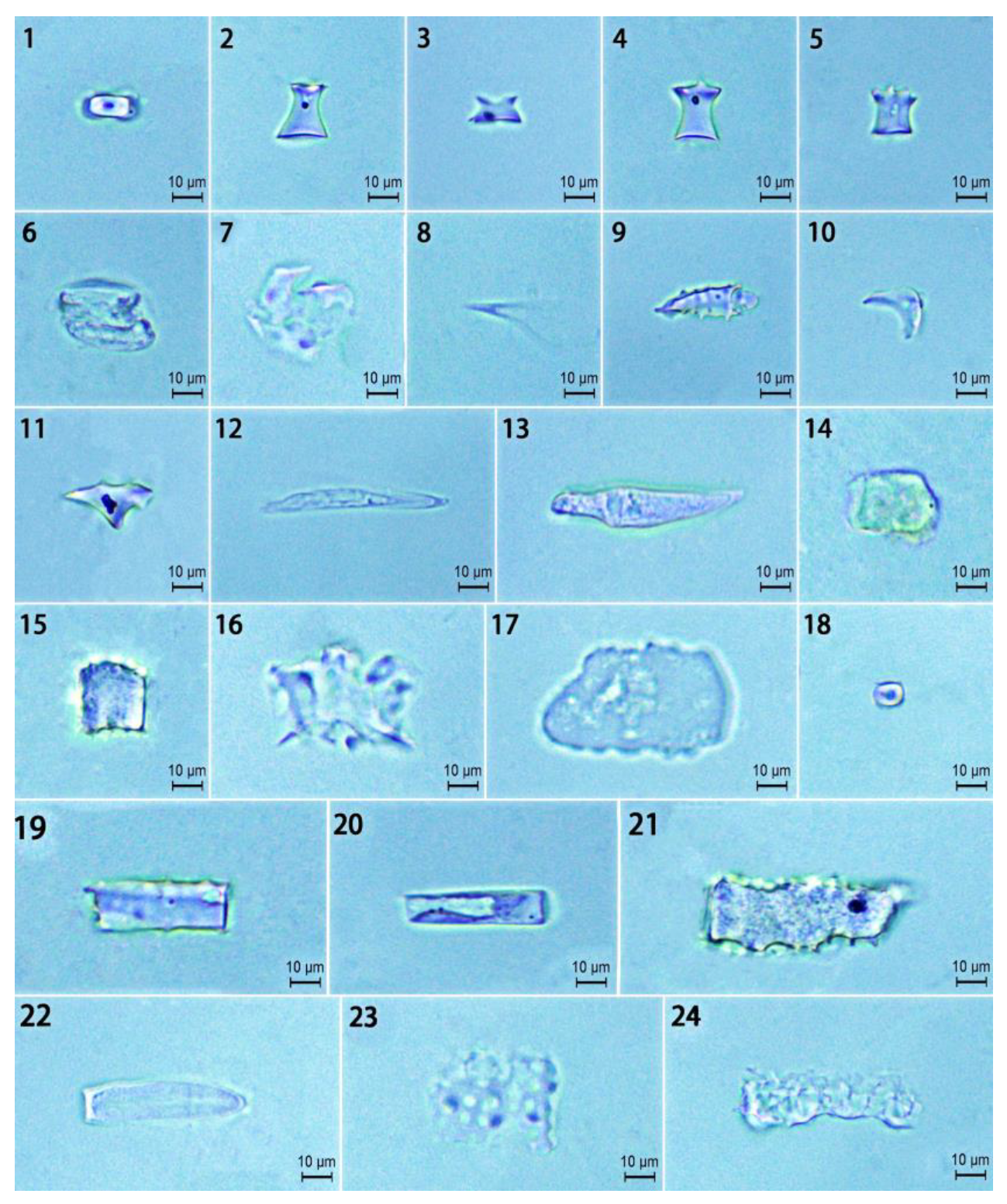
4.3. Phytolith Extraction and Observation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S. Bamboo sheath—A modified branch based on the anatomical observations. Sci. Rep. 2017, 7, 16132. [Google Scholar] [CrossRef]
- Janzen, D.H. Why Bamboos Wait So Long to Flower. Annu. Rev. Ecol. Syst. 1976, 7, 347–391. [Google Scholar] [CrossRef]
- Kaack, K.; Schwarz, K. Morphological and mechanical properties of Miscanthus in relation to harvesting, lodging, and growth conditions. Ind. Crop Prod. 2001, 14, 145–154. [Google Scholar] [CrossRef]
- Wang, S.; He, W.; Zhan, H. Culm sheaths affect height growth of bamboo shoots in Fargesia yunnanensis. Rev. Bras. Botânica 2018, 41, 255–266. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, H.; Li, P.; Chu, C.; Li, J.; Wang, C. Physiological mechanism of internode bending growth after the excision of shoot sheath in Fargesia yunnanensis and its implications for understanding the rapid growth of bamboos. Front. Plant Sci. 2020, 11, 418. [Google Scholar] [CrossRef]
- Wong, K.M. Bamboo-The Amazing Grass: A Guide to the Diversity and Study of Bamboos in South East Asia; Ipgri Apo: Serdang, Malaysia, 2004. [Google Scholar]
- Liese, W.; Köhl, M. Bamboo-The Plant and Its Uses; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- An, X.; Xie, B. Phytoliths from woody plants: A review. Diversity 2022, 14, 339. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H. The Study of Phytolith and its Application; Haiyang Pres: Beijing, China, 2008. [Google Scholar]
- Wu, Y.; You, H.; Li, X. Dinosaur-associated Poaceae epidermis and phytoliths from the Early Cretaceous of China. Natl. Sci. Rev. 2018, 5, 721–727. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, H.; Wang, H.; Li, R.; Yu, J. Phytoliths as a method of identification for three genera of woody bamboos (Bambusoideae) in tropical southwest China. J. Archaeol. Sci. 2016, 68, 46–53. [Google Scholar] [CrossRef]
- Zhan, H.; Juan, L.; Zhao-hui, N.; Li, M.-B.; Wang, C.-M.; Wang, S.-G. Silicon variation and phytolith morphology in different organs of Dendrocalamus brandisii (Munro) Kurz (Bambusoideae). Rev. Bras. Botânica 2019, 42, 529–541. [Google Scholar] [CrossRef]
- Dai, C.; Xu, R.; Yu, L.; Zhu, F.; Li, M.; Li, J.; Wang, S.; Wang, C.; Zhan, H. Silicon Uptake and Phytolith Morphology in Dendrocalamus brandisii Seedling Leaf from Different Rearing Methods. Forests 2023, 14, 1877. [Google Scholar] [CrossRef]
- Gu, Y.; Yan, H.; Zheng, M.; Liu, H.; Ji, Y.; Zhang, Y. Phytolith records from 15 continuously growing Bambusa emeiensis leaves and its climatic significance. Rev. Palaeobot. Palyno 2022, 300, 104620. [Google Scholar] [CrossRef]
- Zhu, F.; Niu, Z.; Li, J.; Yu, L.; Wang, S.; Wang, C.; Zhan, H. Variation of the phytolith content and morphology of Dendrocalamus giganteus at different phenological periods. J. Southwest. For. Univ. 2022, 42, 71–77. [Google Scholar]
- Xu, R.; He, H.; Guo, H.; Zhu, F.; Wang, S.; Dai, C.; Zheng, X.; Xie, D.; Li, H.; Wang, C.; et al. Characteristics of silicon and phytolith distribution in bamboo (Ferrocalamus strictus): Variations between different organs and ages. Rev. Palaeobot. Palyno 2023, 311, 104817. [Google Scholar] [CrossRef]
- Li, D.; Wang, Z.; Zhu, Z.; Xia, N.; Jia, L.; Guo, Z.; Yang, G.; Chris, S. Bambuseae. In Flora of China. 22, Poaceae; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2006. [Google Scholar]
- Madella, M.; Alexandre, A.; Ball, T. International code for phytolith nomenclature 1.0. Ann. Bot. 2005, 96, 253–260. [Google Scholar] [CrossRef]
- Neumann, K.; Strömberg, C.A.E.; Ball, T.; Albert, R.M.; Vrydaghs, L.; Cummings, L.S. International Code for Phytolith Nomenclature (ICPN) 2.0. Ann. Bot. 2019, 124, 189–199. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, I.; Gupta, S. Classification of Bamboo Species by Fourier and Legendre Moment. Int. J. Adv. Sci. Technol. 2013, 50, 61–70. [Google Scholar]
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell Mol. Life Sci. 2008, 65, 3049–3057. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Paasch, S.; Brunner, E.; Bäucker, E.; Dudel, E.G. Silica uptake from nanoparticles and silica condensation state in different tissues of Phragmites australis. Sci. Total Environ. 2013, 442, 6–9. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Gessner, M.O.; Bäuker, E.; Dudel, E.G. Silicon supply modifies C:N:P stoichiometry and growth of Phragmites australis. Plant Biol. 2012, 14, 392–396. [Google Scholar] [CrossRef]
- Pearsall, D.M.; Piperno, D.R.; Dinan, E.H.; Umlauf, M.; Zhao, Z.; Benfer, R.A. Distinguishing rice (Oryza sativa Poaceae) from wild Oryza species through phytolith analysis: Results of preliminary research. Econ. Bot. 1995, 49, 183–196. [Google Scholar] [CrossRef]
- Ball, T.; Chandler-Ezell, K.; Dickau, R.; Duncan, N.; Hart, T.C.; Iriarte, J.; Lentfer, C.; Logan, A.; Lu, H.; Madella, M.; et al. Phytoliths as a tool for investigations of agricultural origins and dispersals around the world. J. Archaeol. Sci. 2016, 68, 32–45. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, Z.; Pearsall, D.M. Phytolith morphology research on wild and domesticated rice species in East Asia. Quatern Int. 2013, 287, 141–148. [Google Scholar] [CrossRef]
- Lu, H.Y.; Liu, K.B. Morphological variations of lobate phytoliths from grasses in China and the southeastern USA. Divers. Distrib. 2003, 9, 73e87. [Google Scholar] [CrossRef]
- Piperno, D.R.; Pearsall, D.M. The silica bodies of tropical American grasses: Morphology, taxonomy, and implications for grass systematics and fossil phytolith identification. Smithson. Contrib. Bot. 1998, 85, 1e40. [Google Scholar] [CrossRef]
- Lu, H.; Yang, X.; Ye, M.; Liu, K.-B.; Xia, Z.; Ren, X.; Cai, L.; Wu, N.; Liu, T.-S. Millet noodles in Late Neolithic China. Nature 2005, 437, 967–968. [Google Scholar] [CrossRef]
- Piperno, D.R. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists; Rowman Altamira: Lanham, MD, USA, 2006; ISBN 0759103852. [Google Scholar]
- Zhang, X.; Gélin, U.; Spicer, R.A.; Wu, F.; Farnsworth, A.; Chen, P.; Del Rio, C.; Li, S.; Liu, J.; Huang, J.; et al. Rapid Eocene diversification of spiny plants in subtropical woodlands of central Tibet. Nat. Commun. 2022, 13, 3787. [Google Scholar] [CrossRef]
- Bremond, L.; Alexandre, A.; Peyron, O.; Guiot, J. Grass water stress estimated from phytoliths in West Africa. J. Biogeogr. 2013, 40, 311–327. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Johnson, D.M.; Lachenbruch, B.; McCulloh, K.A.; Woodruff, D.R. Xylem hydraulic safety margins in woody plants: Coordination of stomatal control of xylem tension with hydraulic capacitance. Funct. Ecol. 2009, 23, 922–930. [Google Scholar] [CrossRef]
- Zou, Q. Experiment Instruction of Plant Physiology; China Agricultural Press: Beijing, China, 2008. [Google Scholar]
- Pearsall, D.M. Paleoethnobotany: A Handbook of Procedures; Routledge: Oxford, UK, 2016; ISBN 131542309X. [Google Scholar]

| Stages | Silicon | Phytolith |
|---|---|---|
| Shooting | 32.92 ± 8.05 b | 25.53 ± 1.38 c |
| Culm | 127.18 ± 6.12 b | 40.38 ± 23.33 b |
| Shedding | 150.55 ± 2.72 a | 103.44 ± 3.03 a |
| Means | 103.55 | 56.45 |
| Stages | Position | Silicon | Phytolith |
|---|---|---|---|
| Shooting | Upper | 21.97 ± 1.97 a | 16.21 ± 5.33 b |
| Middle | 25.38 ± 2.73 a | 23.84 ± 2.75 a | |
| Base | 23.92 ± 0.61 a | 23.48 ± 1.76 a | |
| Mean | 23.75 ± 1.71 B | 21.18 ± 4.31 B | |
| Culm | Upper | 32.44 ± 2.27 a | 27.45 ± 1.05 a |
| Middle | 31.37 ± 6.33 a | 26.39 ± 3.38 a | |
| Base | 39.41 ± 7.46 a | 26.60 ± 7.93 a | |
| Mean | 34.41 ± 4.37 A | 26.81 ± 0.56 A | |
| Shedding | Upper | 38.05 ± 5.21 a | 30.92 ± 2.58 a |
| Middle | 25.44 ± 4.64 b | 22.90 ± 2.30 b | |
| Base | 33.27 ± 1.50 ab | 23.10 ± 2.69 b | |
| Mean | 32.25 ± 6.37 A | 25.96 ± 4.57 A | |
| Means | 30.14 | 24.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.; Li, M.; Xie, D.; Xu, R.; Wang, S.; Wang, C.; Zhan, H. Silicon Deposition and Phytolith Morphological Variation in Culm Sheaths of Dendrocalamus brandisii at Different Growth Stages. Plants 2025, 14, 841. https://doi.org/10.3390/plants14060841
Duan S, Li M, Xie D, Xu R, Wang S, Wang C, Zhan H. Silicon Deposition and Phytolith Morphological Variation in Culm Sheaths of Dendrocalamus brandisii at Different Growth Stages. Plants. 2025; 14(6):841. https://doi.org/10.3390/plants14060841
Chicago/Turabian StyleDuan, Siyuan, Maobiao Li, Dongbo Xie, Rui Xu, Shuguang Wang, Changming Wang, and Hui Zhan. 2025. "Silicon Deposition and Phytolith Morphological Variation in Culm Sheaths of Dendrocalamus brandisii at Different Growth Stages" Plants 14, no. 6: 841. https://doi.org/10.3390/plants14060841
APA StyleDuan, S., Li, M., Xie, D., Xu, R., Wang, S., Wang, C., & Zhan, H. (2025). Silicon Deposition and Phytolith Morphological Variation in Culm Sheaths of Dendrocalamus brandisii at Different Growth Stages. Plants, 14(6), 841. https://doi.org/10.3390/plants14060841






