Identifications of Genes Involved in ABA and MAPK Signaling Pathways Positively Regulating Cold Tolerance in Rice
Abstract
1. Introduction
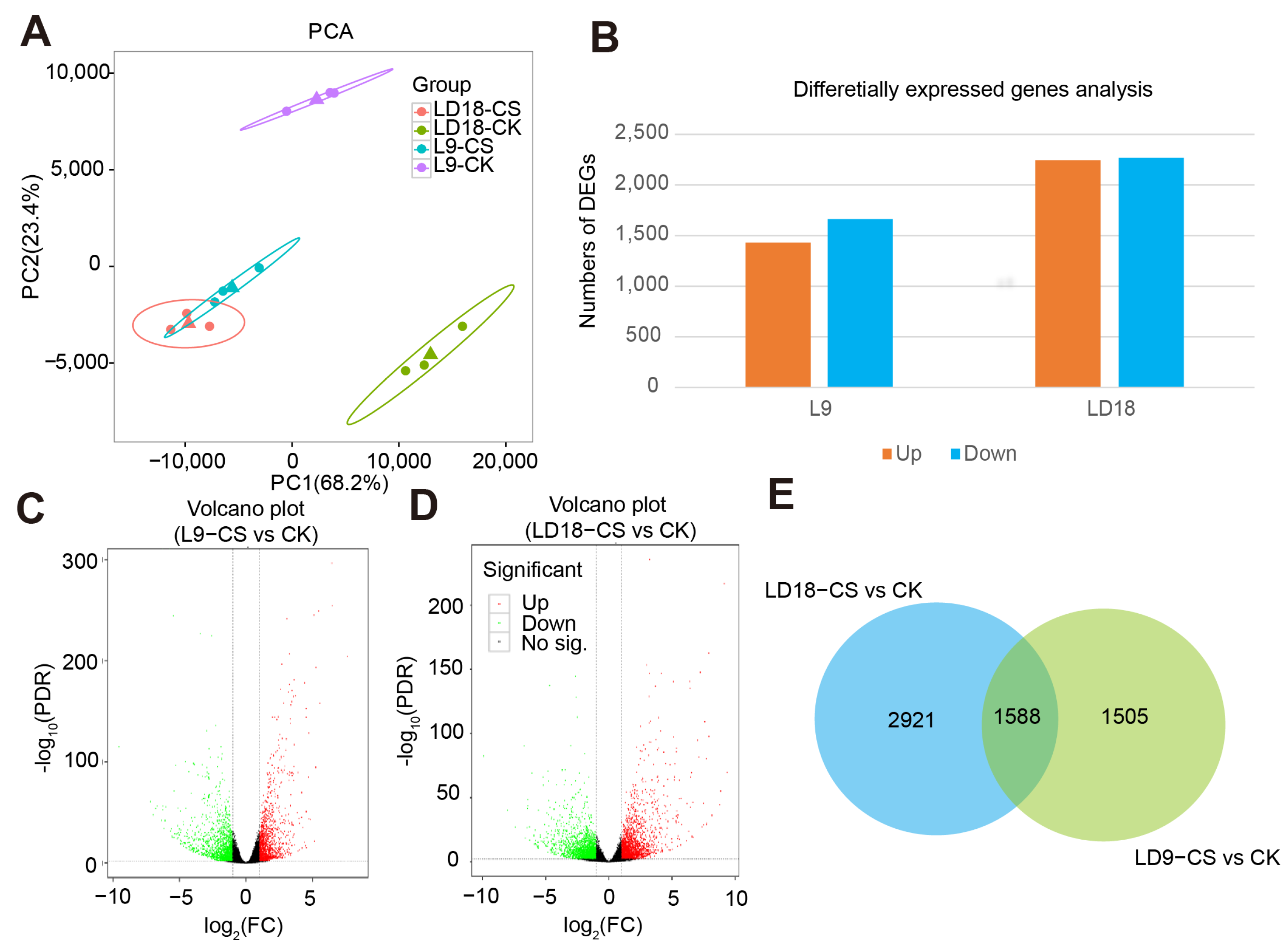
2. Results
3. Discussion
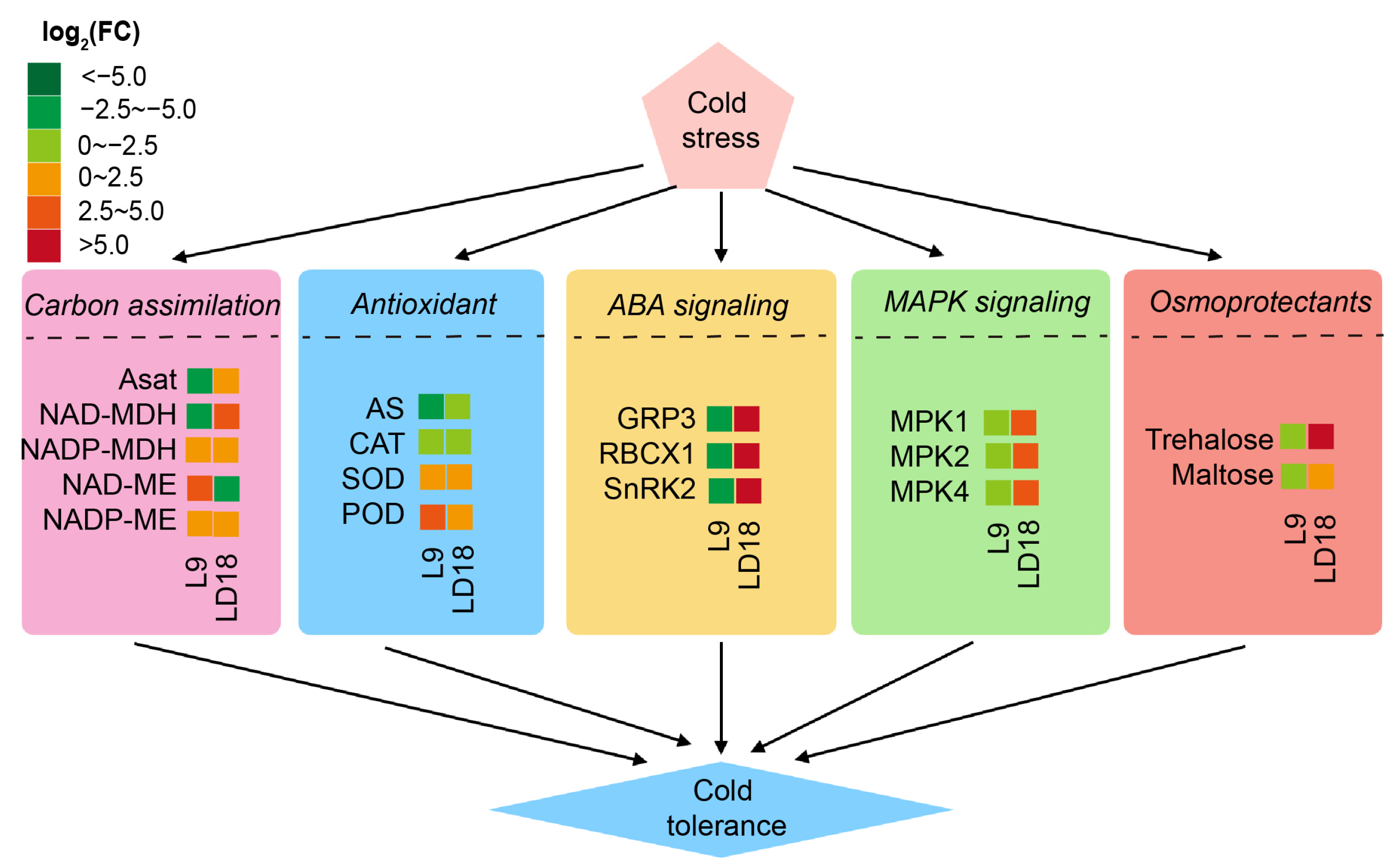
3.1. Physiology Responses to Cold Stress
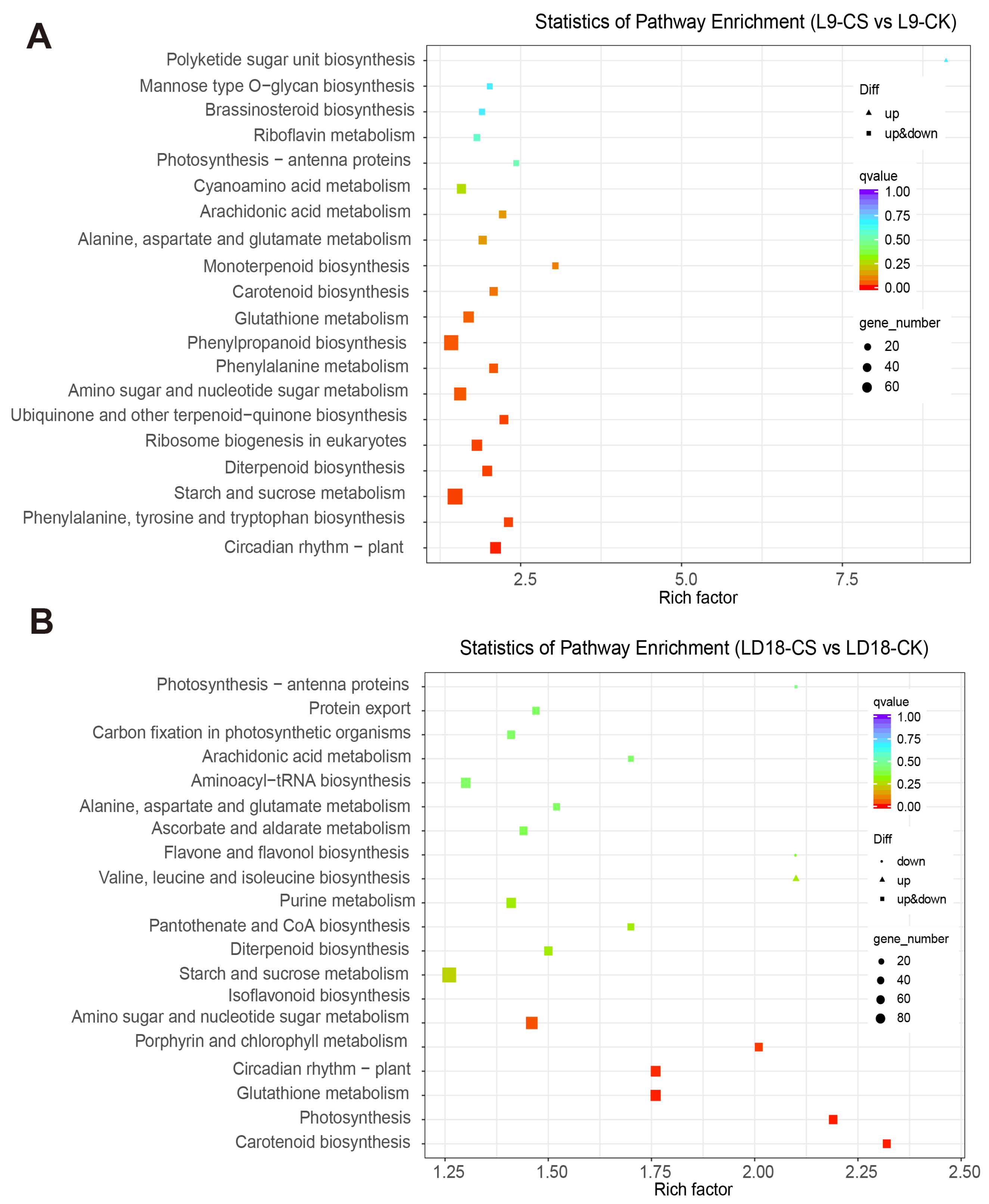
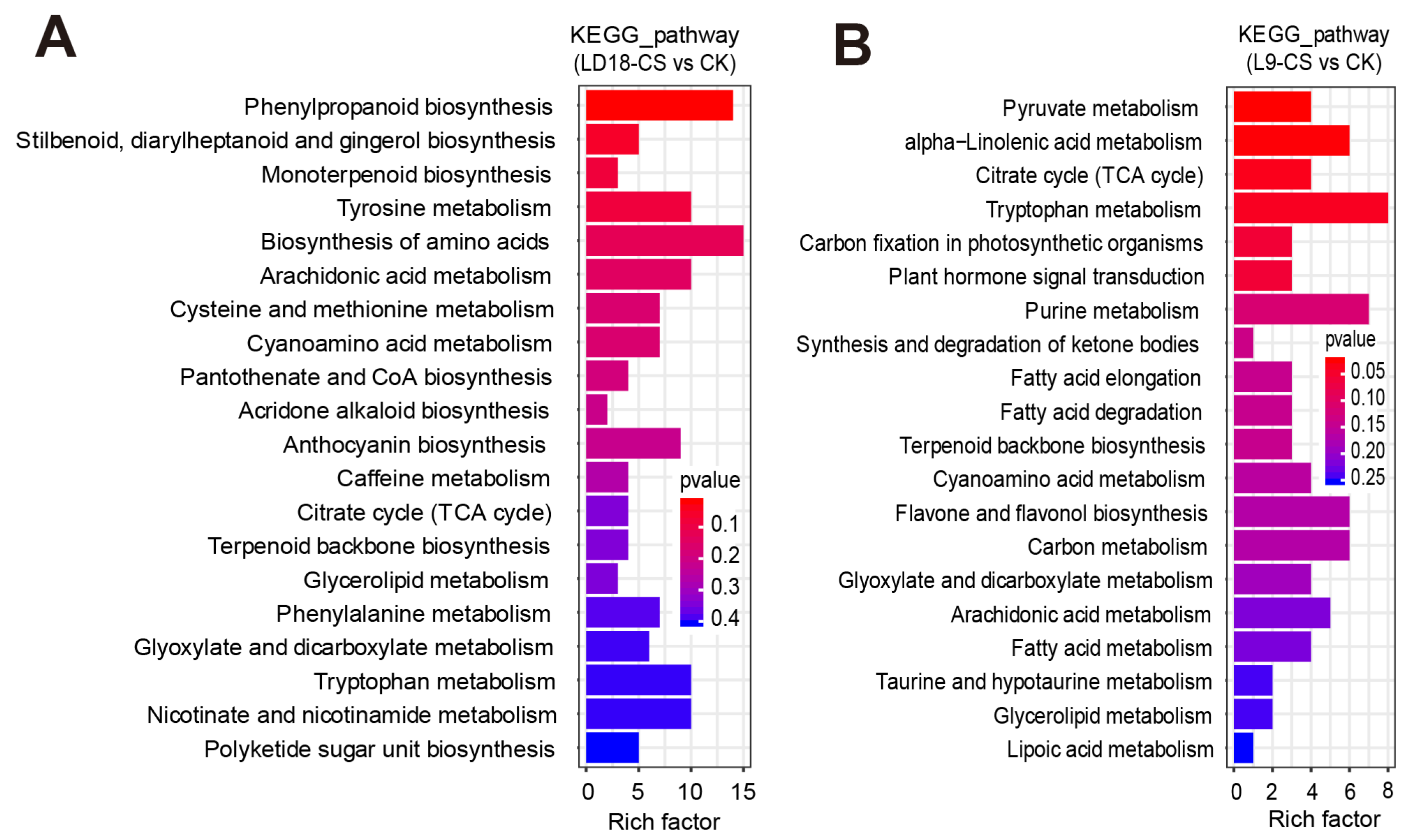
3.2. DEGs Involved in the Pathway of Photosynthesis
3.3. ABA Signaling in Cold Stress Response
3.4. MAPK Signaling in Cold Stress Response
3.5. Metabolite’s Roles in ROS Scavenging During Cold Stress Response
3.6. Collaborative Roles of MAPK and ABA Signaling in CS
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Cold Stress Treatments
4.3. Photosynthetic Measurements
4.4. Agronomic Traits
4.5. Antioxidant and Oxidoreductase Measurements
4.6. Transmitted Electron Microscopic Analysis
4.7. RNA Extraction and RNA Sequencing
4.8. Gene Ontology and KEGG Analysis
4.9. Quantitative Transcript Measurements
4.10. Metabolism Determinations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Li, J.; Pan, Y.; Li, J.; Zhou, L.; Shi, H.; Zeng, Y.; Guo, H.; Yang, S.; Zheng, W.; et al. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nat. Commun. 2017, 8, 14788. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, J.; Wang, X.; Essemine, J.; Jin, J.; Qu, M.; Xiang, Y.; Chen, W. Cold-induced inhibition of photosynthesis-related genes integrated by a TOP6 complex in rice mesophyll cells. Nucleic Acids Res. 2023, 51, 1823–1842. [Google Scholar] [CrossRef]
- Taghavi, T.; Aghajani, M. Effect of chilling duration on runner production and vegetative growth of ‘Pajaro’ strawberry. In Proceedings of the VIII International Strawberry Symposium 1156, Québec City, QC, Canada, 13 August 2016; pp. 505–508. [Google Scholar]
- Zhang, X.; Liu, T.; Zhu, S.; Wang, D.; Sun, S.; Xin, L. Short-term hypobaric treatment alleviates chilling injury by regulating membrane fatty acids metabolism in peach fruit. J. Food Biochem. 2022, 46, e14113. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Xin, Y.; Tan, Y.; Hu, X.; Bai, J.; Liu, Z.-Y.; Yu, Y.; Li, L.; Peng, C.; Fan, T.; et al. Natural variation in the HAN1 gene confers chilling tolerance in rice and allowed adaptation to a temperate climate. Proc. Natl. Acad. Sci. USA 2019, 116, 3494–3501. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, J.; Prerostova, S.; Černý, M.; Dobrev, P.; Gaudinova, A.; Knirsch, V.; Kobzová, E.; Müller, K.; Fiala, R.; Benczúr, K.; et al. Hormonal responses of rice to organ-targeted cold stress. Environ. Exp. Bot. 2024, 222, 105739. [Google Scholar] [CrossRef]
- Shahzad, N.; Nabi, H.G.; Qiao, L.; Li, W. The Molecular Mechanism of Cold-Stress Tolerance: Cold Responsive Genes and Their Mechanisms in Rice (Oryza sativa L.). Biology 2024, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants. Plant Physiol. Biochem. 2023, 197, 107646. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhu, S.; Li, H.; Zhang, B.; Liu, J.; Song, F. Further enhancement of cold tolerance in rice seedlings by Piriformospora indica collaborating with plant growth-promoting bacteria: Evidence from the antioxidant defense, osmoregulation, photosynthesis, and related genes. Plant Stress 2024, 14, 100656. [Google Scholar] [CrossRef]
- Mehrotra, S.; Verma, S.; Kumar, S.; Kumari, S.; Mishra, B.N. Transcriptional regulation and signalling of cold stress response in plants: An overview of current understanding. Environ. Exp. Bot. 2020, 180, 104243. [Google Scholar] [CrossRef]
- Ma, Q.; Dai, X.; Xu, Y.; Guo, J.; Liu, Y.; Chen, N.; Xiao, J.; Zhang, D.; Xu, Z.; Zhang, X.; et al. Enhanced tolerance to chilling stress in OsMYB3R-2 transgenic rice is mediated by alteration in cell cycle and ectopic expression of stress genes. Plant Physiol. 2009, 150, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, J.; Xu, Q.; Feng, Y.; Yuan, X.; Yu, H.; Wang, Y.; Wei, X.; Yang, Y. Genome-wide association study of cold tolerance of Chinese indica rice varieties at the bud burst stage. Plant Cell Rep. 2018, 37, 529–539. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Vo, K.T.X.; Nguyen, C.D.; Jeong, D.-H.; Lee, S.-K.; Kumar, M.; Kim, S.-R.; Park, S.-H.; Kim, J.-K.; Jeon, J.-S. Functional analysis of a cold-responsive rice WRKY gene, OsWRKY71. Plant Biotechnol. Rep. 2016, 10, 13–23. [Google Scholar] [CrossRef]
- Su, C.-F.; Wang, Y.-C.; Hsieh, T.-H.; Lu, C.-A.; Tseng, T.-H.; Yu, S.-M. A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol. 2010, 153, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, N.; Sato, Y.; Tanabe, S.; Chujo, T.; Shimizu, T.; Okada, K.; Yamane, H.; Shimono, M.; Sugano, S.; Takatsuji, H.; et al. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 2013, 64, 5085–5097. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, C.; Zhu, Y.; Zhang, L.; Chen, T.; Zhou, F.; Chen, H.; Lin, Y. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. J. Integr. Plant Biol. 2018, 60, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Essemine, J.; Bunce, J.A.; Shang, C.; Zhang, H.; Sun, D.; Chen, G.; Qu, M. Roles of heat shock protein and reprogramming of photosynthetic carbon metabolism in thermotolerance under elevated CO2 in maize. Environ. Exp. Bot. 2019, 168, 103869. [Google Scholar] [CrossRef]
- Zhou, S.; He, L.; Lin, W.; Su, Y.; Liu, Q.; Qu, M.; Xiao, L. Integrative analysis of transcriptome and metabolism reveals potential roles of carbon fixation and photorespiratory metabolism in response to drought in Shanlan upland rice. BMC Genom. 2022, 23, 862. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Feng, Y.; Wang, H.; Zhan, X.; Shen, X.; Wu, W.; Zhang, Y.; Chen, D.; Dai, G.; Yang, Z.; et al. Transcriptome analysis of rice root heterosis by RNA-Seq. BMC Genom. 2013, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhu, X.; Elling, A.A.; Chen, L.; Wang, X.; Guo, L.; Liang, M.; He, H.; Zhang, H.; Chen, F.; et al. Global epigenetic and transcriptional trends among two rice subspecies and their reciprocal hybrids. Plant Cell 2010, 22, 17–33. [Google Scholar] [CrossRef]
- Oono, Y.; Kawahara, Y.; Kanamori, H.; Mizuno, H.; Yamagata, H.; Yamamoto, M.; Hosokawa, S.; Ikawa, H.; Akahane, I.; Zhu, Z. mRNA-Seq reveals a comprehensive transcriptome profile of rice under phosphate stress. Rice 2011, 4, 50–65. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; He, S.; Huang, M.; Tan, J.; Zhao, L.; Yan, S.; Li, H.; Zhou, K.; Liang, Y.; et al. Cold stress selectively unsilences tandem repeats in heterochromatin associated with accumulation of H3K9ac. Plant Cell Environ. 2012, 35, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhong, Y.; Guo, A.; Zhu, Q.; Tang, W.; Zheng, W.; Gu, X.; Wei, L.; Luo, J. DRTF: A database of rice transcription factors. Bioinformatics 2006, 22, 1286–1287. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Yang, T.; Zeng, Z.; Huang, Z.; Liu, Q.; Wang, X.; Leach, J.; Leung, H.; Liu, B. Global transcriptional profiling of a cold-tolerant rice variety under moderate cold stress reveals different cold stress response mechanisms. Physiol. Plant. 2015, 154, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Shimono, H.; Hasegawa, T.; Fujimura, S.; Iwama, K. Responses of leaf photosynthesis and plant water status in rice to low water temperature at different growth stages. Field Crops Res. 2004, 89, 71–83. [Google Scholar] [CrossRef]
- Ariizumi, T.; Kishitani, S.; Inatsugi, R.; Nishida, I.; Murata, N.; Toriyama, K. An increase in unsaturation of fatty acids in phosphatidylglycerol from leaves improves the rates of photosynthesis and growth at low temperatures in transgenic rice seedlings. Plant Cell Physiol. 2002, 43, 751–758. [Google Scholar] [CrossRef]
- Yahmed, J.B.; de Oliveira, T.M.; Novillo, P.; Quinones, A.; Forner, M.-A.; Salvador, A.; Froelicher, Y.; Mimoun, M.B.; Talon, M.; Ollitrault, P.; et al. A simple, fast and inexpensive method to assess salt stress tolerance of aerial plant part: Investigations in the mandarin group. J. Plant Physiol. 2016, 190, 36–43. [Google Scholar] [CrossRef]
- Shi, Z.; Chang, T.; Chen, G.; Song, Q.; Wang, Y.; Zhou, Z.; Wang, M.; Qu, M.; Wang, B.; Zhu, X.G. Dissection of mechanisms for high yield in two elite rice cultivars. F. Crops Res. 2019, 241, 107563. [Google Scholar] [CrossRef]
- Cui, H. Challenges and approaches to crop improvement through C3-to-C4 engineering. Front. Plant Sci. 2021, 12, 715391. [Google Scholar] [CrossRef] [PubMed]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Stern, D.B. Increased Rubisco content in maize mitigates chilling stress and speeds recovery. Plant Biotechnol. J. 2020, 18, 1409–1420. [Google Scholar] [CrossRef]
- Fowler, S.; Thomashow, M.F. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 2002, 14, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Caselles, V.; Casadesús, A.; Munné-Bosch, S. A dual role for abscisic acid integrating the cold stress response at the whole-plant level in Iris pseudacorus L. growing in a natural wetland. Front. Plant Sci. 2021, 12, 722525. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cang, J.; Lu, Q.; Fan, B.; Xu, Q.; Li, W.; Wang, X. ABA enhanced cold tolerance of wheat ‘dn1′ via increasing ROS scavenging system. Plant Signal. Behav. 2020, 15, 1780403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, J.; Qu, G.; Chen, S. The cold-responsive C-repeat binding factors in Betula platyphylla Suk. positively regulate cold tolerance. Plant Sci. 2024, 341, 112012. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Lee, S.C. Arabidopsis SnRK2.3/SRK2I plays a positive role in seed germination under cold stress conditions. Environ. Exp. Bot. 2023, 212, 105399. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef] [PubMed]
- Vlad, F.; Rubio, S.; Rodrigues, A.; Sirichandra, C.; Belin, C.; Robert, N.; Leung, J.; Rodriguez, P.L.; Lauriere, C.; Merlot, S. Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 2009, 21, 3170–3184. [Google Scholar] [CrossRef]
- Fujita, Y.; Nakashima, K.; Yoshida, T.; Katagiri, T.; Kidokoro, S.; Kanamori, N.; Umezawa, T.; Fujita, M.; Maruyama, K.; Ishiyama, K.; et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Zhang, J.; Xie, H.; Zhang, L.; Zhang, H.; Feng, L.; Zhou, J.; Zhao, Y.; Rong, J. Identification and analysis of MAPK cascade gene families of Camellia oleifera and their roles in response to cold stress. Mol. Biol. Rep. 2024, 51, 602. [Google Scholar] [CrossRef]
- Teige, M.; Scheikl, E.; Eulgem, T.; Doczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant mitogen-activated protein kinase cascades in environmental stresses. Int. J. Mol. Sci. 2021, 22, 1543. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yamaguchi, K.; Yoshimura, S.; Terauchi, A.; Kawasaki, T. Conservation of chitin-induced MAPK signaling pathways in rice and Arabidopsis. Plant Cell Physiol. 2017, 58, 993–1002. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Zhang, C.; Zhu, P.; Dai, H.; Yu, N.; He, Z.; Xu, L.; Wang, E. OsCERK1-mediated chitin perception and immune signaling requires receptor-like cytoplasmic kinase 185 to activate an MAPK cascade in rice. Mol. Plant 2017, 10, 619–633. [Google Scholar] [CrossRef]
- Khan, N.; Choi, S.H.; Lee, C.H.; Qu, M.; Jeon, J.S. Photosynthesis: Genetic strategies adopted to gain higher efficiency. Int. J. Mol. Sci. 2024, 25, 8933. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ye, X.; Li, M.; Li, J.; Qi, H.; Hu, X. H2O2 and NO are involved in trehalose-regulated oxidative stress tolerance in cold-stressed tomato plants. Environ. Exp. Bot. 2020, 171, 103961. [Google Scholar] [CrossRef]
- Zhang, K.; Tian, G.; Li, X.; Zhang, Z.; Liu, J.; Li, Y.; Xie, J.; Wang, P. ROS produced via BsRBOHD plays an important role in low temperature-induced anthocyanin biosynthesis in begonia semperflorens. Russ. J. Plant Physiol. 2020, 67, 250–258. [Google Scholar] [CrossRef]
- Luo, Y.; Li, W.-M.; Wang, W. Trehalose: Protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environ. Exp. Bot. 2008, 63, 378–384. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, X.; Zhang, Y.; Huang, C.; Lin, D. Metabolomic Analysis of Trehalose Alleviating Oxidative Stress in Myoblasts. Int. J. Mol. Sci. 2023, 24, 13346. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.D.; Ebenezer, C.; Solomon, R.V.; Iyyappan, E. Deciphering the structure-property relationship and antioxidant mechanisms of trehalose—An in-silico approach. J. Mol. Struct. 2023, 1291, 135957. [Google Scholar] [CrossRef]
- Qu, M.; Essemine, J.; Li, M.; Chang, S.; Chang, T.; Chen, G.Y.; Zhu, X.G. Genome-wide association study unravels LRK1 as a dark respiration regulator in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 4930. [Google Scholar] [CrossRef]
- Wurzinger, B.; Nukarinen, E.; Nägele, T.; Weckwerth, W.; Teige, M. The SnRK1 kinase as central mediator of energy signaling between different organelles. Plant Physiol. 2018, 176, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Emanuelle, S.; Doblin, M.S.; Stapleton, D.I.; Bacic, A.; Gooley, P.R. Molecular insights into the enigmatic metabolic regulator, SnRK1. Trends Plant Sci. 2016, 21, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.; Nunes-Nesi, A.; Hajirezaei, M.R.; Hofmann, J.; Sonnewald, U.; Fernie, A.R.; Börnke, F. Altering trehalose-6-phosphate content in transgenic potato tubers affects tuber growth and alters responsiveness to hormones during sprouting. Plant Physiol. 2011, 156, 1754–1771. [Google Scholar] [CrossRef]
- Nunes, C.; Primavesi, L.F.; Patel, M.K.; Martinez-Barajas, E.; Powers, S.J.; Sagar, R.; Fevereiro, P.S.; Davis, B.G.; Paul, M.J. Inhibition of SnRK1 by metabolites: Tissue-dependent effects and cooperative inhibition by glucose 1-phosphate in combination with trehalose 6-phosphate. Plant Physiol. Biochem. 2013, 63, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef]
- Tsai, A.Y.-L.; Gazzarrini, S. Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: The emerging picture. Front. Plant Sci. 2014, 5, 119. [Google Scholar] [CrossRef]
- Qu, M.; Zheng, G.; Hamdani, S.; Essemine, J.; Song, Q.; Wang, H.; Chu, C.; Sirault, X.; Zhu, X.-G. Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey. Plant Physiol. 2017, 175, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Rossatto, T.; Amaral, M.N.D.; Benitez, L.C.; Vighi, I.L.; Braga, E.J.B.; Júnior, A.M.d.M.; Maia, M.A.C.; Pinto, L.d.S. Gene expression and activity of antioxidant enzymes in rice plants, cv. BRS AG, under saline stress. Physiol. Mol. Biol. Plants 2017, 23, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, T.; Zhao, Y.; Wang, X.; Wang, K.; Shen, Y.; Ding, Y.; Tang, S. Effects of high temperature on rice grain development and quality formation based on proteomics comparative analysis under field warming. Front. Plant Sci. 2021, 12, 746180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hugenholtz, J.; Abee, T.; Molenaar, D. Glutathione protects Lactococcus lactis against oxidative stress. Appl. Environ. Microbiol. 2003, 69, 5739–5745. [Google Scholar] [CrossRef]
- Li, L.; Singh, B.R. Role of zinc binding in type A botulinum neurotoxin light chain’s toxic structure. Biochemistry 2000, 39, 10581–10586. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Li, G.; Liu, L.; Zhang, Y.; Li, J.; Yang, M.; Chen, S.; Lin, Q.; Fu, G.; Zheng, D.; et al. OsGRF4AA compromises heat tolerance of developing pollen grains in rice. Front. Plant Sci. 2023, 14, 1121852. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, Z.; Ansah, E.O.; Peng, W.; Huang, L.; Xiong, F.; Li, P.; An, G.; Wang, W.; Wu, Y. Rice NADP-dependent malate dehydrogenase gene OsMDH8.2 is involved in heat tolerance. Fundam. Res. 2024, in press. [CrossRef]
- Qu, M.; Chen, G.; Bunce, J.A.; Zhu, X.; Sicher, R.C. Systematic biology analysis on photosynthetic carbon metabolism of maize leaf following sudden heat shock under elevated CO2. Sci. Rep. 2018, 8, 7849. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Thumma, B.R.; Sharma, N.; Southerton, S.G. Transcriptome sequencing of Eucalyptus camaldulensis seedlings subjected to water stress reveals functional single nucleotide polymorphisms and genes under selection. BMC Genom. 2012, 13, 364. [Google Scholar] [CrossRef]
- Kyndt, T.; Denil, S.; Haegeman, A.; Trooskens, G.; De Meyer, T.; Van Criekinge, W.; Gheysen, G. Transcriptome analysis of rice mature root tissue and root tips in early development by massive parallel sequencing. J. Exp. Bot. 2012, 63, 2141–2157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Essemine, J.; Shang, C.; Zhang, H.; Zhu, X.; Yu, J.; Chen, G.; Qu, M.; Sun, D. Combined proteomics and metabolism analysis unravels prominent roles of antioxidant system in the prevention of alfalfa (Medicago sativa L.) against salt stress. Int. J. Mol. Sci. 2020, 21, 909. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, G.; Li, Z.; Iqbal, Z.; Zhao, M.; Cui, Z.; Cao, L.; Zhou, J.; Lei, L.; Luo, Y.; Bai, L.; et al. Identifications of Genes Involved in ABA and MAPK Signaling Pathways Positively Regulating Cold Tolerance in Rice. Plants 2025, 14, 498. https://doi.org/10.3390/plants14040498
Ding G, Li Z, Iqbal Z, Zhao M, Cui Z, Cao L, Zhou J, Lei L, Luo Y, Bai L, et al. Identifications of Genes Involved in ABA and MAPK Signaling Pathways Positively Regulating Cold Tolerance in Rice. Plants. 2025; 14(4):498. https://doi.org/10.3390/plants14040498
Chicago/Turabian StyleDing, Guohua, Zhugang Li, Zubair Iqbal, Minghui Zhao, Zhibo Cui, Liangzi Cao, Jinsong Zhou, Lei Lei, Yu Luo, Liangming Bai, and et al. 2025. "Identifications of Genes Involved in ABA and MAPK Signaling Pathways Positively Regulating Cold Tolerance in Rice" Plants 14, no. 4: 498. https://doi.org/10.3390/plants14040498
APA StyleDing, G., Li, Z., Iqbal, Z., Zhao, M., Cui, Z., Cao, L., Zhou, J., Lei, L., Luo, Y., Bai, L., Yang, G., Wang, R., Li, K., Wang, X., Liu, K., Qu, M., & Sun, S. (2025). Identifications of Genes Involved in ABA and MAPK Signaling Pathways Positively Regulating Cold Tolerance in Rice. Plants, 14(4), 498. https://doi.org/10.3390/plants14040498






