Gymnema sylvestre as a Potential Anti-Inflammatory and Anti-Biofilm Agent Against Anaerobic Infections: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis by Gymnema sylvestre Extract
2.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Anaerobic Microorganisms
Antimicrobial Action on Monotypic Biofilms
2.3. Cytotoxicity by MTT Assay
2.4. Anti-Inflammatory Analysis by ELISA
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Equipment
4.3. Extract and Phytochemical Analysis
4.3.1. Soluble Solids Content in Ethanol
4.3.2. Determination of Total Phenol Content
4.3.3. Determination of the Total Flavonoid Content Expressed as Quercetin
4.4. Evaluation of Antioxidant Activity
4.5. Liquid Chromatographic Analysis
Liquid Chromatography Coupled to Mass Spectrometry
4.6. Antimicrobial Analysis
4.6.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Anaerobic Microorganisms
4.6.2. Antimicrobial Action on Monotypic Biofilms
4.7. Cell Testing
4.7.1. Cell Viability Test
4.7.2. Anti-Inflammatory Potential in Mouse Macrophages (RAW 264.7)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plantderived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska, W.; Bazylko, A. The Potential of Medicinal Plants and Natural Products in the Treatment of Burns and Sunburn—A Review. Pharmaceutics 2023, 15, 633. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Ansari, M.H.R.; Parveen, R.; Khan, W.; Ahmad, S.; Husain, S.A. Chromatography Based Metabolomics and In Silico Screening of Gymnema sylvestre Leaf Extract for Its Antidiabetic Potential. Evid.-Based Complement. Altern. Med. 2019, 2019, 7523159. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, D.D. Anatomical studies on the leaf of Gymnema sylvestre (Retz.) R. Br. ex Schult. (Apocynaceae): A magical herbal medicine for diabetes. Int. J. Herb. Med. 2016, 4, 70–72. [Google Scholar]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and Pharmacological Properties of Gymnema sylvestre: An Important Medicinal Plant. Biomed. Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef] [PubMed]
- Arun, L.B.; Arunachalam, A.M.; Arunachalam, K.D.; Annamalai, S.K.; Kumar, K.A. In vivo anti-ulcer, anti-stress, anti-allergic, and functional properties of Gymnemic Acid Isolated from Gymnema sylvestre R Br. BMC Complement. Altern. Med. 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Jangam, A.; Tirunavalli, S.K.; Adimoolam, B.M.; Kasireddy, B.; Patnaik, S.S.; Erukkambattu, J.; Thota, J.R.; Andugulapati, S.B.; Addlagatta, A. Anti-inflammatory and antioxidant activities of Gymnema sylvestre extract rescue acute respiratory distress syndrome in rats via modulating the NF-κB/MAPK pathway. Inflammopharmacology 2023, 31, 823–844. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, R.S.; Prashanth, K.V.H.; Manonmani, H.K. In Vitro Antidiabetic Effects of Isolated Triterpene Glycoside Fraction from Gymnema sylvestre. Evid. -Based Complement. Altern. Med. 2018, 2018, 7154702. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ong, S.T.; Annamalai, S.K.; Kamruddin, M.; Verma, N.K.; Ramakrishna, S.; Lakshminarayanan, R.; Arunachalam, K.D. Antimicrobial properties, and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mater. Sci. Eng. C. 2019, 98, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Kumar, N.; Sachan, A.; Lakhani, P.; Tutu, S.; Nath, R.; Sachan, A.K.; Dixit, R.K. Hypolipidaemic effects of Gymnema sylvestre on high fat diet induced dyslipidaemia in wistar rats. J. Clin. Diagn. Res. 2017, 11, FF01–FF05. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.R.; Alsayari, A.; Habib, A.H.; Wahab, S.; Nadig, A.P.R.; Rafeeq, M.M.; Binothman, N.; Aljadani, M.; Al-Dhuayan, I.S.; Alaqeel, N.K.; et al. Anti-Tumor Potential of Gymnema sylvestre Saponin Rich Fraction on In Vitro Breast Cancer Cell Lines and In Vivo Tumor-Bearing Mouse Models. Antioxidants 2023, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, H.; Qamar, I.; Bashir, M.; Jabeen, F.; Irfan, S.; Anwar, H. Gymnema sylvestre Supplementation Restores Normoglycemia, Corrects Dyslipidemia, and Transcriptionally Modulates Pancreatic and Hepatic Gene Expression in Alloxan-Induced Hyperglycemic Rats. Metabolites 2023, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Sarker, M.R.; Ming, L.C.; Mohamed, I.N.; Zhao, C.; Sheikh, B.Y.; Tsong, H.F.; Rashid, M.A. Comprehensive review on phytochemicals, pharmacological and clinical potentials of Gymnema sylvestre. Front. Pharmacol. 2019, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R. Pharmacognostic and phytochemical valuation of Gymnema sylvestre leaf. World J. Pharm. Pharm. Sci. 2017, 6, 1532–1538. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, T.-H.; Zhang, M.-Q.; Li, X.; Xu, Y.-J.; Jiang, H.-Y.; Liu, T.-H.; Xu, D.-M. Chemical constituents from the stems of Gymnema sylvestre. Chin. J. Nat. Med. 2014, 12, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Sood, H. In vitro antimicrobial potential of extracts and phytoconstituents from Gymnema sylvestre R.Br. leaves and their biosafety evaluation. AMB Express. 2017, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- David, B.C.; Sudarsanam, G. Antimicrobial activity of Gymnema sylvestre (Asclepiadaceae). J. Acute Dis. 2013, 2, 222–225. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm, and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Byun, J.; Kim, M.; Lee, Y.; Lee, K.; Chong, Y. Antimicrobial Susceptibility Patterns of Anaerobic Bacterial Clinical Isolates from 2014 to 2016, Including Recently Named or Renamed Species Bacterial isolates. Ann. Lab. Med. 2019, 39, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Rasheed, M.A.; Niaz, Q.; Ashraf, M.; Anjum, A.A.; Ahmed, M.U. Evaluation of antibacterial effect of Gymnema sylvestre R.Br. species cultivated in Pakistan. Pak. Vet. J. 2017, 37, 245–250. [Google Scholar]
- Liu, M.; Zhou, T.; Zhang, J.; Liao, G.; Lu, R.; Yang, X. Identification of C21 steroidal glycosides from Gymnema sylvestre (Retz.) and evaluation of their glucose uptake activities. Molecules 2021, 26, 6549. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Cecere, M.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Tchorz, J.P.; Al-Ahmad, A. Compounds from Olea europaea and Pistacia lentiscus inhibit oral microbial growth. BMC Complement. Altern. Med. 2019, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Shekar, B.R.C.; Nagarajappa, R.; Jain, R.; Singh, R.; Suma, S.; Thakur, R. Antimicrobial Efficacy of Acacia nilotica, Murraya koenigii L. Sprengel, Eucalyptus hybrid, Psidium guajava extracts and their combinations on Fusobacterium nucleatum and Porphyromonas gingivalis. Indian. J. Dent. Res. 2018, 29, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Kohli, D.; Hugar, S.M.; Bhat, K.G.; Shah, P.; Mundada, M.V.; Badakar, C.M. Comparative evaluation of the antimicrobial susceptibility and cytotoxicity of husk extract of Cocos nucifera and chlorhexidine as irrigating solutions against Enterococcus Faecalis, Prevotella Intermedia and Porphyromonas Gingivalis—An in-vitro study. J. Indian. Soc. Pedod. Prev. Dent. 2018, 36, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.R.; Olsen, J.; Cuesta, A.; Silva-Vetri, M.; Hernández, M.; Romanos, G.; Santosh, A.B.R.; Palma, P. In vitro microbiological analysis on antibacterial, anti-inflammatory, and inhibitory action on matrix metalloproteinases-8 of commercially available chlorhexidine digluconate mouth rinses. Indian. J. Dent. Res. 2018, 29, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Takase, H.; Nakamura, M.; Makino, T. Effect of Lonicera caerulea var. emphyllocalyx Fruit on Biofilm Formed by Porphyromonas gingivalis. Biomed. Res. Int. 2019, 2019, 3547858. [Google Scholar] [CrossRef]
- González, O.A.; Escamilla, C.; Danaher, R.J.; Dai, J.; Ebersole, J.L.; Mumper, R.J.; Miller, C.S. Antibacterial effects of blackberry extract target periodontopathogens. J. Periodontal Res. 2013, 48, 80–86. [Google Scholar] [CrossRef]
- Mangalekar, S.B.; Reddy, H.; Shetty, Y.S.; Shankarapillai, R.; Vivekanandan, G.; Reddy, C.S. Evaluation of the efficacy of guava extract as an antimicrobial agent on periodontal pathogens. J. Contemp. Dent. Pract. 2018, 19, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Yiemwattana, I.; Chaisomboon, N.; Jamdee, K. Antibacterial and Anti-inflammatory Potential of Morus alba Stem Extract. Open Dent. J. 2018, 12, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial Activity of the Phenolic Compounds of Prunus mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.B.; de Almeida-Apolonio, A.A.; Alfredo, T.M.; Dantas, F.G.d.S.; Campos, J.F.; Cardoso, C.A.L.; Souza, K.d.P.; de Oliveira, K.M.P. Chemical Composition, Antimicrobial Activity, and Antioxidant Activity of Ocotea minarum (Nees & Mart.) Mez. Oxidative Med. Cell. Longev. 2019, 2019, 5736919. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Mendoza, L.; Cotoras, M. Alteration of oxidative phosphorylation as a possible mechanism of the antifungal action of p-coumaric acid against Botrytis cinerea. J. Appl. Microbiol. 2017, 123, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Pernin, A.; Guillier, L.; Dubois-brissonnet, F. Inhibitory activity of phenolic acids against Listeria monocytogenes: Deciphering the mechanisms of action using three different models. J. Food Microbiol. 2019, 80, 18–24. [Google Scholar] [CrossRef]
- Zulkarnain, M.Z.; Tong, W.Y.; Tan, W.-N.; Leong, C.R.; Yusof, F.A.M.; Wahidin, S.; Attifah, N.R.; Zubaidah, S. p-Coumaric acid quantum dots inhibit beta lactam resistant foodborne microorganisms. Mater. Today Proc. 2020, 31, 48–53. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control. 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Merlani, M.; Barbakadze, V.; Amiranashvili, L.; Gogilashvili, L.; Poroikov, V.; Petrou, A.; Geronikaki, A.; Ciric, A.; Glamoclija, J.; Sokovic, M. New Caffeic Acid Derivatives as Antimicrobial Agents: Design, Synthesis, Evaluation and Docking. Curr. Top. Med. Chem. 2019, 19, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Sekita, K.; Umemura, T.; Saito, M.; Ono, A.; Kawasaki, Y.; Uchida, O.; Matsushima, Y.; Inoue, T.; Kanno, J. Gymnema sylvestre leaf extract: A 52-week dietary toxicity study in Wistar rats. Shokuhin Eiseigaku Zasshi 2004, 45, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Anjum, M.; Tanveer, M.Z.; Iqbal, M. Evaluation of Antibacterial and Cytotoxic Effects of Gymnema sylvestre R.BR. Species Cultivated in Pakistan Against Common Resistant Pathogens. Value Health 2016, 19, 831. [Google Scholar] [CrossRef]
- Shim, J.H.; Cho, K.J.; Lee, K.A.; Kim, S.H.; Myung, P.K.; Choe, Y.K.; Yoon, D.Y. E7-expressing HaCaT keratinocyte cells are resistant to oxidative stress-induced cell death via the induction of catalase. Proteomics 2005, 5, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Ghosh, S.; Bishayee, K.; Mukherjee, A.; Sikdar, S.; Khuda-Bukhsh, A.R. Antihyperglycemic Drug Gymnema sylvestre Also Shows Anticancer Potentials in Human Melanoma A375 Cells via Reactive Oxygen Species Generation and Mitochondria-Dependent Caspase Pathway. Integr. Cancer Ther. 2013, 12, 433–441. [Google Scholar] [CrossRef]
- Vannini, S.; Villarini, M.; Levorato, S.; Salvatori, T.; Fatigoni, C.; Pagiotti, R.; Moretti, M. In vitro evalutation of cytotoxic, genotoxic, and apoptotic properties of herbal products from leaves of Gymnema sylvestre. Int. J. Herb. Med. 2017, 5, 33–38. [Google Scholar]
- Malik, J.K.; Manvi, F.V.; Alagawadi, K.R.; Noolvi, M. Evaluation of anti-inflammatory activity of Gymnema sylvestre leaves extract in rats. Int. J. Green. Pharm. 2008, 2, 114–115. [Google Scholar] [CrossRef]
- Yasukawa, K.; Okuda, S.; Nobushi, Y. Inhibitory Effects of Gymnema (Gymnema sylvestre) Leaves on Tumour Promotion in Two-Stage Mouse Skin Carcinogenesis. Evid. Based Complement. Altern. Med. 2014, 2014, 13–17. [Google Scholar] [CrossRef]
- Aleisa, A.M.; Al-rejaie, S.S.; Abuohashish, H.M.; Ola, M.S.; Parmar, M.Y.; Ahmed, M.M. Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement. Altern. Med. 2014, 14, 49. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Frani, M.; Butera, A. Ozonized gels vs chlorhexidine in non-surgical periodontal treatment: A randomized clinical trial. Oral. Dis. 2023; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Elbay, M.; Elbay, Ü.Ş.; Kaya, E.; Kalkan, Ö.P. Effects of photobiomodulation with different application parameters on injection pain in children: A randomized clinical trial. J. Clin. Pediatr. Dent. 2023, 47, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Fatani, A.J.; Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Assaf, A.; Parmar, M.Y.; Ola, M.S.; Ahmed, M.M. Neuroprotective effects of Gymnema sylvestre on streptozotocin-induced diabetic neuropathy in rats. Exp. Ther. Med. 2015, 9, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Abreu, V.G.; Correa, G.M.; Silva, T.M.; Fontoura, H.S.; Cara, D.C.; Piló-Veloso, D.; Alcântara, A.F. Anti-inflammatory effects in muscle injury by transdermal application of gel with Lychnophora pinaster aerial parts using phonophoresis in rats. BMC Complement. Altern. Med. 2013, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.J.; Ngoc, T.M.; Bae, K.; Cho, H.-J.; Kim, D.-D.; Chun, J.; Khan, S.; Kim, Y.S. Anti-inflammatory properties of anthraquinones and their relationship with the regulation of P-glycoprotein function and expression. Eur. J. Pharm. Sci. 2013, 48, 272–281. [Google Scholar] [CrossRef]
- Ortega, Y.H.; Foubert, K.; Berghe, W.V.; Chirumamilla, C.S.; Pieters, L.; Mosquera, D.M.G.; Apers, S. Flavonol glycosides from the leaves of Boldoa purpurascens and their anti-inflammatory properties. Phytochem. Lett. 2017, 19, 71–76. [Google Scholar] [CrossRef]
- Somensi, L.B.; Costa, P.; Boeing, T.; Mariano, L.N.B.; Longo, B.; Magalhães, C.G.; Duarte, L.P.; e Silva, A.T.M.; de Souza, P.; de Andrade, S.F.; et al. Gastroprotective properties of Lupeol-derived ester: Pre-clinical evidences of Lupeol-stearate as a potent antiulcer agent. Chem. Biol. Interact. 2020, 108964. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 7th ed.; Approved Standard; CLSI: Wayne, PA, USA, 2007; Volume 27, no. 2 M11-A7. [Google Scholar]
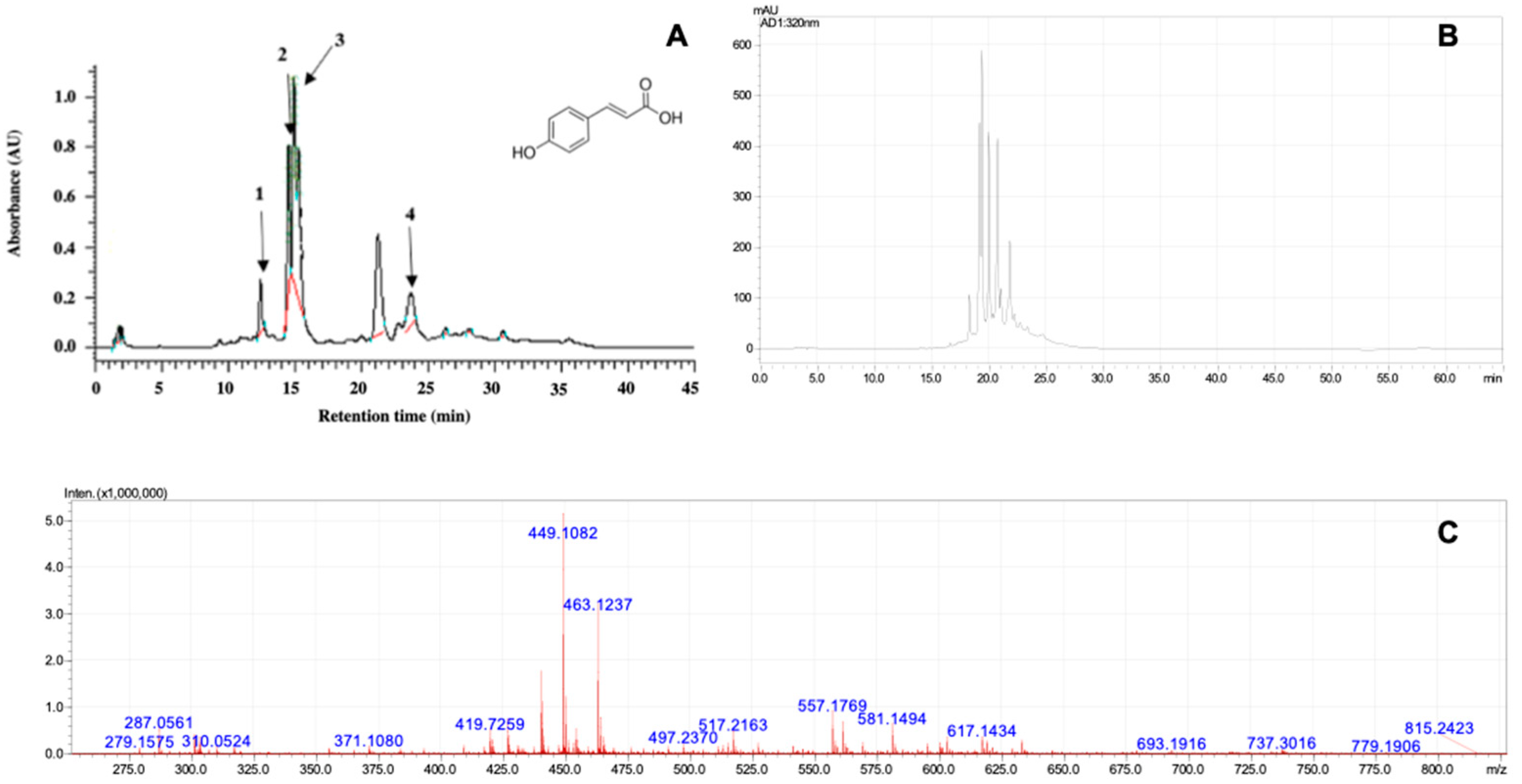
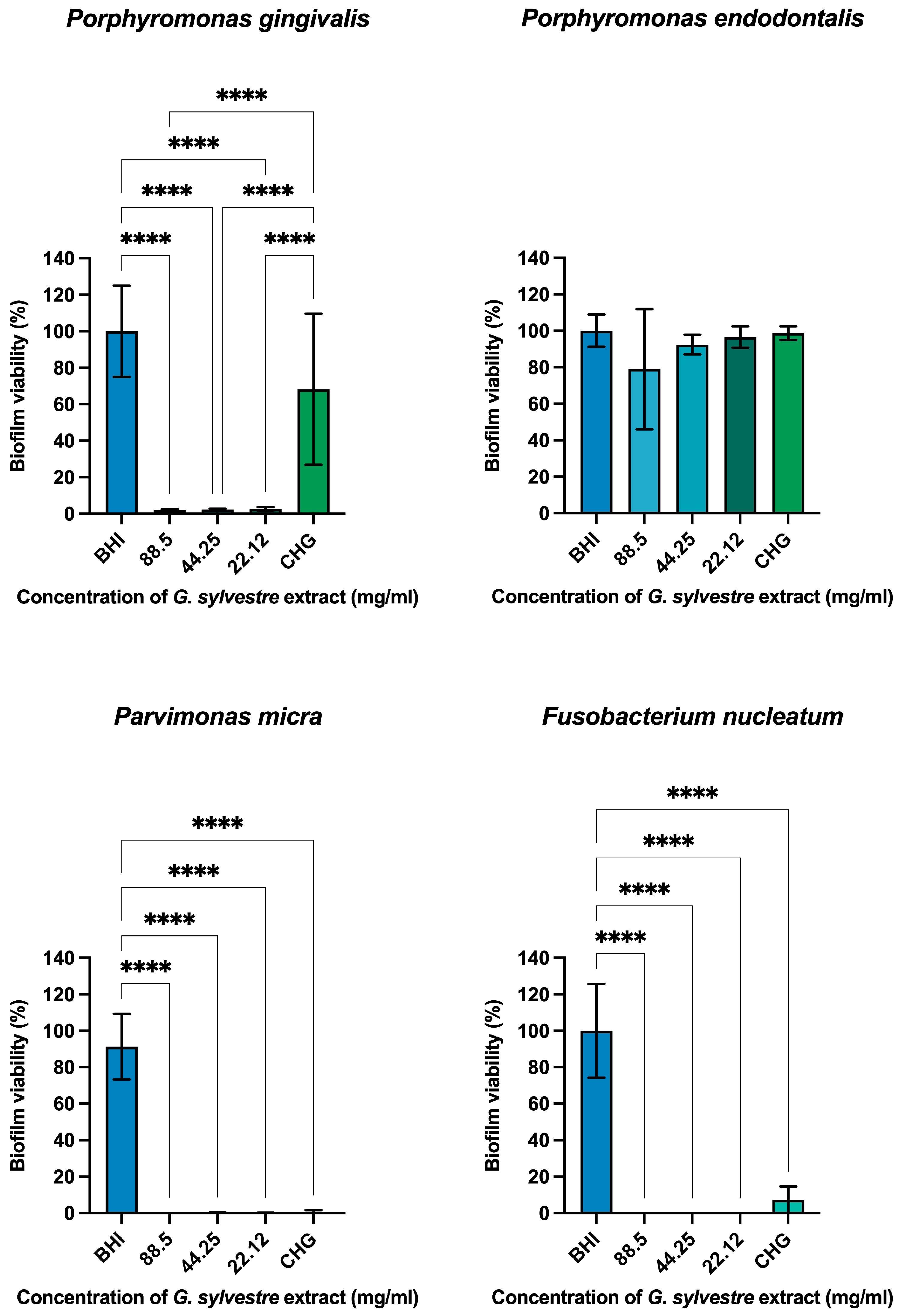
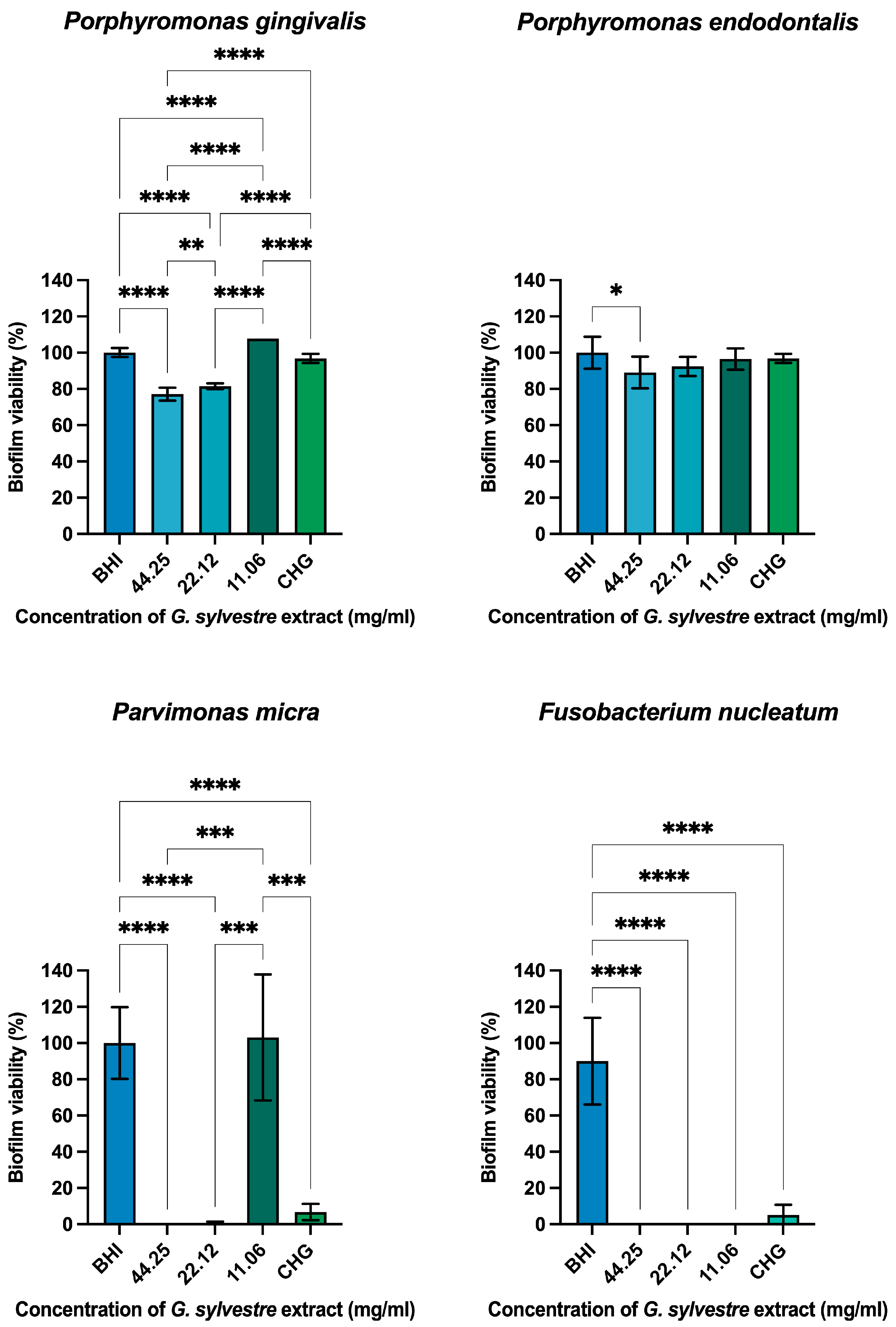
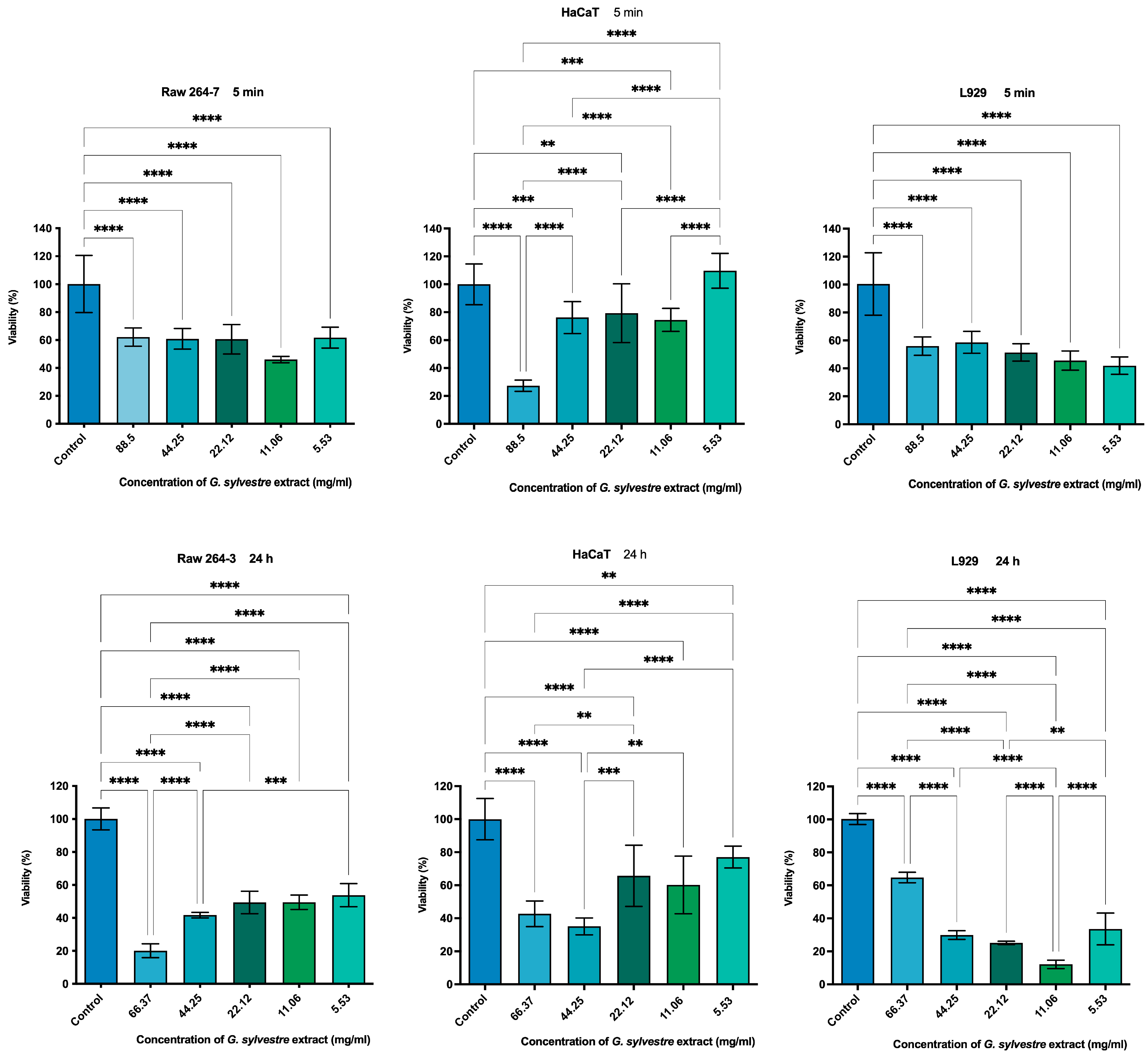

| Microorganism | MBC |
|---|---|
| Porphyromonas gingivalis | 22.12 mg/mL |
| Porphyromonas endodontalis | 22.12 mg/mL |
| Parvimonas micra | 11.06 mg/mL |
| Fusobacterium nucleatum | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, D.G.; Tomé, F.M.; Miguel, M.M.V.; Liberato, S.F.d.S.; Marcucci, M.C.; Vigerelli, H.; Rodrigues, F.P.; Pacheco-Soares, C.; Godoi, B.H.; Carrouel, F.; et al. Gymnema sylvestre as a Potential Anti-Inflammatory and Anti-Biofilm Agent Against Anaerobic Infections: An In Vitro Study. Plants 2025, 14, 497. https://doi.org/10.3390/plants14040497
Miranda DG, Tomé FM, Miguel MMV, Liberato SFdS, Marcucci MC, Vigerelli H, Rodrigues FP, Pacheco-Soares C, Godoi BH, Carrouel F, et al. Gymnema sylvestre as a Potential Anti-Inflammatory and Anti-Biofilm Agent Against Anaerobic Infections: An In Vitro Study. Plants. 2025; 14(4):497. https://doi.org/10.3390/plants14040497
Chicago/Turabian StyleMiranda, Diego Garcia, Fernanda Malagutti Tomé, Manuela Maria Viana Miguel, Sabrina Ferreira dos Santos Liberato, Maria Cristina Marcucci, Hugo Vigerelli, Flavia Pires Rodrigues, Cristina Pacheco-Soares, Bruno Henrique Godoi, Florence Carrouel, and et al. 2025. "Gymnema sylvestre as a Potential Anti-Inflammatory and Anti-Biofilm Agent Against Anaerobic Infections: An In Vitro Study" Plants 14, no. 4: 497. https://doi.org/10.3390/plants14040497
APA StyleMiranda, D. G., Tomé, F. M., Miguel, M. M. V., Liberato, S. F. d. S., Marcucci, M. C., Vigerelli, H., Rodrigues, F. P., Pacheco-Soares, C., Godoi, B. H., Carrouel, F., de Oliveira, L. D., & Ramos, L. d. P. (2025). Gymnema sylvestre as a Potential Anti-Inflammatory and Anti-Biofilm Agent Against Anaerobic Infections: An In Vitro Study. Plants, 14(4), 497. https://doi.org/10.3390/plants14040497










