Comprehensive Analysis of Boron-Induced Changes in Cell Expansion and Phytohormone During Early Ovary Development in Pear (Pyrus sinkiangensis Yu)
Abstract
1. Introduction
2. Results
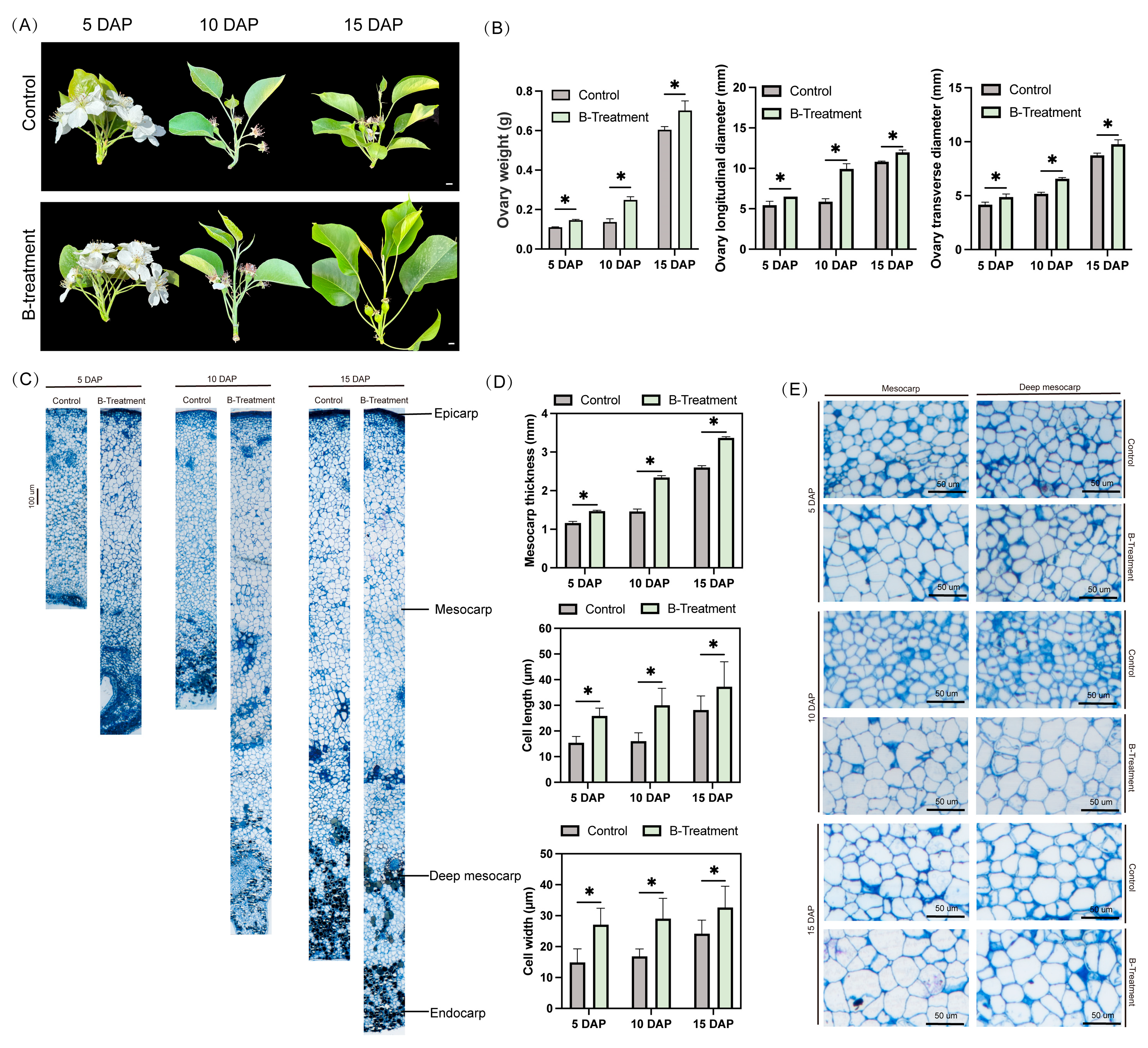
2.1. Exogenous Boron Increases Ovary Weight and Promotes Cell Expansion
2.2. Effect of Exogenous Boron Application on Internal Boron Concentration and Fruit Set Rate
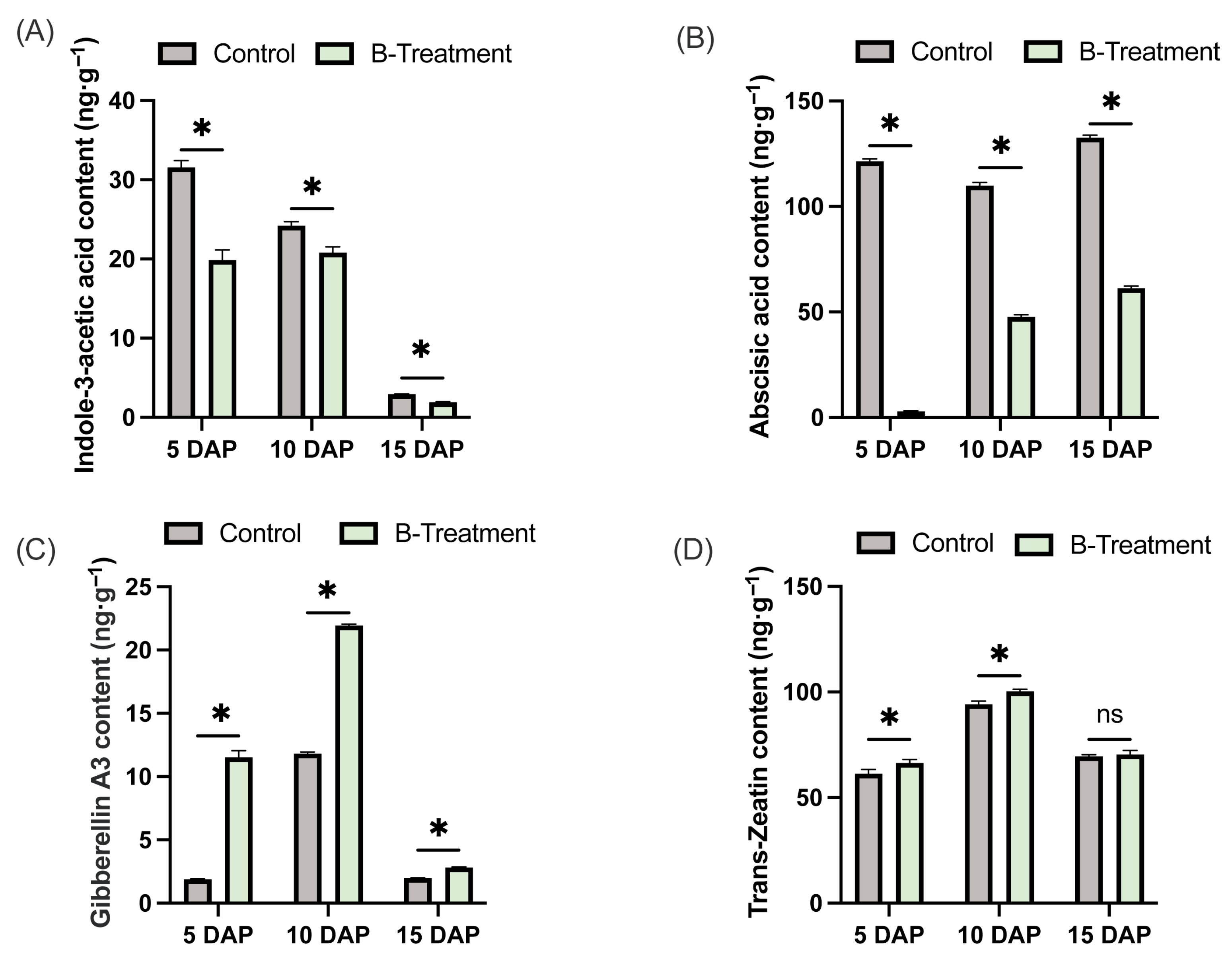
2.3. Changes in Phyhormone Content in Pear Ovaries with Boron Treatment
2.4. Boron-Mediated Phyhormone Signaling-Related Gene Expression in Ovary
2.4.1. Effect of Boron on Auxin Signaling-Related Gene Expression
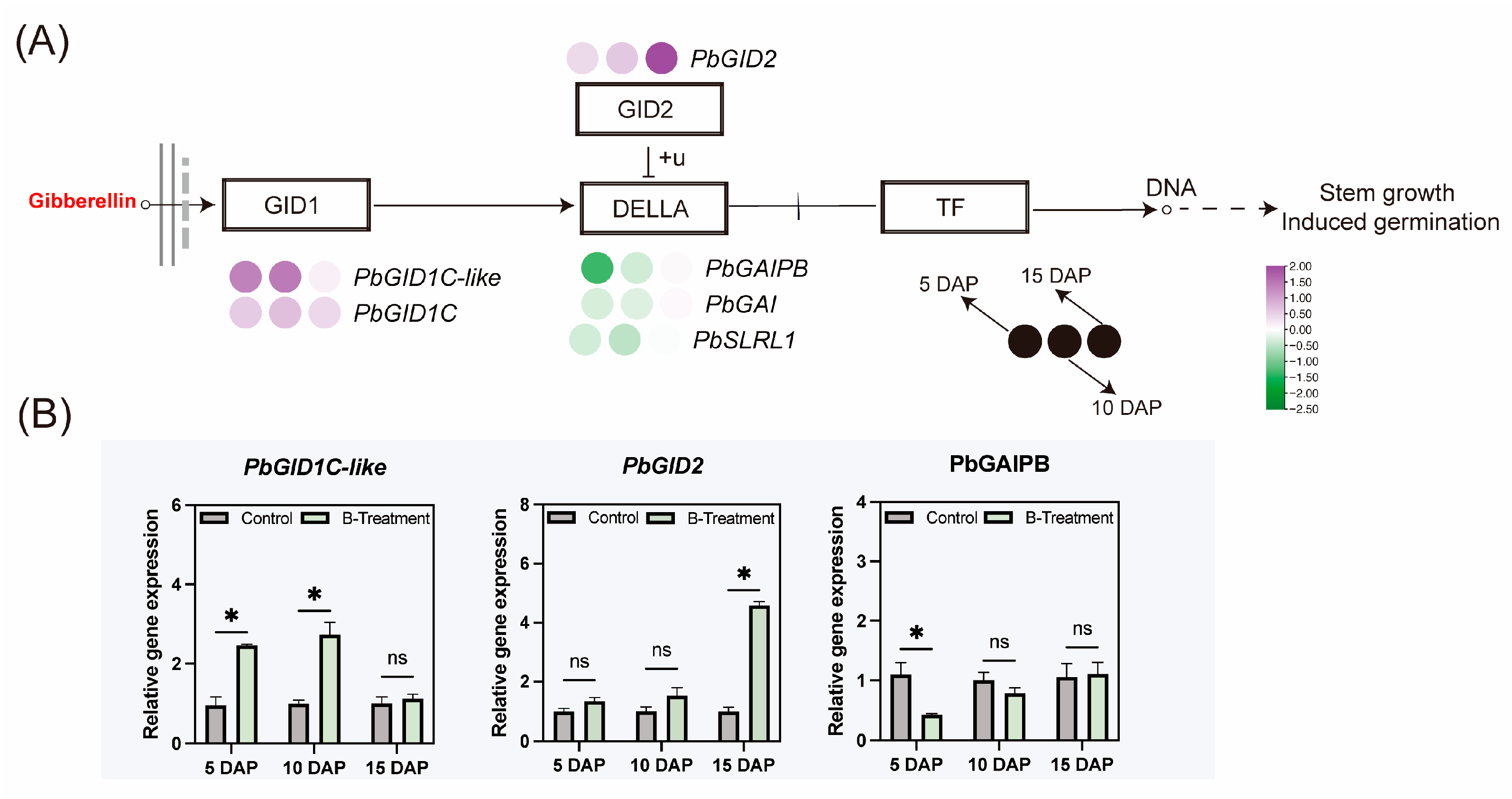
2.4.2. Effect of Boron on Gibberellin Signaling-Related Gene Expression
2.4.3. Effect of Boron on ABA Signaling-Related Gene Expression
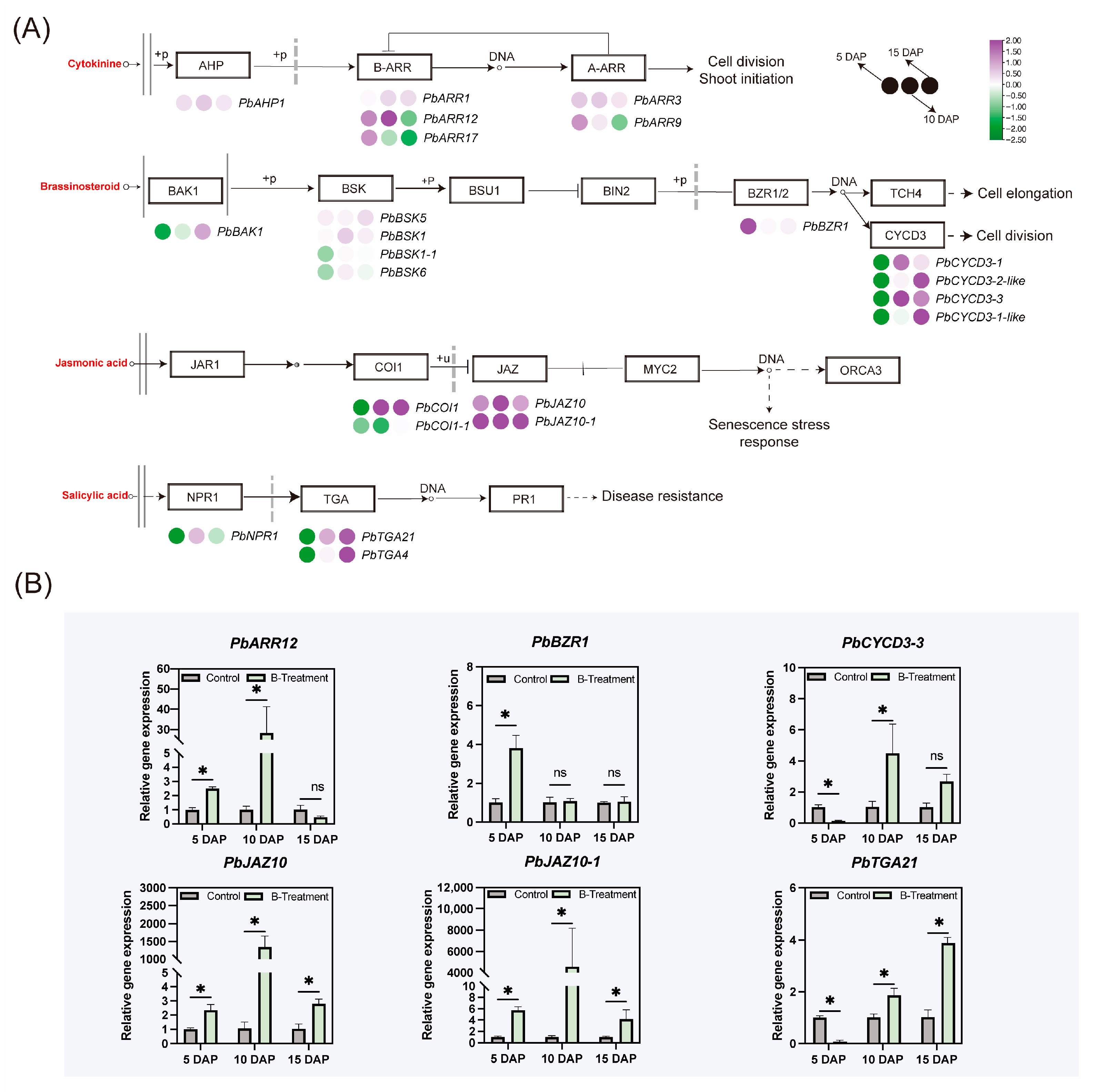
2.4.4. Effect of Boron on Other Phytohormones Signaling-Related Gene Expression
3. Discussion
3.1. Boron Treatment Promoted Cell Expansion in the Ovaries of ‘Kuerle Xiangli’
3.2. Effects of Boron Application on Fruit Set Rate in ‘Kuerle Xiangli’
3.3. Boron-Induced Hormonal Alterations in the Ovary
3.4. Boron-Mediated Regulation of Hormonal Signaling Pathways in the Ovary
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Boric Acid Treatments
4.3. Fruit Set Rate
4.4. Determination of Hormone Content
4.5. Histological Analysis
4.6. RNA Extraction and Quantitative Real-Time PCR
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAP | Days After Pollination |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| HPLC-MS/MS | High-Performance Liquid Chromatography–Tandem Mass Spectrometry |
| GA | Gibberellin |
| ABA | Abscisic Acid |
| IAA | Indole-3-Acetic Acid |
| tZ | Trans-Zeatin |
| cZ | Cis-Zeatin |
| iP | Isopentenyladenine |
| BR | Brassinosteroid |
| JA | Jasmonic Acid |
| SA | Salicylic Acid |
| ROS | Reactive Oxygen Species |
| DAA | Days After Anthesis |
| EBK/CS | Epicastasterone/Castasterone |
| DW | Dry Weight |
References
- Garratt, M.P.D.; O’Connor, R.S.; Carvell, C.; Fountain, M.T.; Breeze, T.D.; Pywell, R.; Redhead, J.W.; Kinneen, L.; Mitschunas, N.; Truslove, L.; et al. Addressing pollination deficits in orchard crops through habitat management for wild pollinators. Ecol. Appl. 2023, 33, e2743. [Google Scholar] [CrossRef]
- Sabir, I.A.; Liu, X.; Jiu, S.; Whiting, M.; Zhang, C. Plant Growth Regulators Modify Fruit Set, Fruit Quality, and Return Bloom in Sweet Cherry. HortScience 2021, 56, 922–931. [Google Scholar] [CrossRef]
- Iwai, H.; Hokura, A.; Oishi, M.; Chida, H.; Ishii, T.; Sakai, S.; Satoh, S. The gene responsible for borate cross-linking of pectin Rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization. Proc. Natl. Acad. Sci. USA 2006, 103, 16592–16597. [Google Scholar] [CrossRef]
- Abd El-wahed, A.E.-w.N.; Khalifa, S.M.; Alqahtani, M.D.; Abd Alrazik, A.M.; Abdel-Aziz, H.; Mancy, A.; Elnaggar, I.A.; Alharbi, B.M.; Hamdy, A.; Elkelish, A. Nano-enhanced growth and resilience strategies for Pomegranate cv. Wonderful: Unveiling the impact of zinc and boron nanoparticles on fruit quality and abiotic stress management. J. Agric. Food Res. 2024, 15, 100908. [Google Scholar] [CrossRef]
- Portela, E.; Ferreira-Cardoso, J.; Louzada, J.; Gomes-Laranjo, J. Assessment of Boron Application in Chestnuts: Nut Yield and Quality. J. Plant Nutr. 2015, 38, 973–987. [Google Scholar] [CrossRef]
- Paula Silva, A.; Eduardo, R.; Haneklaus, S.H. Influence of Foliar Boron Application on Fruit Set and Yield of Hazelnut. J. Plant Nutr. 2003, 26, 561–569. [Google Scholar] [CrossRef]
- Chen, X.; Smith, S.M.; Shabala, S.; Yu, M. Phytohormones in plant responses to boron deficiency and toxicity. J. Exp. Bot. 2022, 74, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Godoy, F.; Kühn, N.; Muñoz, M.; Marchandon, G.; Gouthu, S.; Deluc, L.; Delrot, S.; Lauvergeat, V.; Arce-Johnson, P. The role of auxin during early berry development in grapevine as revealed by transcript profiling from pollination to fruit set. Hortic. Res. 2021, 8, 140. [Google Scholar] [CrossRef]
- Israeli, A.; Schubert, R.; Man, N.; Teboul, N.; Serrani Yarce, J.C.; Rosowski, E.E.; Wu, M.-F.; Levy, M.; Efroni, I.; Ljung, K.; et al. Modulating auxin response stabilizes tomato fruit set. Plant Physiol. 2023, 192, 2336–2355. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Beauvoit, B.P.; Takahara, M.; Hao, S.; Ezura, K.; Andrieu, M.-H.; Nishida, K.; Mori, K.; Suzuki, Y.; Kuhara, S.; et al. Fruit setting rewires central metabolism via gibberellin cascades. Proc. Natl. Acad. Sci. USA 2020, 117, 23970–23981. [Google Scholar] [CrossRef]
- de Jong, M.; Mariani, C.; Vriezen, W.H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 2009, 60, 1523–1532. [Google Scholar] [CrossRef]
- Kohli, A.; Sreenivasulu, N.; Lakshmanan, P.; Kumar, P.P. The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep. 2013, 32, 945–957. [Google Scholar] [CrossRef]
- Tao, L.; Zhu, H.; Huang, Q.; Xiao, X.; Luo, Y.; Wang, H.; Li, Y.; Li, X.; Liu, J.; Jásik, J.; et al. PIN2/3/4 auxin carriers mediate root growth inhibition under conditions of boron deprivation in Arabidopsis. Plant J. 2023, 115, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Cristóbal, J.J.; Martín Rejano, E.M.; Herrera Rodríguez, M.B.; Navarro Gochicoa, M.T.; Rexach, J.; González Fontes, A. Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings. J. Exp. Bot. 2015, 66, 3831–3840. [Google Scholar] [CrossRef] [PubMed]
- Poza-Viejo, L.; Abreu, I.; González-García, M.P.; Allauca, P.; Bonilla, I.; Bolaños, L.; Reguera, M. Boron deficiency inhibits root growth by controlling meristem activity under cytokinin regulation. Plant Sci. 2018, 270, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, S.; Wang, C.; Ding, G.; Cai, H.; Shi, L.; Xu, F. Induction of jasmonic acid biosynthetic genes inhibits Arabidopsis growth in response to low boron. J. Integr. Plant Biol. 2021, 63, 937–948. [Google Scholar] [CrossRef]
- Zhang, C.; He, M.; Wang, S.; Chu, L.; Wang, C.; Yang, N.; Ding, G.; Cai, H.; Shi, L.; Xu, F. Boron deficiency-induced root growth inhibition is mediated by brassinosteroid signalling regulation in Arabidopsis. Plant J. 2021, 107, 564–578. [Google Scholar] [CrossRef]
- Zhou, D.; Shen, W.; Cui, Y.; Liu, Y.; Zheng, X.; Li, Y.; Wu, M.; Fang, S.; Liu, C.; Tang, M.; et al. APICAL SPIKELET ABORTION (ASA) Controls Apical Panicle Development in Rice by Regulating Salicylic Acid Biosynthesis. Front. Plant Sci. 2021, 12, 636877. [Google Scholar] [CrossRef]
- Martínez-Mazón, P.; Bahamonde, C.; Herrera-Rodríguez, M.B.; Fernández-Ocaña, A.M.; Rexach, J.; González-Fontes, A.; Camacho-Cristóbal, J.J. Role of ABA in the adaptive response of Arabidopsis plants to long-term boron toxicity treatment. Plant Physiol. Biochem. 2023, 202, 107965. [Google Scholar] [CrossRef]
- Hegazi, E.S.; El-Motaium, R.A.; Yehia, T.A.; Hashim, M.E. Effect of foliar boron application on boron, chlorophyll, phenol, sugars and hormones concentration of olive (Olea europaea L.) buds, leaves, and fruits. J. Plant Nutr. 2018, 41, 749–765. [Google Scholar] [CrossRef]
- Michailidis, M.; Bazakos, C.; Kollaros, M.; Adamakis, I.-D.S.; Ganopoulos, I.; Molassiotis, A.; Tanou, G. Boron stimulates fruit formation and reprograms developmental metabolism in sweet cherry. Physiol. Plant. 2023, 175, e13946. [Google Scholar] [CrossRef]
- Abobatta, W.F.; El-Enin, M.M.S.A.; El-Zawily, H.M.A.E.-M.; Saif, M.I. Effect of foliar application of boric acid and ammonium molybdate on the productivity and fruit quality of Navel orange. Sci. Hortic. 2024, 334, 113290. [Google Scholar] [CrossRef]
- Jiang, T.; Luo, C.; Mo, X.; Zhang, X.; Li, X.; Li, J.; He, X. Transcriptome analysis to identify candidate genes that response to GA3 and CPPU treatments for mango fruit development. Plant Growth Regul. 2024, 104, 885–897. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Guan, P.; Hu, J.; Sun, L. Interaction of VvDELLA2 and VvCEB1 Mediates Expression of Expansion-Related Gene during GA-Induced Enlargement of Grape Fruit. Int. J. Mol. Sci. 2023, 24, 14870. [Google Scholar] [CrossRef]
- Loomis, W.D.; Durst, R.W. Chemistry and biology of boron. Biofactors 1992, 3, 229–239. [Google Scholar]
- Ali, M.S.; Elhamahmy, M.A.; El-Shiekh, A.F. Mango trees productivity and quality as affected by Boron and Putrescine. Sci. Hortic. 2017, 216, 248–255. [Google Scholar] [CrossRef]
- Boldingh, H.L.; Alcaraz, M.L.; Thorp, T.G.; Minchin, P.E.H.; Gould, N.; Hormaza, J.I. Carbohydrate and boron content of styles of ‘Hass’ avocado (Persea americana Mill.) flowers at anthesis can affect final fruit set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Ferrán, X.; Tous, J.; Romero, A.; Lloveras, J.; Pericón, J.R. Boron Does Not Increase Hazelnut Fruit Set and Production. HortScience 1997, 32, 1053–1055. [Google Scholar] [CrossRef]
- Usenik, V. Effect of late season boron spray on boron accumulation and fruit set of ‘Summit’ and ‘Hedelfinger’ sweet cherry (Prunus avium L.). Acta Agric. Slov. 2007, 89, 51–58. [Google Scholar] [CrossRef]
- De Silva, A.L.; Kämper, W.; Wallace, H.M.; Ogbourne, S.M.; Hosseini Bai, S.; Nichols, J.; Trueman, S.J. Boron Effects on Fruit Set, Yield, Quality and Paternity of Macadamia. Agronomy 2022, 12, 684. [Google Scholar] [CrossRef]
- Eggert, K.; von Wirén, N. Response of the plant hormone network to boron deficiency. New Phytol. 2017, 216, 868–881. [Google Scholar] [CrossRef]
- Bohnsack, C.W.; Albert, L.S. Early Effects of Boron Deficiency on Indoleacetic Acid Oxidase Levels of Squash Root Tips. Plant Physiol. 1977, 59, 1047–1050. [Google Scholar] [CrossRef]
- Husted, S.; Persson, D.; Laursen, K.; Hansen, T.; Pedas, P.; Stokholm, M.; Hegelund, J.; Schjoerring, J. Review: The role of atomic spectrometry in plant science. J. Anal. At. Spectrom. 2010, 26, 52–79. [Google Scholar] [CrossRef]
- Serrani, J.C.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J. 2008, 56, 922–934. [Google Scholar] [CrossRef]
- Hu, J.; Israeli, A.; Ori, N.; Sun, T.-p. The Interaction between DELLA and ARF/IAA Mediates Crosstalk between Gibberellin and Auxin Signaling to Control Fruit Initiation in Tomato. Plant Cell 2018, 30, 1710–1728. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Sun, T.-p. Four class A AUXIN RESPONSE FACTORs promote tomato fruit growth despite suppressing fruit set. Nat. Plants 2023, 9, 706–719. [Google Scholar] [CrossRef] [PubMed]
- García-Hurtado, N.; Carrera, E.; Ruiz-Rivero, O.; López-Gresa, M.P.; Hedden, P.; Gong, F.; García-Martínez, J.L. The characterization of transgenic tomato overexpressing gibberellin 20-oxidase reveals induction of parthenocarpic fruit growth, higher yield, and alteration of the gibberellin biosynthetic pathway. J. Exp. Bot. 2012, 63, 5803–5813. [Google Scholar] [CrossRef]
- Martínez-Bello, L.; Moritz, T.; López-Díaz, I. Silencing C19-GA 2-oxidases induces parthenocarpic development and inhibits lateral branching in tomato plants. J. Exp. Bot. 2015, 66, 5897–5910. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Hao, S.; Kojima, M.; Sakakibara, H.; Ozeki-Iida, Y.; Zheng, Y.; Fei, Z.; Zhong, S.; Giovannoni, J.J.; Rose, J.K.C.; et al. Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. Plant J. 2015, 83, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Ge, T.; Zhang, J.; Pan, X.; Ma, Y.; Yi, S.; Zheng, Y. The molecular events of IAA inhibiting citrus fruitlet abscission revealed by digital gene expression profiling. Plant Physiol. Biochem. 2018, 130, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lu, S.; Xie, M.; Wu, M.; Ding, S.; Khaliq, A.; Ma, Z.; Mao, J.; Chen, B. Identification and expression analysis of the small auxin-up RNA (SAUR) gene family in apple by inducing of auxin. Gene 2020, 750, 144725. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, M.; Wang, J.; Zhang, B.; Li, Y.; Wang, J.; El Sappah, A.H.; Liang, Y. Comparative Transcriptomic Analysis of the Development of Sepal Morphology in Tomato (Solanum Lycopersicum L.). Int. J. Mol. Sci. 2020, 21, 5914. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Wightman, R.; Jönsson, H.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef]
- Chapman, E.J.; Estelle, M. Mechanism of Auxin-Regulated Gene Expression in Plants. Annu. Rev. Genet. 2009, 43, 265–285. [Google Scholar] [CrossRef]
- Bargmann, B.O.R.; Vanneste, S.; Krouk, G.; Nawy, T.; Efroni, I.; Shani, E.; Choe, G.; Friml, J.; Bergmann, D.C.; Estelle, M.; et al. A map of cell type—Specific auxin responses. Mol. Syst. Biol. 2013, 9, 688. [Google Scholar] [CrossRef]
- Frigerio, M.n.; Alabadí, D.; Pérez-Gómez, J.; García-Cárcel, L.; Phillips, A.L.; Hedden, P.; Blázquez, M.A. Transcriptional Regulation of Gibberellin Metabolism Genes by Auxin Signaling in Arabidopsis. Plant Physiol. 2006, 142, 553–563. [Google Scholar] [CrossRef]
- Ezura, K.; Nomura, Y.; Ariizumi, T. Molecular, hormonal, and metabolic mechanisms of fruit set, the ovary-to-fruit transition, in horticultural crops. J. Exp. Bot. 2023, 74, 6254–6268. [Google Scholar] [CrossRef]
- Schäfer, M.; Brütting, C.; Meza-Canales, I.D.; Großkinsky, D.K.; Vankova, R.; Baldwin, I.T.; Meldau, S. The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J. Exp. Bot. 2015, 66, 4873–4884. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Faber, M.; Hajirezaei, M.-R.; von Wirén, N.; Bienert, G.P. Cytokinins as boron deficiency signals to sustain shoot development in boron-efficient oilseed rape. Physiol. Plant. 2022, 174, e13776. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 2016, 6, 28298. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; van der Does, D.; Ladwig, F.; Sticht, C.; Kolbeck, A.; Schürholz, A.-K.; Augustin, S.; Keinath, N.; Rausch, T.; Greiner, S.; et al. A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 15261–15266. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Bao, F.; Li, J. Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 2000, 24, 693–701. [Google Scholar] [CrossRef]
- Collins, C.; Maruthi, N.M.; Jahn, C.E. CYCD3 D-type cyclins regulate cambial cell proliferation and secondary growth in Arabidopsis. J. Exp. Bot. 2015, 66, 4595–4606. [Google Scholar] [CrossRef]
- Zhao, W.; Baldwin, E.A.; Bai, J.; Plotto, A.; Irey, M. Comparative analysis of the transcriptomes of the calyx abscission zone of sweet orange insights into the huanglongbing-associated fruit abscission. Hortic. Res. 2019, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Gil-Amado, J.A.; Gomez-Jimenez, M.C. Transcriptome Analysis of Mature Fruit Abscission Control in Olive. Plant Cell Physiol. 2013, 54, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tao, S.; Qi, K.; Zhang, H.; Zhang, Q.; Zhang, S. Effects of foliar application with H3BO3 on contents of boron and calcium and sclereid development related enzymes in pear fruits. Plant Nutr. Fertil. Sci. 2011, 17, 1250–1255. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, M.; Zhou, Y.; Wang, Y.; Shen, J.; Chen, H.; Zhang, L.; Lü, B.; Liang, G.; Liang, J. The Rice G Protein γ Subunit DEP1/qPE9–1 Positively Regulates Grain-Filling Process by Increasing Auxin and Cytokinin Content in Rice Grains. Rice 2019, 12, 91. [Google Scholar] [CrossRef]
- Kojima, M.; Sakakibara, H. Highly Sensitive High-Throughput Profiling of Six Phytohormones Using MS-Probe Modification and Liquid Chromatography–Tandem Mass Spectrometry. In High-Throughput Phenotyping in Plants: Methods and Protocols; Normanly, J., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 151–164. [Google Scholar]
- Pappas, D.; Giannoutsou, E.; Panteris, E.; Gkelis, S.; Adamakis, I.-D.S. Microcystin-LR and cyanobacterial extracts alter the distribution of cell wall matrix components in rice root cells. Plant Physiol. Biochem. 2022, 191, 78–88. [Google Scholar] [CrossRef]
- Meidani, C.; Ntalli, N.G.; Giannoutsou, E.; Adamakis, I.-D.S. Cell Wall Modifications in Giant Cells Induced by the Plant Parasitic Nematode Meloidogyne incognita in Wild-Type (Col-0) and the fra2 Arabidopsis thaliana Katanin Mutant. Int. J. Mol. Sci. 2019, 20, 5465. [Google Scholar] [CrossRef]
- Bai, S.; Sun, Y.; Qian, M.; Yang, F.; Ni, J.; Tao, R.; Li, L.; Shu, Q.; Zhang, D.; Teng, Y. Transcriptome analysis of bagging-treated red Chinese sand pear peels reveals light-responsive pathway functions in anthocyanin accumulation. Sci. Rep. 2017, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Qiao, Z.-W.; Ren, Y.-X.; An, J.-P.; Xu, S.-Z.; Gu, K.-D.; Zheng, P.-F.; Chen, S.-L.; Xue, C.; Wu, J. MYB20, an R2R3-type MYB transcription factor, negatively regulates salt and cold stress tolerance in pears. Plant Physiol. Biochem. 2025, 229, 110065. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Yang, X.a.; Wang, S.; Jiang, Z.; Zhang, C.; Zhao, L.; Cui, Y. Comprehensive Physiology, Cytology, and Transcriptomics Studies Reveal the Regulatory Mechanisms Behind the High Calyx Abscission Rate in the Bud Variety of Korla Pear (Pyrus sinkiangensis ‘Xinnonglinxiang’). Plants 2024, 13, 3504. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, Y.; Wei, J.; Zhang, X.; Guo, Z.; Lu, X. Comprehensive Analysis of Boron-Induced Changes in Cell Expansion and Phytohormone During Early Ovary Development in Pear (Pyrus sinkiangensis Yu). Plants 2025, 14, 3619. https://doi.org/10.3390/plants14233619
Chen J, Li Y, Wei J, Zhang X, Guo Z, Lu X. Comprehensive Analysis of Boron-Induced Changes in Cell Expansion and Phytohormone During Early Ovary Development in Pear (Pyrus sinkiangensis Yu). Plants. 2025; 14(23):3619. https://doi.org/10.3390/plants14233619
Chicago/Turabian StyleChen, Jiuhong, Yongfeng Li, Jie Wei, Xiaoyun Zhang, Zhihua Guo, and Xiaoyan Lu. 2025. "Comprehensive Analysis of Boron-Induced Changes in Cell Expansion and Phytohormone During Early Ovary Development in Pear (Pyrus sinkiangensis Yu)" Plants 14, no. 23: 3619. https://doi.org/10.3390/plants14233619
APA StyleChen, J., Li, Y., Wei, J., Zhang, X., Guo, Z., & Lu, X. (2025). Comprehensive Analysis of Boron-Induced Changes in Cell Expansion and Phytohormone During Early Ovary Development in Pear (Pyrus sinkiangensis Yu). Plants, 14(23), 3619. https://doi.org/10.3390/plants14233619






