Growth and Physiological Responses and Selection of Tedera (Bituminaria bituminosa L.) Genotypes Under Salt Stress Conditions
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Area
4.2. Plant Growth Measurements
4.3. Photosynthetic Pigments
4.4. Lipid Peroxidation (MDA Content)
4.5. Proline Content
4.6. Crude Protein Analysis
4.7. Determination of Na, K, Ca, and Mg Content
4.8. Total Phosphorus
4.9. Chlorine Determination
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PH | Plant height |
| SFW | Stem fresh weight |
| SDW | Stem dry weight |
| RFW | Root fresh weight |
| RDW | Root dry weight |
| CH-A | Chlorophyll a |
| CH-B | Chlorophyll b |
| CTS | Carotenoids |
| LP | Lipid peroxidation |
| PL | Proline |
| CP | Crude protein |
| P | Phosphorus content |
| Cl | Chlorine content |
| Na | Sodium content |
| Mg | Magnesium content |
| Ca | Calcium content |
| K | Potassium content |
| ROS | Reactive oxygen species |
References
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid. Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- FAO. Global Assessment of Salt-Affected Soils; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; Available online: https://www.fao.org/global-soil-partnership/resources/highlights/detail/en/c/1412475/ (accessed on 10 June 2025).
- Zarat, L.; Alfaiz, C.; Chiadmi, N.; Kallida, R.; Gaboun, F.; Ibriz, M. Plant growth, physiological response and osmotic adjustment of wild Bituminaria bituminosa (L.) stirton accessions under salt stress. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 59–69. [Google Scholar]
- Foster, K.; Lambers, H.; Real, D.; Ramankutty, P.; Cawthray, G.R.; Ryan, M.H. Drought resistance and recovery in mature Bituminaria bituminosa var. albomarginata. Ann. Appl. Biol. 2014, 165, 147–160. [Google Scholar] [CrossRef]
- Sternberg, M.; Gishri, N.; Mabjeesh, S.J. Effects of grazing on Bituminaria bituminosa (L.) Stirton: A potential forage crop in Mediterranean grasslands. J. Agron. Crop Sci. 2006, 192, 399–407. [Google Scholar] [CrossRef]
- Barbera, M.; Muñoz, M.C.; Rodriguez-Ponce, E.; Ventura, M.R. Potential value of tedera (Bituminaria bituminosa) as high protein resource for poultry feed. Trop. Anim. Health Prod. 2019, 51, 465–468. [Google Scholar] [CrossRef]
- Beard, C.; Nichols, P.; Loo, C.; Michael, P. Establishment of Tedera (Bituminaria bituminosa var. albomarginata); Future Farm Industries CRC Technical Report; Curtin University of Technology, Centre for Crop Disease Management: Perth, Australia, 2014. [Google Scholar]
- Gül, V.; Dinler, B.S.; Taşci, E. Effects of pre-application with gibberellic acid on germination of soybean under salt stress. J. Stress Physiol. Biochem. 2019, 15, 86–92. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Bian, Y.M.; Chen, S.Y.; Xie, M.Y. Effects of HF on proline of some plants. Plant Physiol. Commun. 1988, 6, 19–21. [Google Scholar]
- Bai, X.; Dai, L.; Sun, H.; Chen, M.; Sun, Y. Effects of moderate soil salinity on osmotic adjustment and energy strategy in soybean under drought stress. Plant Physiol. Biochem. 2019, 139, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Sahoo, K.K.; Tripathi, A.K.; Kumar, R.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Knockdown of an inflorescence meristem-specific cytokinin oxidase–OsCKX2 in rice reduces yield penalty under salinity stress condition. Plant Cell Environ. 2018, 41, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Li, Y.; Li, X.; Zhang, J. Proline metabolism-related gene expression in four potato genotypes in response to drought stress. Biol. Plant. 2019, 63, 757–764. [Google Scholar] [CrossRef]
- Kara, E.; Taşkın, H.; Baktemur, G. Determination of the effects of different concentrations of salt (NaCl) added to the nutrient medium under in vitro conditions on the development of tomato (Solanum lycopersicum L.). ISPEC J. Agric. Sci. 2024, 8, 301–309. [Google Scholar]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M. Effect of salt stress on growth and ion accumulation of alfalfa (Medicago sativa L.) cultivars. J. Plant Nutr. 2018, 41, 818–831. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A new insight of salt stress signaling in plants. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Seven, S.; Akıncı, Ş. Effects of humic acid and salt stress on growth, physiological parameters and mineral substance uptake in Artemisia dracunculus L. (Tarragon). Turk. J. Bot. 2025, 49, 64–79. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Adav, S.P.; Bharadwaj, R.; Nayak, H.; Mahto, R.; Singh, R.K.; Prasad, S.K. Impact of salt stress on growth, productivity and physicochemical properties of plants: A review. Int. J. Chem. Stud. 2019, 7, 1793–1798. [Google Scholar]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Díaz-Vivancos, P.; Sánchez-Blanco, M.J.; Hernández, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Hussein, M.A.A.; Alqahtani, M.M.; Alwutayd, K.M.; Aloufi, A.S.; Osama, O.; Azab, E.S.; Abdelsattar, M.; Hassanin, A.A.; Okasha, S.A. Exploring salinity tolerance mechanisms in diverse wheat genotypes using physiological, anatomical, agronomic and gene expression analyses. Plants 2023, 12, 3330. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Breeding for salinity tolerance in plants. Crit. Rev. Plant Sci. 1994, 13, 17–42. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; Glenn, K.; McDonald, K. The response of barley to salinity stress differs between hydroponic and soil systems. Funct. Plant Biol. 2011, 37, 621–633. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Sanjani, S.; Nikkhah-Chamanabad, H.; Mehrvar, M.R.; Asadi, A.; Amini, A. Identification of salt-tolerant barley genotypes using multiple-traits index and yield performance at early growth and maturity stages. Bull. Natl. Res. Cent. 2021, 45, 117. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Chourasia, K.N.; More, S.J.; Kumar, A.; Kumar, D.; Singh, B.; Bhardwaj, V.; Lal, M.K. Salinity responses and tolerance mechanisms in underground vegetable crops: An integrative review. Planta 2022, 255, 68. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Cha-um, S.; Batin, C.B.; Samphumphung, T.; Kidmanee, C. Physio-morphological changes of cowpea (Vigna unguiculata Walp.) and jack bean (Canavalia ensiformis (L.) DC.) in responses to soil salinity. Aust. J. Crop Sci. 2013, 7, 2128–2135. [Google Scholar]
- Sharma, S.; Verslues, P.A. Proline metabolism and its implications for plant development and stress tolerance. Plant Biol. 2011, 157, 292–302. [Google Scholar]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Ahmad, P.; Jhon, R.; Sarwat, M.; Umar, S. Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int. J. Plant Prod. 2008, 2, 353–366. [Google Scholar]
- Kaymak, G.; Acar, Z. Determination of salinity tolerance levels of tedera (Bituminaria bituminosa L.) genotypes. Anadolu J. Agric. Sci. 2020, 35, 51–58. [Google Scholar] [CrossRef]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; Shabala, S., Ed.; CAB International: Wallingford, UK, 2012; pp. 59–93. [Google Scholar]
- Finck, A. Pflanzenernährung in Stich Worten; Hirt: Kiel, Germany, 1976; pp. 1–200. [Google Scholar]
- Arnon, G.L. Copper enzyme in isolated chloroplasts: Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Claussen, W. Proline as a measure of stress in tomato plants. Plant Sci. 2005, 168, 241–248. [Google Scholar] [CrossRef]
- Kacar, B.; Inal, A. Plant analysis. Nobel. Pres. 2008, 1241, 891. [Google Scholar]
- Johnson, C.M.; Ulrich, A. Analytical Methods for Use in Plant Analysis; California Agricultural Experiment Station Bulletin No. 766; University of California: Berkeley, CA, USA, 1959; pp. 25–78. [Google Scholar]

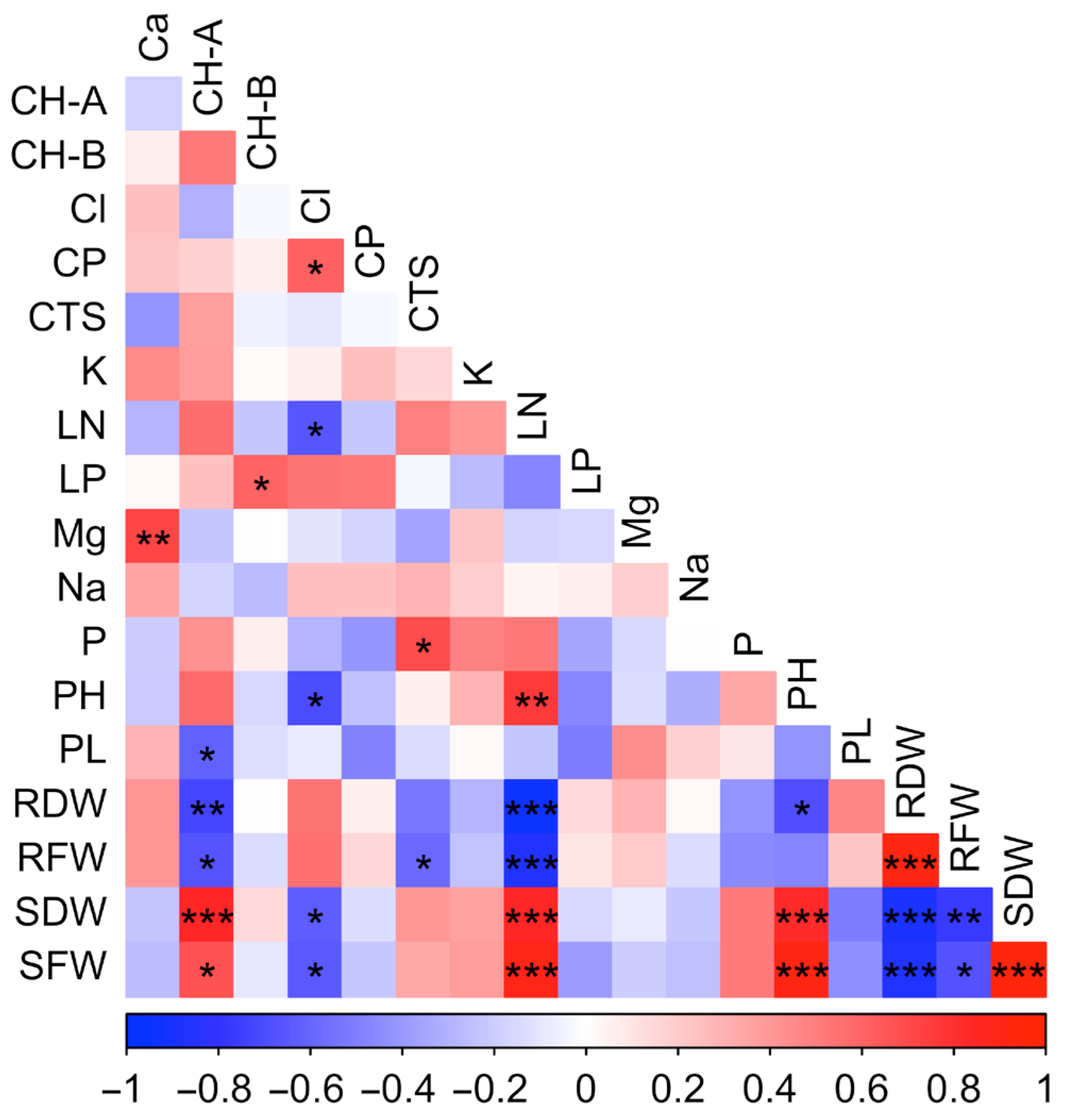

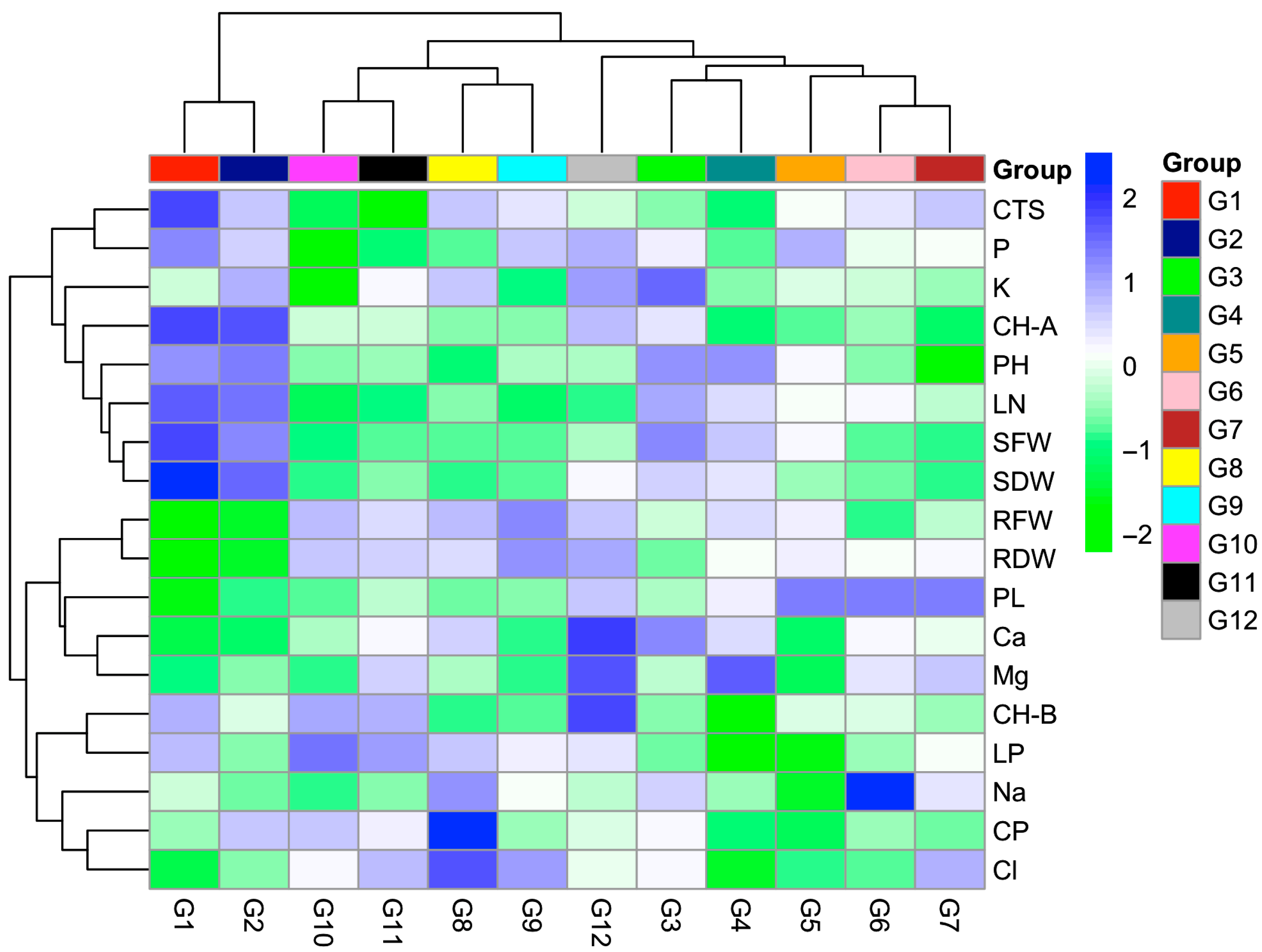
| Genotypes | Plant | Leaf | Stem Fresh | Stem Dry | Root Fresh | Root Dry |
|---|---|---|---|---|---|---|
| Height (cm) | Number | Weight (g) | Weight (g) | Weight (g) | Weight (g) | |
| G1 | 52.23 ± 9.04 a | 53.66 ± 15.75 a | 25.74 ± 4.27 a | 16.78 ± 3.46 a | 16.34 ± 0.90 d | 13.50 ± 0.32 d |
| G2 | 52.96 ± 11.09 a | 51.53 ± 15.25 ab | 24.56 ± 3.81 ab | 16.26 ± 1.08 ab | 16.73 ± 1.61 cd | 13.68 ± 0.57 cd |
| G3 | 52.06 ± 8.30 a | 47.53 ± 7.44 abc | 24.44 ± 2.98 ab | 15.54 ± 0.84 abc | 17.65 ± 2.13 abc | 13.94 ± 0.81 bc |
| G4 | 52.30 ± 12.82 a | 42.93 ± 9.28 bcd | 23.39 ± 1.96 bc | 15.40 ± 0.55 bc | 18.10 ± 1.70 ab | 14.13 ± 0.75 ab |
| G5 | 47.16 ± 8.34 ab | 39.86 ± 9.39 cde | 22.38 ± 3.77 bcd | 14.78 ± 3.16 c | 17.92 ± 1.30 abc | 14.18 ± 0.64 ab |
| G6 | 43.10 ± 13.43 ab | 40.60 ± 10.36 cde | 20.49 ±2.40 d | 14.64 ± 0.62 c | 17.19 ± 1.59 bcd | 14.13 ± 0.56 ab |
| G7 | 36.86 ± 13.99 c | 36.26 ± 9.27 def | 20.25 ± 1.69 d | 14.46 ± 0.43 c | 17.58 ± 1.43 abc | 14.17 ± 0.49 ab |
| G8 | 40.46 ±12.97 ab | 33.26 ± 9.89 def | 20.44 ± 2.10 d | 14.48 ± 0.55 c | 18.28 ± 2.23 ab | 14.24 ± 0.71 ab |
| G9 | 43.96 ± 7.63 ab | 28.53 ± 8.08 f | 20.60 ± 2.62 d | 14.50 ± 0.61 c | 18.60 ± 4.10 a | 14.42 ± 2.77 a |
| G10 | 42.73 ± 15.46 ab | 27.13 ± 8.27 f | 20.18 ± 1.34 d | 14.48 ± 0.86 c | 18.27 ± 1.02 ab | 14.30 ± 0.52 ab |
| G11 | 43.26 ± 13.31 ab | 29.60 ± 10.41 f | 20.44 ± 1.54 d | 14.69 ± 0.44 c | 18.06 ± 1.85 ab | 14.26 ± 0.72 ab |
| G12 | 43.96 ± 10.25 ab | 30.93 ± 7.21 ef | 21.25 ± 2.21 cd | 15.28 ± 0.69 bc | 18.19 ± 1.25 ab | 14.37 ± 0.49 ab |
| Dose | ||||||
| 0 mM | 50.31 ± 10.59 | 39.81 ± 15.31 a | 21.46 ± 3.98 | 15.72 ± 3.13 a | 17.21 ± 0.92 b | 14.04 ± 0.45 |
| 25 mM | 45.19 ± 12.63 | 34.72 ± 13.28 ab | 22.28 ± 3.87 | 15.00 ± 1.04 ab | 18.30 ± 3.06 a | 14.60 ± 1.89 |
| 50 mM | 42.77 ± 12.89 | 30.91 ± 10.04 b | 21.17 ± 3.09 | 14.76 ± 0.75 ab | 18.28 ± 1.71 a | 14.25 ± 0.60 |
| 75 mM | 41.58 ± 14.86 | 30.33 ± 10.67 b | 21.13 ± 2.87 | 14.96 ± 0.84 ab | 18.49 ± 1.59 a | 14.38 ± 0.62 |
| 100 mM | 41.66 ± 13.96 | 28.38 ± 12.47 b | 19.97 ± 2.61 | 14.21 ± 0.86 b | 19.01 ± 2.17 a | 14.59 ± 0.91 |
| Genotype | ** | ** | * | * | * | ** |
| NaCl | ns | * | ns | * | * | ns |
| GxD | ** | ** | ns | ns | ns | ns |
| Genotypes | Calcium (%) | Magnesium (%) | Potassium (%) |
|---|---|---|---|
| G1 | 1.19 ± 0.282 c | 0.27 ± 0.05 b | 0.30 ± 0.07 bcd |
| G2 | 1.24 ± 0.39 c | 0.31 ± 0.05 b | 0.35 ± 0.05 abc |
| G3 | 1.91 ± 1.34 ab | 0.34 ± 0.02 ab | 0.37 ± 0.09 a |
| G4 | 1.70 ± 0.55 abc | 0.54 ± 0.35 a | 0.28 ± 0.07 cd |
| G5 | 1.23 ± 0.22 c | 0.24 ± 0.05 b | 0.30 ± 0.03 bcd |
| G6 | 1.62 ± 0.50 abc | 0.40 ± 0.27 ab | 0.30 ± 0.04 bcd |
| G7 | 1.56 ± 0.54 abc | 0.44 ± 0.26 ab | 0.29 ± 0.05 bcd |
| G8 | 1.71 ± 0.41 abc | 0.32 ± 0.04 b | 0.34 ± 0.07 abc |
| G9 | 1.33 ± 0.29 bc | 0.28 ± 0.03 b | 0.26 ± 0.06 de |
| G10 | 1.46 ± 0.65 bc | 0.28 ± 0.10 b | 0.21 ± 0.10 e |
| G11 | 1.61 ± 0.43 abc | 0.42 ± 0.18 ab | 0.32 ± 0.07 a–d |
| G12 | 2.08 ± 0.51 a | 0.54 ± 0.21 a | 0.36 ± 0.05 ab |
| Dose | |||
| 0 mM | 1.17 ± 0.21 b | 0.29 ± 0.05 | 0.34 ± 0.05 a |
| 25 mM | 1.73 ± 0.90 a | 0.43 ± 0.25 | 0.36 ± 0.08 a |
| 50 mM | 1.28 ± 0.36 b | 0.34 ± 0.19 | 0.28 ± 0.06 b |
| 75 mM | 1.78 ± 0.57 a | 0.39 ± 0.15 | 0.27 ± 0.08 b |
| 100 mM | 1.79 ± 0.53 a | 0.39 ± 0.23 | 0.27 ± 0.08 b |
| Genotype | * | * | * |
| NaCl | ** | ns | ** |
| GxD | ns | ns | ns |
| Genotype Number | Collection Site | Coordinates | |
|---|---|---|---|
| G1 | Spain | - | - |
| G2 | Kastamonu İnebolu | 41°58′32.8″ | 33°46′10.4′ |
| G3 | Kastamonu-Çatalzeytin | 41°57′48.4″ | 34°09′07.8′ |
| G4 | Sinop Kanlıçay | 41°40′40.3″ | 35°22′22.8′ |
| G5 | Samsun-Kozağzı | 41°28′05.1″ | 35°49′56.8′ |
| G6 | Samsun-Çarşamba | 41°04′35.1″ | 36°40′09.0′ |
| G7 | Samsun-Bağkur | 41°18′39.0″ | 36°20′02.5′ |
| G8 | Samsun-Baruthane | 41°19′08.5″ | 36°19′13.6′ |
| G9 | Samsun-Nebyan | 41°23′35.9″ | 35°59′06.2′ |
| G10 | Samsun-Kurupelit | 41°22′16.0″ | 36°11′46.7′ |
| G11 | Sinop-Tıngıroğlu | 41°47′41″ | 35°00′23″ |
| G12 | Samsun-Kavak | 41°03′14.35″ | 35°56′59.84″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaymak Bayram, G.; Can, M.; Tunalı, U.; Acar, Z.; Ayan, İ. Growth and Physiological Responses and Selection of Tedera (Bituminaria bituminosa L.) Genotypes Under Salt Stress Conditions. Plants 2025, 14, 3618. https://doi.org/10.3390/plants14233618
Kaymak Bayram G, Can M, Tunalı U, Acar Z, Ayan İ. Growth and Physiological Responses and Selection of Tedera (Bituminaria bituminosa L.) Genotypes Under Salt Stress Conditions. Plants. 2025; 14(23):3618. https://doi.org/10.3390/plants14233618
Chicago/Turabian StyleKaymak Bayram, Gülcan, Mehmet Can, Utku Tunalı, Zeki Acar, and İlknur Ayan. 2025. "Growth and Physiological Responses and Selection of Tedera (Bituminaria bituminosa L.) Genotypes Under Salt Stress Conditions" Plants 14, no. 23: 3618. https://doi.org/10.3390/plants14233618
APA StyleKaymak Bayram, G., Can, M., Tunalı, U., Acar, Z., & Ayan, İ. (2025). Growth and Physiological Responses and Selection of Tedera (Bituminaria bituminosa L.) Genotypes Under Salt Stress Conditions. Plants, 14(23), 3618. https://doi.org/10.3390/plants14233618








