Abstract
Invasive seed predators can severely affect the reproduction of long-lived trees, especially when host range expansion occurs. The beetle Specularius impressithorax (Chrysomelidae: Bruchinae), native to Africa, has become established in Hawaiʻi where it attacks the endemic coral tree (Erythrina sandwicensis; Wiliwili). Here, we report the infestation of an African coral tree (E. livingstoniana) by this beetle and assess its performance and oviposition patterns on native and non-native hosts. Field surveys showed that eggs were aggregated on both hosts but more abundant on E. sandwicensis than on E. livingstoniana. Laboratory assays revealed no difference in larva-to-adult survival between the two hosts, although adults emerging from E. sandwicensis were larger. Choice tests indicated no oviposition preference between the two Erythrina species, despite the larger seed size of E. sandwicensis. To explore potential host range expansion, trials were run on economic legumes with varying phylogenetic distance from Erythrina, which showed oviposition on peanut (Arachis hypogaea) with low but successful survival (10.3%), while no development occurred on broad bean or pigeon pea. More E. sandwicensis seeds germinated when infested by a single early-stage larva (70% germination) than when uninfested (20%), suggesting that minimal seed predation may facilitate germination because previously reported greater damage induced by infestation through adulthood reduces germination. Our findings highlight the ecological flexibility of an invasive bruchine, its potential to exploit other Faboideae plants, and the dual role of seed predators as both threats and facilitators of seed germination. These results have implications for conservation of endemic coral trees and for understanding invasion dynamics of shared seed predators. Additionally, we examined non-botanical substrate filled with seed powder for oviposition and compiled global host records of S. impressithorax to contextualize its host range expansion.
1. Introduction
Shared insect predators can enhance plant diversity by suppressing relatively abundant plant species, thereby reducing competition among plants [1]. By contrast, they can also induce apparent competition among plant species by preferentially feeding on abundant plants, which indirectly reduces other host plants through the increased abundance of the predator’s offspring [2]. Invasive seed predators may severely affect the reproduction of long-lived trees, especially when host range expansion occurs. Seed predators of Fabaceae belong mainly to the subfamily Bruchinae (Coleoptera: Chrysomelidae) [3,4,5,6] alongside five other beetle families and a few Lepidoptera. Bruchines are specious in all continents except Australia and Antarctica [6]. The subfamily Bruchinae includes 1346 valid species, 84% of which attack Fabaceae hosts [3,4]. Most species are host specific at the genus or tribe level [5,6,7,8,9], and several specialist species are used as biological control agents of noxious plants [7,10].
Plants of the genus Erythrina (Fabaceae: Phaseoleae), commonly known as coral trees, are flowering plants of Gondwanan origin broadly distributed in tropical and subtropical regions worldwide [11]. Neill (1993) recognized 113 Erythrina species globally—70 Neotropical, 31 African, and 12 Asian or Oceania species [12]. Twenty-two Erythrina species are endangered or critically endangered (IUCN Red List [13]). Many species are used as ornamental street or park trees [14], and in tropical regions they serve as shade trees in coffee and cocoa plantations [15]. Their seeds contain erythrina alkaloids [16].
Wiliwili, Erythrina sandwicensis O.Deg., a tree up to 15 m tall, with 1–3 red or yellow-orange seeds per pod [17] (Figure 1a), is the dominant species in lowland dry forests and the only endemic Erythrina species in the Hawai’ian Islands [11]. Traditionally, it was important to Native Hawai’ians for producing canoe outriggers, surfboards, and wreaths [18]. Today, Wiliwili is listed as Vulnerable by the IUCN [19], largely due to damage from the Erythrina gall wasp, Quadrastichus erythrinae Kim (Hymenoptera: Eulophidae) [20,21]. The internal seed-predatory bruchine beetle (feeding within seeds), Specularius impressithorax (Pic) (hereafter, SI; Figure 1c) (Coleoptera: Chrysomelidae: Bruchinae), native to Africa, arrived in the Hawai’ian Islands in 2001 [22,23]. SI females deposit eggs directly on seeds after pods dehisce but not on the pod surface [24] (Figure 1b); the hatching larvae bore into the seeds through the testa and develop into adulthood to emerge out through the excised exit hole (Figure 1b). Fully developed larvae likely damage radicles during the development, which eventually reduces germination success of E. sandwicensis (2.5% germination for seeds with one adult exit hole, and 0% for seeds with two or more adult exit holes, versus 68.5% germination for seeds with zero adult exit holes) [22]. As is typical for seed predators, SI likely entered Hawai’i in imported Erythrina seeds from Africa. Initial establishment probably occurred on the widely cultivated non-native host E. variegata [22]. Within three years of its first detection, SI caused a 77.4% mean seed crop loss in E. sandwicensis populations, posing a major threat. Adults feed on pollen and nectar.

Figure 1.
(a,b) Erythrina sandwicensis or Wiliwili, (c–e) E. livingstoniana. (a) Mature dehisced and indehiscent pods with seeds (11 February 2020). (b) A seed with hatched eggs, a dead embryo (circled, egg length = 0.77 mm), an adult emergence hole (top), and excised testa before adult emergence (center) (13 February 2020, O’ahu Island). (c) Mature dehisced pod with seeds; inset: adult Specularius impressithorax. (d) Tree trunk and seed pod. Ho’omaluhia Botanical Garden, O’ahu Island (11 February 2020). (e) Seeds with hatched eggs and one infertile egg.
Erythrina livingstoniana Baker, known as the aloe coral tree, grows up to 25 m tall [25] (Figure 1d). Its seed pod bears 6–8 red seeds (Figure 1c,d). The species is native to southeastern Africa—Malawi, Mozambique, Zambia, Zimbabwe, and South Africa—where it grows in woodlands. It is cultivated as an ornamental in tropical gardens and parks and is also used in traditional medicine for wound treatment [16].
This study compares the performance (survival of immature stages) and oviposition preference of SI on the newly found host E. livingstoniana (Figure 1e) and on its established host E. sandwicensis. To explore potential host range expansion, economic legumes (peanut, broad bean, and pigeon pea) with varying phylogenetic distance from Erythrina were tested for oviposition and larval development. Furthermore, we hypothesized that infestation by an early-instar larva may promote seed germination, contrary to previous findings that infestation through later stage (adult) inhibits germination [22]. Additionally, we tested whether non-botanical substrate (gelatine capsules) encapsulating seed powder can induce oviposition and complete larval development. We also compiled literature records of host associations to assess the global host range and invasion potential of this beetle.
2. Results
2.1. Plant Suitability and Seed Size Effects on Specularius impressithorax Egg Deposition
The onset of oviposition on raw peanuts A. hypogaea (seed volume = 724 ± 20 mm3, mean ± SE, n = 32) was delayed by one day. Development from hatched eggs to the last-instar larva proceeded successfully on A. hypogaea (survival = 0.199 ± 0.034, n = 50) (Figure 2A,B), while survival from hatched eggs to adulthood reached 0.103 ± 0.026 (n = 50) (Figure 3b). On V. faba (seed volume = 833 ± 35 mm3, n = 40), SI laid eggs on 18 seeds (45%) with a two-day delay, with up to 30 hatched eggs per seed (Figure 2C). Although larvae hatched, none developed further (Figure 3b). On C. cajan, eggs were laid on 22 seeds (44%), larvae hatched, but development did not proceed beyond the first instar (0.82 ± 0.10 eggs per seed, range 0–2, n = 50, Figure 2D and Figure 3b).
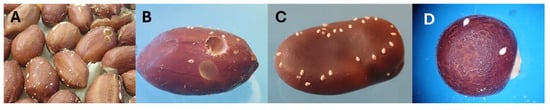
Figure 2.
Rearing of Specularius impressithorax on economic non-Erythrina plants. (A) Peanut, Arachis hypogaea: oviposition and partial development; (B) peanut showing adult emergence holes; (C) broad bean, Vicia faba; (D) pigeon pea, Cajanus cajan.
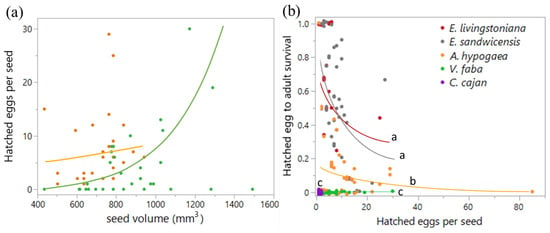
Figure 3.
Specularius impressithorax oviposition and survival on different plant seeds. (a) Number of hatched eggs per seed increased with seed volume on broad bean V. faba (p = 0.004) but not on peanut A. hypogaea (p = 0.355). (b) Survival from hatched eggs to adulthood was different among plant species (p < 0.0001) and decreased with increasing hatched-egg density per seed (p < 0.0001) with plant species × hatched-egg density interaction (p < 0.0001). Shared letters indicate no significant difference. C. cajan: Cajanus cajan (pigeon pea). Line: model prediction. Jittered points in (b).
Hatched-egg density differed between plant species (Wald χ21 = 22.58, p < 0.0001) and changed with seed volume (Wald χ21 = 17.69, p < 0.0001) on peanuts and broad beans (Figure 3a). The plant species × seed volume interaction was marginally significant (Wald χ21 = 3.62, p = 0.057). When analyzed separately, hatched egg density on A. hypogaea did not correlate with seed volume (Wald χ21 = 0.86, p = 0.355), whereas on V. faba it increased significantly with seed volume (Wald χ21 = 8.29, p = 0.004).
Survival from hatched eggs to adulthood differed among the five plant species (likelihood ratio (LR) χ24 = 865.23, p < 0.0001). Survival decreased with hatched-egg density per seed (LR χ21 = 401.10, p < 0.0001), and the interaction was significant (LR χ24 = 458.31, p < 0.0001). Post hoc comparisons showed that survival was lower on non-hosts than on the Erythrina hosts (E. livingstoniana and E. sandwicensis), but among non-hosts, survival was higher on A. hypogaea than on V. faba or C. cajan (Figure 3b).
2.2. Egg Distribution, Egg and Larval Density Effects, and Infestation Capacity
More eggs were laid on Wiliwili (E. sandwicensis) than on E. livingstoniana in the field (Table 1): up to 33 eggs per E. sandwicensis seed and 26 per E. livingstoniana seed, comparable to a previous report of up to 38 eggs per field-collected E. sandwicensis seed [22]. Egg distributions on both Erythrina species followed negative-binomial rather than Poisson distributions, indicating aggregation (Table 1). Hatched-egg distributions were also negative-binomial for both Erythrina hosts and for peanuts and broad beans, but Poisson-distributed for pigeon pea (Figure A3).

Table 1.
Specularius impressithorax field egg infestation per seed of Erythrina livingstoniana and Erythrina sandwicensis (Wiliwili) (February 2020, O’ahu), and consequent laboratory rearing results. n: number of seeds. Unless otherwise noted, there was no significant plant × egg density interaction, which was then excluded from a model. p-values < 0.05 are bolded. 0.05 < p < 0.06 are italicized. Goodness of fit tests on egg distributions.
No difference was detected between the two Erythrina species in mortality traits or hatchability, except first-instar mortality, which was higher on E. sandwicensis (p = 0.023; Table 1, Figure 4 and Figure A1). Egg-density effects were significant for infertility + embryo mortality (negative; p < 0.0001), predation (host × egg density interaction p = 0.0002; negative only on E. livingstoniana p = 0.0002, on E. sandwicensis p = 0.266), first-instar mortality (positive; p = 0.013), and hatchability (positive; p < 0.0001) (Table 1, Figure 4 and Figure A1). Survival from hatched eggs to adults did not correlate with hatched-egg density per seed (p = 0.200; Table 1, Figure 3b). The number of emerged adults per seed did not differ between the two Erythrina hosts (Wald χ21 = 1.05, p = 0.305) but increased with hatched-egg density (Wald χ21 = 16.46, p < 0.0001) (Figure 5). Maximum adult emergence per seed was 18 for E. sandwicensis and 11 for E. livingstoniana.
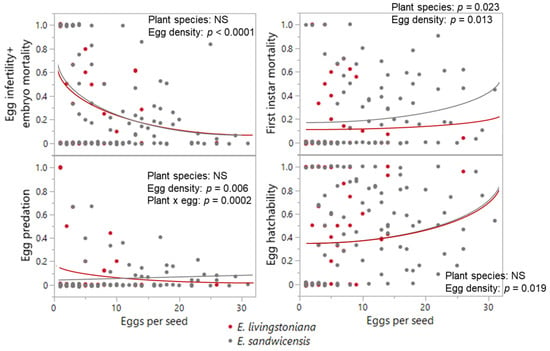
Figure 4.
Effects of Erythrina species and egg density on the egg survival in Specularius impressithorax. No difference in mortality traits was found between Erythrina livingstoniana and E. sandwicensis (Wiliwili), except in the first instar mortality (higher on Wiliwili). Overall egg survival (hatchability) was positively correlated with egg density per seed. Line: model prediction. Data points are jittered along the y-axis.
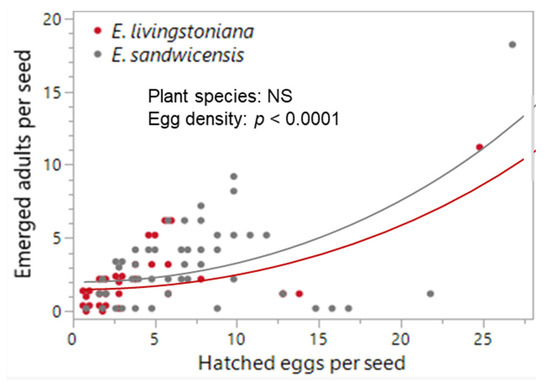
Figure 5.
Effects of Erythrina plants and hatched-egg density on the number of emerged adults per seed in Specularius impressithorax. Number of emerged adults was not different between the two Erythrina species (p = 0.305) but increased with increasing hatched-egg density per seed (p < 0.0001). Line: model prediction. Data points are jittered.
Seed width and length were significantly greater and the volume was marginally greater in E. sandwicensis compared to E. livingstoniana, whereas height and weight did not differ (Table 1).
2.3. Body Size
Elytron width, elytron area, and width/length ratio were significantly larger in beetles reared on E. sandwicensis than on E. livingstoniana, with no effect of sex (Table 2, Figure 6). Elytron length did not differ between host plants but was significantly greater in females than in males.

Table 2.
Elytron size of Specularius impressithorax reared on Erythrina livingstoniana and E. sandwicensis (Wiliwili) in the laboratory environment. Significant factors and p-values (<0.05) are in bold.
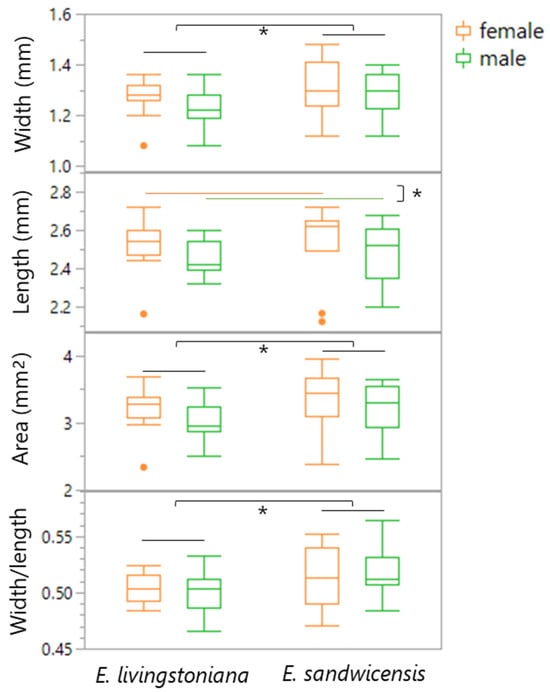
Figure 6.
Box plots of adult morphological traits (elytron width, length, area, and width/length ratio) by sex of Specularius impressithorax emerging from Erythrina livingstoniana and E. sandwicensis (Wiliwili). Elytral width, area, and width/length ratio were different between rearing plant species, while elytral length was different between sexes, but not between rearing plants. *: p < 0.05.
2.4. Choice Experiment
One replicate yielded no oviposition and was removed. No difference occurred in egg number between E. livingstoniana and E. sandwicensis (t = 0.23, df = 4, p = 0.829).
2.5. Seed Germination
Time to germination differed significantly between treatments (LR χ21 = 12.52, p = 0.0004), with infested seeds (by single early-stage larva) germinating earlier than uninfested controls (Figure 7). Replicate effects were non-significant (LR χ26 = 4.23, p = 0.646). Final cumulative germination (day 70) reached 70.0 ± 5.77% for infested seeds, which was higher than 20.0 ± 8.16% for uninfested seeds (F1,6 = 25.00, p = 0.0025). All larvae died at the second instar, probably due to drowning.
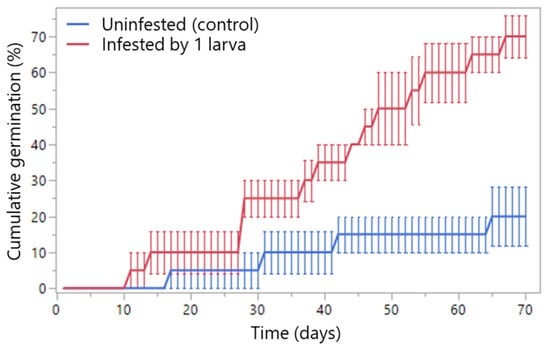
Figure 7.
Cumulative germination (%) of infested (by a single early-stage larva) and uninfested Wiliwili seeds (Erythrina sandwicensis) over time (mean ± SE).
2.6. Literature Review of Native Host Plant Range
A literature review identified 22 Erythrina species used by SI (Table A1): 11 African/pantropical, 7 Neotropical, 1 native Hawaiian (E. sandwicensis), 2 from Indonesia/Philippines (E. eudiphylla, and E. microcarpa), and 1 from Australia (E. sykesii). The proportion of native Erythrina species utilized by SI was high in Asia/Oceania (41.7%) and Africa (35.5%), and low in the Americas (10.0%) (Figure A4). Non-Erythrina Fabaceae hosts have also been reported: the wooly wild bean Strophostyles sarmentosa (eastern North America) [26] and Calabar bean Physostigma mesoponticum (Angola) [27], both in Phaseoleae.
3. Discussion
Specularius impressithorax (SI), an African internal seed predator, is known to infest native Hawaiian E. sandwicensis and seven introduced Erythrina species in Hawai’i (Table A1). By comparing E. livingstoniana (native to southeastern Africa) with E. sandwicensis, we evaluated field infestation, laboratory survival, and host preference. Beetles reared from E. livingstoniana were smaller than those from E. sandwicensis. SI is best described as oligophagous: specialized on Erythrina but capable of opportunistic oviposition on non-host substrates. It develops in mature dry seeds, either still in dehisced pods on the plant or in fallen seeds. The use of dry mature seeds is likely an adaptation to arid native environments [28].
SI readily oviposited on non-host seeds and even inert objects (e.g., gelatin capsules), consistent with previous observations of oviposition on plastic beads [22]. The smooth surface and curvature may elicit oviposition as in other bruchine beetles [29,30]. Its ability to complete development on peanuts (A. hypogaea, tribe Dalbergieae), but not on broad bean (V. faba, tribe Fabeae) or pigeon pea (C. cajan, tribe Phaseoleae), indicates that factors beyond phylogenetic relatedness among plants influence the beetle’s developmental success. Ecologically, peanuts are inaccessible because they develop underground, consistent with their absence from natural host records (Table A1). SI’s specialization on Erythrina likely reflects larval digestive limitation against unadapted toxic chemicals such as alkaloids present in other Fabaceae [31,32]. Erythrina seeds disperse near maternal trees, producing dense local seed banks vulnerable to attack by multivoltine bruchines [31].
Our work provides the first confirmed record of SI infestation on E. livingstoniana [26,33]. Despite similar oviposition preference and immature survival between the two hosts and E. livingstoniana being native to SI’s range, E. sandwicensis received more SI eggs in the field. Several explanations are plausible. (1) Seed size [34]: E. sandwicensis seeds are marginally larger, which can promote oviposition, although the laboratory choice test did not support a strong preference. (2) Seed quality: Larger adult body size when reared on E. sandwicensis may indicate higher larval suitability for development. (3) Resource abundance [35]: On O’ahu, E. sandwicensis is substantially more abundant (131 trees) than E. livingstoniana (four cultivated trees) (botanical gardens; N.F. Hoffman, pers. comm.), offering more frequent oviposition opportunities for a multivoltine species. (4) Phenology: E. sandwicensis exhibits variable flowering and seed-set periods among trees [36], whereas E. livingstoniana flowers within a narrower window (our observations), reducing temporal availability.
Egg survival (hatchability) increased with egg density on both two Erythrina, although high larval density reduced survival to adulthood. Nevertheless, adult emergence often remained high, suggesting selective pressure favoring egg aggregation. Egg aggregation behavior is observed in other bruchines such as Zabrotes subfasciatus and Acanthoscelides obtectus that utilize Phaseolus seeds [37,38]. Hard, large seeds such as those of Erythrina and Phaseolus may favor group foraging by endophagous larvae.
Infestation by a single early-instar larva increased E. sandwicensis germination (from 20% of uninfested control to 70%), probably by enhancing water permeability via larval entry holes [6,39]. Germination success depends on the developmental stage of the seed predator: late-stage larval feeding or adult emergence suppresses germination, with only 2.5% germination after an adult exit hole compared to >68% of undamaged seeds [22] (or 73% [40]). The low germination of unin-fested controls (20%) in the present study likely reflects prolonged cold storage. Effects of seed predators on germination vary widely across plant taxa, seed sizes, and seed-predator densities [6,41,42,43,44], as observed for the bruchine Acanthoscelides macrophthalmus on Leucaena leucocephala seeds [42]. Higher germination percentage (66.6%) was recorded for E. americana seeds with a single SI adult emergence hole [45]. Furthermore, plant–insect coevolutionary history, interactions with mammalian seed predators, water availability, and environmental factors that aid seed scarification (e.g., forest fire) would all determine whether insect seed predation enhances germination [6,41,46,47,48]. Hawaii’s February rains may amplify early-damage-induced germination.
Use of introduced Erythrina species (e.g., E. variegata and E. livingstoniana) may elevate SI abundance, indirectly increasing seed predation on the endemic E. sandwicensis via apparent competition induced by shared predation [2]. Similar patterns occur globally in other invaded ecosystems: introduced hosts can subsidize populations of seed predators that then spill over onto native plants (e.g., scolytine beetles on endemic and non-native palms in Mediterranean coastal systems [49]; Neotropical A. macrophthalmus expanding from its native host L. leucocephala to the introduced Falcataria moluccana in Taiwan [50]; Megabruchidius dorsalis expanding from native Gleditsia species to introduced North American Gleditsia triacanthos and Gymnocladus dioica in Europe [51]. Collectively, such cross-host predator spillover highlights the potential for altered community composition to reshape native plant recruitment.
4. Materials and Methods
All experiments were conducted under laboratory conditions of 26 ± 1 °C during the day and 24 ± 1 °C at night, 60–80% RH, and 12 L:12 D h photocycle (under fluorescent and natural light).
4.1. Field Collection of Erythrina and Laboratory Rearing
Seeds of Erythrina livingstoniana and Wiliwili (E. sandwicensis) were collected at Ho’omaluhia Botanical Garden, Kaneohe, O’ahu Island, Hawai’i on 11 February 2020 (21°22′55.32″ N, 157°48′03.47″ W, elevation 96.9 m). Fallen ripe pods and seeds dropped from pods onto grass were collected randomly. Infestation was observed in fallen open pods, pods still attached to trees, and dropped seeds.
The number of hatched eggs and dead eggs or larvae (still within eggs) per seed due to infertility, predation, embryo death, or first-instar larval death was recorded (Figure A1), as were the numbers of emerged adults per seed. Observations continued until all adults emerged. These monitoring procedures were conducted under the laboratory conditions described above.
Width and length of the right elytron of emerged adults were measured post-mortem for 10 females and 10 males from each Erythrina species, using a calibrated ocular micrometer on a Leica 125 microscope. Sex of SI was determined both by antennal morphology (males possess stouter and more expanded serrate funicles) and confirmed by dissection for genital observation [24,26].
4.2. Host Plant Range, Egg and Larval Density Effects, and Seed Size
Three non-Erythrina economic legumes, peanut Arachis hypogaea L. (Fabaceae: Dalbergieae) (78 seeds), Vicia faba L. (Fabaceae: Fabeae) (40 seeds), and pigeon pea Cajanus cajan (L.) Huth (Fabaceae: Phaseoleae) (50 seeds), were tested as potential hosts. They were each exposed to 40, 20, and 20 SI adults, respectively, for oviposition and development. Dead adults were removed, and hatched eggs were counted. Seeds were held for 90 days from initial exposure to record adult emergence. When no adults emerged, seeds bearing hatched eggs were dissected to determine developmental stages of dead individuals.
Seed width, length, and height of both Erythrina species, and of A. hypogaea and V. faba were measured to the nearest 0.5 mm. Because seed size of C. cajan was relatively uniform, we did not measure it. Seed volume was estimated as
4.3. Development Using Seed Powder
To test whether oviposition could be elicited by non-botanical material encapsulating seed material, powdered seeds of Erythrina variegata and A. hypogaea, prepared using an electric grinder, were placed into gelatin capsules. Each capsule was then exposed to adult SI for potential oviposition.
4.4. Choice Experiment
Oviposition choice between the two Erythrina hosts was tested using three seeds each of E. livingstoniana and E. sandwicensis placed on opposite sides of a Petri dish (9 cm diameter, 1.5 cm height) within a smaller dish (4 cm diameter, 0.6 cm height) each. Three unmated females and two unmated males (6 days old) reared on E. variegata—therefore previously unexposed to both test species—were confined together in a plastic cylinder (8 cm height, 2 cm diameter) for 24 h to allow mating. Only females were subsequently introduced to the Petri dish and allowed to oviposit for 48 h. Eggs were then counted. The test was replicated six times.
4.5. Seed Germination
Mature, undamaged Wiliwili seeds (collected from Koko Crater Botanical Garden, O’ahu in December 2008 and stored refrigerated) were exposed to five SI adults for 2 h in April 2025. Seeds bearing one egg were transferred to a Petri dish and incubated for 7 d to allow larval development under laboratory conditions (26 ± 1 °C light phase, 24 ± 1 °C dark phase, 12 L:12 D). Equal numbers (five) of infested or uninfested seeds were evenly spaced and allowed to germinate in between two filter papers moistened with distilled water in a Petri dish (9 cm diameter, 1.5 cm height) with four replications per treatment (20 seeds per treatment and eight dishes total). Dishes were enclosed in a polyethylene bag to minimize evaporation and kept under the same laboratory conditions. Germination was scored when the radicle had emerged ≥ 3 mm. Germination was recorded every 24 h for 70 d. Infested seeds were dissected at the end of the experiment to confirm larval death and developmental stages.
All procedures complied with relevant U.S. guidelines and regulations.
4.6. Literature Search for Host Plant Associations
A literature survey was conducted to compile host plant records of SI from global field observation and percentage utilization by SI was calculated.
4.7. Statistical Analysis and Vouchers
A generalized linear model (GLM) with a negative-binomial distribution and log-link function was applied to hatched eggs per seed, with plant species and seed volume, and their interaction effect as explanatory variables. A GLM with an exponential distribution and reciprocal link function was used for survival from hatched egg to emerged adults, with plant species, hatched eggs per seed, and their interaction effect as an explanatory variable. Likewise, GLMs with an exponential distribution and reciprocal link were used to examine the effects of host plant (two Erythrina), egg density, and their interaction effect on egg mortality (infertility + embryo mortality, egg predation, and first-instar larval mortality), egg hatchability (1—egg mortality), and survival from hatched eggs to adults. A GLM with a negative-binomial distribution and log-link function was applied to compare egg numbers per seed between the two host Erythrina species. A GLM with a negative-binomial distribution and log-link function was applied to emerged adults per seed, with plant species (two Erythrina) and hatched eggs per seed, and their interaction as an explanatory variable. A nonsignificant interaction effect was excluded from all models.
Seed size and mass between the two Erythrina species were compared by one-way ANOVA. Nonparametric one-sided Wilcoxon tests (with normal distribution approximation) were applied to assess effects of host plant and sex on elytron traits (width, length, area (=width × length), and width/length ratio) of emerged adults. A paired t-test was used to compare egg numbers in the choice experiment. Egg (on the two Erythrina hosts) and hatched-egg (on all five plants) distributions were fitted to Poisson and negative-binomial distributions. Time to germination with censoring was analyzed using a parametric survival analysis with a best-fit Weibull distribution (among Weibull, log-normal, exponential, Frechet and log-logistic distributions) with treatment and replicate nested within treatment. All statistical analyses were performed using JMP18.2.1 (SAS Institute, Cary, NC, USA). Voucher specimens are deposited in the insect collection of the Hawaii Department of Agriculture, Honolulu, Hawai’i.
5. Conclusions
The invasive African bruchine Specularius impressithorax continues to threaten native Erythrina populations in Hawai‘i through intensive seed predation and potential host range expansion. Our discovery of its successful development on the African Erythrina livingstoniana underscores its ecological flexibility and the risk of apparent competition across co-occurring Erythrina species. Beyond reporting a new host record, this study emphasizes the dual ecological role of seed predators: while the full development of beetle poses a threat to plant regeneration through extensive seed damage, early-stage larval infestation may facilitate germination by enhancing water absorption via larval entry holes, with minimal harm to the seed. Future work on host–parasitoid interactions [52] and chemical ecology [53,54] will be key to managing this invasive seed predator and conserving the native Erythrina flora.
Author Contributions
Conceptualization, M.M.R. and M.T.; Methodology, M.M.R.; Validation, M.T.; Formal Analysis, M.M.R. and M.T.; Investigation, M.M.R. and M.T.; Data Curation, M.M.R.; Writing—Original Draft Preparation, M.M.R. and M.T.; Writing—Review and Editing, M.T.; Visualization, M.M.R. and M.T.; Funding Acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded in part by KAKENHI (19K06840) from JSPS and the Realizing Diversity Initiative in the Research Environment at Kyushu University from the Ministry of Education, Science and Technology (MEXT) to M.T.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding authors.
Acknowledgments
The authors thank the members of Plant Pest Control Branch, especially Darcy E. Oishi.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SI | Specularius impressithorax |
Appendix A
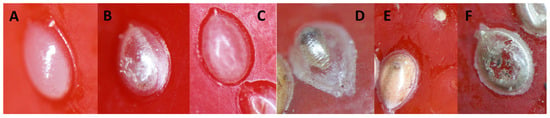
Figure A1.
Specularius impressithorax egg categories on E. livingstoniana seeds: (A) newly deposited egg (oval; length: 0.77 ± 0.01 mm, n = 10), (B) infertile egg, (C) dead eggs with developed zygote, (D) dead first instar, and, (E) a hatched egg with first instar entered the seed (left) and a larval entry hole (top-right, Ø = 194.0 ± 6.7 µm, n = 10), (F) an egg predated upon with chewed chorion.

Figure A2.
Gelatin capsules containing (A) ground Erythrina variegata seed powder with emergence holes, (B) adults failed to emerge from the hole, and (C) ground peanut (Arachis hypogaea) seed powder as an oviposition and growing medium for Specularius impressithorax. White arrows indicate laid eggs hatched on the surface of capsules.
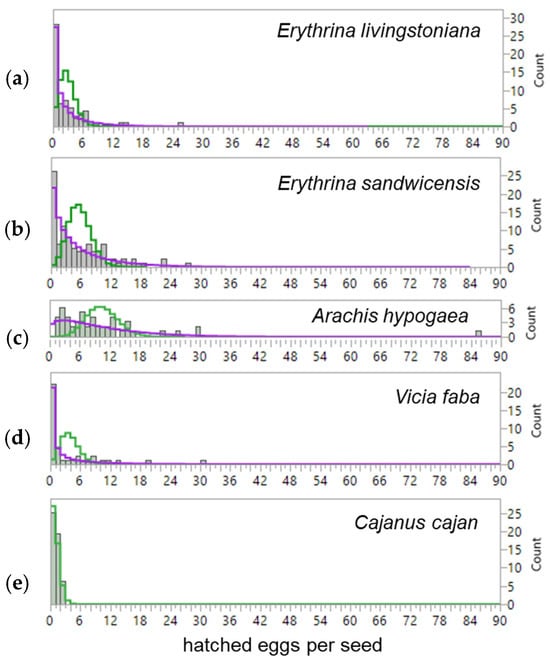
Figure A3.
Distribution of number of hatched eggs per seed collected from the field for Erythrina and under the laboratory conditions for three economic Fabaceae species. Green line: Poisson distribution. Purple line: negative binomial (NB) distribution. (a) Poisson distribution, χ2 = 301.21, n = 60, p < 0.0001; NB distribution, χ2 = 2.74, n = 60, p = 0.434. (b) Poisson distribution, χ2 = 217.01, n = 98, p < 0.0001; NB distribution, χ2 = 8.22, n = 98, p = 0.412. (c) Poisson distribution, χ2 = 65.74, df = 5, n = 50, p < 0.0001; NB distribution, χ2 = 3.74, df = 5, p = 0.588. (d) Poisson distribution, χ2 = 102.95, df = 4, n = 40, p < 0.0001; NB distribution, χ2 = 1.58, df = 1, n = 40, p = 0.208. (e) Poisson distribution, χ2 = 0.59, df = 1, n = 50, p = 0.442; NB distribution failed to be fitted.
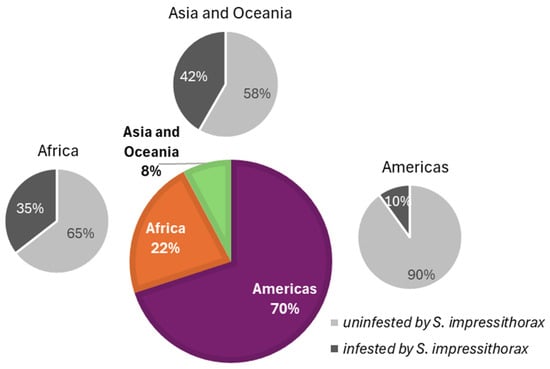
Figure A4.
World Erythrina species distribution and utilization by Specularius impressithorax.

Table A1.
Compiled host plant association of Specularius impressithorax from native and introduced regions.
Table A1.
Compiled host plant association of Specularius impressithorax from native and introduced regions.
| Species | Native Regions | Field Infestation | References |
|---|---|---|---|
| Erythrina abyssinica Lam. ex DC. | Eritrea, Ethiopia, Kenya, Republic of the Congo, Rhodesia, South Africa, Sudan, Tanzania, Mozambique, Nyasaland, Uganda, Zaire, Zimbabwe | Field | [25,55,56] |
| Erythrina americana Mill. | Mexico, C. America, Belize, Guatemala, southern N. America | Field, Hawaii 2006 | [22,23,57] |
| Erythrina berteroana Urb. | Central America, Colombia, Guatemala, Mexico, Venezuela | Field, Hawaii 2017, Honduras 2024 | [58] |
| Erythrina caffra Thunb. | Mozambique, South Africa | Field | [51] |
| Erythrina coralloides DC. | Mexico, and Central America | Field | [59] |
| Erythrina crista-galli L. | Argentina, Bolivia, Brazil, Paraguay, United States of America, Uruguay | Field, Hawaii 2006 | [22] |
| Erythrina eudiphylla Hassk. ex Backh | Indonesia | Field, Hawaii 2006 | [22,23] |
| Erythrina folkersii Krukoff & Moldenke | Belize, Guatemala, Honduras, Mexico Gulf, Mexico Southeast, Mexico Southwest | Field, Kaluakauila, Coll. Mexico | [52] |
| Erythrina humeana Spreng. | South Africa, Mozambique | Field, Mozambique 2007 | [12,22,52] |
| Erythrina latissima E.Mey. | South Africa | Field | [52] |
| Erythrina livingstoniana Baker | East Africa, Zimbabwe, Mozambique, Malawi, Tanzania, Zambia | Field, Hawaii 2020 | This study |
| Erythrina lysistemon Hutch. | South Africa, Mozambique | Field, Mozambique 2007 | [22,52] |
| Erythrina microcarpa Koord. & Valeton | Jawa, Philippines | Field, Hawaii 2006 | [22,23] |
| Erythrina orophila Ghesq. | Burundi, Democratic Republic of Congo, Zaïre | [12,59] | |
| Erythrina pallida Britton & Rose | Tobago, Trinidad, Venezuela, Venezuelan Antilles | Field | [59] |
| Erythrina sacleuxii Hua | Kenya, Tanzania | Field | [52] |
| Erythrina sandwicensis O.Deg. | Hawaii | Field, Hawaii 2001 | [22,52] |
| Erythrina senegalensis DC. | Angola, Cameroon, Chad, Ghana, Nigeria, West Africa, Mauritania | Field | [12] |
| Erythrina sykesii Barneby & Krukoff | (as hybrid: sykesii × variegata) native to Australia | Field, Hawaii 2006 | [27] |
| Erythrina variegata L. | Africa, Australia, coast of India, east Asia, India, Malaysia, Oceania | Field, Hawaii 2006 | [12,22,25,52] |
| Strophostyles sarmentosa Elliott | United States of America | Field | [26] |
| Physostigmana mesoponticum Taub. | Angola | Field | [27] |
References
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 940. [Google Scholar] [CrossRef]
- Lewis, O.T.; Gripenberg, S. Insect seed predators and environmental change. J. Appl. Ecol. 2008, 45, 1593–1599. [Google Scholar] [CrossRef]
- Borowiec, L. The genera of seed-beetles (Coleoptera, Bruchidae). Pol. Pismo Entomol. 1987, 57, 3–207. [Google Scholar]
- Kingsolver, J.M. Handbook of the Bruchidae of the United States and Canada (Insecta, Coleoptera); Technical Bulletin Number 1912; United States Department of Agriculture: Washington, DC, USA, 2004; p. 324. [Google Scholar]
- Johnson, C.D. Seed beetles host specificity and the systematics of the Leguminosae. In Advances in Leguminosae Systematics; Polhill, R.M., Raven, P.H., Eds.; Royal Botanic Gardens: London, UK, 1981; Volume 2, pp. 995–1027. [Google Scholar]
- Southgate, B.J. Biology of the Bruchidae. Annu. Rev. Entomol. 1979, 24, 449–473. [Google Scholar] [CrossRef]
- Tuda, M.; Chou, L.-Y.; Niyomdham, C.; Buranapanichpan, S.; Tateishi, Y. Ecological factors associated with pest status in Callosobruchus (Coleoptera: Bruchidae): High host specificity of non-pests to Cajaninae (Fabaceae). J. Stored Prod. Res. 2005, 41, 31–45. [Google Scholar] [CrossRef]
- Kergoat, G.J.; Silvain, J.-F.; Delobel, A.; Tuda, M.; Anton, K.-W. Defining the limits of taxonomic conservatism in host-plant use for phytophagous insects: Molecular systematics and evolution of host-plant associations in the seed-beetle genus Bruchus Linnaeus (Coleoptera: Chrysomelidae: Bruchinae). Mol. Phylogenet. Evol. 2007, 43, 251–269. [Google Scholar] [CrossRef]
- Kergoat, G.J.; Silvain, J.-F.; Buranapanichpan, S.; Tuda, M. When insects help to resolve plant phylogeny: Evidence for a paraphyletic genus Acacia from the systematics and host-plant range of their seed-predators. Zool. Scr. 2007, 36, 143–152. [Google Scholar] [CrossRef]
- Beenen, R. Translocation in Leaf beetles (Coleoptera: Chrysomelidae). Bonn. Zool. Beitr. 2005, 54, 179–199. [Google Scholar]
- Wagner, W.L.; Herbst, D.R.; Sohmer, S.H. Manual of the Flowering Plants of Hawai’i, 2nd ed.; Bishop Museum: Honolulu, HI, USA, 1999; p. 1952. [Google Scholar]
- Neill, D.A. The Genus Erythrina: Taxonomy, Distribution and Ecological Differentiation. In Erythrina in the New and Old Worlds Nitrogen Fixing Tree Research Reports—Special Issue; Westley, S.B., Powell, M.H., Eds.; Nitrogen Fixing Tree Association: Morrilton, AR, USA, 1993; pp. 15–27. [Google Scholar]
- IUCN 2024. The IUCN Red List of Threatened Species. Version 2024-2. Available online: https://www.iucnredlist.org/search/list?query=erythrina&searchType=species (accessed on 17 December 2024).
- Ikabanga, D.U.; Bidault, E.; Paradis, A.-H.; Texier, N.; Stévart, T. Erythrina wieringae. The IUCN Red List of Threatened Species 2020, e.T172511949A172511959. Available online: https://www.iucnredlist.org/species/172511949/172511959 (accessed on 24 November 2025). [CrossRef]
- Russo, R.; Budowski, G. Effect of pollarding frequency on biomass of Erythrina poeppigiana as a coffee shade tree. Agrofor. Syst. 1986, 4, 145–162. [Google Scholar] [CrossRef]
- Bedane, K.G.; Kusari, S.; Masesane, I.B.; Spiteller, M.; Majinda, R.R.T. Flavanones of Erythrina livingstoniana with antioxidant properties. Fitoterapia 2016, 108, 48–54. [Google Scholar] [CrossRef]
- Carlquist, S. The biota of long distance dispersal. III. Loss of dispersibility in the Hawaiian flora. Brittonia 1966, 18, 310–335. [Google Scholar] [CrossRef]
- Loope, L.; VanGelder, E. Appendix E: Invasive species report. In Pacific Island Network Vital Signs Monitoring Plan,(Natural Resource Report NPS/PACN/NRR—2006/003); HaySmith, L., Klasner, F.L., Stephens, S.H., Dicus, G.H., Eds.; National Park Service: Fort Collins, CO, USA, 2006. [Google Scholar]
- Grave, E.; Kroessig, T. Erythrina sandwicensis. The IUCN Red List of Threatened Species 2020, e.T120702630A120702761. Available online: https://www.iucnredlist.org/species/120702630/120702761 (accessed on 24 November 2025). [CrossRef]
- Kim, I.K.; Delvare, G.; LaSalle, J. A new species of Quadrastichus (Hymenoptera: Eulophidae): A gall-inducing pest on Erythrina spp. (Fabaceae). J. Hymenopt. Res. 2004, 13, 243–249. [Google Scholar]
- Heu, R.A.; Tsuda, D.M.; Nagamine, W.T.; Yalemar, J.A.; Suh, T.H. Erythrina Gall Wasp Quadrastichus erythrinae Kim (Hymenoptera: Eulophidae); New Pest Advisory no. 05–03; State of Hawaii Department of Agriculture: Honolulu, HI, USA, 2008. [Google Scholar]
- Medeiros, A.C.; Vonallmen, E.; Fukada, M.; Samuelson, A.; Lau, T. Impact of the newly arrived seed-predating beetle Specularius impressithorax (Coleoptera: Chrysomelidae: Bruchinae) in Hawaii. Pac. Conserv. Biol. 2008, 14, 7–12. [Google Scholar] [CrossRef]
- Samuelson, G.A.; Medeiros, A.C. Specularius impressithorax, an adventive bean weevil on Erythrina new to the Hawaiian Islands (Coleoptera: Chrysomelidae: Bruchinae). In Bishop Museum Occasional Papers No. 88; Bishop Museum Press: Honolulu, HI, USA, 2006; pp. 45–47. [Google Scholar]
- Bridwell, J.C. Specularius erythrinae, a new bruchid affecting seeds of Erythrina (Coleoptera). J. Wash. Acad. Sci. 1938, 28, 69–76. [Google Scholar]
- GBIF Secretariat 2023. Erythrina livingstoniana Baker, GBIF Backbone Taxonomy. Checklist Dataset. Available online: https://www.gbif.org/dataset/d7dddbf4-2cf0-4f39-9b2a-bb099caae36c (accessed on 9 November 2024). [CrossRef]
- Kingsolver, J.M.; Decelle, J.E. Host associations of Specularius impressithorax (Pic) (Insecta: Coleoptera: Bruchidae) with species of Erythrina (Fabales: Fabaceae). Ann. Mo. Bot. Gard. 1979, 66, 528–532. [Google Scholar] [CrossRef]
- Decelle, J. Les Coleopteres Bruchides d’Angola; Publicacoes culturais da Companhia de Diamantes de Angola: Lisboa, Portugal, 1975; Volume 98, pp. 13–32. [Google Scholar]
- Tuda, M.; Ronn, J.; Buranapanichpan, S.; Wasano, N.; Arnqvist, G. Evolutionary diversification of the bean beetle genus Callosobruchus (Coleoptera: Bruchidae): Traits associated with stored-product pest status. Mol. Ecol. 2006, 15, 3541–3551. [Google Scholar] [CrossRef]
- Nwanze, K.F.; Horber, E. Seed Coats of Cowpeas Affect Oviposition and Larval Development of Callosobruchus maculatus. Environ. Entomol. 1976, 5, 213–218. [Google Scholar] [CrossRef]
- Honda, H.; Ohsawa, K. Chemical ecology for stored product insects. J. Pestic. Sci. 1990, 15, 263–270. [Google Scholar] [CrossRef][Green Version]
- Ernst, W.H.O. Food consumption, life history and determinants of host range in the bruchid beetle Specularius impressithorax (Coleoptera: Bruchidae). J. Stored Prod. Res. 1993, 29, 53–62. [Google Scholar] [CrossRef]
- Janzen, D.H. The ecology and evolutionary biology of seed chemistry as relates to seed predation. In Biochemical Aspects of Plant and Animal Coevolution; Harborne, J.B., Ed.; Academic Press: London, UK, 1978; pp. 163–206. [Google Scholar]
- Raven, P.H. Erythrina (Fabaceae): Achievements and opportunities. Lloydia 1974, 37, 321–331. [Google Scholar]
- Cope, J.M.; Fox, C.W. Oviposition decisions in the seed beetle, Callosobruchus maculatus (Coleoptera: Bruchidae): Effects of seed size on superparasitism. J. Stored Prod. Res. 2003, 39, 355–365. [Google Scholar] [CrossRef]
- Feeny, P. Plant apparency and chemical defense. Recent Adv. Phytochem. 1976, 10, 140. [Google Scholar]
- Weissich, P.R. Hawaiian native plants in the landscape. Comb. Proc. Int. Plant Propagators’ Soc. 1995, 44, 332–335. [Google Scholar]
- Sari, L.T.; Ribeiro-Costa, C.S.; da Silva Pereira, P.R.V. Biological aspects of Zabrotes subfasciatus (Bohemann, 1833) (Coleoptera, Bruchidae) on Phaseolus vulgaris L., cv. Carioca (Fabaceae), under laboratory conditions. Rev. Bras. Entomol. 2003, 47, 621–624. [Google Scholar] [CrossRef]
- Pouzat, J. Egg distribution by Acanthoscelides obtectus on identical seeds of Phaseolus vulgaris. Biol. Behav. 1983, 8, 215–230. [Google Scholar]
- Nakai, Z.; Kondo, T.; Akimoto, S. Parasitoid attack of the seed-feeding beetle Bruchus loti enhances the germination success of Lathyrus japonicus seeds. Arthropod-Plant Interact. 2011, 5, 227–234. [Google Scholar] [CrossRef]
- Grave, E.F.; Kroessig, T.I.; Ticktin, T. Pollination Biology of an Endemic Hawaiian Tree, Erythrina sandwicensis (Fabaceae: Papilionoideae), in a Novel Ecosystem. Pac. Sci. 2021, 75, 289–308. [Google Scholar] [CrossRef]
- Takakura, K. The specialist seed predator Bruchidius dorsalis (Coleoptera: Bruchidae) plays a crucial role in the seed germination of its host plant, Gleditsia japonica (Leguminosae). Funct. Ecol. 2002, 16, 252–257. [Google Scholar] [CrossRef]
- Da Silva, A.; Rossi, M.N. When a seed-feeding beetle is a predator and also increases the speed of seed germination: An intriguing interaction with an invasive plant. Evol. Ecol. 2019, 33, 211–232. [Google Scholar] [CrossRef]
- Horvat, E.; Sajna, N. Exploring the impact of a non-native seed predator on the seed germination of its non-native host. Biol. Invas. 2021, 23, 3703–3717. [Google Scholar] [CrossRef]
- Fox, C.W.; Wallin, W.G.; Bush, M.L.; Czesak, M.E.; Messina, F.J. Effects of seed beetles on the performance of desert legumes depend on host species, plant stage, and beetle density. J. Arid Environ. 2012, 80, 10–16. [Google Scholar] [CrossRef]
- Parra-Gil, P.d.J.; Espinosa-Vásquez, G.; Lucio-Cruz, C.Y.; Romero-Nápoles, J.; Arce-Cervantes, O. Damage to the seeds of Erythrina americana Mill. (Leguminosae: Faboideae: Erythrininae) by the bruchid Specularius impressithorax (Pic, 1932) (Coleoptera: Bruchidae) and its effect on germination. Acta Zool. Mex. 2023, 39, e2523. [Google Scholar] [CrossRef]
- Center, T.; Johnson, C.D. Coevolution of some Seed beetles (Coleoptera: Bruchidae) and their hosts. Ecology 1974, 55, 1096–1103. [Google Scholar] [CrossRef]
- Aguirre-Salcedo, C.; Montaño-Arias, S.A.; Jansson, R. Restoration implications of the germination ecology of six dry-forest woody Fabaceae species in Mexico. Trees 2025, 39, 33. [Google Scholar] [CrossRef]
- Auld, T.D.; O’Connell, M.A. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Aust. J. Ecol. 1991, 16, 53–70. [Google Scholar] [CrossRef]
- Rodríguez, M.; Delibes, M.; Fedriani, J.M. Hierarchical levels of seed predation variation by introduced beetles on an endemic Mediterranean palm. PLoS ONE 2014, 9, e109867. [Google Scholar] [CrossRef]
- Tuda, M.; Wu, L.-H.; Tateishi, Y.; Niyomdham, C.; Buranapanichpan, S.; Morimoto, K.; Wu, W.-J.; Wang, C.-P.; Chen, Z.; Zhu, H.; et al. A novel host shift and invaded range of a seed predator, Acanthoscelides macrophthalmus (Coleoptera: Chrysomelidae: Bruchinae), of an invasive weed, Leucaena leucocephala. Entomol. Sci. 2009, 12, 1–8. [Google Scholar] [CrossRef]
- Gyorgy, Z.; Tuda, M. Host plant range expansion to Gymnocladus dioica by an introduced seed predatory beetle Megabruchidius dorsalis. Entomol. Sci. 2020, 23, 28–32. [Google Scholar] [CrossRef]
- Gumovsky, A.V.; Ramadan, M.M. Biology, immature and adult morphology, and molecular characterization of a new species of the genus Entedon (Hymenoptera: Eulophidae) associated with the invasive pest Specularius impressithorax (Coleoptera: Chrysomelidae: Bruchinae) on Erythrina plants. Bull. Entomol. Res. 2011, 101, 715–739. [Google Scholar] [CrossRef]
- Ribeiro-Costa, C.S.; Almeida, L.M. Seed-Chewing Beetles (Coleoptera: Chrysomelidae, Bruchinae). In Insect Bioecology and Nutrition for Integrated Pest Management; Panizzi, A.R., Parra, J.R.P., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 325–352. [Google Scholar]
- Tuda, M.; Wu, L.-H.; Yamada, N.; Wang, C.-P.; Wu, W.-J.; Buranapanichpan, S.; Kagoshima, K.; Chen, Z.-Q.; Teramoto, K.K.; Kumashiro, B.R.; et al. Host shift capability of a specialist seed predator of an invasive plant: Roles of competition, population genetics and plant chemistry. Biol. Inv. 2014, 16, 303–313. [Google Scholar] [CrossRef]
- Bridwell, J.C. Notes on the Bruchidae (Coleoptera) and their Parasites in the Hawaiian Islands, 3rd Paper. Proc. Hawaii. Entomol. Soc. 1920, 4, 403–409. [Google Scholar]
- Gillett, J.B. The Fruit and seeds of Erythrina brucei and the identity of E. abyssinica. Kew Bull. 1962, 15, 425–429. [Google Scholar] [CrossRef]
- Ruiz-Montiel, C.; Martínez Hernández Mde, J.; Romero Nápoles, J.; Rios Reyes, A.V. Primer reporte de Specularius impressithorax (Pic.) (Coleoptera: Bruchidae) alimentándose de semillas de Erythrina americana miller en los estados de Veracruz y Morelos, México. Acta Zool. Mex. 2012, 28, 635–639. [Google Scholar] [CrossRef][Green Version]
- Lagos-Ordoñez, M.E.; Romero-Nápoles, J.; González-Hernández, H.; Pérez-Panduro, A.; Zetina, D.A.H. New Distribution Records of Specularius impressithorax (Pic) in Honduras on Erythrina berteroana. Southwest. Entomol. 2024, 49, 542–546. [Google Scholar] [CrossRef]
- Romero, N.J.; Johnson, C.D. BRUCOL, a database for Bruchidae (Insecta, Coleoptera). Entomol. Mex. 2002, 1, 519–526. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).