Integrated Soil Amendments Alleviate Subsoil Acidification and Enhance Ponkan Seedling Growth in a Column Experiment
Abstract
1. Introduction
2. Results
2.1. Effects of Different Soil Amendments on Soil Acidity
2.2. Effects of Different Amendments on Soil Exchangeable Base Cation Content
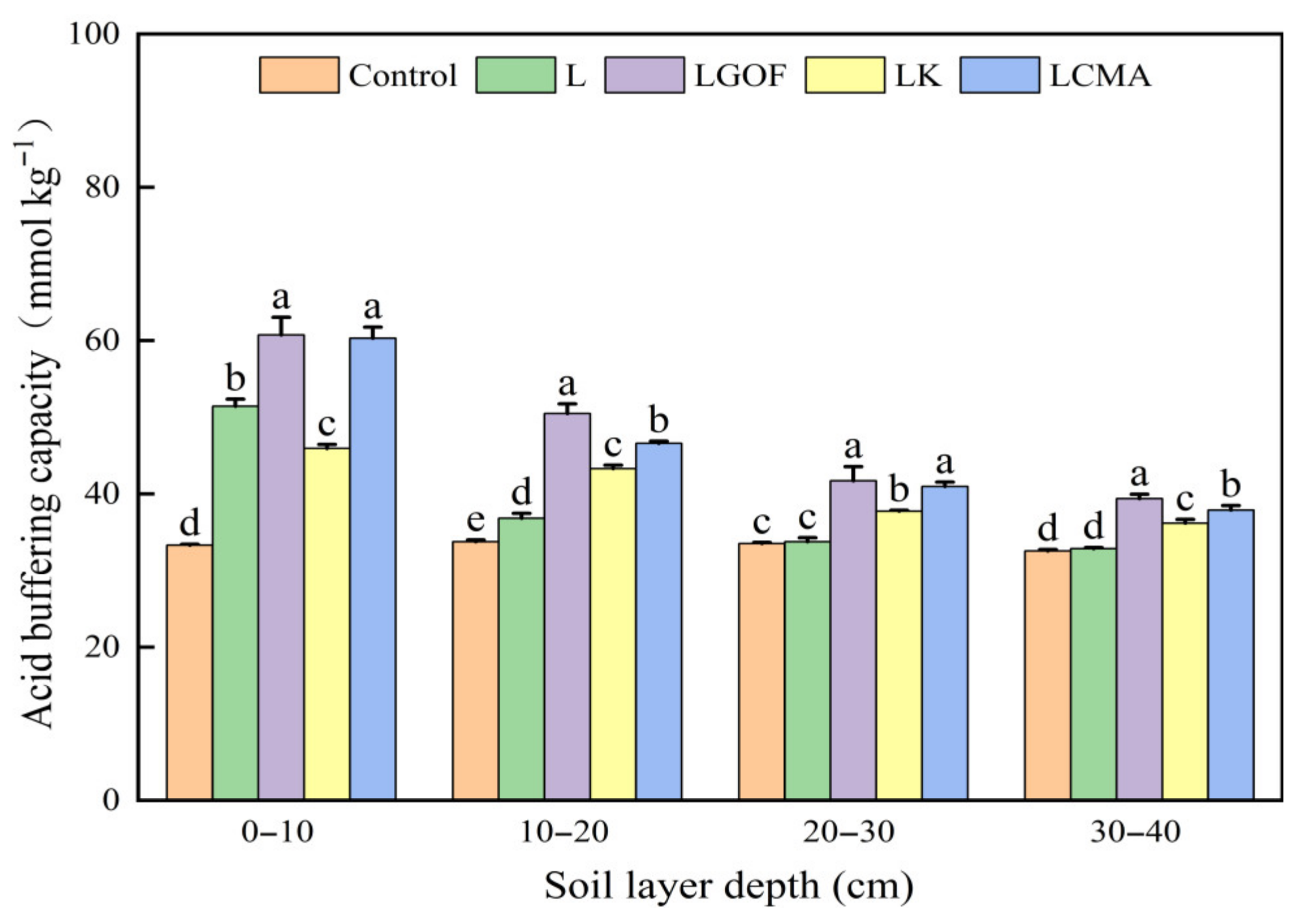
2.3. Effects of Different Amendments on Soil Acid Buffering Capacity
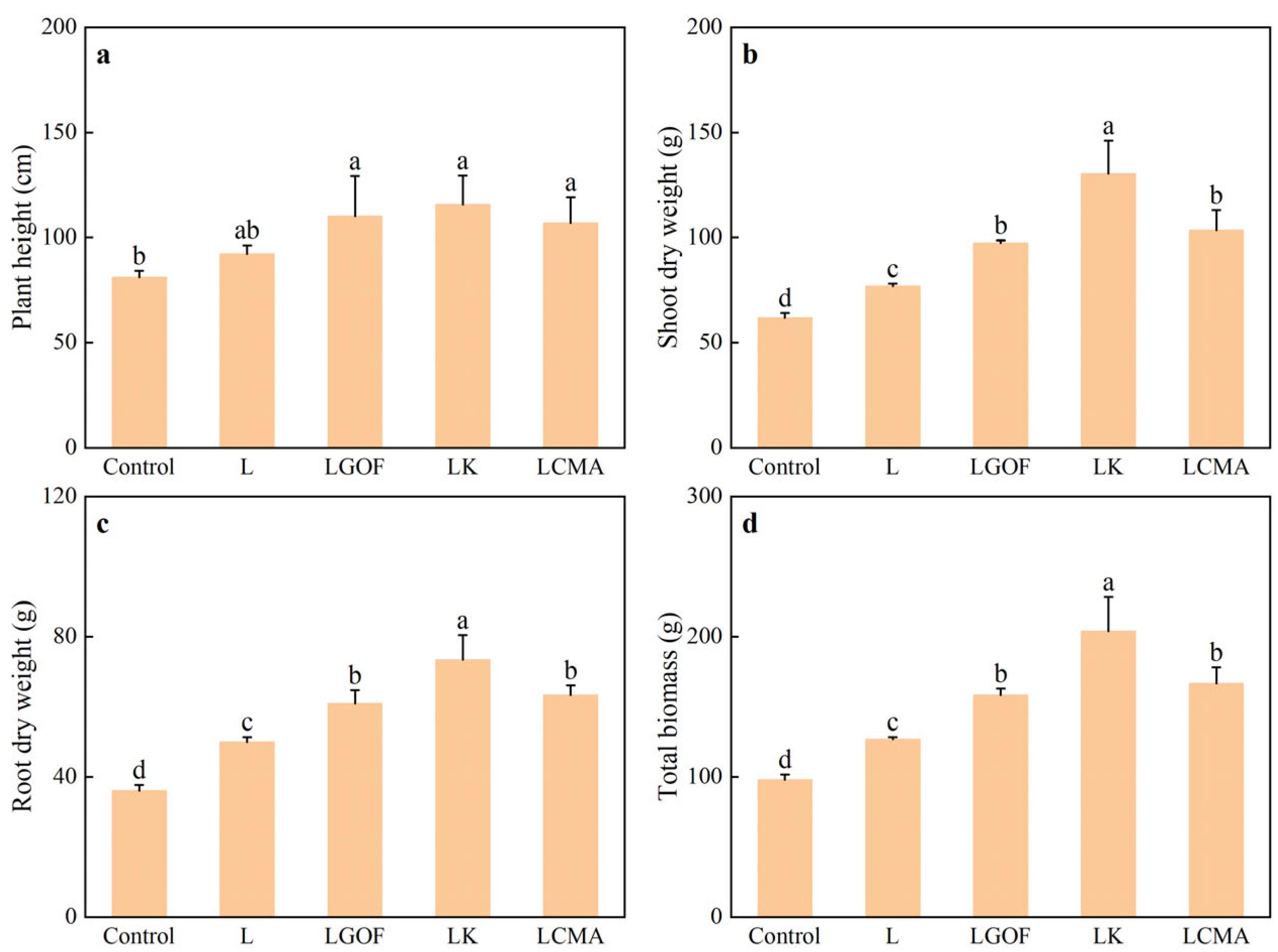
2.4. Effects of Different Soil Amendments on Ponkan Seedling Growth
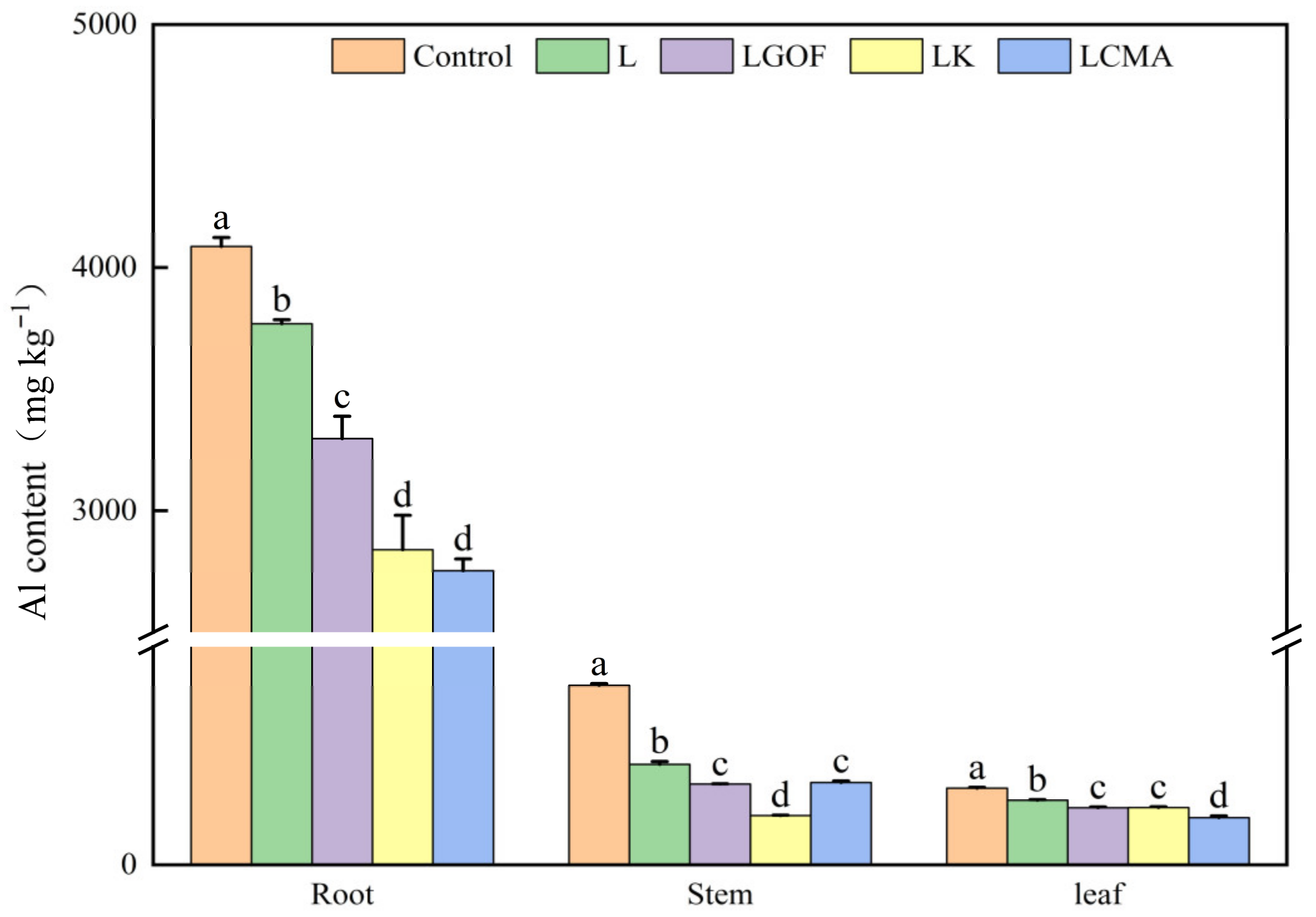
2.5. Al Content in Plants
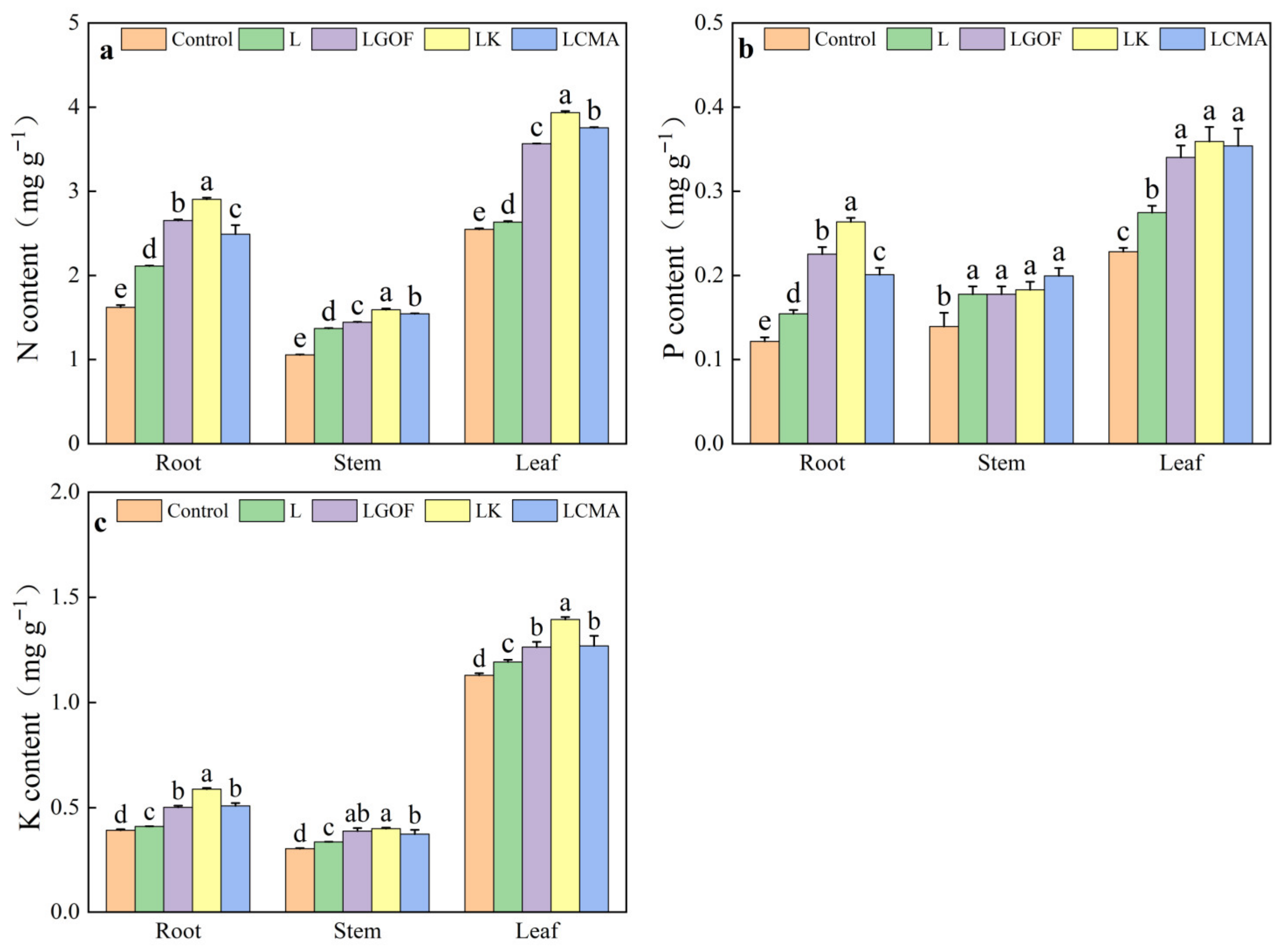
2.6. Effects of Different Soil Amendments on Nutrient Uptake in Ponkan Seedlings
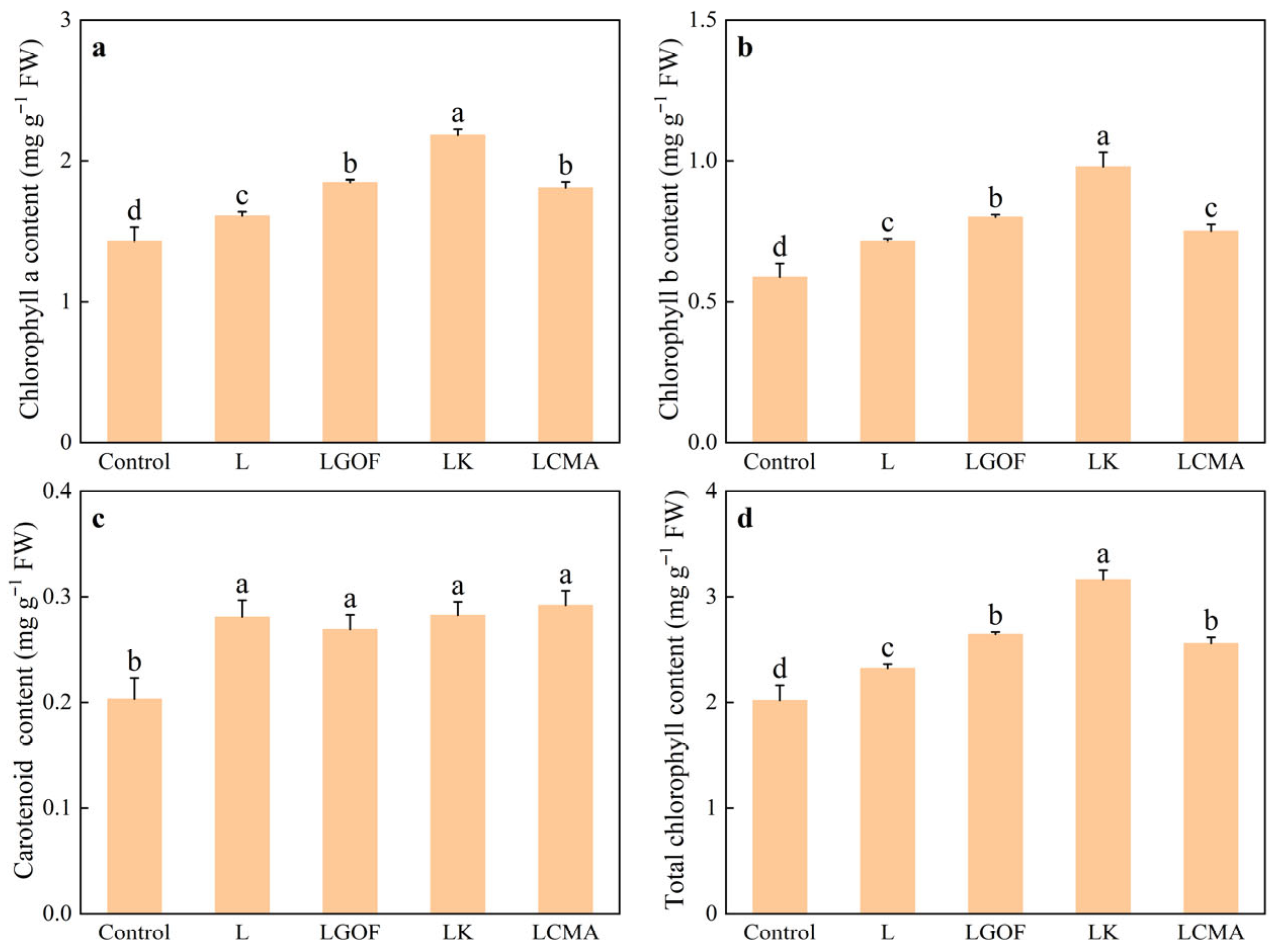
2.7. Effects of Different Soil Amendments on Photosynthetic Pigment Content in Ponkan Seedling
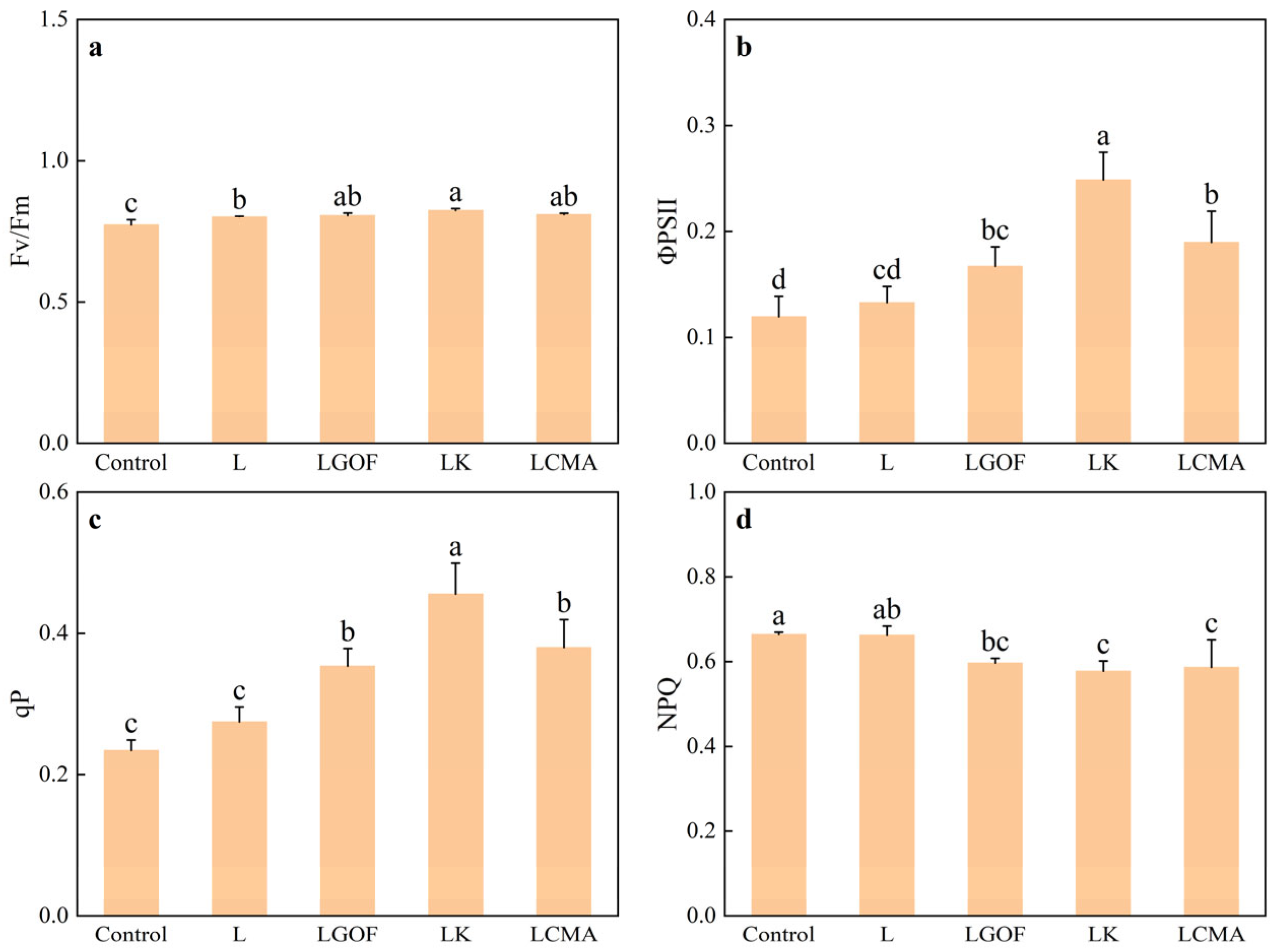
2.8. Effects of Different Soil Amendments on Chlorophyll Fluorescence Parameters and Photosynthetic Characteristics
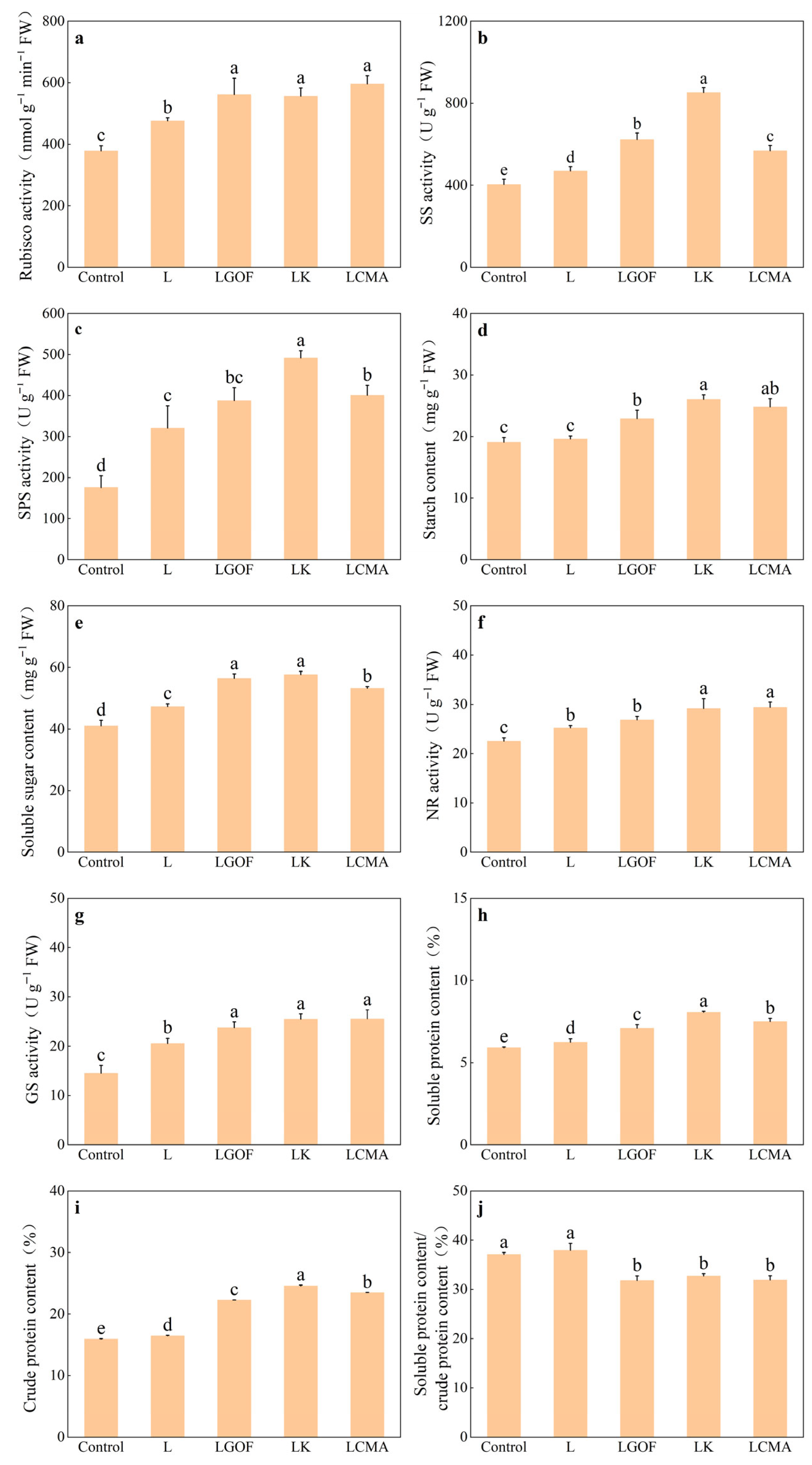
2.9. Effects of Different Soil Amendments on Key Enzyme Activities and Product Accumulation in Photosynthesis and Nitrogen Metabolism
3. Discussion
3.1. Ameliorative Effects of Soil Amendments on Soil Acidification and Al Toxicity
3.2. Effects of Soil Amendments on Alleviating Aluminum Toxicity and Improving Root Architecture and Nutrient Uptake in Ponkan
3.3. Synergistic Enhancement of Photosynthetic Characteristics and Carbon–Nitrogen Metabolism by Soil Amendments
4. Materials and Methods
4.1. Experimental Site and Materials
4.2. Experimental Design
4.3. Determination of Soil Acidity and Exchangeable Base Cation Content
4.4. Determination of Plant Growth Indicators
4.5. Determination of N, P, K, and Al Contents in Ponkan Seedlings
4.6. Photosynthetic Pigment Content
4.7. Chlorophyll Fluorescence Parameters and Photosynthesis Characteristics
4.8. Determination of Carbon and Nitrogen Metabolism-Related Enzyme Activities and Product Content in Ponkan Seedlings
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil. 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.L.; Zhao, X.R.; Yang, S.H.; Zhang, G.L. Contribution of different proton sources to the acidification of red soil with maize cropping in subtropical China. Geoderma 2021, 392, 114995. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Piñeros, M.A.; Magalhaes, J.V.; Carvalho Alves, V.M.; Kochian, L.V. The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize. Plant Physiol. 2002, 129, 1194–1206. [Google Scholar] [CrossRef]
- Yang, M.; Tan, L.; Xu, Y.Y.; Zhao, Y.H.; Cheng, F.; Ye, S.M.; Jiang, W.X. Effect of low pH and aluminum toxicity on the photosynthetic characteristics of different fast-growing Eucalyptus vegetatively propagated clones. PLoS ONE 2015, 10, e0130963. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Wolf, W.M. The impact of soil pH on heavy metals uptake and photosynthesis efficiency in Melissa officinalis, Taraxacum officinalis, Ocimum basilicum. Molecules 2022, 27, 4671. [Google Scholar] [CrossRef]
- Dong, D.H.; Deng, Q.L.; Zhang, J.L.; Jia, C.Y.; Gao, M.; Wang, Y.R.; Zhang, L.; Zhang, N.; Guo, Y.D. Transcription factor SlSTOP1 regulates Small Auxin-Up RNA Genes for tomato root elongation under aluminum stress. Plant Physiol. 2024, 196, 2654–2668. [Google Scholar] [CrossRef]
- Long, A.; Zhang, J.; Yang, L.T.; Ye, X.; Lai, N.W.; Tan, L.L.; Lin, D.; Chen, L.S. Effects of low pH on photosynthesis, related physiological parameters, and nutrient profiles of Citrus. Front. Plant Sci. 2017, 8, 185. [Google Scholar] [CrossRef]
- Zhu, S.P.; Nong, J.F.; Luo, G.T.; Li, Q.P.; Wang, F.S.; Jiang, D.; Zhao, X.C. Varied tolerance and different responses of five citrus rootstocks to acid stress by principle component analysis and orthogonal analysis. Sci. Hortic. 2021, 278, 109853. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Myhre, D.L. Citrus root-growth as affected by soil aluminum level under field conditions. Soil Sci. Soc. Am. J. 1990, 54, 1340–1344. [Google Scholar] [CrossRef]
- Yang, T.Y.; Huang, W.T.; Zhang, J.; Yang, L.T.; Huang, Z.R.; Wu, B.S.; Lai, N.W.; Chen, L.S. Raised pH conferred the ability to maintain a balance between production and detoxification of reactive oxygen species and methylglyoxal in aluminum-toxic Citrus sinensis leaves and roots. Environ. Pollut. 2021, 268, 115676. [Google Scholar] [CrossRef]
- Chen, X.H.; Yu, W.H.; Cai, Y.Y.; Zhang, S.W.; Muneer, M.A.; Zhu, Q.C.; Xu, D.H.; Ma, C.C.; Yan, X.J.; Li, Y.; et al. How to identify and adopt cleaner strategies to improve the continuous acidification in orchard soils? J. Clean. Prod. 2022, 330, 129826. [Google Scholar] [CrossRef]
- Adriano, E.; Laclau, J.P.; Rodrigues, J.D. Deep rooting of rainfed and irrigated orange trees in Brazil. Trees 2017, 31, 285–297. [Google Scholar] [CrossRef]
- Huang, X.M.; Muneer, M.A.; Li, J.; Hou, W.; Ma, C.C.; Jiao, J.B.; Cai, Y.Y.; Chen, X.H.; Wu, L.Q.; Zheng, C.Y. Integrated nutrient management significantly improves pomelo (Citrus grandis) root growth and nutrients uptake under acidic soil of southern China. Agronomy 2021, 11, 1231. [Google Scholar] [CrossRef]
- Zhang, S.W.; Chen, X.H.; Ji, Z.J.; Yan, X.J.; Kong, K.P.; Cai, Y.Y.; Zhu, Q.C.; Muneer, M.A.; Zhang, F.S.; Wu, L.Q. Reducing aluminum is the key nutrient management strategy for ameliorating soil acidification and improving root growth in an acidic citrus orchard. Land Degrad. Dev. 2022, 34, 1681–1693. [Google Scholar] [CrossRef]
- Holland, J.E.; Bennett, A.E.; Newton, A.C.; White, P.J.; McKenzie, B.M.; George, T.S.; Pakeman, R.J.; Bailey, J.S.; Fornara, D.A.; Hayes, R.C. Liming impacts on soils, crops and biodiversity in the UK: A review. Sci. Total Environ. 2018, 610–611, 316–332. [Google Scholar] [CrossRef]
- Jouichat, H.; Khiari, L.; Gallichand, J.; Ismail, M. Modeling temporal variation of soil acidity after the application of liming materials. Soil Till. Res. 2024, 240, 106050. [Google Scholar] [CrossRef]
- Azam, G.; Gazey, C. Slow movement of alkali from surface-applied lime warrants the introduction of strategic tillage for rapid amelioration of subsurface acidity in south-western Australia. Soil Res. 2021, 59, 97–106. [Google Scholar] [CrossRef]
- Arshad, M.A.; Soon, Y.K.; Azooz, R.H.; Lupwayi, N.Z.; Chang, S.X. Soil and crop response to wood ash and lime application in acidic soils. Agron. J. 2012, 104, 715–721. [Google Scholar] [CrossRef]
- Li, G.D.; Conyers, M.K.; Refshauge, G.; Ataollahi, F.; Hayes, R.C. Long-term liming changes pasture mineral profile. Sci. Rep. 2024, 14, 3539. [Google Scholar] [CrossRef]
- Sonsri, K.; Watanabe, A. Insights into the formation and stability of soil aggregates in relation to the structural properties of dissolved organic matter from various organic amendments. Soil Till. Res. 2023, 232, 105774. [Google Scholar] [CrossRef]
- Regasa, A.; Haile, W.; Abera, G. Effects of lime and vermicompost application on soil physicochemical properties and phosphorus availability in acidic soils. Sci. Rep. 2025, 15, 25544. [Google Scholar] [CrossRef] [PubMed]
- De Souza, I.M.D.; Borges, W.L.B.; Juliano, P.H.G.; Modesto, V.C.; Girardi, V.A.M.; de Souza Júnior, N.C.; Ribeiro, N.A.A.; Matos, A.M.S.; Galindo, F.S.; Andreotti, M. Methodologies for agricultural gypsum application recommendations in no-tillage systems on tropical sandy soils. Agronomy 2025, 15, 416. [Google Scholar] [CrossRef]
- Sumner, M.E.; Shahandeh, H.; Bouton, J.; Hammel, J. Amelioration of an acid soil profile through deep liming and surface application of gypsum. Soil Sci. Soc. Am. J. 1986, 50, 1254–1258. [Google Scholar] [CrossRef]
- Curtin, D.; Syers, J.K. Mechanism of sulphate adsorption by two tropical soils. Eur. J. Soil Sci. 1990, 41, 295–304. [Google Scholar] [CrossRef]
- Shi, R.Y.; Liu, Z.D.; Li, Y.; Jiang, T.M.; Xu, M.G.; Li, J.Y.; Xu, R.K. Mechanisms for increasing soil resistance to acidification by long-term manure application. Soil Till. Res. 2019, 185, 77–84. [Google Scholar] [CrossRef]
- Dai, P.G.; Cong, P.; Wang, P.; Dong, J.X.; Dong, Z.R.; Song, W.J. Alleviating soil acidification and increasing the organic carbon pool by long-term organic fertilizer on tobacco planting soil. Agronomy 2021, 11, 2135. [Google Scholar] [CrossRef]
- Bernal, M.P.; Álvarez-Robles, M.J.; Bernal-Molina, P.; Clemente, R. Bottom ash from combustion of chicken manure as a fertiliser material. Front. Sustain. Food Syst. 2024, 8, 1392445. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, Z.D.; Zhao, W.Z.; Masud, M.M.; Xu, R.K. Alkaline slag is more effective than phosphogypsum in the amelioration of subsoil acidity in an Ultisol profile. Soil Till. Res. 2015, 149, 21–32. [Google Scholar] [CrossRef]
- Shi, R.Y.; Ni, N.; Nkoh, J.N.; Dong, Y.; Zhao, W.R.; Pan, X.Y.; Li, J.Y.; Xu, R.K.; Qian, W. Biochar retards Al toxicity to maize (Zea mays L.) during soil acidification: The effects and mechanisms. Sci. Total Environ. 2020, 719, 137448. [Google Scholar] [CrossRef]
- Wang, T.Q.; Yu, L.X.; Wang, Z.; Yang, C.; Dong, F.Y.; Yang, D.W.; Xi, H.J.; Sun, Z.P.; Bol, R.; Awais, M.; et al. Effect of simulated acidification on soil properties and plant nutrient uptake of eggplant in greenhouse. Front. Plant Sci. 2025, 16, 1558458. [Google Scholar] [CrossRef]
- Yang, T.Y.; Cai, L.Y.; Qi, Y.P.; Yang, L.T.; Lai, N.W.; Chen, L.S. Increasing nutrient solution pH alleviated aluminum-induced inhibition of growth and impairment of photosynthetic electron transport chain in Citrus sinensis seedlings. Biomed Res. Int. 2019, 2019, 9058715. [Google Scholar] [CrossRef]
- Du, L.X.; Zhang, Z.Y.; Chen, Y.Q.; Wang, Y.; Zhou, C.X.; Yang, H.Y.; Zhang, W. Heterogeneous impact of soil acidification on crop yield reduction and its regulatory variables: A global meta-analysis. Field Crops Res. 2024, 319, 109643. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995, 107, 315–321. [Google Scholar] [CrossRef]
- Hu, Y.L.; Chen, J.; Hui, D.F.; Li, J.L.; Yao, X.Y.; Zhang, D.Q.; Deng, Q. Soil acidification suppresses phosphorus supply through enhancing organomineral association. Sci. Total Environ. 2023, 905, 167105. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Qi, Y.P.; Smith, B.R.; Liu, X.H. Aluminum-induced decrease in CO2 assimilation in citrus seedlings is unaccompanied by decreased activities of key enzymes involved in CO2 assimilation. Tree Physiol. 2005, 25, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Q.; Qi, Y.P.; Huang, W.L.; Yang, L.T.; Lai, N.W.; Ye, X.; Chen, L.S. Low pH-responsive proteins revealed by a 2-DE based MS approach and related physiological responses in Citrus leaves. BMC Plant Biol. 2018, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, R.J.; Salvucci, M.E. Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–475. [Google Scholar] [CrossRef]
- William, B.J.; Costa, C.C.A.; Gustavo, M.L.; Ariani, G.; Roberto, P.J.; Leila, B.; Gonçalves, V.R.; Fávero, C.E.; Carneiro, A.T.J.; Carlos, C.J.; et al. Improving soil fertility with lime and phosphogypsum enhances soybean yield and physiological characteristics. Agron. Sustain. Dev. 2022, 42, 26. [Google Scholar] [CrossRef]
- Huang, W.T.; Zheng, Z.C.; Hua, D.; Chen, X.F.; Zhang, J.; Chen, H.H.; Ye, X.; Guo, J.X.; Yang, L.T.; Chen, L.S. Adaptive responses of carbon and nitrogen metabolisms to nitrogen-deficiency in Citrus sinensis seedlings. BMC Plant Biol. 2022, 22, 370. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yue, X.L.; Fang, S.Z.; Qian, M.Y.; Zhou, S.T.; Shang, X.L.; Yang, W.X. Responses of nitrogen metabolism, photosynthetic parameter and growth to nitrogen fertilization in Cyclocarya paliurus. For. Ecol. Manag. 2021, 502, 119715. [Google Scholar] [CrossRef]
- Aitken, R.L.; Moody, P.W. The effect of valence and ionic-strength on the measurement of pH buffer capacity. Aust. J. Soil Res. 1994, 32, 975–984. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.Q.; Lin, F.; Ten, Y.; Lin, J.; Zhu, D.H.; Guo, P.; Weng, Y.B.; Chen, L.S. Soil chemical properties, ‘Guanximiyou’ pummelo leaf mineral nutrient status and fruit quality in the southern region of Fujian province, China. J. Soil Sci. Plant Nutr. 2015, 15, 615–628. [Google Scholar] [CrossRef]
- Yang, W.J.; Li, X.; Chang, F.; Qiu, X.; Huang, X.L.; Feng, Z.; Yan, J.; Wu, Q.H.; Wen, F.Y.; Pei, J.; et al. Low light reduces saffron corm yield by inhibiting starch synthesis. Front. Plant Sci. 2025, 16, 1544054. [Google Scholar] [CrossRef]
- Rao, R.Y.; Huang, W.L.; Yang, H.; Shen, Q.; Huang, W.T.; Lu, F.; Ye, X.; Yang, L.T.; Huang, Z.R.; Chen, L.S. Raising pH reduces manganese toxicity in Citrus grandis (L.) Osbeck by efficient maintenance of nutrient homeostasis to enhance photosynthesis and growth. Plants 2025, 14, 2390. [Google Scholar] [CrossRef]


| Soil Depth (cm) | Treatment | pH | Exchangeable H (cmol kg−1) | Exchangeable Al (cmol kg−1) |
|---|---|---|---|---|
| 0–10 | Control | 3.80 ± 0.03 d | 1.77 ± 0.06 a | 2.35 ± 0.10 a |
| L | 7.76 ± 0.06 a | 0.12 ± 0.03 b | 0.08 ± 0.03 b | |
| LGOF | 7.56 ± 0.07 b | 0.12 ± 0.03 b | 0.07 ± 0.03 b | |
| LK | 7.21 ± 0.11 c | 0.13 ± 0.03 b | 0.12 ± 0.03 b | |
| LCMA | 7.45 ± 0.13 b | 0.13 ± 0.03 b | 0.08 ± 0.03 b | |
| 10–20 | Control | 3.74 ± 0.01 d | 2.02 ± 0.08 a | 2.58 ± 0.06 a |
| L | 5.02 ± 0.04 c | 1.12 ± 0.03 b | 1.32 ± 0.08 b | |
| LGOF | 6.25 ± 0.03 a | 0.52 ± 0.06 c | 0.29 ± 0.05 e | |
| LK | 6.11 ± 0.02 b | 0.48 ± 0.03 c | 0.72 ± 0.03 c | |
| LCMA | 6.17 ± 0.09 ab | 0.47 ± 0.03 c | 0.47 ± 0.03 d | |
| 20–30 | Control | 3.65 ± 0.02 c | 2.27 ± 0.06 a | 2.52 ± 0.10 a |
| L | 3.70 ± 0.04 c | 2.27 ± 0.03 a | 2.55 ± 0.10 a | |
| LGOF | 4.94 ± 0.11 b | 1.47 ± 0.06 b | 1.52 ± 0.08 b | |
| LK | 5.11 ± 0.07 a | 1.33 ± 0.06 c | 1.55 ± 0.09 b | |
| LCMA | 5.05 ± 0.10 ab | 1.38 ± 0.03 bc | 1.42 ± 0.08 b | |
| 30–40 | Control | 3.57 ± 0.01 d | 2.58 ± 0.10 a | 2.90 ± 0.15 a |
| L | 3.58 ± 0.02 d | 2.52 ± 0.07 a | 2.82 ± 0.08 a | |
| LGOF | 4.45 ± 0.03 c | 2.17 ± 0.08 b | 2.20 ± 0.05 b | |
| LK | 4.62 ± 0.01 a | 1.92 ± 0.03 c | 1.97 ± 0.06 c | |
| LCMA | 4.52 ± 0.04 b | 2.15 ± 0.05 b | 2.17 ± 0.08 b |
| Soil Depth (cm) | Treatment | Exchangeable K+(mg kg−1) | Exchangeable Ca2+ (mg kg−1) | Exchangeable Mg2+ (mg kg−1) |
|---|---|---|---|---|
| 0–10 | Control | 961.35 ± 20.30 a | 152.07 ± 11.74 e | 34.90 ± 0.92 c |
| L | 986.11 ± 58.69 a | 2520.79 ± 71.10 b | 33.32 ± 0.56 c | |
| LGOF | 712.24 ± 16.15 b | 3511.99 ± 173.87 a | 179.04 ± 6.87 b | |
| LK | 527.21 ± 24.07 d | 1004.56 ± 44.77 d | 35.73 ± 0.28 c | |
| LCMA | 630.13 ± 15.72 c | 1538.99 ± 40.73 c | 286.37 ± 20.79 a | |
| 10–20 | Control | 587.45 ± 11.91 b | 167.85 ± 4.00 e | 37.78 ± 1.34 c |
| L | 584.23 ± 60.24 b | 619.53 ± 37.77 c | 32.01 ± 7.38 c | |
| LGOF | 666.06 ± 43.98 a | 1930.62 ± 134.75 a | 72.43 ± 1.81 b | |
| LK | 710.59 ± 30.43 a | 379.33 ± 11.08 d | 36.30 ± 0.54 c | |
| LCMA | 644.79 ± 15.31 ab | 827.50 ± 23.87 b | 104.36 ± 3.22 a | |
| 20–30 | Control | 381.17 ± 73.00 c | 163.59 ± 11.12 c | 35.24 ± 0.59 c |
| L | 348.95 ± 72.34 c | 163.03 ± 3.31 c | 34.82 ± 0.87 c | |
| LGOF | 509.00 ± 57.69 ab | 901.52 ± 14.87 a | 45.07 ± 1.36 b | |
| LK | 523.37 ± 56.57 a | 150.78 ± 7.19 c | 34.73 ± 0.52 c | |
| LCMA | 460.61 ± 60.61 b | 383.74 ± 19.05 b | 62.15 ± 3.35 a | |
| 30–40 | Control | 286.66 ± 29.86 d | 154.99 ± 2.32 c | 35.61 ± 0.46 c |
| L | 277.15 ± 11.27 d | 155.12 ± 8.72 c | 34.69 ± 0.64 c | |
| LGOF | 352.39 ± 11.35 c | 354.58 ± 15.17 a | 37.84 ± 0.53 b | |
| LK | 500.35 ± 20.51 a | 142.80 ± 3.99 c | 35.05 ± 0.17 c | |
| LCMA | 416.22 ± 17.39 b | 225.23 ± 22.61 b | 40.90 ± 0.45 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Duan, X.; Sun, P.; Zheng, F.; Ma, X.; Yu, Y.; Li, Y.; Wang, P. Integrated Soil Amendments Alleviate Subsoil Acidification and Enhance Ponkan Seedling Growth in a Column Experiment. Plants 2025, 14, 3613. https://doi.org/10.3390/plants14233613
Zhang J, Duan X, Sun P, Zheng F, Ma X, Yu Y, Li Y, Wang P. Integrated Soil Amendments Alleviate Subsoil Acidification and Enhance Ponkan Seedling Growth in a Column Experiment. Plants. 2025; 14(23):3613. https://doi.org/10.3390/plants14233613
Chicago/Turabian StyleZhang, Jiacheng, Xiaoya Duan, Pengxiao Sun, Fei Zheng, Xiaochuan Ma, Yuan Yu, Yan Li, and Ping Wang. 2025. "Integrated Soil Amendments Alleviate Subsoil Acidification and Enhance Ponkan Seedling Growth in a Column Experiment" Plants 14, no. 23: 3613. https://doi.org/10.3390/plants14233613
APA StyleZhang, J., Duan, X., Sun, P., Zheng, F., Ma, X., Yu, Y., Li, Y., & Wang, P. (2025). Integrated Soil Amendments Alleviate Subsoil Acidification and Enhance Ponkan Seedling Growth in a Column Experiment. Plants, 14(23), 3613. https://doi.org/10.3390/plants14233613






