Soil Phosphorus Availability Modulates Host Selectivity of Pedicularis kansuensis Between Legumes and Grasses
Abstract
1. Introduction
2. Results
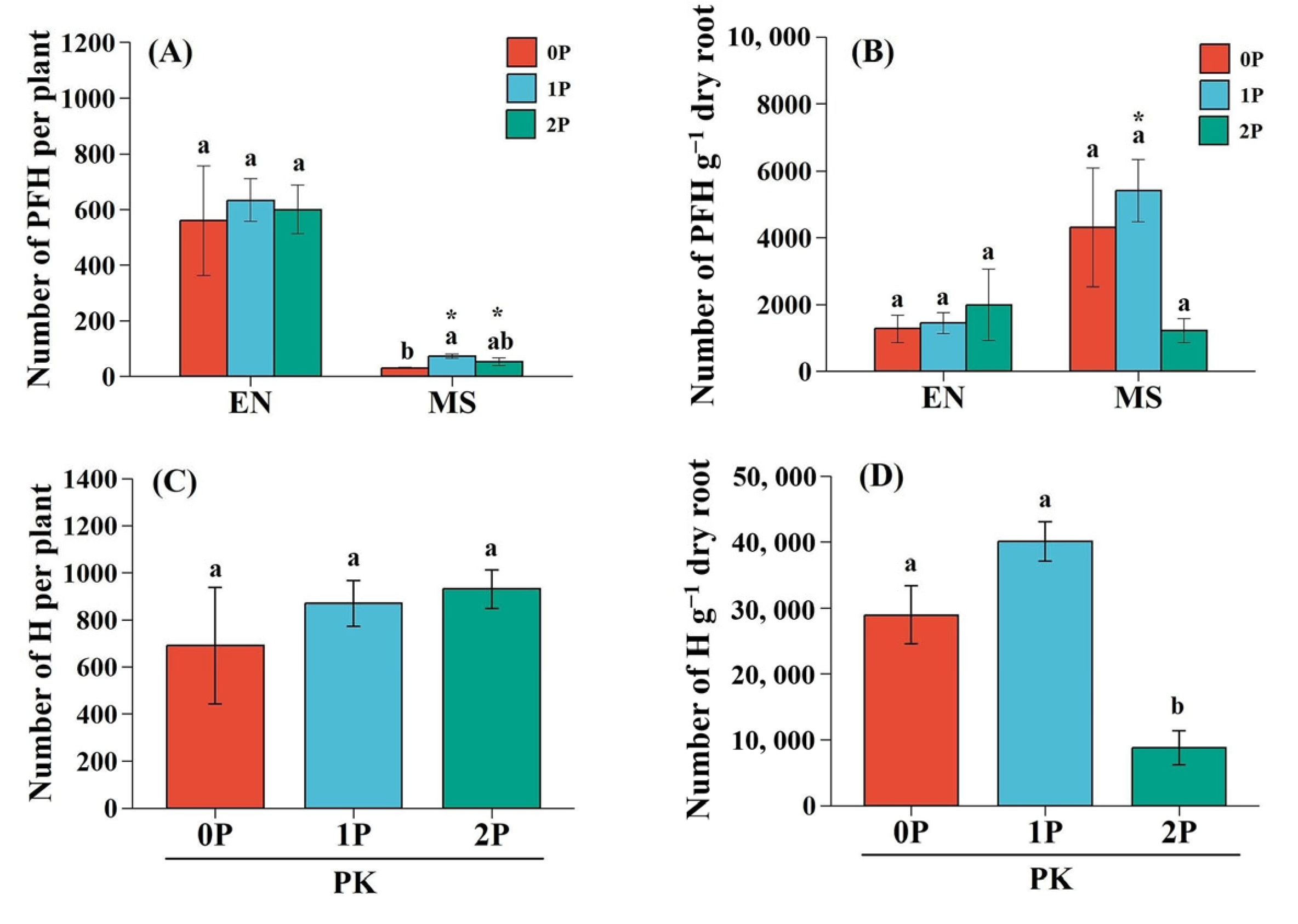
2.1. Haustorium Formation of the Hemiparasite
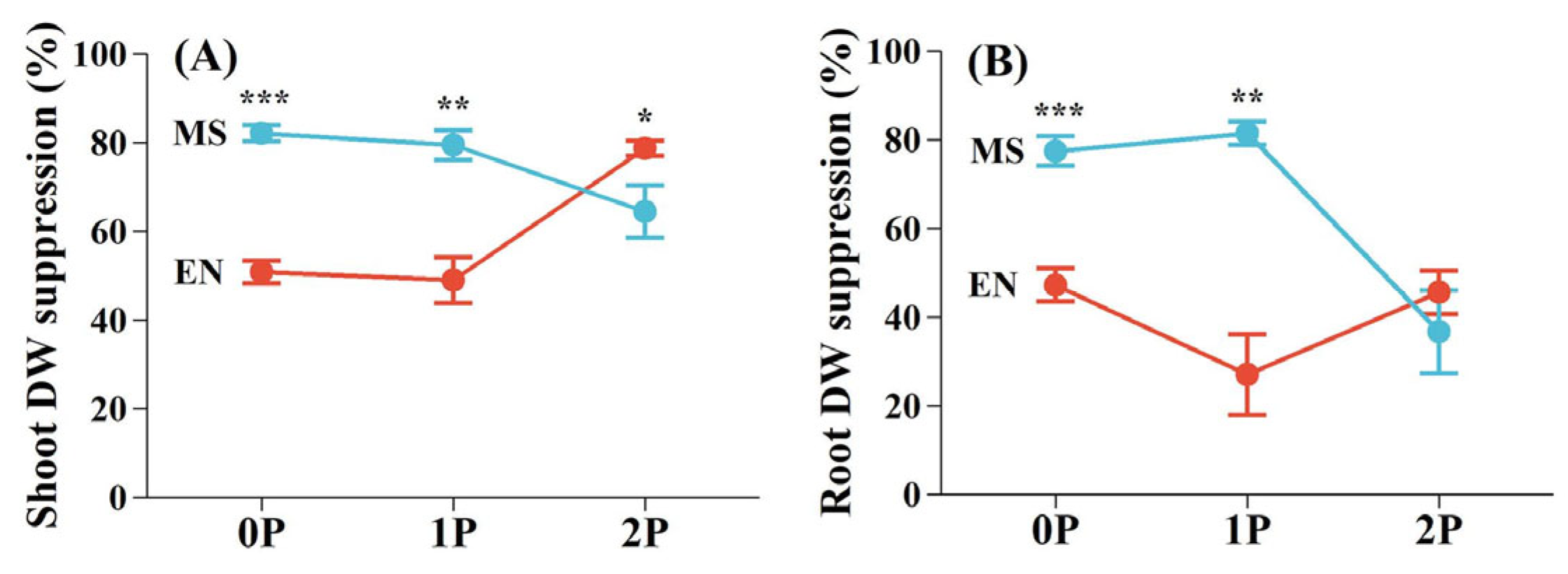
2.2. Growth Performance and Biomass Allocation of Hosts and the Hemiparasite
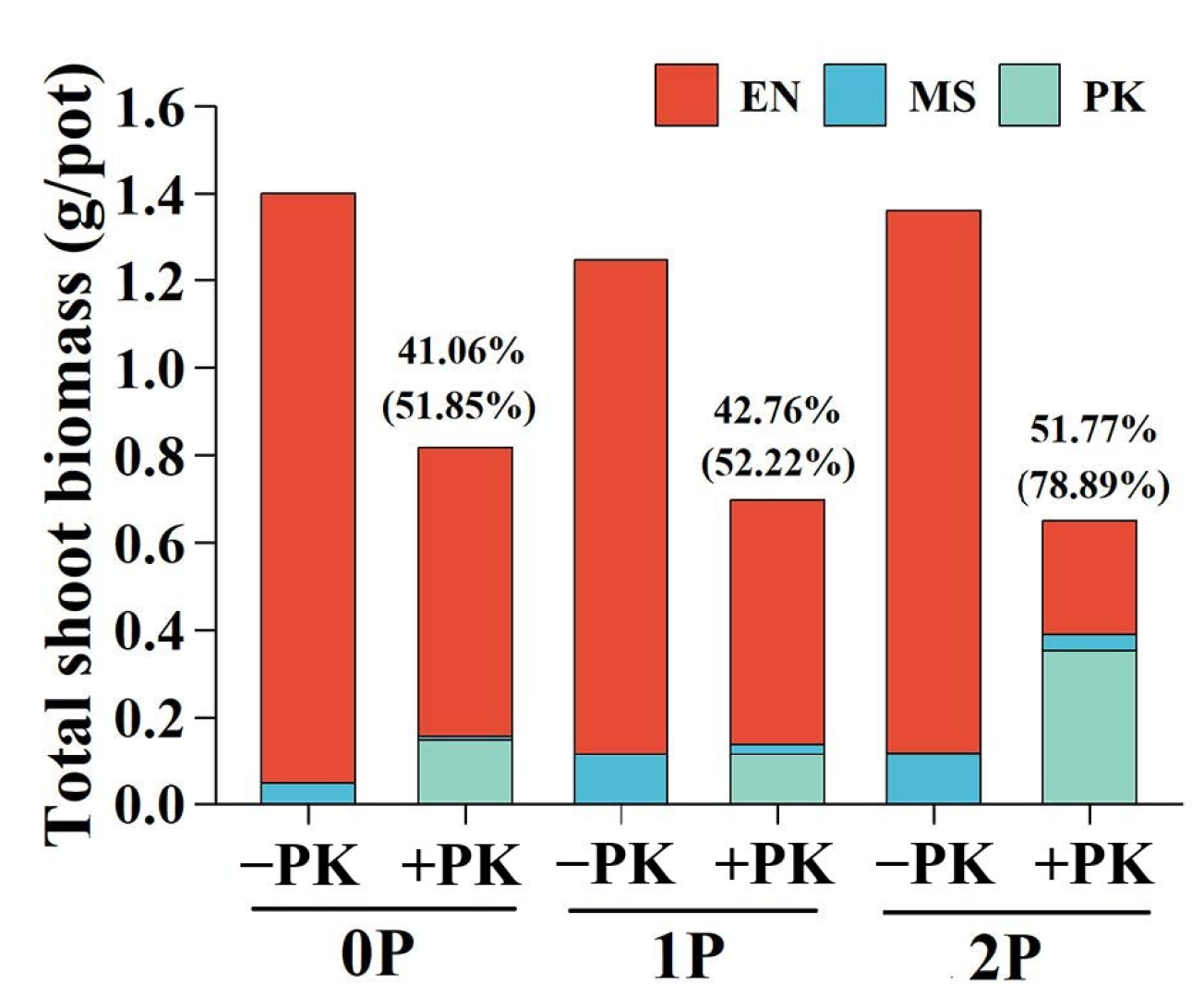
2.3. Biomass Proportions of Host and Hemiparasite per Pot
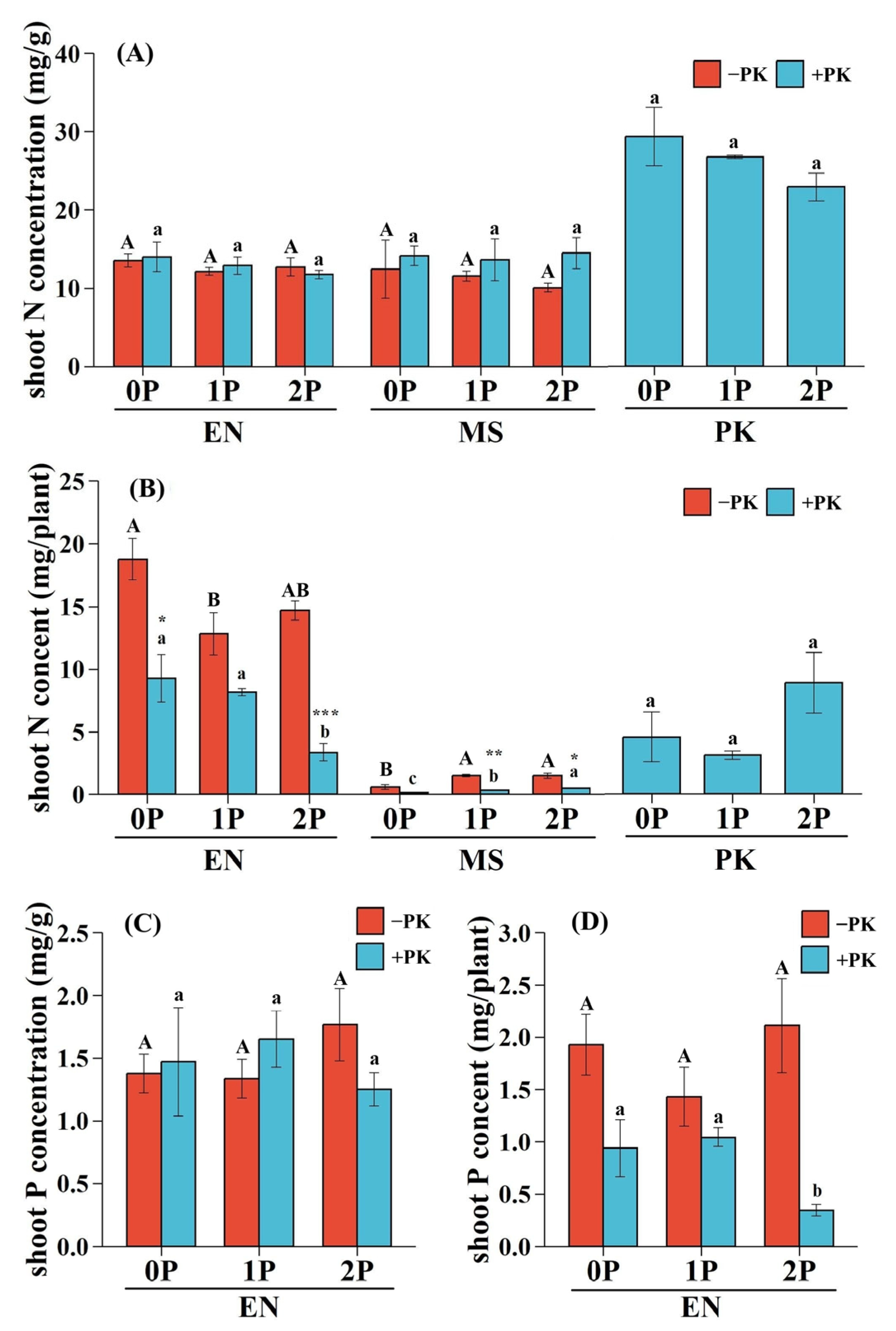
2.4. Shoot N and P Status of Hosts and Hemiparasite
3. Discussion
3.1. P Affected Host Selectivity of Root Hemiparasitic Plants Between Grass and Legume
3.2. Potential Mechanisms of P Affect Host Selectivity in Root Hemiparasitic Plants
3.3. Impact of Soil P Availability on Growth Performance of Hemiparasites
3.4. Implications for Management of Parasitic Plants
4. Materials and Methods
4.1. Experimental Design
4.2. Plant Materials
4.3. Planting and Growth Conditions
4.4. Harvest and Sampling
4.5. Measurement of Shoot N and P Concentrations
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Těšitel, J. Functional biology of parasitic plants: A review. Plant Ecol. Evol. 2016, 149, 5–20. [Google Scholar] [CrossRef]
- Westwood, J.H.; Yoder, J.I.; Timko, M.P.; dePamphilis, C.W. The evolution of parasitism in plants. Trends Plant Sci. 2010, 15, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Irving, L.J.; Cameron, D.D. You are what you eat: Interactions between root parasitic plants and their hosts. Adv. Bot. Res. 2009, 50, 87–138. [Google Scholar]
- Matthies, D. Parasitic and competitive interactions between the hemiparasites Rhinanthus serotinus and Odontites rubra and their host Medicago sativa. J. Ecol. 1995, 83, 245–251. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef]
- Fibich, P.; Leps, J.; Chytry, M.; Tesitel, J. Root hemiparasitic plants are associated with high diversity in temperate grasslands. J. Veg. Sci. 2017, 28, 184–191. [Google Scholar] [CrossRef]
- Těšitel, J.; Li, A.R.; Knotkova, K.; McLellan, R.; Bandaranayake, P.C.G.; Watson, D.M. The bright side of parasitic plants: What are they good for? Plant Physiol. 2021, 185, 1309–1324. [Google Scholar] [CrossRef]
- Sandner, T.M.; Schoppan, L.; Matthies, D. Seedlings of a hemiparasite recognize legumes, but do not distinguish good from poor host species. Folia Geobot. 2022, 57, 117–126. [Google Scholar] [CrossRef]
- Matthies, D. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: Heterotrophic benefit and parasite-mediated competition. Oikos 1996, 75, 118–124. [Google Scholar] [CrossRef]
- Cechin, I.; Press, M.C. Nitrogen relations of the sorghum-Striga hermonthica host-parasite association: Germination, attachment and early growth. New Phytol. 1993, 124, 681–687. [Google Scholar] [CrossRef]
- Rowntree, J.K.; Craig, H. The contrasting roles of host species diversity and parasite population genetic diversity in the infection dynamics of a keystone parasitic plant. J. Ecol. 2019, 107, 23–33. [Google Scholar] [CrossRef]
- Westbury, D.B.; Dunnett, N.P. The promotion of grassland forb abundance: A chemical or biological solution? Basic Appl. Ecol. 2008, 9, 653–662. [Google Scholar] [CrossRef]
- Ameloot, E.; Verheyen, K.; Hermy, M. Meta-analysis of standing crop reduction by Rhinanthus spp. and its effect on vegetation structure. Folia Geobot. 2005, 40, 289–310. [Google Scholar] [CrossRef]
- Fisher, J.P.; Phoenix, G.K.; Childs, D.Z.; Press, M.C.; Smith, S.W.; Pilkington, M.G.; Cameron, D.D. Parasitic plant litter input: A novel indirect mechanism influencing plant community structure. New Phytol. 2013, 198, 222–231. [Google Scholar] [CrossRef]
- Ameloot, E.; Verlinden, G.; Boeckx, P.; Verheyen, K.; Hermy, M. Impact of hemiparasitic Rhinanthus angustifolius and R. minor on nitrogen availability in grasslands. Plant Soil 2008, 311, 255–268. [Google Scholar] [CrossRef]
- Matthies, D. Interactions between a root hemiparasite and 27 different hosts: Growth, biomass allocation and plant architecture. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 118–137. [Google Scholar] [CrossRef]
- Chaudron, C.; Mazalova, M.; Kuras, T.; Malenovsky, I.; Mladek, J. Introducing ecosystem engineers for grassland biodiversity conservation: A review of the effects of hemiparasitic Rhinanthus species on plant and animal communities at multiple trophic levels. Perspect. Plant Ecol. Evol. Syst. 2021, 52, 125633. [Google Scholar] [CrossRef]
- Irving, L.J.; Kim, D.; Schwier, N.; Vaughan, J.K.E.; Ong, G.; Hama, T. Host nutrient supply affects the interaction between the hemiparasite Phtheirospermum japonicum and its host Medicago sativa. Environ. Expe. Bot. 2019, 162, 125–132. [Google Scholar] [CrossRef]
- Jiang, F.; Jeschke, W.D.; Hartung, W.; Cameron, D.D. Interactions between Rhinanthus minor and its hosts: A review of water, mineral nutrient and hormone flows and exchanges in the hemiparasitic association. Folia Geobot. 2010, 45, 369–385. [Google Scholar] [CrossRef]
- Li, A.R.; Li, Y.J.; Smith, S.E.; Smith, F.A.; Guan, K.Y. Nutrient requirements differ in two Pedicularis species in the absence of a host plant: Implication for driving forces in the evolution of host preference of root hemiparasitic plants. Ann. Bot. 2013, 112, 1099–1106. [Google Scholar] [CrossRef]
- Těšitel, J.; Těšitelová, T.; Fisher, J.P.; Lepš, J.; Cameron, D.D. Integrating ecology and physiology of root-hemiparasitic interaction: Interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytol. 2015, 205, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Cirocco, R.M.; Facelli, E.; Delean, S.; Facelli, J.M. Does phosphorus influence performance of a native hemiparasite and its impact on a native legume? Physiol. Plant 2021, 173, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Cirocco, R.M.; Watling, J.R.; Facelli, J.M. The combined effects of water and nitrogen on the relationship between a native hemiparasite and its invasive host. New Phytol. 2021, 229, 1728–1739. [Google Scholar] [CrossRef]
- Korell, L.; Sandner, T.M.; Matthies, D.; Ludewig, K. Effects of drought and N level on the interactions of the root hemiparasite Rhinanthus alectorolophus with a combination of three host species. Plant Biol. 2020, 22, 84–92. [Google Scholar] [CrossRef]
- Jiang, F.; Jeschke, W.D.; Hartung, W. Solute flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite nutrient relations. Funct. Plant Biol. 2004, 31, 633–643. [Google Scholar] [CrossRef]
- Seel, W.E.; Parsons, A.N.; Press, M.C. Do inorganic solutes limit growth of the facultative hemiparasite Rhinanthus minor L in the absence of a host. New Phytol. 1993, 124, 283–289. [Google Scholar] [CrossRef]
- He, Y.; Bi, Y.; Yu, H.; Zhang, Y.; Struik, P.C.; Jing, J. Positive legacy effects of grass-legume mixture leys on phosphorus uptake and yield of maize weaken over the growing season. Field Crop Res. 2024, 314, 109434. [Google Scholar] [CrossRef]
- Zhu, S.G.; Tao, H.Y.; Li, W.B.; Zhou, R.; Gui, Y.W.; Zhu, L.; Zhang, X.L.; Wang, W.; Wang, B.Z.; Mei, F.J.; et al. Phosphorus availability mediates plant-plant interaction and field productivity in maize-grass pea intercropping system: Field experiment and its global validation. Agric. Syst. 2023, 205, 103584. [Google Scholar] [CrossRef]
- Tawaraya, K.; Horie, R.; Shinano, T.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of soybean root exudates under phosphorus deficiency. Soil Sci. Plant Nutr. 2014, 60, 679–694. [Google Scholar] [CrossRef]
- Xiong, C.; Li, X.; Wang, X.; Wang, J.; Lambers, H.; Vance, C.P.; Shen, J.; Cheng, L. Flavonoids are involved in phosphorus-deficiency-induced cluster-root formation in white lupin. Ann. Bot. 2022, 129, 101–112. [Google Scholar] [CrossRef]
- Yoneyama, K.; Yoneyama, K.; Takeuchi, Y.; Sekimoto, H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 2007, 225, 1031–1038. [Google Scholar] [CrossRef]
- Ogawa, S.; Cui, S.K.; White, A.R.F.; Nelson, D.C.; Yoshida, S.; Shirasu, K. Strigolactones are chemoattractants for host tropism in Orobanchaceae parasitic plants. Nat. Commun. 2022, 13, 4653. [Google Scholar] [CrossRef]
- Sui, X.L.; Kuss, P.; Li, W.J.; Yang, M.Q.; Guan, K.Y.; Li, A.R. Identity and distribution of weedy Pedicularis kansuensis Maxim. (Orobanchaceae) in Tianshan Mountains of Xinjiang: Morphological, anatomical and molecular evidence. J. Arid Land 2016, 8, 453–461. [Google Scholar] [CrossRef]
- Li, W.J.; Sui, X.L.; Kuss, P.; Liu, Y.Y.; Li, A.R.; Guan, K.Y. Long-Distance Dispersal after the Last Glacial Maximum (LGM) led to the disjunctive distribution of Pedicularis kansuensis (Orobanchaceae) between the Qinghai-Tibetan Plateau and Tianshan Region. PLoS ONE 2016, 11, e0165700. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Taxipulati, T.; Gong, Y.M.; Sui, X.L.; Wang, X.Z.; Parent, S.E.; Hu, Y.K.; Guan, K.Y.; Li, A.R. N-P fertilization inhibits growth of root hemiparasite Pedicularis kansuensis in natural grassland. Front. Plant Sci. 2017, 8, 2088. [Google Scholar] [CrossRef]
- Bao, G.S.; Suetsugu, K.; Wang, H.S.; Yao, X.; Liu, L.; Ou, J.; Li, C.J. Effects of the hemiparasitic plant Pedicularis kansuensis on plant community structure in a degraded grassland. Ecol. Res. 2015, 30, 507–515. [Google Scholar] [CrossRef]
- Tesitel, J.; Mladek, J.; Hornik, J.; Tesitelova, T.; Adamec, V.; Tichy, L. Suppressing competitive dominants and community restoration with native parasitic plants using the hemiparasitic Rhinanthus alectorolophus and the dominant grass Calamagrostis epigejos. J. Appl. Ecol. 2017, 54, 1487–1495. [Google Scholar] [CrossRef]
- Těšitel, J.; Mladek, J.; Fajmon, K.; Blazek, P.; Mudrak, O. Reversing expansion of Calamagrostis epigejos in a grassland biodiversity hotspot: Hemiparasitic Rhinanthus major does a better job than increased mowing intensity. Appl. Veg. Sci. 2018, 21, 104–112. [Google Scholar] [CrossRef]
- Sandner, T.M.; Matthies, D. Multiple choice: Hemiparasite performance in multi-species mixtures. Oikos 2018, 127, 1291–1303. [Google Scholar] [CrossRef]
- Davies, D.M.; Graves, J.D. The impact of phosphorus on interactions of the hemiparasitic angiosperm Rhinanthus minor and its host Lolium perenne. Oecologia 2000, 124, 100–106. [Google Scholar] [CrossRef]
- Goyet, V.; Wada, S.; Cui, S.; Wakatake, T.; Shirasu, K.; Montiel, G.; Simier, P.; Yoshida, S. Haustorium inducing factors for parasitic Orobanchaceae. Front. Plant Sci. 2019, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.J.; Liu, Y.X.; Zhang, H.K.; Wang, J.P.; Chen, Z.J.; Luo, L.J.; Liu, G.D.; Liu, P.D. Metabolic alterations provide insights into Stylosanthes roots responding to phosphorus deficiency. BMC Plant Biol. 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Malusà, E.; Russo, M.; Mozzetti, C.; Belligno, A. Modification of secondary metabolism and flavonoid biosynthesis under phosphate deficiency in bean roots. J. Plant Nutr. 2006, 29, 245–258. [Google Scholar] [CrossRef]
- Mo, X.H.; Zhang, M.K.; Liang, C.Y.; Cai, L.Y.; Tian, J. Integration of metabolome and transcriptome analyses highlights soybean roots responding to phosphorus deficiency by modulating phosphorylated metabolite processes. Plant Physiol. Biochem. 2019, 139, 697–706. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Borowicz, V.A.; Armstrong, J.E. Resource limitation and the role of a hemiparasite on a restored prairie. Oecologia 2012, 169, 783–792. [Google Scholar] [CrossRef]
- Hautier, Y.; Hector, A.; Vojtech, E.; Purves, D.; Turnbull, L.A. Modelling the growth of parasitic plants. J. Ecol. 2010, 98, 857–866. [Google Scholar] [CrossRef]
- Těšitel, J.; Fibich, P.; de Bello, F.; Chytry, M.; Leps, J. Habitats and ecological niches of root-hemiparasitic plants: An assessment based on a large database of vegetation plots. Preslia 2015, 87, 87–108. [Google Scholar]
- Fibich, P.; Leps, J.; Berec, L. Modelling the population dynamics of root hemiparasitic plants along a productivity gradient. Folia Geobot. 2010, 45, 425–442. [Google Scholar] [CrossRef]
- Sui, X.L.; Zhang, T.; Tian, Y.T.; Xue, R.J.; Li, A.R. A neglected alliance in battles against parasitic plants: Arbuscular mycorrhizal and rhizobial symbioses alleviate damage to a legume host by root hemiparasitic Pedicularis species. New Phytol. 2019, 221, 470–481. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2008. [Google Scholar]
- Wang, X.R.; Zhao, S.P.; Bucking, H. Arbuscular mycorrhizal growth responses are fungal specific but do not differ between soybean genotypes with different phosphate efficiency. Ann. Bot. 2016, 118, 11–21. [Google Scholar] [CrossRef]
- Wang, G.; Sheng, L.; Zhao, D.; Sheng, J.; Wang, X.; Liao, H. Allocation of nitrogen and carbon is regulated by nodulation and mycorrhizal networks in soybean/maize intercropping system. Front. Plant Sci. 2016, 7, 1901. [Google Scholar] [CrossRef]
- Lekberg, Y.; Arnillas, C.A.; Borer, E.T.; Bullington, L.S.; Fierer, N.; Kennedy, P.G.; Leff, J.W.; Luis, A.D.; Seabloom, E.W.; Henning, J.A. Nitrogen and phosphorus fertilization consistently favor pathogenic over mutualistic fungi in grassland soils. Nat. Commun. 2021, 12, 3484. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1966. [Google Scholar]
- Li, A.R.; Guan, K.Y. Arbuscular mycorrhizal fungi may serve as another nutrient strategy for some hemiparasitic species of Pedicularis (Orobanchaceae). Mycorrhiza 2008, 18, 429–436. [Google Scholar] [CrossRef]
- Li, A.R.; Smith, S.E.; Smith, F.A.; Guan, K.Y. Inoculation with arbuscular mycorrhizal fungi suppresses initiation of haustoria in the root hemiparasite Pedicularis tricolor. Ann. Bot. 2012, 109, 1075–1080. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of data Manipulation. R package Version 1.1.4. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 31 October 2024).
- Lenth, R. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.10.6. 2024. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 12 December 2024).
- Ben-Shachar, M.S.; Lüdecke, D.; Makowski, D. Effectsize: Estimation of effect size indices and standardized parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Kassambara, A. gpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 31 October 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
| Species | P Level | Shoot Dry Weight (g) | Root Dry Weight (g) | Root–Shoot Ratio | |||
|---|---|---|---|---|---|---|---|
| −PK | +PK | −PK | +PK | −PK | +PK | ||
| E. nutans | 0P | 1.35 ± 0.07 Aa | 0.66 ± 0.11 Ab | 0.89 ± 0.06 Aa | 0.46 ± 0.09 Ab | 0.66 ± 0.04 Aa | 0.73 ± 0.16 Aa |
| 1P | 1.13 ± 0.11 Aa | 0.56 ± 0.08 ABb | 0.71 ± 0.08 Aa | 0.49 ± 0.08 Aa | 0.62 ± 0.02 Aa | 0.88 ± 0.06 Aa | |
| 2P | 1.25 ± 0.08 Aa | 0.26 ± 0.04 Bb | 0.74 ± 0.06 Aa | 0.39 ± 0.12 Aa | 0.60 ± 0.05 Ab | 1.46 ± 0.42 Aa | |
| M. sativa | 0P | 0.05 ± 0.01 Ba | 0.01 ± 0.00 Bb | 0.05 ± 0.01 Ba | 0.01 ± 0.00 Bb | 1.02 ± 0.08 Aa | 1.26 ± 0.58 Aa |
| 1P | 0.12 ± 0.02 Aa | 0.02 ± 0.00 Bb | 0.10 ± 0.01 Aa | 0.02 ± 0.00 Bb | 0.89 ± 0.10 ABa | 0.77 ± 0.08 Aa | |
| 2P | 0.12 ± 0.02 Aa | 0.04 ± 0.01 Ab | 0.08 ± 0.01 ABa | 0.05 ± 0.01 Aa | 0.72 ± 0.05 Bb | 1.31 ± 0.12 Aa | |
| P. kansuensis | 0P | -- | 0.15 ± 0.07 AB | -- | 0.03 ± 0.01 B | -- | 0.18 ± 0.02 B |
| 1P | -- | 0.12 ± 0.01 B | -- | 0.02 ± 0.00 B | -- | 0.17 ± 0.02 B | |
| 2P | -- | 0.35 ± 0.08 A | -- | 0.13 ± 0.02 A | -- | 0.38 ± 0.07 A | |
| N Concentration | P Concentration | N Content | P Content | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EN | MS | EN | MS | EN | MS † | EN | MS | ||||||||
| df | F | ηP2 | F | ηP2 | F | ηP2 | F | ηP2 | F | ηP2 | F | ηP2 | |||
| P | 2, 12 | 1.091 | 0.15 | 0.117 | 0.02 | 0.063 | 0.01 | -- | 7.717 ** | 0.56 | 30.153 *** | 0.83 | 0.358 | 0.06 | -- |
| Pa | 1, 12 | 0.010 | 0.00 | 2.484 | 0.17 | 0.028 | 0.00 | -- | 63.166 ** | 0.84 | 101.813 *** | 0.89 | 21.840 ** | 0.65 | -- |
| P * Pa | 2, 12 | 0.334 | 0.05 | 0.239 | 0.04 | 1.440 | 0.19 | -- | 3.479 | 0.37 | 1.057 | 0.15 | 3.169 | 0.35 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, X.; Xue, R.; Li, A. Soil Phosphorus Availability Modulates Host Selectivity of Pedicularis kansuensis Between Legumes and Grasses. Plants 2025, 14, 2356. https://doi.org/10.3390/plants14152356
Sui X, Xue R, Li A. Soil Phosphorus Availability Modulates Host Selectivity of Pedicularis kansuensis Between Legumes and Grasses. Plants. 2025; 14(15):2356. https://doi.org/10.3390/plants14152356
Chicago/Turabian StyleSui, Xiaolin, Ruijuan Xue, and Airong Li. 2025. "Soil Phosphorus Availability Modulates Host Selectivity of Pedicularis kansuensis Between Legumes and Grasses" Plants 14, no. 15: 2356. https://doi.org/10.3390/plants14152356
APA StyleSui, X., Xue, R., & Li, A. (2025). Soil Phosphorus Availability Modulates Host Selectivity of Pedicularis kansuensis Between Legumes and Grasses. Plants, 14(15), 2356. https://doi.org/10.3390/plants14152356






