Origanum vulgare subsp. virens (Hoffmanns. & Link) Bonnier & Layens Essential Oils: Chemotypes and Bioactivity as Antifungal, Antifeeding and Enzyme Inhibitors
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield and EO Phytochemical Profile
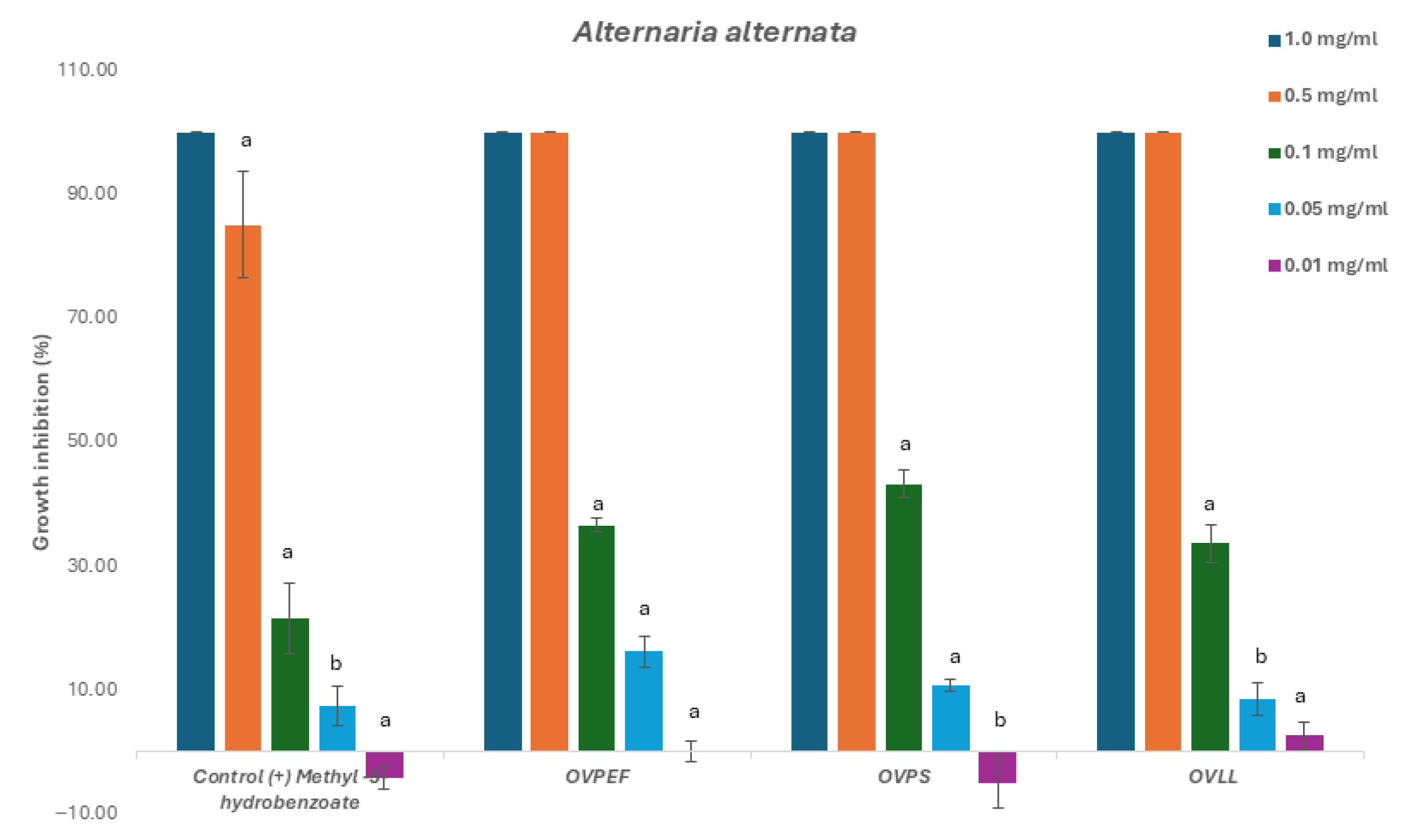
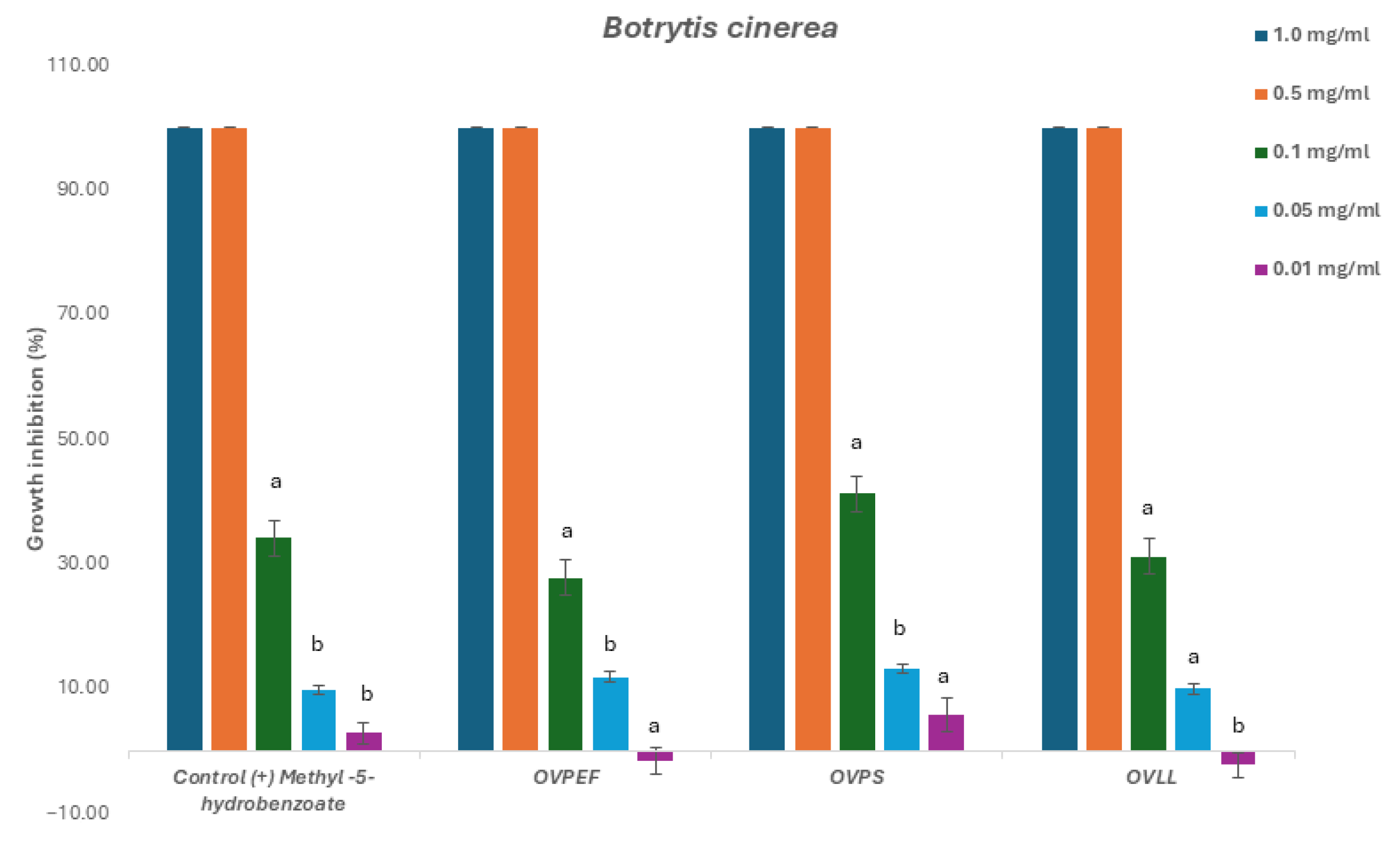
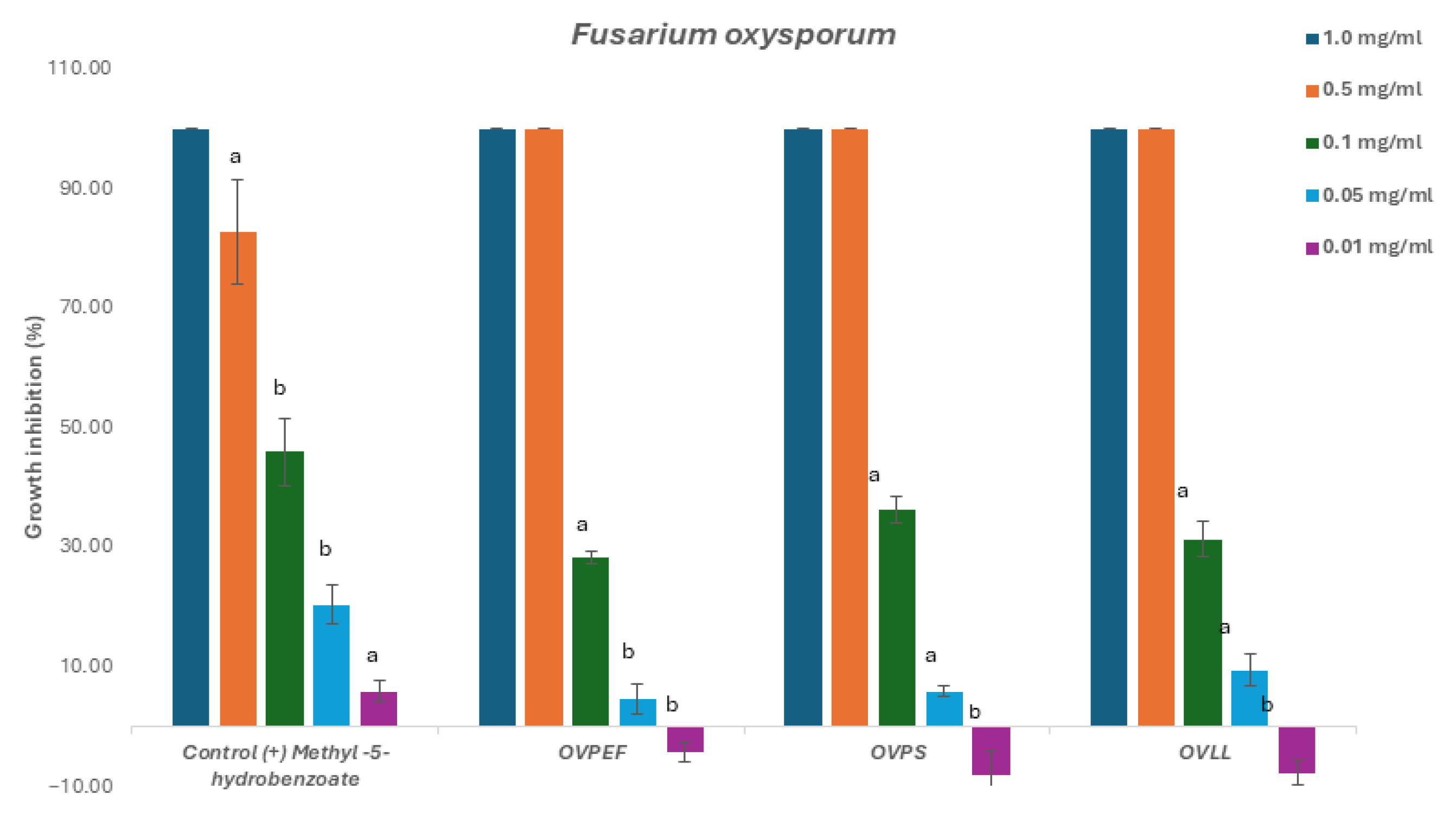
2.2. Fungicide and Fungistatic Activity
2.2.1. Phytopathogenic Fungi
2.2.2. Obligate Biotrophic Fungi
2.3. Antifeeding Bioassay
2.4. Mortality and Oviposition Bioassay
2.5. In Vitro Inhibition Assays
2.5.1. Acetylcholinesterase Inhibitory Assay
2.5.2. α- and β-Glucosidase Inhibitory Assays
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Sample Preparation
3.3. Essential Oil Extraction
3.4. Phytochemical Analysis
3.4.1. Gas Chromatography–Flame Ionization Detector (GC–FID) Conditions
3.4.2. Gas Chromatography–Mass Spectrometry (GC–MS) Conditions
3.5. Fungicide and Fungistatic Activity
3.5.1. Phytopathogenic Fungi
3.5.2. Obligate Biotrophic Fungi
3.6. Insect Bioassay
3.6.1. Breeding and Maintenance of Insects
3.6.2. Antifeeding Bioassay
3.6.3. Mortality and Oviposition Bioassay
3.7. In Vitro Inhibition Assays
3.7.1. Acetylcholinesterase Inhibition Assay
3.7.2. α-GLU Inhibitory Assay
3.7.3. β-GLU Inhibitory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing Softly: A Roadmap of Botrytis Cinerea Pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis Cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- Fagodiya, R.K.; Trivedi, A.; Fagodia, B.L. Impact of Weather Parameters on Alternaria Leaf Spot of Soybean Incited by Alternaria Alternata. Sci. Rep. 2022, 12, 6131. [Google Scholar] [CrossRef] [PubMed]
- Chulze, S.N.; Tittlemier, S.; Torres, A.M. Editorial: Fusarium Species as Plant and Human Pathogens, Mycotoxin Producers, and Biotechnological Importance. Front. Fungal Biol. 2023, 4, 1320198. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A. Mycotoxigenic Fungi. In Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Springer New York: New York, NY, USA, 2017; Volume 1542, ISBN 978-1-4939-6705-6. [Google Scholar]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current Progress on Pathogenicity-related Transcription Factors in Fusarium Oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
- Kusch, S.; Qian, J.; Loos, A.; Kümmel, F.; Spanu, P.D.; Panstruga, R. Long-term and Rapid Evolution in Powdery Mildew Fungi. Mol. Ecol. 2023, 33, e16909. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide Resistance in Powdery Mildew Fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef]
- CABI Chrysodeixis Chalcite Datasheet. Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.13243 (accessed on 5 February 2025).
- Fuentes, E.G.; Hernández-Suárez, E.; Simón, O.; Williams, T.; Caballero, P. Chrysodeixis Chalcites, a Pest of Banana Crops on the Canary Islands: Incidence, Economic Losses and Current Control Measures. Crop Prot. 2018, 108, 137–145. [Google Scholar] [CrossRef]
- Del Pino, M.; Carnero, A.; Hernandez, E. Tomas Cabello La Lagarta o Bicho Camello, Chrysodeixis Chalcites (Esper, 1189), Una Plaga Emergente En Los Cultivos de Platanera de Canarias. Phytoma 2011, 225, 1–6. [Google Scholar]
- EPPO. Ceratitis Capitata. EPPO Bull. 1987, 17, 429–440. [Google Scholar] [CrossRef]
- Thomas, M.C.; Heppner, J.B.; Woodruff, R.E.; Weems, H.V., Jr.; Steck, G.J.; Fasulo, T.R. Mediterranean Fruit Fly, Ceratitis Capitata (Wiedemann) (Insecta: Diptera: Tephritidae). Edis 1969, 2004, 1–15. [Google Scholar] [CrossRef]
- Tavares, W.R.; Jiménez, I.A.; Oliveira, L.; Kuhtinskaja, M.; Vaher, M.; Rosa, J.S.; Seca, A.M.L.; Bazzocchi, I.L.; Barreto, M.D.C. Macaronesian Plants as Promising Biopesticides against the Crop Pest Ceratitis Capitata. Plants 2023, 12, 4122. [Google Scholar] [CrossRef] [PubMed]
- Kuek, M.; McLean, S.K.; Palombo, E.A. Application of Bacteriophages in Food Production and Their Potential as Biocontrol Agents in the Organic Farming Industry. Biol. Control 2022, 165, 104817. [Google Scholar] [CrossRef]
- Tavares, W.R.; Barreto, M.D.C.; Seca, A.M.L. Aqueous and Ethanolic Plant Extracts as Bio-Insecticides— Establishing a Bridge between Raw Scientific Data and Practical Reality. Plants 2021, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Magaña, C.; Hernández-Crespo, P.; Brun-Barale, A.; Couso-Ferrer, F.; Bride, J.M.; Castañera, P.; Feyereisen, R.; Ortego, F. Mechanisms of Resistance to Malathion in the Medfly Ceratitis Capitata. Insect Biochem. Mol. Biol. 2008, 38, 756–762. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Pavela, R. History, Presence and Perspective of Using Plant Extracts as Commercial Botanical Insecticides and Farm Products for Protection against Insects—A Review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical Diversity in Leaf and Stem Essential Oils of Origanum vulgare L. and Their Effects on Microbicidal Activities. AMB Express 2019, 9, 176. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Zinno, P.; Guantario, B.; Lombardi, G.; Ranaldi, G.; Finamore, A.; Allegra, S.; Mammano, M.M.; Fascella, G.; Raffo, A.; Roselli, M. Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the Carvacrol and Thymol Chemotypes. Plants 2023, 12, 1344. [Google Scholar] [CrossRef]
- Askin, H.; Yildiz, M.; Ayar, A. Effects of Thymol and Carvacrol on Acetylcholinesterase from Drosophila Melanogaster. Acta Phys. Pol. A 2017, 132, 720–722. [Google Scholar] [CrossRef]
- Chen, W.N.; Chin, K.W.; Tang, K.S.; Agatonovic-Kustrin, S.; Yeong, K.Y. Neuroprotective, Neurite Enhancing, and Cholinesterase Inhibitory Effects of Lamiaceae Family Essential Oils in Alzheimer’s Disease Model. J. Herb. Med. 2023, 41, 100696. [Google Scholar] [CrossRef]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M.; Milos, M. In Vitro Acetylcholinesterase Inhibitory Properties of Thymol, Carvacrol and Their Derivatives Thymoquinone and Thymohydroquinone. Phyther. Res. 2007, 21, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Galiffa, V.; Cásedas, G.; Moliner, C.; Maggi, F.; López, V.; Gómez-Rincón, C. Essential Oils of Two Subspecies of Satureja montana L. against Gastrointestinal Parasite Anisakis Simplex and Acetylcholinesterase Inhibition. Molecules 2024, 29, 4640. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST) NIST Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: https://webbook.nist.gov (accessed on 12 March 2025).
- Azizi, A.; Hadian, J.; Gholami, M.; Friedt, W.; Honermeier, B. Correlations between Genetic, Morphological, and Chemical Diversities in a Germplasm Collection of the Medicinal Plant Origanum vulgare L. Chem. Biodivers. 2012, 9, 2784–2801. [Google Scholar] [CrossRef]
- Novak, J.; Lukas, B.; Franz, B. Temperature Influences Thymol and Carvacrol Differentially in Origanum Spp. (Lamiaceae). J. Essent. Oil Res. 2010, 22, 412–415. [Google Scholar] [CrossRef]
- Andi, S.A.; Maskani, F. Essential Oil Chemical Diversity of Twenty Iranian Origanum vulgare L. Subsp. Viride Populations. Biochem. Syst. Ecol. 2021, 98, 104323. [Google Scholar] [CrossRef]
- De Mastro, G.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential Oil Diversity of Origanum vulgare L. Populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef]
- De Mastro, G.; Ruta, C.; Marzi, V. Agronomic and Technological Assessment of Oregano (Origanum vulgare ssp.) Biotypes. Acta Hortic. 2004, 629, 355–363. [Google Scholar] [CrossRef]
- Lopez-Reyes, J.G.; Spadaro, D.; Prelle, A.; Garibaldi, A.; Gullino, M.L. Efficacy of Plant Essential Oils on Postharvest Control of Rots Caused by Fungi on Different Stone Fruits in Vivo. J. Food Prot. 2013, 76, 631–639. [Google Scholar] [CrossRef]
- Mancini, E.; Camele, I.; Elshafie, H.S.; De Martino, L.; Pellegrino, C.; Grulova, D.; De Feo, V. Chemical Composition and Biological Activity of the Essential Oil of Origanum vulgare ssp. Hirtum from Different Areas in the Southern Apennines (Italy). Chem. Biodivers. 2014, 11, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.T.; Liao, P.; Crocoll, C.; Boachon, B.; Foster, C.; Leidecker, F.; Wiese, N.; Zhao, D.; Wood, J.C.; Buell, C.R.; et al. The Biosynthesis of Thymol, Carvacrol, and Thymohydroquinone in Lamiaceae Proceeds via Cytochrome P450s and a Short-Chain Dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2110092118. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential Oil Diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare Essential Oil and Its Two Main Components, Carvacrol and Thymol, on the Plant Pathogen Botrytis Cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef]
- Adebayo, O.; Dang, T.; Bélanger, A.; Khanizadeh, S. Antifungal Studies of Selected Essential Oils and a Commercial Formulation against Botrytis Cinerea. J. Food Res. 2013, 2, 217. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal Activity of Thymol and Carvacrol against Postharvest Pathogens Botrytis Cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Perina, F.J.; Amaral, D.C.; Fernandes, R.S.; Labory, C.R.G.; Teixeira, G.A.; Alves, E. Thymus Vulgaris Essential Oil and Thymol against Alternaria Alternata (Fr.) Keissler: Effects on Growth, Viability, Early Infection and Cellular Mode of Action. Pest Manag. Sci. 2015, 71, 1371–1378. [Google Scholar] [CrossRef]
- Bounar, R.; Krimat, S.; Boureghda, H.; Dob, T. Chemical Analyses, Antioxidant and Antifungal Effects of Oregano and Thyme Essential Oils Alone or in Combination against Selected Fusarium Species. Int. Food Res. J. 2020, 27, 66–77. [Google Scholar]
- Mirahmadi, S.F.; Shayganfar, A. Inhibitory Effects of Endemic Thyme’s Thymol-Carvacrol Chemotype Essential Oil on Aspergillus Species with Free Radical Scavenging Properties. Chem. Biodivers. 2024, 21, e202302115. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- González-Coloma, A.; Terrero, D.; Perales, A.; Escoubas, P.; Fraga, B.M. Insect Antifeedant Ryanodane Diterpenes from Persea indica. J. Agric. Food Chem. 1996, 44, 296–300. [Google Scholar] [CrossRef]
- Santana, O.; Cabrera, R.; Giménez, C.; González-Coloma, A.; Sánchez-Vioque, R.; de los Mozos-Pascual, M.; Rodríguez-Conde, M.F.; Laserna-Ruiz, I.; Usano-Alemany, J.; Herraiz, D. Perfil Químico y Biológico de Aceites Esenciales de Plantas Aromáticas de Interés Agro-Industrial En Castilha-La Mancha (España). Grasas Aceites 2012, 63, 214–222. [Google Scholar] [CrossRef]
- Hummelbrunner, L.A.; Isman, M.B. Acute, Sublethal, Antifeedant, and Synergistic Effects of Monoterpenoid Essential Oil Compounds on the Tobacco Cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef]
- Nasr, M.; Jalali Sendi, J.; Moharramipour, S.; Zibaee, A. Evaluation of Origanum vulgare L. Essential Oil as a Source of Toxicant and an Inhibitor of Physiological Parameters in Diamondback Moth, Plutella xylustella L. (Lepidoptera: Pyralidae). J. Saudi Soc. Agric. Sci. 2017, 16, 184–190. [Google Scholar] [CrossRef]
- Pavela, R. Acute, Synergistic and Antagonistic Effects of Some Aromatic Compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) Larvae. Ind. Crops Prod. 2014, 60, 247–258. [Google Scholar] [CrossRef]
- Park, B.S.; Choi, W.S.; Kim, J.H.; Kim, K.H.; Lee, S.E. Monoterpenes from Thyme (Thymus vulgaris) as Potential Mosquito Repellents. J. Am. Mosq. Control Assoc. 2005, 21, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Karpouhtsis, I.; Pardali, E.; Feggou, E.; Kokkini, S.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and Genotoxic Activities of Oregano Essential Oils. J. Agric. Food Chem. 1998, 46, 1111–1115. [Google Scholar] [CrossRef]
- Sedy, K.A.; Kosehier, E.H. Bioactivity of Carvacrol and Thymol against Frankliniella occidentalis and Thrips tabaci. J. Appl. Entomol. 2003, 127, 313–316. [Google Scholar] [CrossRef]
- Tabanca, N.; Cloonan, K.R.; Nesterkina, M.; Gill, M.A.; Montgomery, W.S.; Kravchenko, I.; Kendra, P.E. Behavioral and Electrophysiological Responses of the Male Medfly, Ceratitis Capitata, to Thymol and Carvacrol Ethers. Pest Manag. Sci. 2024. [Google Scholar] [CrossRef]
- Öztürk, M. Anticholinesterase and Antioxidant Activities of Savoury (Satureja thymbra L.) with Identified Major Terpenes of the Essential Oil. Food Chem. 2012, 134, 48–54. [Google Scholar] [CrossRef]
- Huber, M.; Roder, T.; Irmisch, S.; Riedel, A.; Gablenz, S.; Fricke, J.; Rahfeld, P.; Reichelt, M.; Paetz, C.; Liechti, N.; et al. A Beta-Glucosidase of an Insect Herbivore Determines Both Toxicity and Deterrence of a Dandelion Defense Metabolite. Elife 2021, 10, e68642. [Google Scholar] [CrossRef]
- Eltalawy, H.M.; El-Fayoumi, H.; Aboelhadid, S.M.; Al-Quraishy, S.; El-Mallah, A.M.; Tunali, F.; Sokmen, A.; Daferera, D.; Abdel-Baki, A.-A.S. Repellency, Fumigant Toxicity, Antifeedent and Residual Activities of Coridothymus capitatus and Its Main Component Carvacrol against Red Flour Beetle. Molecules 2024, 29, 4255. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, M.; Zawitowska, B.; Szumny, A. Insecticidal Activities of Thymus Vulgaris Essential Oil and Its Components (Thymol and Carvacrol) against Larvae of Lesser Mealworm, Alphitobius Diaperinus Panzer (Coleoptera: Tenebrionidae). Allelopath. J. 2012, 30, 129–142. [Google Scholar]
- Mrabet, A.; Annaz, H.; Abdelfattah, B.; Ouabou, M.; Kounnoun, A.; Cacciola, F.; Simou, A.; Bouayad, N.; Rharrabe, K.; Khaddor, M. Antioxidant, Insecticidal, Antifeedant, and Repellent Activities of Oregano (Origanum vulgare). Int. J. Environ. Health Res. 2024, 35, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, F.; Olmeda, A.S.; González, M.G.; Andrés, M.F.; Navarro-Rocha, J.; González-Coloma, A. Acaricidal and Insect Antifeedant Effects of Essential Oils From Selected Aromatic Plants and Their Main Components. Front. Agron. 2021, 3, 662802. [Google Scholar] [CrossRef]
- Griffin, S.G.; Markham, J.L.; Leach, D.N. An Agar Dilution Method for the Determination of the Minimum Inhibitory Concentration of Essential Oils. J. Essent. Oil Res. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Giménez Mariño, C. Productos Bioactivos de Plantas Canarias y Sus Hongos Endófitos: Detección de Actividad y Utilización En El Controlo de Plagas y Enfermidades Agrícolas; Universidad de La Laguna: Tenerife, Spain, 2006. [Google Scholar]
- Krug, J.C. Moist chambers for the development of fungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Academic Press: Cambridge, MA, USA, 2004; pp. 589–593. [Google Scholar]
- Bajonero, J.G.; Parra, J.R.P. Selection and Suitability of an Artificial Diet for Tuta absoluta (Lepidoptera: Gelechiidae) Based on Physical and Chemical Characteristics. J. Insect Sci. 2017, 17, 13. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Valverde-García, P.; Garrido-Jurado, I. The Effect of Temperature and Soil Moisture on the Development of the Preimaginal Mediterranean Fruit Fly (Diptera: Tephritidae). Environ. Entomol. 2012, 41, 966–970. [Google Scholar] [CrossRef]
- Escoubas, P.; Lajide, L.; Mitzutani, J. An Improved Leaf-disk Antifeedant Bioassay and Its Application for the Screening of Hokkaido Plants. Entomol. Exp. Appl. 1993, 66, 99–107. [Google Scholar] [CrossRef]
- Salles, L. Metodologia de Criação de Anastrepha Fraterculus (Wied., 1830) (Diptera: Tephritidae) Em Dieta Artificial Em Laboratório. An. Soc. Entomológica Bras. 1992, 21, 479–486. [Google Scholar] [CrossRef]
- Furtado, R.; Baptista, J.; Lima, E.; Paiva, L.; Barroso, J.G.; Rosa, J.S.; Oliveira, L. Chemical Composition and Biological Activities of Laurus Essential Oils from Different Macaronesian Islands. Biochem. Syst. Ecol. 2014, 55, 333–341. [Google Scholar] [CrossRef]
- Barreto, M.C.; Simões, N. Determination of Biological Activities. A Laboratory Manual; Açoreana, E.-E.G., Ed.; Universidade dos Açores: Ponta Delgada, Portugal, 2012; ISBN 978-972-8612-82-5. [Google Scholar]
- Xiao, Z.; Storms, R.; Tsang, A. A Quantitative Starch-Iodine Method for Measuring Alpha-Amylase and Glucoamylase Activities. Anal. Biochem. 2006, 351, 146–148. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Gouveia-Figueira, S.; Castilho, P.C. Ulex Europaeus: From Noxious Weed to Source of Valuable Isoflavones and Flavanones. Ind. Crops Prod. 2016, 90, 9–27. [Google Scholar] [CrossRef]
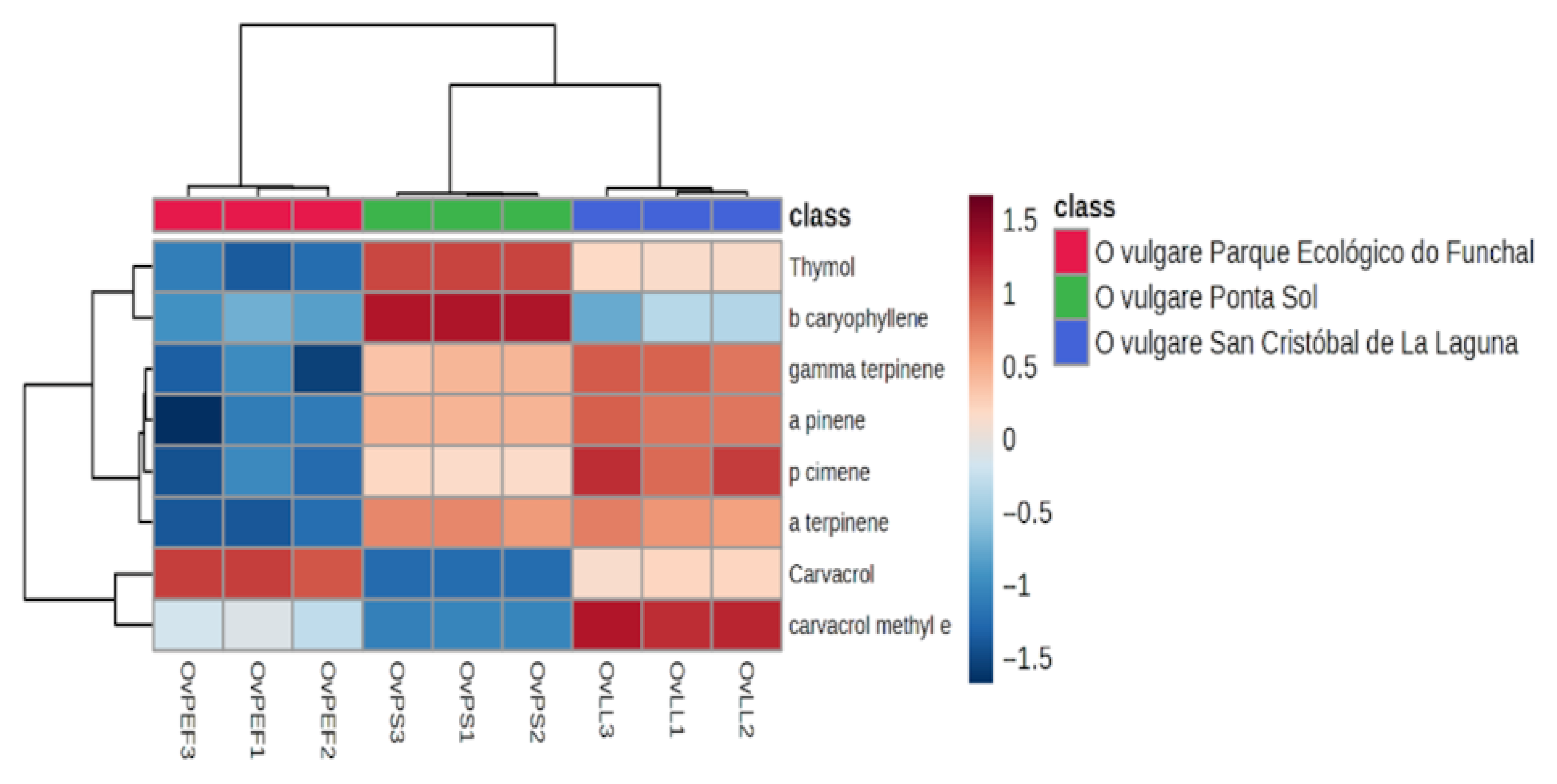
| Classes of Compounds s | I.D. | RI 1 | RI 2 | OVPEF | OVPS | OVLL |
|---|---|---|---|---|---|---|
| Relative% ± SD | Relative% ± SD | Relative% ± SD | ||||
| Monoterpene | α-pinene | 1011 | 910 | 0.40 ± 0.06 a | 1.61 ± 0.09 b | 2.11 ± 0.09 b |
| α-terpinene | 1168 | 1120 | 0.77 ± 0.06 a | n.d. | 2.50 ± 0.12 b | |
| γ-terpinene | 1245 | 1043 | 5.97 ± 0.38 a | 14.81 ± 0.75 b | 18.77 ± 0.75 c | |
| p-cymene | 1272 | 1024 | 2.11 ± 0.29 ab | 3.57 ± 0.21 ab | 5.05 ± 0.30 b | |
| Methyl ether terpenes | Carvacrol methyl ether | 1549 | 1231 | 1.96 ± 0.09 a | 0.87 ± 0.07 b | 4.71 ± 0.15 c |
| Sesquiterpene | β-caryophyllene | 1601 | 1439 | 0.78 ± 0.05 a | 1.12 ± 0.04 a | 0.54 ± 0,20 a |
| Monoterpenoid | Thymol | 2120 | 1288 | 5.65 ± 0.47 a | 59.19 ± 0.90 b | 30.22 ± 0.60 c |
| Carvacrol | 2125 | 1290 | 73.04 ± 1.07 aa | 4.16 ± 0.35 b | 32.18 ± 1.54 c | |
| Other | 9.32 | 14.68 | 3.93 | |||
| Code | Inhibition Concentration IC50 (mg/mL) and Gradient Concentration t = 48 1 and 72 h | |||||
|---|---|---|---|---|---|---|
| Alternaria alternata | Botrytis cinerea | Fusarium oxysporum | ||||
| IC50 (mg/mL) | Pearson Correlation Coefficient r p Value | IC50 (mg/mL) | Pearson Correlation Coefficient r p Value | IC50 (mg/mL) | Pearson Correlation Coefficient r p Value | |
| OVPEF (C) | 0.121 (0.11–0.13) | 0.9667 0.0073 | 0.138 (0.13–0.15) | 0.9536 0.0119 | 0.131 (0.12–0.14) | 0.9504 0.0132 |
| OVPS (T) | 0.109 (0.10–0.11) | 0.9687 0.0066 | 0.113 (0.11–0.12) | 0.9675 0.0070 | 0.118 (0.11–0.12) | 0.9654 0.0077 |
| OVLL (Mix) | 0.126 (0.12- 0.13) | 0.9480 0.0141 | 0.132 (0.12–0.14) | 0.9570 0.0106 | 0.131 (0.12–0.14) | 0.9659 0.0075 |
| Control (+) methyl-4-hydrobenzoate | 0.119 (0.11–0.13) | 0.9613 0.009 | 0.192 (0.18–0.20) | 0.9444 0.016 | 0.124 (0.11–0.14) | 0.9873 0.002 |
| Essential Oil | Mycelial Area per Leaf (%) | |||||
|---|---|---|---|---|---|---|
| Gradient Concentration (µL/mL) | ||||||
| 2.5 µL/mL | 5 µL/mL | 7.5 µL/mL | 10 µL/mL | 15 µL/mL | 20 µL/mL | |
| OVPEF (C) | 83 | 79 | 82 | 37 | 28 | 21 |
| OVPS (T) | 85 | 80 | 83 | 49 | 44 | 26 |
| OVLL (Mix) | 86 | 81 | 84 | 52 | 42 | 35 |
| Control (+) methyl-4-hydrobenzoate | 31 | 26 | 28 | 22 | 28 | 18 |
| Control (+) ARAW | 41 | 42 | 38 | 33 | 31 | 32 |
| Control (−) EtOH 96% | 81 | 86 | 88 | 83 | 71 | 62 |
| Code | Refusal Rate (% FR 1) 50 µg/cm2, t = 10 h | |
|---|---|---|
| Chrysodeixis chalcites | ||
| Election Assay | Non-Election Assay | |
| OVPEF (C) | 36.42 ± 1.87 b | 65.93 ± 2.97 a |
| OVPS (T) | 25.54 ± 2.05 c | 73.85 ± 8.46 a |
| OVLL (Mix) | 31.89 ± 2.04 bc | 68.71 ± 4.68 a |
| Code | Concentration (mg/mL) | Mortality Rate (% 1) | Oviposition Deterrent Rate (% OD 2) | ||
|---|---|---|---|---|---|
| Abbott 24 h | Abbott 48 h | Abbott 72 h | |||
| OVPEF (C) | 50 | −0.64 ± 0.00 a | 1.34 ± 2.65 a | 8.11 ± 4.27 a | −177.78 ± 83.87 b |
| OVPS (T) | 0.65 ± 0.87 a | 4.03 ± 3.36 a | 6.75 ± 3.80 a | −94.09 ± 40.53 a | |
| α-pinene | 0.65 ± 1.29 a | 3.36 ± 3.97 a | 7.43 ± 5.13 a | 59.34 ± 8.02 d | |
| Carvacrol | 5.16 ± 3.03 a | 14.10 ± 6.45 a | 16.21 ± 6.72 a | −172.44 ± 78.52 ab | |
| Carvacrol methyl ether | 0.65 ± 1.29 a | 7.39 ± 6.23 a | 10.13 ± 6.65 a | 20.17 ± 21.41 abcd | |
| p-cymene | −0.64 ± 0.00 a | −1.34 ± 2.09 a | −0.00 ± 2.51 a | 28.48 ± 14.28 bc | |
| Thymol | 2.58 ± 1.49 a | 11.41 ± 3.97 b | 14.86 ± 3.67 a | −48.09 ± 35.22 abc | |
| Control (-) H2O | 0.00 ± 0.65 a | 0.00 ± 1.55 a | 0.00 ± 1.52 a | 0.00 ± 19.38 abc | |
| Control (+) azadirachtin | 0.88 | 0.00 ± 0.65 a | −0.67 ± 1.85 a | 4.73 ± 3.75 a | 49.25 ± 11.89 cd |
| Code | IC50 (mg/mL) | Gradient Concentration R2 |
|---|---|---|
| Carvacrol | 0.027 ± 0.000 a | y = 46.56x + 123.14 0.981 |
| Thymol | 2.531 ± 0.515 d | y = 22.56x + 45.52 0.936 |
| Carvacrol/thymol (1:1) | 0.070 ± 0.002 b | y = 33.43x + 88.09 0.945 |
| OVPEF (C) | 0.086 ± 0.004 b | y = 39.40x + 92.85 0.962 |
| OVPS (T) | 0.135 ± 0.003 c | y = 35.40x + 80.77 0.945 |
| OVLL (Mix) | 0.109 ± 0.015 c | y = 53.32x + 104.56 0.997 |
| Control (+) galantamine hydrobromide | 0.002 ± 0.000 e | y= 35.33x + 146.62 0.984 |
| Code | α-GLU | β-GLU | ||
|---|---|---|---|---|
| IC50 (mg/mL) | Gradient Concentration R2 | IC50 (mg/mL) | Gradient Concentration R2 | |
| Carvacrol | 109.34 ± 23.12 c | y = 12.43x + 23.89 0.954 | - | y = 9.86x + 23.04 0.966 |
| Thymol | 0.304 ± 0.025 a | y = 38.36x + 70.47 0.972 | - | y = 2.70x + 8.10 0.979 |
| Carvacrol/thymol (1:1) | 0.497 ± 0.091 ab | y = 32.50x + 62.49 0.965 | - | y = 5.66x + 20.23 0.990 |
| OVPEF (C) | - | y = 8.89x + 17.39 0.991 | - | y = 5.32x + 16.15 0.974 |
| OVPS (T) | 16.33 ± 8.15 b | y = 18.73x + 33.83 0.976 | - | y = 3.34x + 10.98 0.987 |
| OVLL (mix) | 90.25 ± 30.05 c | y = 12.38x + 25.67 0.899 | - | y = 6.41x + 12.18 0.981 |
| Control (+) 1-DNJ | 0.231 ± 0.005 a | y = 34.11x + 71.96 0.955 | 0.036 ± 0.001 a | y = 40.60x + 108.62 0.984 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.; Martins, M.; Santos, V.; Sardinha, D.; Tavares, W.R.; Sabina, S.; Espinel, G.; Barreto, M.C.; Oliveira, L.; Cabrera, R.; et al. Origanum vulgare subsp. virens (Hoffmanns. & Link) Bonnier & Layens Essential Oils: Chemotypes and Bioactivity as Antifungal, Antifeeding and Enzyme Inhibitors. Plants 2025, 14, 3001. https://doi.org/10.3390/plants14193001
Ferreira R, Martins M, Santos V, Sardinha D, Tavares WR, Sabina S, Espinel G, Barreto MC, Oliveira L, Cabrera R, et al. Origanum vulgare subsp. virens (Hoffmanns. & Link) Bonnier & Layens Essential Oils: Chemotypes and Bioactivity as Antifungal, Antifeeding and Enzyme Inhibitors. Plants. 2025; 14(19):3001. https://doi.org/10.3390/plants14193001
Chicago/Turabian StyleFerreira, Rui, Mariana Martins, Vanessa Santos, Duarte Sardinha, Wilson R. Tavares, Samuel Sabina, Guacimara Espinel, Maria Carmo Barreto, Luísa Oliveira, Raimundo Cabrera, and et al. 2025. "Origanum vulgare subsp. virens (Hoffmanns. & Link) Bonnier & Layens Essential Oils: Chemotypes and Bioactivity as Antifungal, Antifeeding and Enzyme Inhibitors" Plants 14, no. 19: 3001. https://doi.org/10.3390/plants14193001
APA StyleFerreira, R., Martins, M., Santos, V., Sardinha, D., Tavares, W. R., Sabina, S., Espinel, G., Barreto, M. C., Oliveira, L., Cabrera, R., & Castilho, P. (2025). Origanum vulgare subsp. virens (Hoffmanns. & Link) Bonnier & Layens Essential Oils: Chemotypes and Bioactivity as Antifungal, Antifeeding and Enzyme Inhibitors. Plants, 14(19), 3001. https://doi.org/10.3390/plants14193001











