Analysis of the Response of Chlamydomonas reinhardtii to Cobalt Ions Reveals the Protective Role of Thiols, Ascorbate, and Prenyllipid Antioxidants, and the Negative Impact of Cobalt Toxicity on Photoprotective Mechanisms
Abstract
1. Introduction
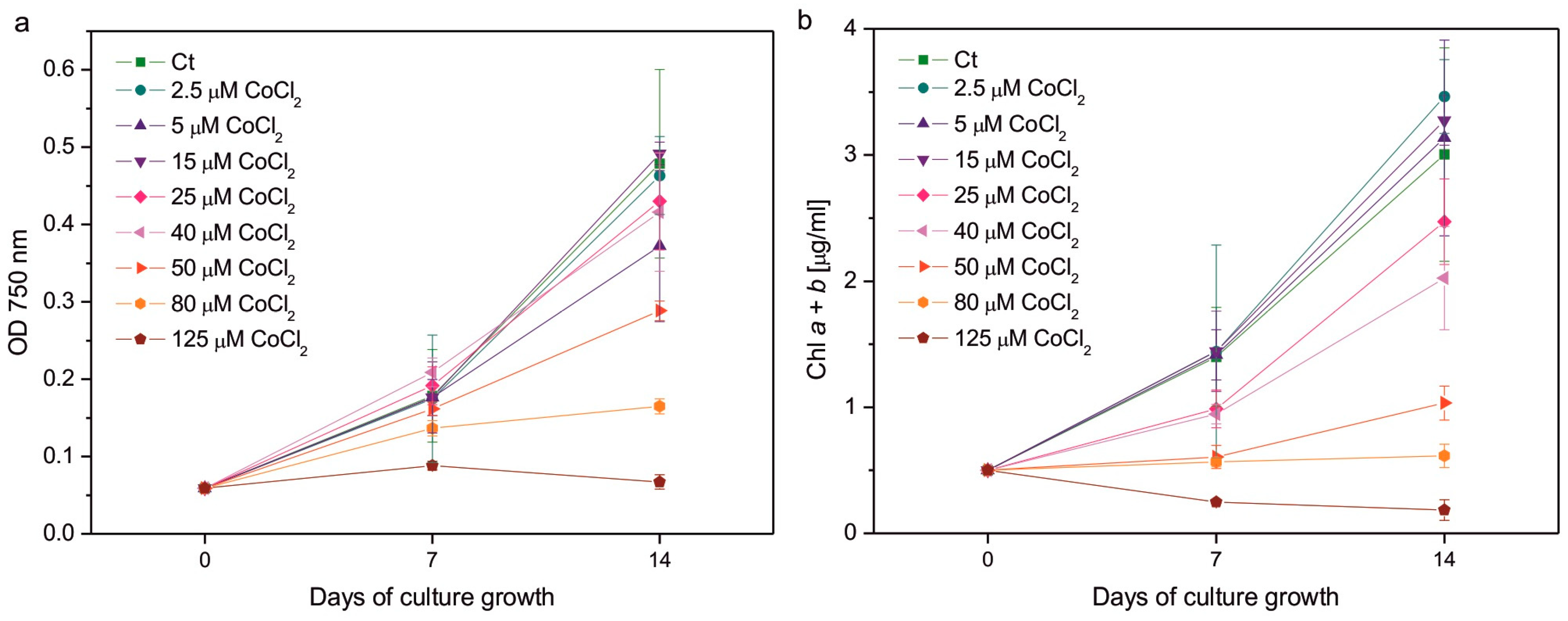
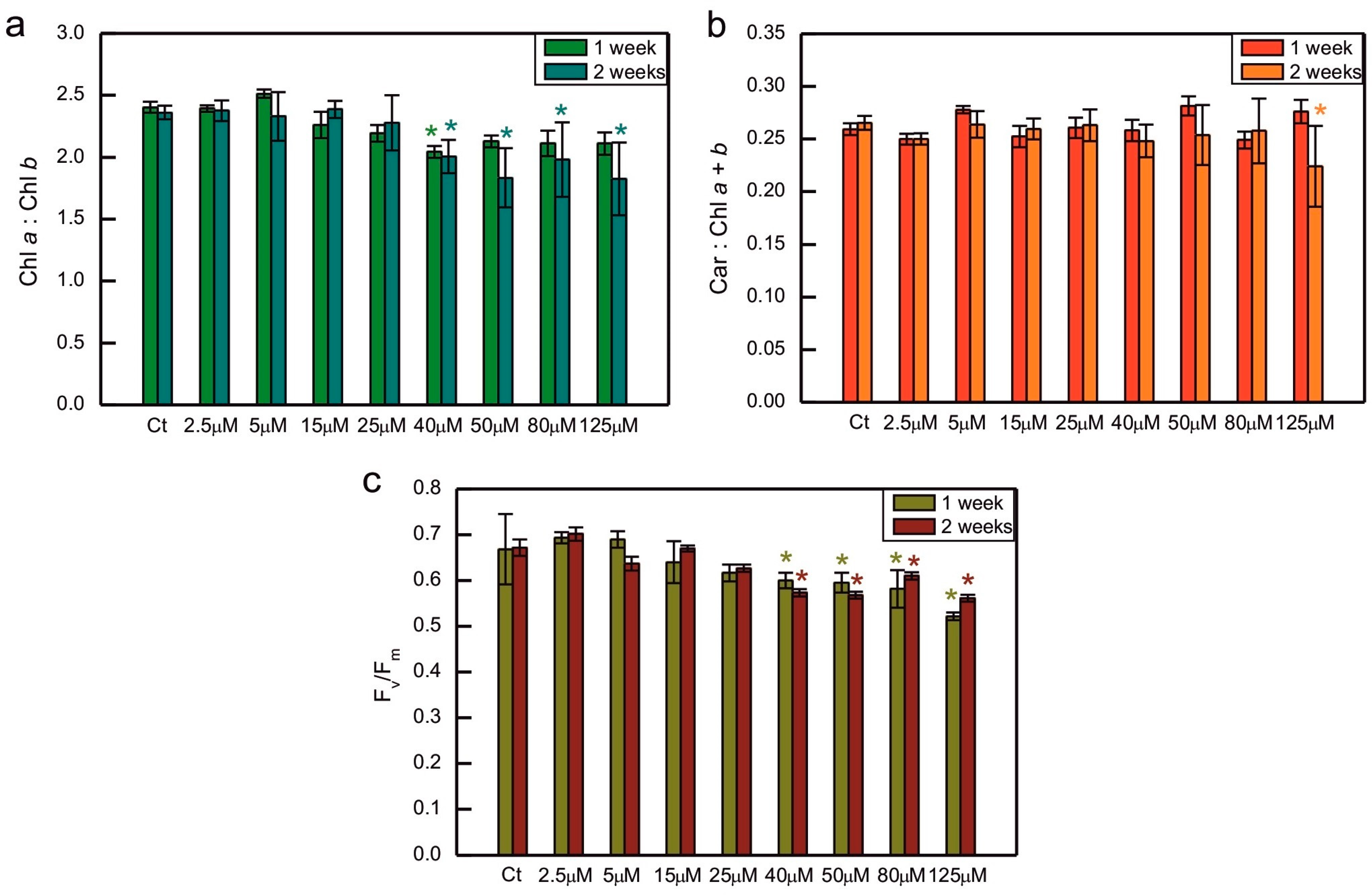
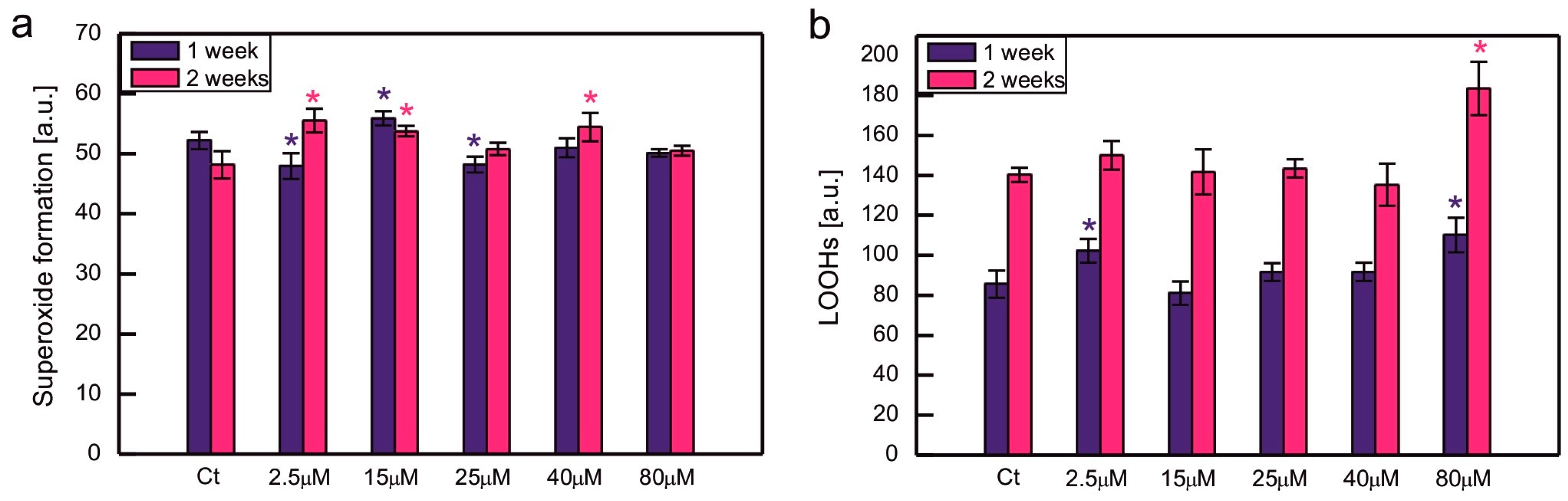
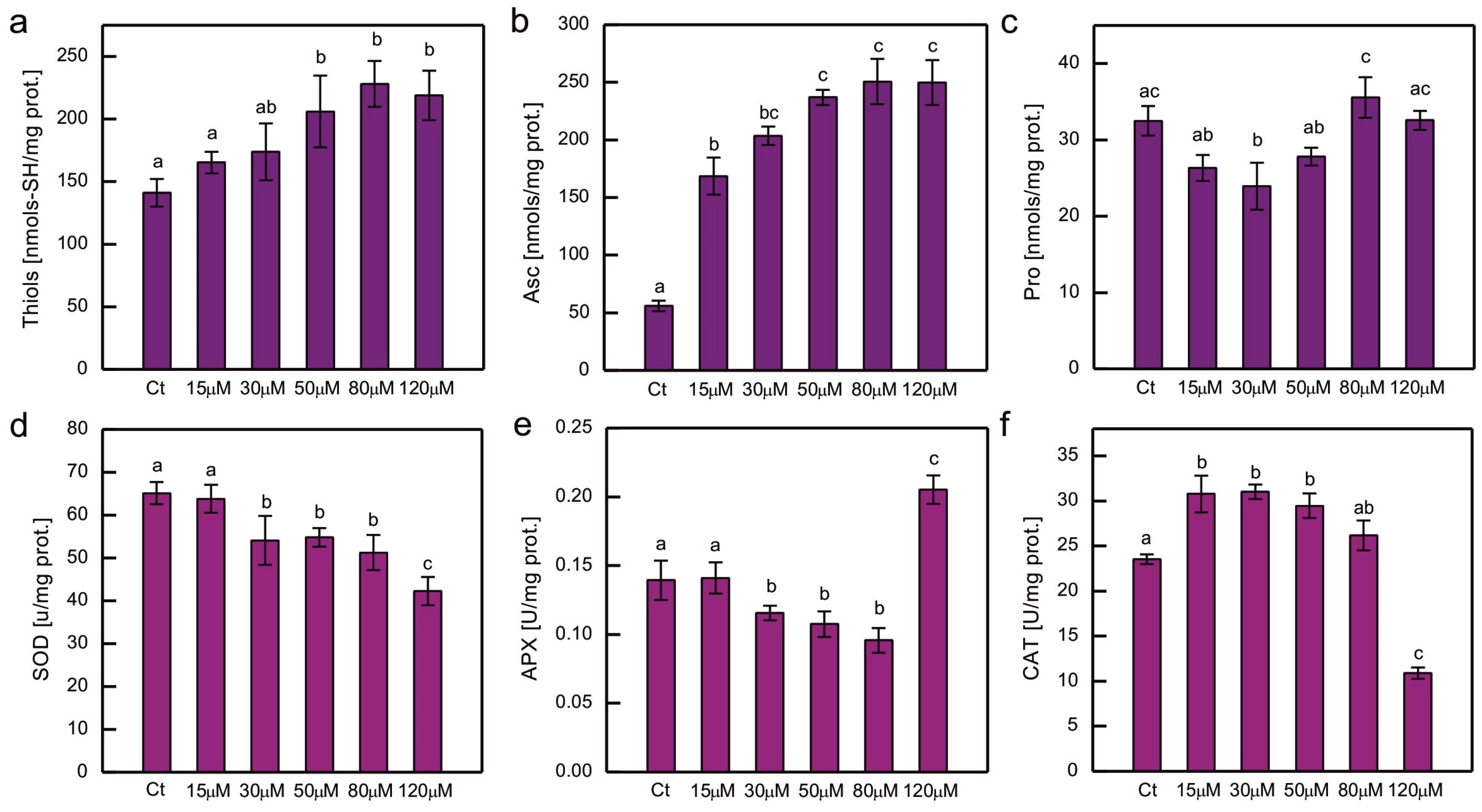
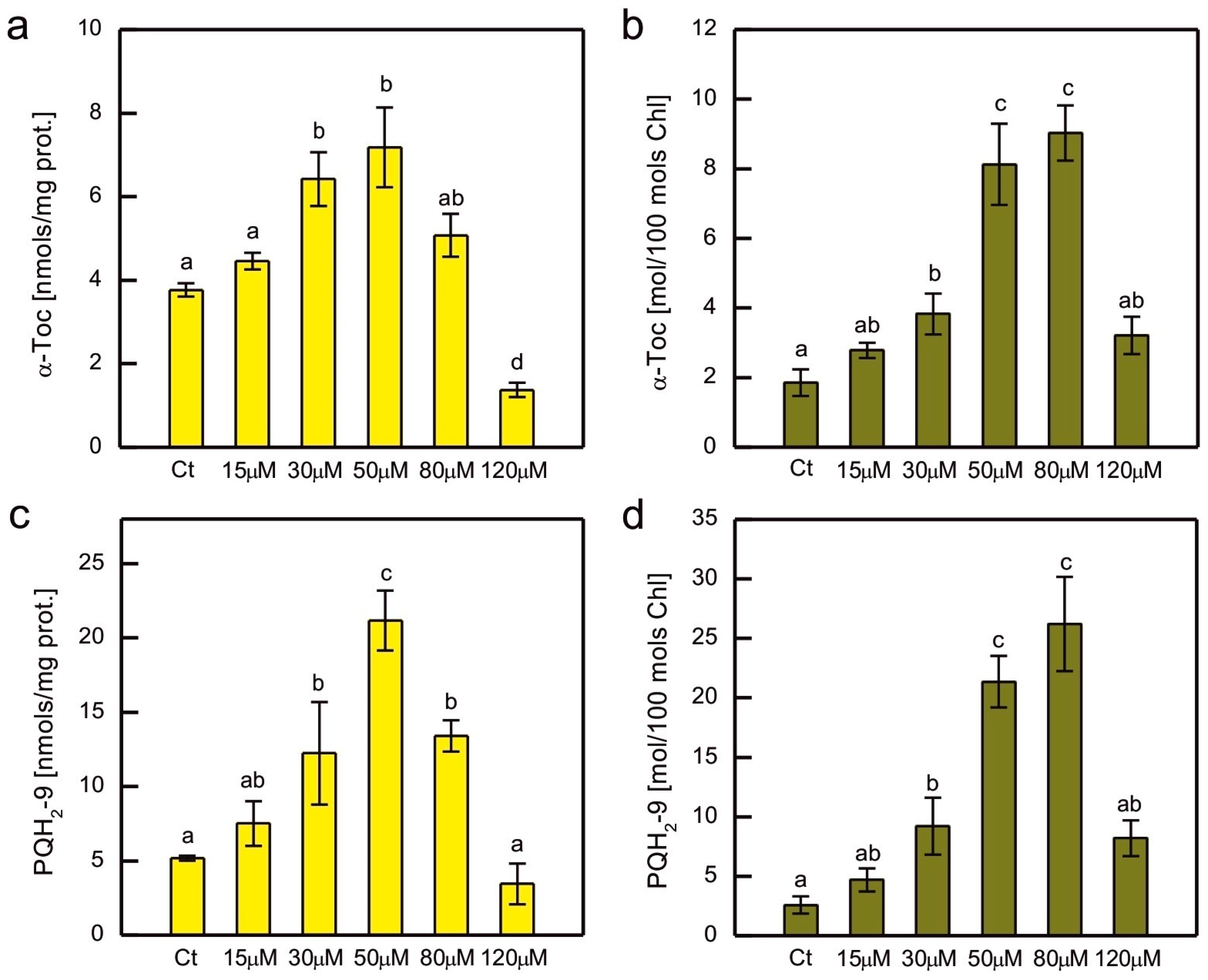
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourret, O.; Hursthouse, A. It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae—Mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G. Heavy metal toxicity in plants. In Plants and Heavy Metals; Furini, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–25. [Google Scholar]
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal-induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Li, X.; Xu, B.; Sahito, Z.A.; Chen, S.; Liang, Z. Transcriptome analysis reveals cadmium exposure enhanced the isoquinoline alkaloid biosynthesis and disease resistance in Coptis chinensis. Ecotoxicol. Environ. Saf. 2024, 271, 115940. [Google Scholar] [CrossRef]
- Yu, S.; Sahito, Z.A.; Lu, M.; Huang, Q.; Du, P.; Chen, D.; Lian, J.; Feng, Y.; He, Z.; Yang, X. Soil water stress alters differentially relative metabolic pathways affecting growth performance and metal uptake efficiency in a cadmium hyperaccumulator ecotype of Sedum alfredii. Environ. Sci. Pollut. Res. 2023, 30, 88986–88997. [Google Scholar] [CrossRef]
- Kalaivanan, D.; Ganeshamurthy, A.N. Mechanisms of heavy metal toxicity in plants. In Abiotic Stress Physiology of Horticultural Crops; Srinivasa Rao, N.K., Shivashankara, K.S., Laxman, R.H., Eds.; Springer: New Delhi, India, 2016; pp. 85–102. ISBN 9788132227250. [Google Scholar]
- Huang, B.; Huang, Y.; Shen, C.; Fan, L.; Fu, H.; Liu, Z.; Sun, Y.; Wu, B.; Zhang, J.; Xin, J. Roles of boron in preventing cadmium uptake by Capsicum annuum root tips: Novel insights from ultrastructural investigation and single-cell RNA sequencing. Sci. Total Environ. 2024, 957, 177858. [Google Scholar] [CrossRef]
- Shen, C.; Fu, H.; Huang, B.; Liao, Q.; Huang, Y.; Wang, Y.; Wang, Y.; Xin, J. Physiological and molecular mechanisms of boron in alleviating cadmium toxicity in Capsicum annuum. Sci. Total Environ. 2023, 903, 166264. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Mahey, S.; Kumar, R.; Sharma, M.; Kumar, V.; Bhardwaj, R. A critical review on toxicity of cobalt and its bioremediation strategies. SN Appl. Sci. 2020, 2, 1279. [Google Scholar] [CrossRef]
- Liu, J.; Reid, R.J.; Smith, F.A. The mechanism of cobalt toxicity in mung beans. Physiol. Plant. 2000, 110, 104–110. [Google Scholar] [CrossRef]
- Palit, S.; Sharma, A.; Talukder, G. Effects of cobalt on plants. Bot. Rev. 1994, 60, 149–181. [Google Scholar] [CrossRef]
- Kashyap, M.; Anand, V.; Ghosh, A.; Kiran, B. Superintending Scenedesmus and Chlorella sp. with lead and cobalt tolerance governed via stress biomarkers. Water Supply 2021, 21, 2387–2399. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Q.; Hu, C.-W.; Chen, L.; Liu, Z.-L.; Kong, Z.-M. Cobalt and manganese stress in the microalga Pavlova viridis (Prymnesiophyceae): Effects on lipid peroxidation and antioxidant enzymes. J. Environ. Sci. 2007, 19, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Trela-Makowej, A.; Latowski, D.; Strzalka, K.; Szymańska, R. Antioxidant and signaling role of plastid-derived isoprenoid quinones and chromanols. Int. J. Mol. Sci. 2021, 22, 2950. [Google Scholar] [CrossRef]
- Edreva, A. Generation and scavenging of reactive oxygen species in chloroplasts: A submolecular approach. Agric. Ecosyst. Environ. 2005, 106, 119–133. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef]
- Magdaong, N.C.M.; Blankenship, R.E. Photoprotective, excited-state quenching mechanisms in diverse photosynthetic organisms. J. Biol. Chem. 2018, 293, 5018–5025. [Google Scholar] [CrossRef]
- El-Din, S.M. Effects of heavy metals (copper, cobalt and lead) on the growth and photosynthetic pigments of the green alga Chlorella pyrenoidosa H. Chick. Catrina Int. J. Environ. Sci. 2016, 15, 1–10. [Google Scholar]
- Liu, C.; Wen, X.; Pan, H.; Luo, Y.; Zhou, J.; Wu, Y.; Zeng, Z.; Sun, T.; Chen, J.; Hu, Z.; et al. Bioremoval of Co(II) by a novel halotolerant microalgae Dunaliella sp. FACHB-558 from saltwater. Front. Microbiol. 2024, 15, 1256814. [Google Scholar] [CrossRef] [PubMed]
- Hanikenne, M. Chlamydomonas reinhardtii as a eukaryotic photosynthetic model for studies of heavy metal homeostasis and tolerance. New Phytol. 2003, 159, 331–340. [Google Scholar] [CrossRef]
- Nowicka, B. Practical aspects of the measurements of non-photochemical chlorophyll fluorescence quenching in green microalgae Chlamydomonas reinhardtii using Open FluorCam. Physiol. Plant. 2020, 168, 617–629. [Google Scholar] [CrossRef]
- Nowicka, B. Gaining insight into mechanisms of nonphotochemical quenching of chlorophyll fluorescence in Chlamydomonas reinhardtii via the observation of dark-induced state transitions. J. Bot. Res. 2024, 6, 1–9. [Google Scholar] [CrossRef]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef]
- Arunakumara, K.K.I.U.; Zhang, X. Effects of heavy metals (Pb2+ and Cd2+) on the ultrastructure, growth and pigment contents of the unicellular cyanobacterium Synechocystis sp. PCC 6803. Chin. J. Oceanol. Limnol. 2009, 27, 383–388. [Google Scholar] [CrossRef]
- Rama Devi, S.; Prasad, M.N.V. Copper toxicity in Ceratophyllum demersum L. (Coontail), a floating macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Hattab, S.; Dridi, B.; Chouba, L.; Ben Kheder, M.; Bousetta, H. Photosynthesis and growth responses of pea Pisum sativum L. under heavy metals stress. J. Environ. Sci. 2009, 21, 1552–1556. [Google Scholar] [CrossRef]
- Vavilin, D.V.; Polynov, V.A.; Matorin, D.N.; Venediktov, P.S. Sublethal concentrations of copper stimulate photosystem II photoinhibition in Chlorella pyrenoidosa. J. Plant Physiol. 1995, 146, 609–614. [Google Scholar] [CrossRef]
- Wong, P.K.; Chang, L. Effects of copper, chromium and nickel on growth, photosynthesis and chlorophyll a synthesis of Chlorella pyrenoidosa 251. Environ. Pollut. 1991, 72, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Gillet, S.; Decottignies, P.; Chardonnet, S.; Le Maréchal, P. Cadmium response and redoxin targets in Chlamydomonas reinhardtii: A proteomic approach. Photosynth. Res. 2006, 89, 201–211. [Google Scholar] [CrossRef]
- Collard, J.M.; Matagne, R.F. Isolation and genetic analysis of Chlamydomonas reinhardtii strains resistant to cadmium. Appl. Environ. Microbiol. 1990, 56, 2051–2055. [Google Scholar] [CrossRef]
- Weiss-Magasic, C.; Lustigman, B.; Lee, L.H. Effect of mercury on the growth of Chlamydomonas reinhardtii. Bull. Environ. Contam. Toxicol. 1997, 59, 828–833. [Google Scholar] [CrossRef]
- Zheng, C.; Aslam, M.; Liu, X.; Du, H.; Xie, X.; Jia, H.; Huang, N.; Tang, K.; Yang, Y.; Li, P. Impact of Pb on Chlamydomonas reinhardtii at physiological and transcriptional levels. Front. Microbiol. 2020, 11, 1443. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Y.; Hu, Z.; Lei, A.; Wang, J. Towards elucidation of the toxic mechanism of copper on the model green alga Chlamydomonas reinhardtii. Ecotoxicology 2016, 25, 1417–1425. [Google Scholar] [CrossRef]
- Luis, P.; Behnke, K.; Toepel, J.; Wilhelm, C. Parallel analysis of transcript levels and physiological key parameters allows the identification of stress phase gene markers in Chlamydomonas reinhardtii under copper excess. Plant Cell Environ. 2006, 29, 2043–2054. [Google Scholar] [CrossRef]
- Nowicka, B.; Pluciński, B.; Kuczyńska, P.; Kruk, J. Physiological characterization of Chlamydomonas reinhardtii acclimated to chronic stress induced by Ag, Cd, Cr, Cu and Hg ions. Ecotoxicol. Environ. Saf. 2016, 130, 133–145. [Google Scholar] [CrossRef]
- Nowicka, B.; Fesenko, T.; Walczak, J.; Kruk, J. The inhibitor-evoked shortage of tocopherol and plastoquinol is compensated by other antioxidant mechanisms in Chlamydomonas reinhardtii exposed to toxic concentrations of cadmium and chromium ions. Ecotoxicol. Environ. Saf. 2020, 191, 110241. [Google Scholar] [CrossRef]
- Nowicka, B.; Zyzik, M.; Kapsiak, M.; Ogrodzińska, W.; Kruk, J. Oxidative stress limits growth of Chlamydomonas reinhardtii (Chlorophyta, Chlamydomonadales) exposed to copper ions at the early stage of culture growth. Phycologia 2021, 60, 303–313. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Drej, K.; Skawińska, A.; Strzałka, K. Toxicity of cadmium and copper in Chlamydomonas reinhardtii wild-type (WT 2137) and cell wall deficient mutant strain (CW 15). Bull. Environ. Contam. Toxicol. 1998, 60, 306–311. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Barsanti, L.; Passarelli, V.; Evangelista, V.; Conforti, V.; Gualtieri, P. Effects of chromium on photosynthetic and photoreceptive apparatus of the alga Chlamydomonas reinhardtii. Environ. Res. 2007, 105, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cheng, Z.Z.; Yang, Z.M. HISN3 mediates adaptive response of Chlamydomonas reinhardtii to excess nickel. Plant Cell Physiol. 2013, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Battah, M.; El-Ayoty, Y.; Abomohra, A.E.F.; El-Ghany, S.A.; Esmael, A. Effect of Mn2+, Co2+ and H2O2 on biomass and lipids of the green microalga Chlorella vulgaris as a potential candidate for biodiesel production. Ann. Microbiol. 2015, 65, 155–162. [Google Scholar] [CrossRef]
- Horvatić, J.; Peršić, V. The effect of Ni2+, Co2+, Zn2+, Cd2+ and Hg2+ on the growth rate of marine diatom Phaeodactylum tricornutum Bohlin: Microplate growth inhibition test. Bull. Environ. Contam. Toxicol. 2007, 79, 494–498. [Google Scholar] [CrossRef]
- Lustigman, B.; Lee, L.H.; Weiss-Magasic, C. Effects of cobalt and pH on the growth of Chlamydomonas reinhardtii. Bull. Environ. Contam. Toxicol. 1995, 55, 65–72. [Google Scholar] [CrossRef]
- Osman, M.E.H.; El-Naggar, A.H.; El-Sheekh, M.M.; El-Mazally, E.E. Differential effects of Co2+ and Ni2+ on protein metabolism in Scenedesmus obliquus and Nitzschia perminuta. Environ. Toxicol. Pharmacol. 2004, 16, 169–178. [Google Scholar] [CrossRef]
- Rachlin, J.W.; Grosso, A. The growth response of the green alga Chlorella vulgaris to combined divalent cation exposure. Arch. Environ. Contam. Toxicol. 1993, 24, 16–20. [Google Scholar] [CrossRef]
- dos Reis, L.L.; de Abreu, C.B.; Gebara, R.C.; Rocha, G.S.; Longo, E.; Mansano, A.d.S.; Melão, M.d.G.G. Isolated and combined effects of cobalt and nickel on the microalga Raphidocelis subcapitata. Ecotoxicology 2024, 33, 104–118. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; El-Naggar, A.H.; Osman, M.E.H.; El-Mazaly, E. Effect of cobalt on growth, pigments and the photosynthetic electron transport in Monoraphidium minutum and Nitzchia perminuta. Braz. J. Plant Physiol. 2003, 15, 159–166. [Google Scholar] [CrossRef]
- Rocha, G.S.; Melão, M.G.G. Does cobalt antagonize P limitation effects on photosynthetic parameters on the freshwater microalgae Raphidocelis subcapitata (Chlorophyceae), or does P limitation acclimation antagonize cobalt effects? More questions than answers. Environ. Pollut. 2024, 341, 122998. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Agrawal, M.; Agrawal, S.B. Impact of heavy metals on physiological processes of plants: With special reference to photosynthetic system. In Plant Responses to Xenobiotics; Singh, A., Prasad, S.M., Singh, R.P., Eds.; Springer: Singapore, 2016; pp. 127–140. ISBN 9789811028601. [Google Scholar]
- Myśliwa-Kurdziel, B.; Strzałka, K. Influence of metals on biosynthesis of photosynthetic pigments. In Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants; Prasad, M.N.V., Strzalka, K., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 201–227. [Google Scholar]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Küpper, H.; Šetlík, I.; Spiller, M.; Küpper, F.C.; Prášil, O. Heavy metal-induced inhibition of photosynthesis: Targets of in vivo heavy metal chlorophyll formation. J. Phycol. 2002, 38, 429–441. [Google Scholar] [CrossRef]
- Drazkiewicz, M. Chlorophyllase: Occurrence, functions, mechanism of action, effects of external and internal factors. Photosynthetica 1994, 30, 321–331. [Google Scholar]
- Csatorday, K.; Gombos, Z.; Szalontai, B. Mn2+ and Co2+ toxicity in chlorophyll biosynthesis. Proc. Natl. Acad. Sci. USA 1984, 81, 476–478. [Google Scholar] [CrossRef]
- Chettri, M.K.; Cook, C.M.; Vardaka, E.; Sawidis, T.; Lanaras, T. The effect of Cu, Zn and Pb on the chlorophyll content of the lichens Cladonia convoluta and Cladonia rangiformis. Environ. Exp. Bot. 1998, 39, 1–10. [Google Scholar] [CrossRef]
- Mallick, N. Copper-induced oxidative stress in the chlorophycean microalga Chlorella vulgaris: Response of the antioxidant system. J. Plant Physiol. 2004, 161, 591–597. [Google Scholar] [CrossRef]
- Rai, U.N.; Singh, N.K.; Upadhyay, A.K.; Verma, S. Chromate tolerance and accumulation in Chlorella vulgaris L.: Role of antioxidant enzymes and biochemical changes in detoxification of metals. Bioresour. Technol. 2013, 136, 604–609. [Google Scholar] [CrossRef]
- Nikookar, K.; Moradshahi, A.; Hosseini, L. Physiological responses of Dunaliella salina and Dunaliella tertiolecta to copper toxicity. Biomol. Eng. 2005, 22, 141–146. [Google Scholar] [CrossRef]
- Moussa, I.D.-B.; Athmouni, K.; Chtourou, H.; Ayadi, H.; Sayadi, S.; Dhouib, A. Phycoremediation potential, physiological, and biochemical response of Amphora subtropica and Dunaliella sp. to nickel pollution. J. Appl. Phycol. 2018, 30, 931–941. [Google Scholar] [CrossRef]
- Boucher, N.; Carpentier, R. Hg2+, Cu2+, and Pb2+-induced changes in photosystem II photochemical yield and energy storage in isolated thylakoid membranes: A study using simultaneous fluorescence and photoacoustic measurements. Photosynth. Res. 1999, 59, 167–174. [Google Scholar] [CrossRef]
- Gan, T.; Zhao, N.; Yin, G.; Chen, M.; Wang, X.; Liu, J.; Liu, W. Optimal chlorophyll fluorescence parameter selection for rapid and sensitive detection of lead toxicity to marine microalgae Nitzschia closterium based on chlorophyll fluorescence technology. J. Photochem. Photobiol. B Biol. 2019, 197, 111551. [Google Scholar] [CrossRef] [PubMed]
- Pluciński, B.; Nowicka, B.; Waloszek, A.; Rutkowska, J.; Strzałka, K. The role of antioxidant response and nonphotochemical quenching of chlorophyll fluorescence in long-term adaptation to Cu-induced stress in Chlamydomonas reinhardtii. Environ. Sci. Pollut. Res. 2023, 30, 67250–67262. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Zheng, Q.; Liu, Z.P.; Yang, Z.M. Regulation of tolerance of Chlamydomonas reinhardtii to heavy metal toxicity by heme oxygenase-1 and carbon monoxide. Plant Cell Physiol. 2011, 52, 1665–1675. [Google Scholar] [CrossRef]
- Zheng, Q.; Meng, Q.; Wei, Y.Y.; Yang, Z.M. Alleviation of copper-induced oxidative damage in Chlamydomonas reinhardtii by carbon monoxide. Arch. Environ. Contam. Toxicol. 2011, 61, 220–227. [Google Scholar] [CrossRef]
- Sabatini, S.E.; Juárez, Á.B.; Eppis, M.R.; Bianchi, L.; Luquet, C.M.; de Molina, M.d.C.R. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol. Environ. Saf. 2009, 72, 1200–1206. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef]
- Gaur, J.P.; Rai, L.C. Heavy metal tolerance in algae. In Algal Adaptation to Environmental Stresses; Gaur, J.P., Rai, L.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 363–388. [Google Scholar]
- Ose, D.E.; Fridovich, I. Manganese-containing superoxide dismutase from Escherichia coli: Reversible resolution and metal replacements. Arch. Biochem. Biophys. 1979, 194, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, M.; Wu, A.J.; Kampf, J.W.; Pecoraro, V.L. Corroborative models of the cobalt(II) inhibited Fe/Mn superoxide dismutases. Inorg. Chem. 2005, 44, 5001–5010. [Google Scholar] [CrossRef]
- Escobar, J.A.; Rubio, M.A.; Lissi, E.A. SOD and catalase inactivation by singlet oxygen and peroxyl radicals. Free Radic. Biol. Med. 1996, 20, 285–290. [Google Scholar] [CrossRef]
- Kuo, E.Y.H.; Cai, M.S.; Lee, T.M. Ascorbate peroxidase 4 plays a role in the tolerance of Chlamydomonas reinhardtii to photo-oxidative stress. Sci. Rep. 2020, 10, 13287. [Google Scholar] [CrossRef]
- Howe, G.; Merchant, S. Heavy metal-activated synthesis of peptides in Chlamydomonas reinhardtii. Plant Physiol. 1992, 98, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, N.; Prasad, M.N.V. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Filová, A.; Fargašová, A.; Molnárová, M. Cu, Ni, and Zn effects on basic physiological and stress parameters of Raphidocelis subcapitata algae. Environ. Sci. Pollut. Res. 2021, 28, 58426–58441. [Google Scholar] [CrossRef] [PubMed]
- Takami, R.; Almeida, J.V.; Vardaris, C.V.; Colepicolo, P.; Barros, M.P. The interplay between thiol-compounds against chromium (VI) in the freshwater green alga Monoraphidium convolutum: Toxicology, photosynthesis, and oxidative stress at a glance. Aquat. Toxicol. 2012, 118–119, 80–87. [Google Scholar] [CrossRef]
- Hamed, S.M.; Zinta, G.; Klöck, G.; Asard, H.; Selim, S.; AbdElgawad, H. Zinc-induced differential oxidative stress and antioxidant responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicol. Environ. Saf. 2017, 140, 256–263. [Google Scholar] [CrossRef]
- Wu, J.-T.; Chang, S.-J.; Chou, T.-L. Intracellular proline accumulation in some algae exposed to copper and cadmium. Bot. Bull. Acad. Sin. 1995, 36, 89–93. [Google Scholar]
- Mehta, S.K.; Gaur, J.P. Heavy metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Phytol. 1999, 143, 253–259. [Google Scholar] [CrossRef]
- Danouche, M.; El Ghachtouli, N.; El Baouchi, A.; El Arroussi, H. Heavy metals phycoremediation using tolerant green microalgae: Enzymatic and non-enzymatic antioxidant systems for the management of oxidative stress. J. Environ. Chem. Eng. 2020, 8, 104460. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Mehta, S.K.; Amar, A.; Gaur, J.P. Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+. Chemosphere 2006, 62, 538–544. [Google Scholar] [CrossRef]
- Dziuba, J.; Nowicka, B. Unravelling the mechanisms of heavy metal tolerance: Enhancement in hydrophilic antioxidants and major antioxidant enzymes is not crucial for long-term adaptation to copper in Chlamydomonas reinhardtii. Plants 2024, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Arora, N.; Pruthi, V.; Poluri, K.M. Elucidating the bioremediation mechanism of Scenedesmus sp. IITRIND2 under cadmium stress. Chemosphere 2021, 283, 131196. [Google Scholar] [CrossRef] [PubMed]
- Collin, V.C.; Eymery, F.; Genty, B.; Rey, P.; Havaux, M. Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ. 2008, 31, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kökten, A.; Nowicka, B. Analysis of the Response of Chlamydomonas reinhardtii to Cobalt Ions Reveals the Protective Role of Thiols, Ascorbate, and Prenyllipid Antioxidants, and the Negative Impact of Cobalt Toxicity on Photoprotective Mechanisms. Plants 2025, 14, 3496. https://doi.org/10.3390/plants14223496
Kökten A, Nowicka B. Analysis of the Response of Chlamydomonas reinhardtii to Cobalt Ions Reveals the Protective Role of Thiols, Ascorbate, and Prenyllipid Antioxidants, and the Negative Impact of Cobalt Toxicity on Photoprotective Mechanisms. Plants. 2025; 14(22):3496. https://doi.org/10.3390/plants14223496
Chicago/Turabian StyleKökten, Aylin, and Beatrycze Nowicka. 2025. "Analysis of the Response of Chlamydomonas reinhardtii to Cobalt Ions Reveals the Protective Role of Thiols, Ascorbate, and Prenyllipid Antioxidants, and the Negative Impact of Cobalt Toxicity on Photoprotective Mechanisms" Plants 14, no. 22: 3496. https://doi.org/10.3390/plants14223496
APA StyleKökten, A., & Nowicka, B. (2025). Analysis of the Response of Chlamydomonas reinhardtii to Cobalt Ions Reveals the Protective Role of Thiols, Ascorbate, and Prenyllipid Antioxidants, and the Negative Impact of Cobalt Toxicity on Photoprotective Mechanisms. Plants, 14(22), 3496. https://doi.org/10.3390/plants14223496










