Herbicidal Formulations with Plant-Based Compounds to Control Amaranthus hybridus, Lolium multiflorum, and Brassica rapa Weeds
Abstract
1. Introduction
2. Materials and Methods
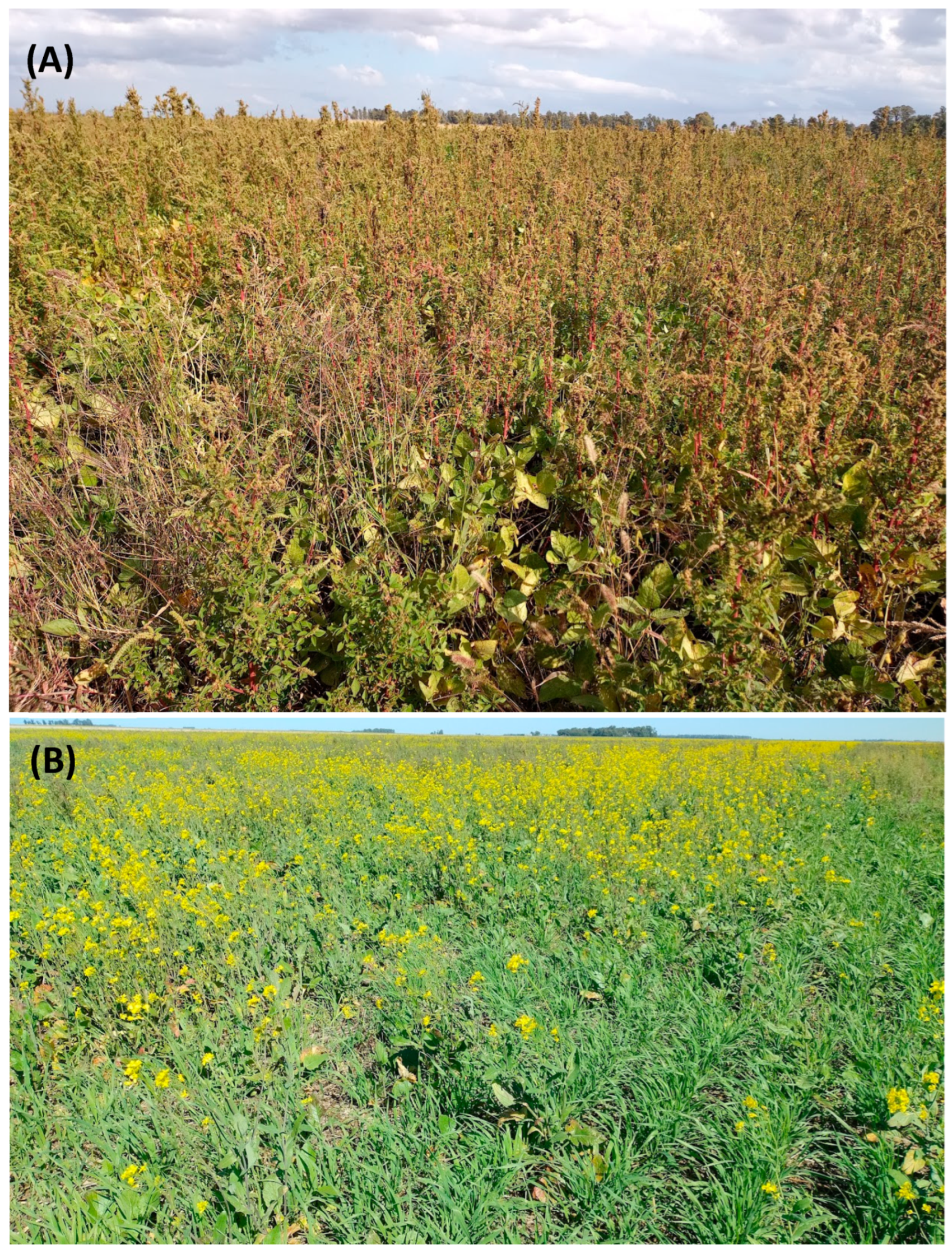
2.1. Plant Materials
2.2. Chemical Compounds
2.3. Formulations
2.4. Chemical Characterization
2.4.1. Fourier Transform Infrared Spectrometry (ATR-FTIR)
2.4.2. Dynamic Light Scattering (DLS) Measurements
2.4.3. Thermogravimetric Analysis (TGA)
2.5. Herbicide Activity
2.5.1. Weed Germination Inhibition Test
2.5.2. Effect of Binary Formulations on Photosystem II (PSII)
2.5.3. Effect of Binary Formulations on Wheat Germination and Seedling Growth
3. Results
3.1. Chemical Characterization
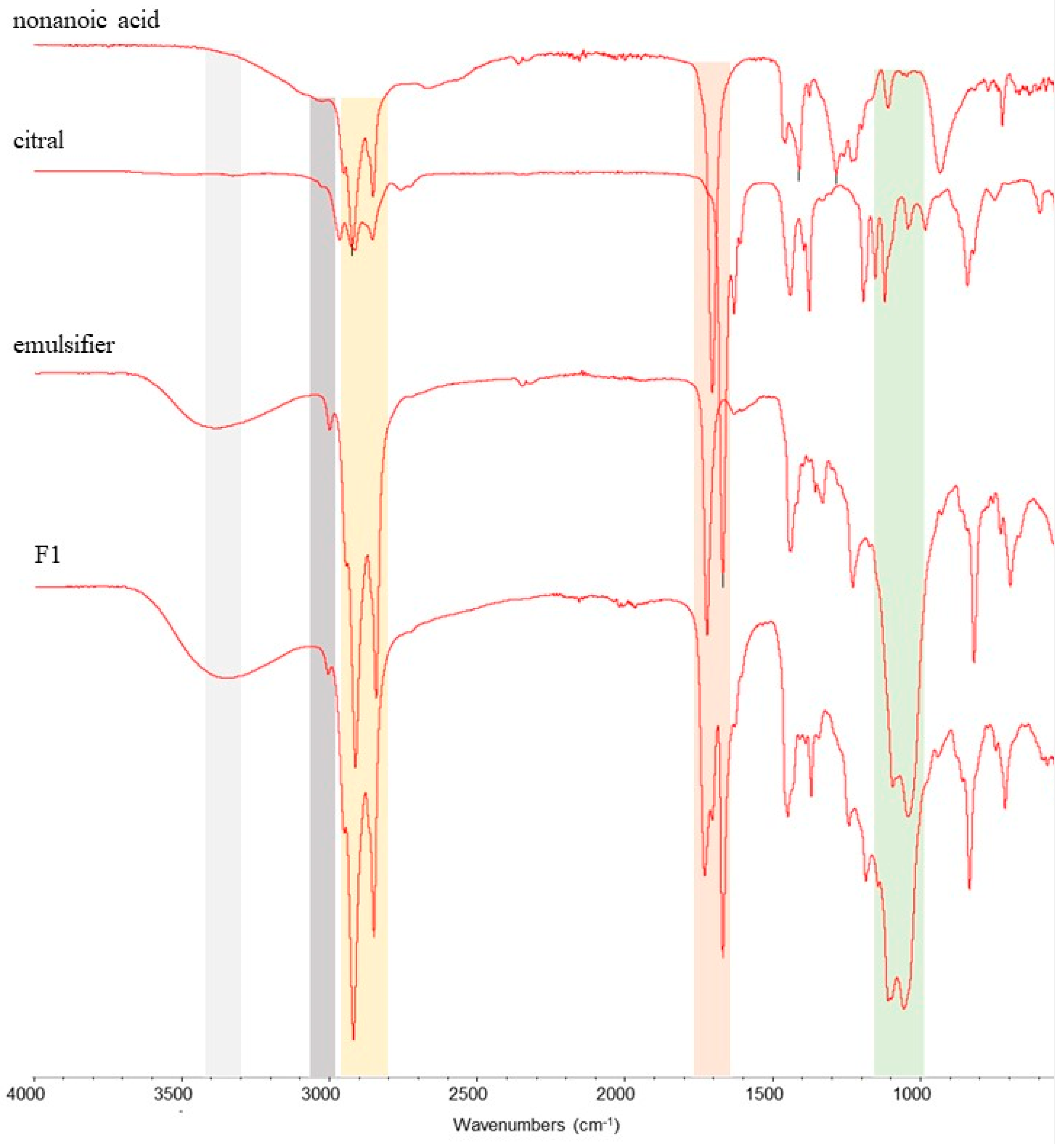
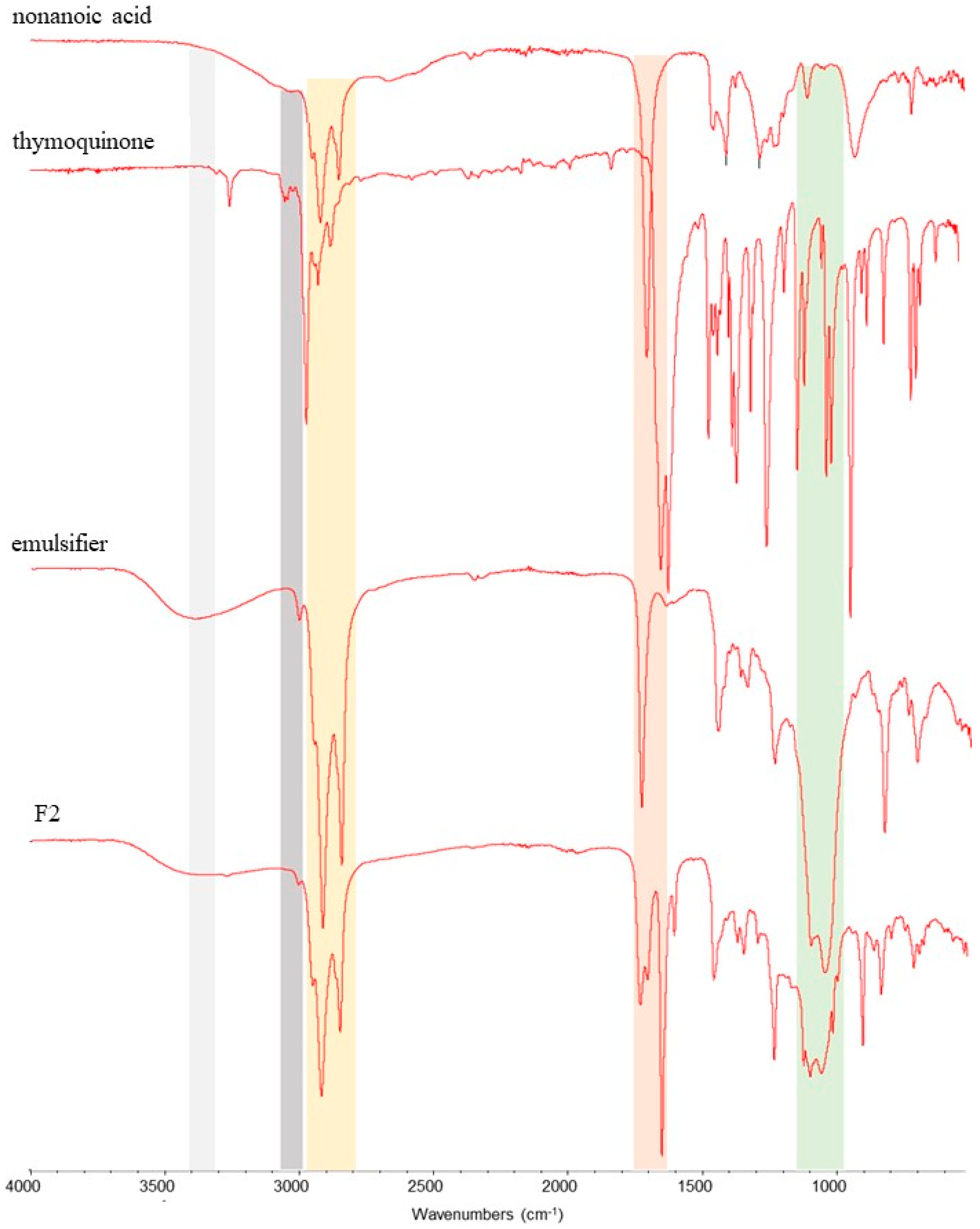
3.1.1. Fourier Transform Infrared Spectrometry (ATR-FTIR)
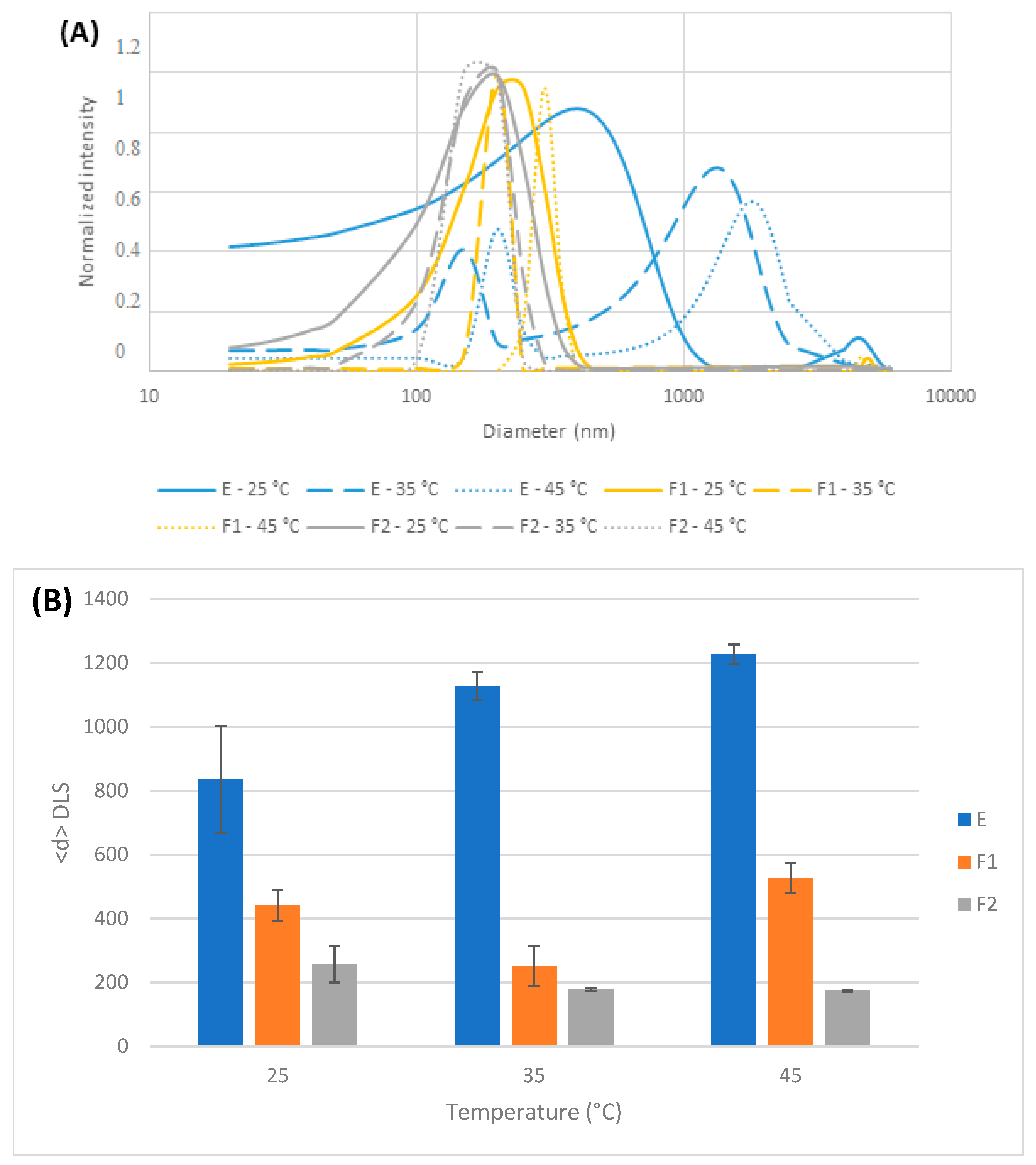
3.1.2. Dynamic Light Scattering (DLS) Measurements
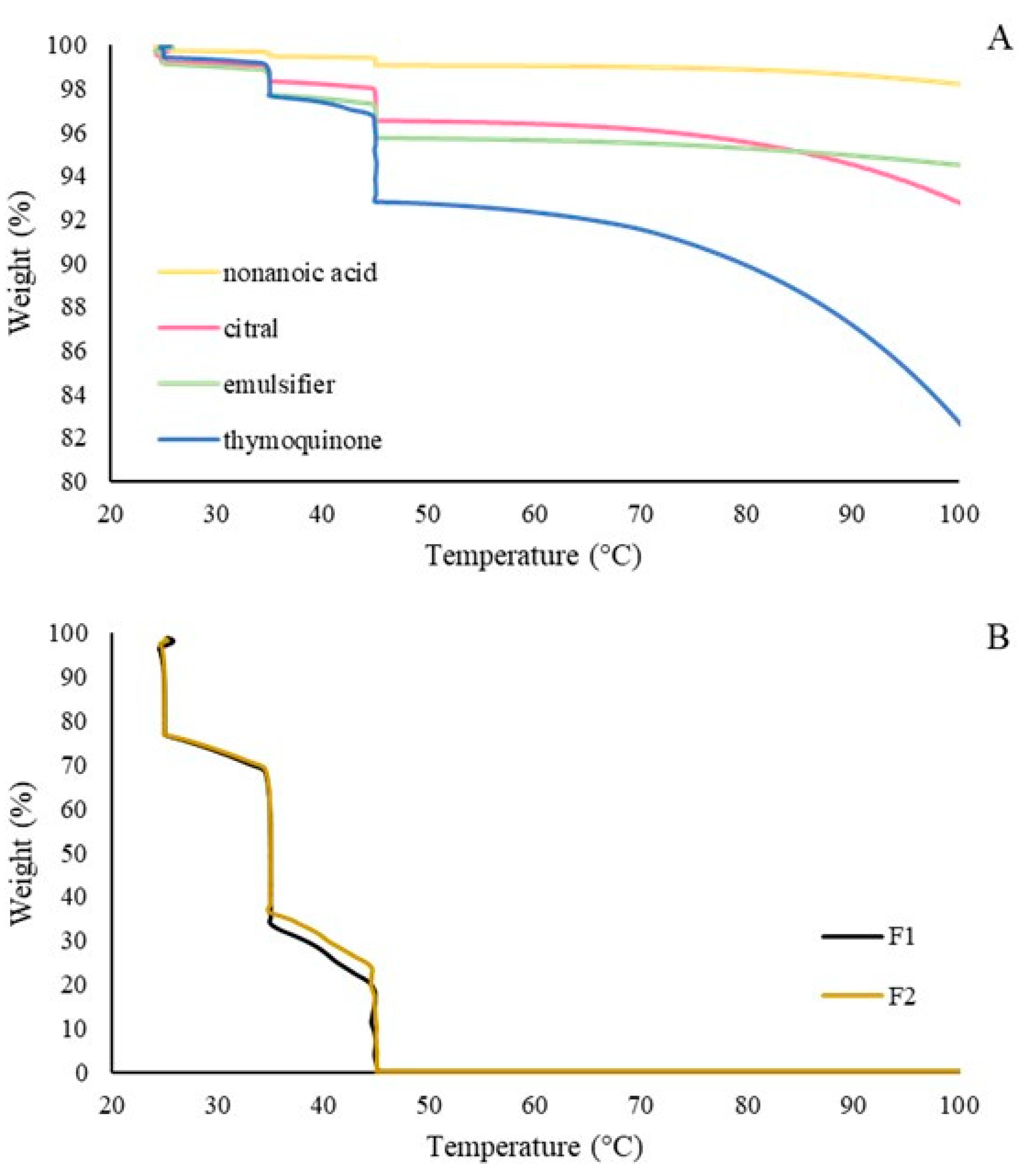
3.1.3. Thermogravimetric Analysis (TGA)
3.2. Herbicide Activity
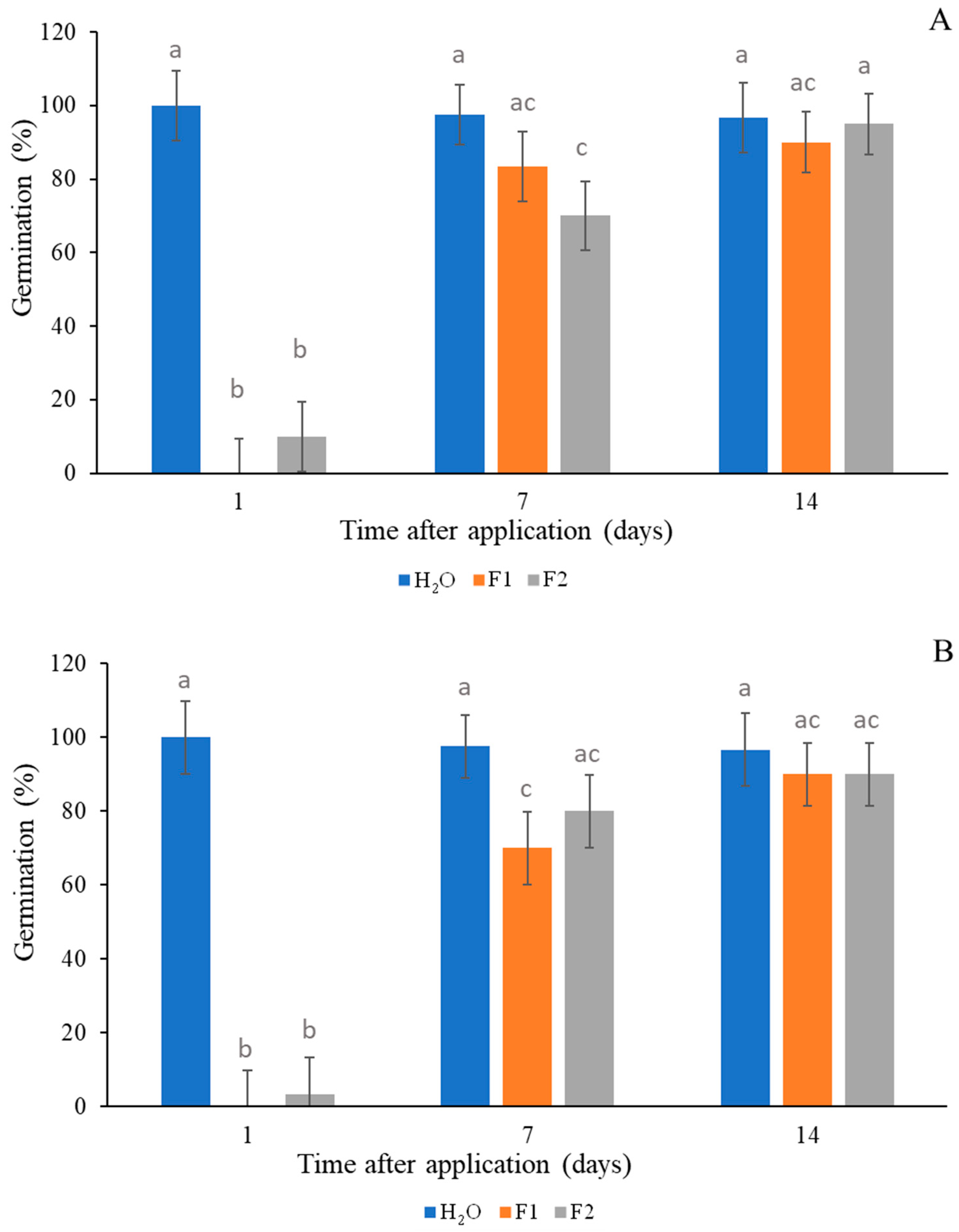
3.2.1. Weed Germination Inhibition Test
3.2.2. Effect of Binary Formulations on Photosystem II (PSII)
3.2.3. Effect of Binary Formulations on Wheat Germination and Seedling Growth
4. Discussion
4.1. Chemical Characterization
4.2. Herbicide Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Intergovernmental Panel on Climate Change. Cambio Climático 2021: Bases Físicas. Contribución del Grupo de Trabajo I al Sexto Informe de Evaluación del Grupo Intergubernamental de Expertos Sobre el Cambio Climático. Available online: http://www.ipcc.ch (accessed on 7 March 2023).
- Oreja, F.; Moreno, N.; Gundel, P.; Vercellino, R.; Pandolfo, C.; Presotto, A.; Perotti, V.; Permingeat, H.; Tuesca, D.; Scursoni, J.; et al. Herbicide-resistant weeds from dryland agriculture in Argentina. Weed Res. 2024, 64, 88–106. [Google Scholar] [CrossRef]
- Yanniccari, M.E.; Istilart, C.; Giménez, D.; Castro, A. Glyphosate resistance in perennial ryegrass (Lolium perenne L.) from Argentina. Crop. Prot. 2012, 32, 12–16. [Google Scholar] [CrossRef]
- Yanniccari, M.E.; Gaines, T.; Scursoni, J.A.; De Prado, R.; Vila-Aiub, M. Global patterns of herbicide resistance evolution in Amaranthus spp.: An analysis comparing species, cropping regions and herbicides. Adv. Weed Sci. 2022, 40, e0202200037. [Google Scholar] [CrossRef]
- Víctor, J.; Núñez Fré, F.; Saint-André, H.; Fernández, R. Responses of 2,4-D resistant Brassica rapa L. biotype to various 2,4-D formulations and other auxinic herbicides. Crop. Prot. 2021, 145, 105621. [Google Scholar]
- Depetri, M.; Muñiz Padilla, E.; Ayala, F.; Tuesca, D.; Breccia, G. Resistance to acetyl-CoA carboxylase (ACCase)-inhibiting herbicides in Lolium multiflorum Lam. populations of Argentina. Pest Manag. Sci. 2024, 80, 6600–6606. [Google Scholar] [CrossRef]
- Ferraro, D.O.; Ghersa, F.; de Paula, R.; Duarte Vera, A.C.; Pessah, S. Historical trends of the ecotoxicological pesticide risk from the main grain crops in Rolling Pampa (Argentina). PLoS ONE 2020, 15, e0238676. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- EPA. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2013-0266-1625 (accessed on 7 March 2023).
- Cotrut, R. Allelopathy and allelochemical interactions among plants. Sci. Pap. 2018, 1, 188–193. [Google Scholar]
- Verdeguer, M.; Castañeda, L.G.; Torres-Pagan, N.; Llorens-Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus essential oils. Molecules 2020, 25, 562. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Azirak, S.; Karaman, S. Allelopathic effect of some essential oils and components on germination of weed species. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2008, 58, 88–92. [Google Scholar] [CrossRef]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance Fatty acids C7 to C18 (approved under Regulation (EC) No 1107/2009 as Fatty acids C7 to C20). EFSA J. 2013, 11, 3023. [Google Scholar] [CrossRef]
- Devi, R.C.; Sim, S.M.; Ismail, R. Spasmolytic effect of citral and extracts of Cymbopogon citratus on isolated rabbit ileum. J. Smooth Muscle Res. 2011, 47, 143–156. [Google Scholar] [CrossRef]
- Sousa, D.G.; Sousa, S.D.; Silva, R.E.; Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; Kerntopf, M.R.; Menezes, I.R.; Leal-Cardoso, J.H.; Barbosa, R. Essential oil of Lippia alba and its main constituent citral block the excitability of rat sciatic nerves. Braz. J. Med. Biol. Res. 2015, 48, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.; Putievsky, E.; Lerner, H. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Herrera, J.M.; Goñi, M.L.; Gañan, N.A.; Zygadlo, J.A. An insecticide formulation of terpene ketones against Sitophilus zeamais and its incorporation into low density polyethylene films. Crop. Prot. 2017, 98, 33–39. [Google Scholar] [CrossRef]
- Isaev, N.K.; Chetverikov, N.S.; Stelmashook, E.V.; Genrikhs, E.E.; Khaspekov, L.G.; Illarioshkin, S.N. Thymoquinone as a potential neuroprotector in acute and chronic forms of cerebral pathology. Biochemistry 2020, 85, 167–176. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Chemspider, Chemical Structure Database. Available online: http://www.chemspider.com/ (accessed on 15 January 2024).
- Sosa, G.M.; Travaini, L.M.; Walter, H.; Cantrell, C.; Duke, S.; Carrillo, N.; Ceccarelli, E. Herbicidal Composition Comprising Chromone Derivatives and a Method for Weed Control. U.S. Patent 11083197B2, 12 December 2021. [Google Scholar]
- YPF Agro. Available online: https://agro.ypf.com (accessed on 12 June 2024).
- Calvimonte, H.; Peschiutta, M.L.; Herrera, J.M.; Zunino, M.P.; Jacquat, A.; Usseglio, V.L.; Zygadlo, J. Allylic and non-allylic alcohols against the maize weevil (Sitophilus zeamais): A promising tool for its control. Agric. Res. 2022, 12, 94–103. [Google Scholar] [CrossRef]
- Mallory-Smith, C.A.; Retzinger, E.J. Revised classification of herbicides by site of action for weed resistance management strategies. Weed Technol. 2003, 17, 605–619. [Google Scholar] [CrossRef]
- Software L-POLO-Plus a User’s Guide to Probit or Logic Analysis; LeOra Software: Berkeley, CA, USA, 2002.
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Torra, J.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2018, 74, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Pooja, M.; Anjana, J.; Sonal, M.; Sudhakar, B. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat; versión 2017; Universidad Nacional de Córdoba: Córdoba, Argentina, 2017. [Google Scholar]
- Peschiutta, M.L.; Brito, V.D.; Achimon, F.; Zunino, M.P.; Usseglio, V.L.; Zygadlo, J. New insecticide delivery method for the control of Sitophilus zeamais in stored maize. J. Stored Prod. Res. 2019, 83, 185–190. [Google Scholar] [CrossRef]
- Van Nieuwenhuyzen, W.; Szuhaj, B.F. Effects of lecithins and proteins on the stability of emulsions. Lipid/Fett 1998, 100, 282–291. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Xie, S.; Ma, G.; Jia, Y. Thermal properties and reliability of a lauric acid/nonanoic acid binary mixture as a phase-change material for thermal energy storage. Energy Technol. 2017, 5, 2309–2316. [Google Scholar] [CrossRef]
- Whittinghill, J.M.; Norton, J.; Proctor, A. Stability determination of soy lecithin-based emulsions by Fourier Transform Infrared Spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 37–42. [Google Scholar] [CrossRef]
- Herrera, J.M.; Peralta, E.; Palacio, M.A.; Mercado Ruiz, J.N.; Strumia, M.C.; Zygadlo, J.A.; Soto Valdez, H. Bioactive silo bag for controlling stored pest: Sithophilus zeamais (Coleoptera: Curculionidae). Rev. Ibero. Tec. Post. 2022, 23, 214–223. [Google Scholar]
- Scholfield, C.R. Composition of soybean lecithin. J. Am. Oil Chem. Soc. 1981, 58, 889–890. [Google Scholar] [CrossRef]
- Taladrid, D.; Marín, D.; Alemán, A.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Effect of chemical composition and sonication procedure on properties of food-grade soy lecithin liposomes with added glycerol. Food Res. Int. 2017, 100, 541–550. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Sui, Z.; Lu, W.; Corke, H. Soybean lecithin-stabilized oil-in-water (O/W) emulsions increase the stability and in vitro bioaccessibility of bioactive nutrients. Food Chem. 2021, 338, 128071. [Google Scholar] [CrossRef]
- Chung, C.; Sher, A.; Rousset, P.; Decker, E.A.; McClements, D.J. Formulation of food emulsions using natural emulsifiers: Utilization of quillaja saponin and soy lecithin to fabricate liquid coffee whiteners. J. Food Eng. 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Zaldivar, G.; Perez Sirkin, Y.A.; Debais, G.; Fiora, M.; Missoni, L.L.; Gonzalez Solveyra, E.; Tagliazucchi, M. Molecular theory: A tool for predicting the outcome of self-assembly of polymers, manoparticles, amphiphiles, and other soft materials. ACS Omega 2022, 7, 38109–38121. [Google Scholar] [CrossRef] [PubMed]
- Grillo, I.; Morfin, I.; Prévost, S. Structural characterization of Pluronic micelles swollen with perfume molecules. Langmuir 2018, 34, 13395–13408. [Google Scholar] [CrossRef] [PubMed]
- Pagola, S.; Benavente, A.; Raschi, A.R.; Molina, M.A.A.; Stephens, P.W. Crystal structure determination of thymoquinone by high-resolution X-ray powder diffraction. Aaps Pharmscitech 2004, 5, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Efficient encapsulation of citral in fast-dissolving polymer-free electrospun nanofibers of cyclodextrin inclusion complexes: High thermal stability, longer shelf-life, and enhanced water solubility of citral. Nanomaterials 2018, 8, 793. [Google Scholar] [CrossRef]
- Senseman, S.A. Herbicide Handbook, 9th ed.; Weed Science Society of America: Champaign, IL, USA, 2007; 458p. [Google Scholar]
- Chaimovitsh, D.; Rogovoy, O.; Altshuler, O.; Belausov, E.; Abu-Abied, M.; Rubin, B.; Sadot, E.; Dudai, N. The relative effect of citral on mitotic microtubules in wheat roots and BY2 cells. Plant Biol. 2012, 14, 354–364. [Google Scholar] [CrossRef]
- Graña Martinez, E. Mode of Action and Herbicide Potential of the Terpenoids Farnesene and Citral on “Arabidopsis thaliana” Metabolism. 2015. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=124599 (accessed on 12 June 2024).
- Herrera, J.M.; Zygadlo, J.A.; Strumia, M.C.; Peralta, E. Biopesticidal silo bag prepared by co-extrusion process. Food Packag. Shelf Life 2021, 28, 100645. [Google Scholar] [CrossRef]
- Govindjee. Chlorophyll a fluorescence: A bit of basics and history. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 1–48. [Google Scholar]
- Slabbert, R.M.; Krüger, G.H. Assessment of changes in photosystem II structure and function as affected by water deficit in Amaranthus hypochondriacus L. and Amaranthus hybridus L. Plant Physiol. Biochem. 2011, 49, 978–984. [Google Scholar] [CrossRef]
- Pino, M.T. Estrés hídrico y térmico en papas, avances y protocolos. Santiago, Chile. Instituto de Investigaciones Agropecuarias; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2016; Boletín INIA N º 331; p. 148. Available online: https://biblioteca.inia.cl/items/7cbd1f1a-aeb4-47a9-b3a5-e432821ad95f (accessed on 12 June 2024).
- Cai, Y.Z.; Corke, H.; Wu, H.X. Amaranth. In Encyclopedia of Grain Science; The University of Hong Kong: Hong Kong, 2004; pp. 1–10. [Google Scholar] [CrossRef]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus essential oils in weeds of Mediterranean summer crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- González Moreno, S.; Perales, V.; Salcedo Alvarez, H.; Martha, O. La fluorescencia de la clorofila a como herramienta en la investigación de efectos tóxicos en el aparato fotosintético de plantas y algas. Rev. Educ. Bioquímica 2008, 27, 119–129. [Google Scholar]
- Amri, I.; Hamrouni, L.; Hanana, M.; Jamoussi, B. Reviews on phytotoxic effects of essential oils and their individual components: News approach for weed management. Int. J. Appl. Biol. Pharm. Technol. 2013, 4, 96–114. [Google Scholar]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A Review Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Synowiec, A.; Możdżeń, K.; Krajewska, A.; Landi, M.; Araniti, F. Carum carvi L. essential oil: A promising candidate for botanical herbicide against Echinochloa crus-galli (L.) P. Beauv. in maize cultivation. Ind. Crops Prod. 2019, 140, 111652. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic effects of commercial essential oils on selected vegetable crops: Cucumber and tomato. Sustain. Chem. Pharm. 2020, 15, 100209. [Google Scholar] [CrossRef]


| Chemical Structures | Boiling Point (°C) | Log P | Solubility in Water at 25 °C (g/L) |
|---|---|---|---|
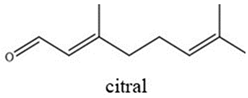 | 225 | 3.17 | 0.08 |
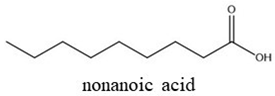 | 254 | 3.43 | 0.21 |
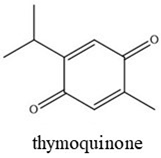 | 230–232 | 2.33 | 0.87 |
| Treatments | A. hybridus | B. rapa | L. multiflorum | L. sativa | S. lycopersicum |
|---|---|---|---|---|---|
| IC50 (mM) 1 (95% Confidence Interval) | |||||
| F1 | 0.07 (0.03–0.09) a | 1.5 (1.3–1.7) a | 0.24 (0.09–0.319) a | 2.54 (2.21–2.9) a | 1.33 (0.89–1.63) a |
| F2 | 0.07 (0.04–0.08) a | 2.3 (1.7–2.9) b | 0.14 (0.08–0.27) a | 4.60 (3.36–10.00) b | 0.99 (0.37–1.38) a |
| Atrazine | 41.0 (25.1–54.1) c | 300 (189.1–368.8) c | 38.9 (25.1–56.1) c | 383.5 (307.1–454.8) c | 37.5 (15.3–49.1) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, J.J.; Soler-Arango, J.; Coustet, M.E.; Moracci, D.B.; Reinoso, S.; Yanniccari, M.E.; Schneider-Teixeira, A.; Herrera, J.M. Herbicidal Formulations with Plant-Based Compounds to Control Amaranthus hybridus, Lolium multiflorum, and Brassica rapa Weeds. Plants 2025, 14, 276. https://doi.org/10.3390/plants14020276
Romero JJ, Soler-Arango J, Coustet ME, Moracci DB, Reinoso S, Yanniccari ME, Schneider-Teixeira A, Herrera JM. Herbicidal Formulations with Plant-Based Compounds to Control Amaranthus hybridus, Lolium multiflorum, and Brassica rapa Weeds. Plants. 2025; 14(2):276. https://doi.org/10.3390/plants14020276
Chicago/Turabian StyleRomero, Juan J., Juliana Soler-Arango, Marcos E. Coustet, Daniela B. Moracci, Sebastián Reinoso, Marcos E. Yanniccari, Aline Schneider-Teixeira, and Jimena M. Herrera. 2025. "Herbicidal Formulations with Plant-Based Compounds to Control Amaranthus hybridus, Lolium multiflorum, and Brassica rapa Weeds" Plants 14, no. 2: 276. https://doi.org/10.3390/plants14020276
APA StyleRomero, J. J., Soler-Arango, J., Coustet, M. E., Moracci, D. B., Reinoso, S., Yanniccari, M. E., Schneider-Teixeira, A., & Herrera, J. M. (2025). Herbicidal Formulations with Plant-Based Compounds to Control Amaranthus hybridus, Lolium multiflorum, and Brassica rapa Weeds. Plants, 14(2), 276. https://doi.org/10.3390/plants14020276








