Exploring Sesquiterpene Lactones from Saussurea lappa: Isolation, Structural Modifications, and Herbicide Bioassay Evaluation
Abstract
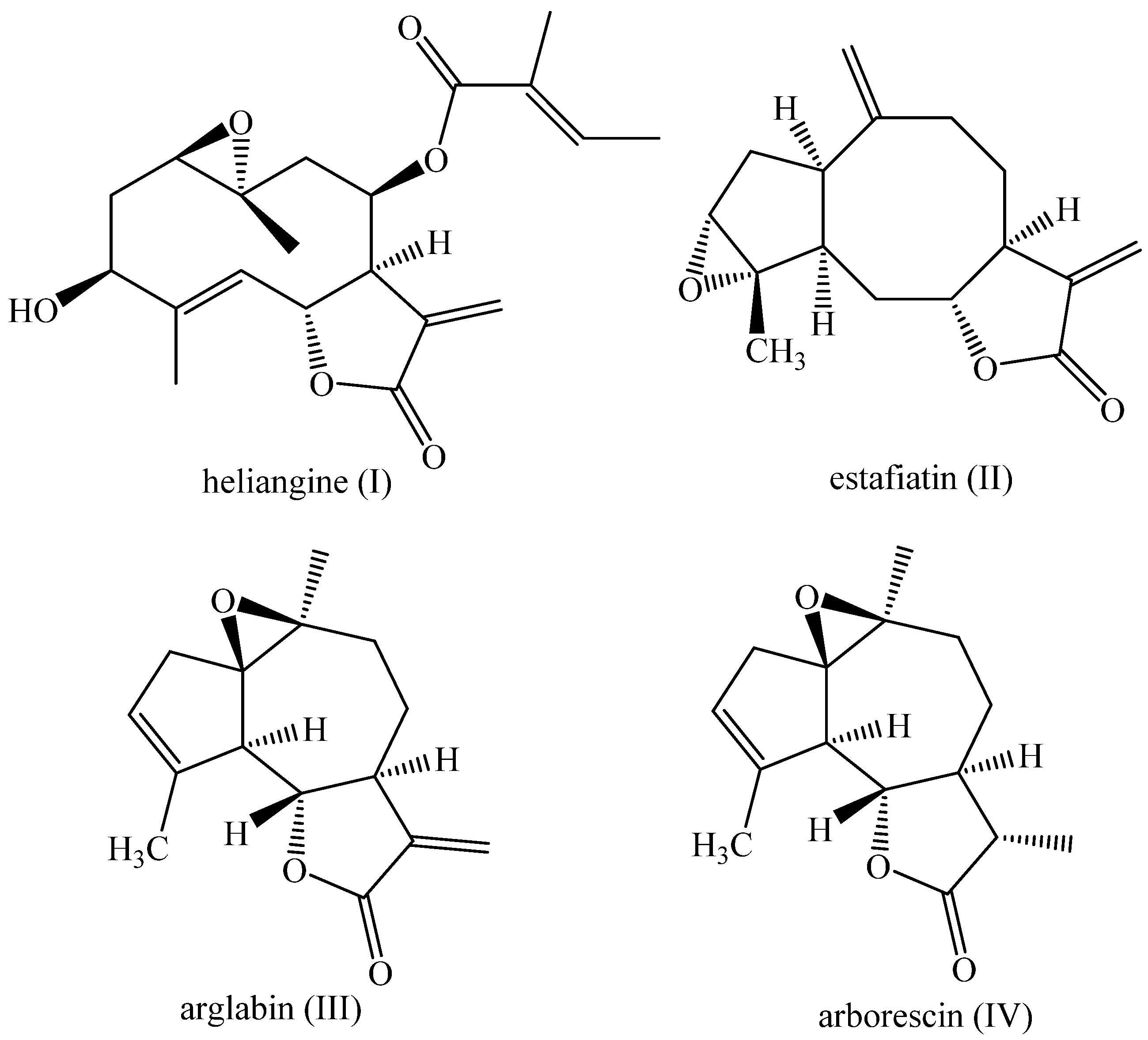
1. Introduction
2. Results and Discussion
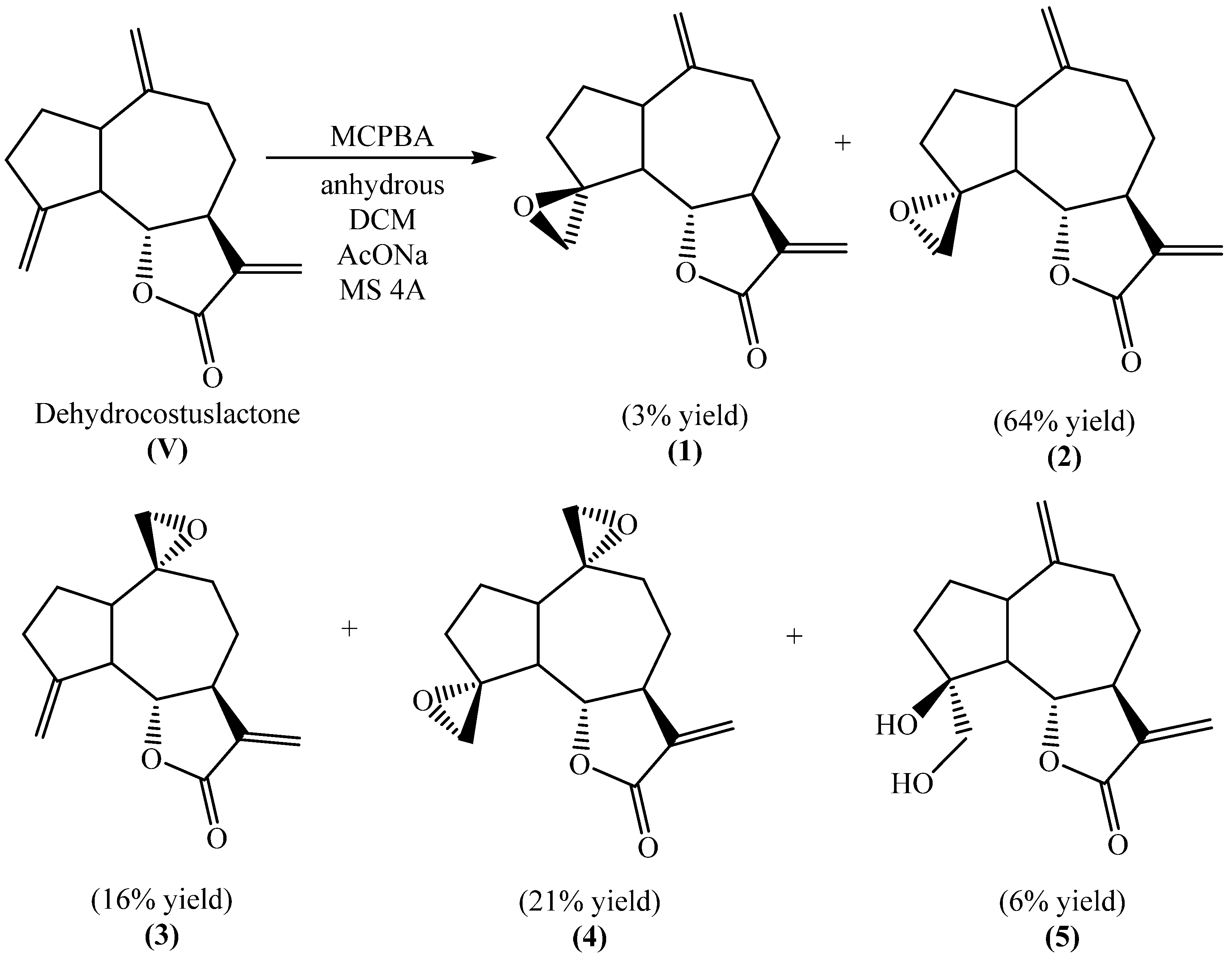
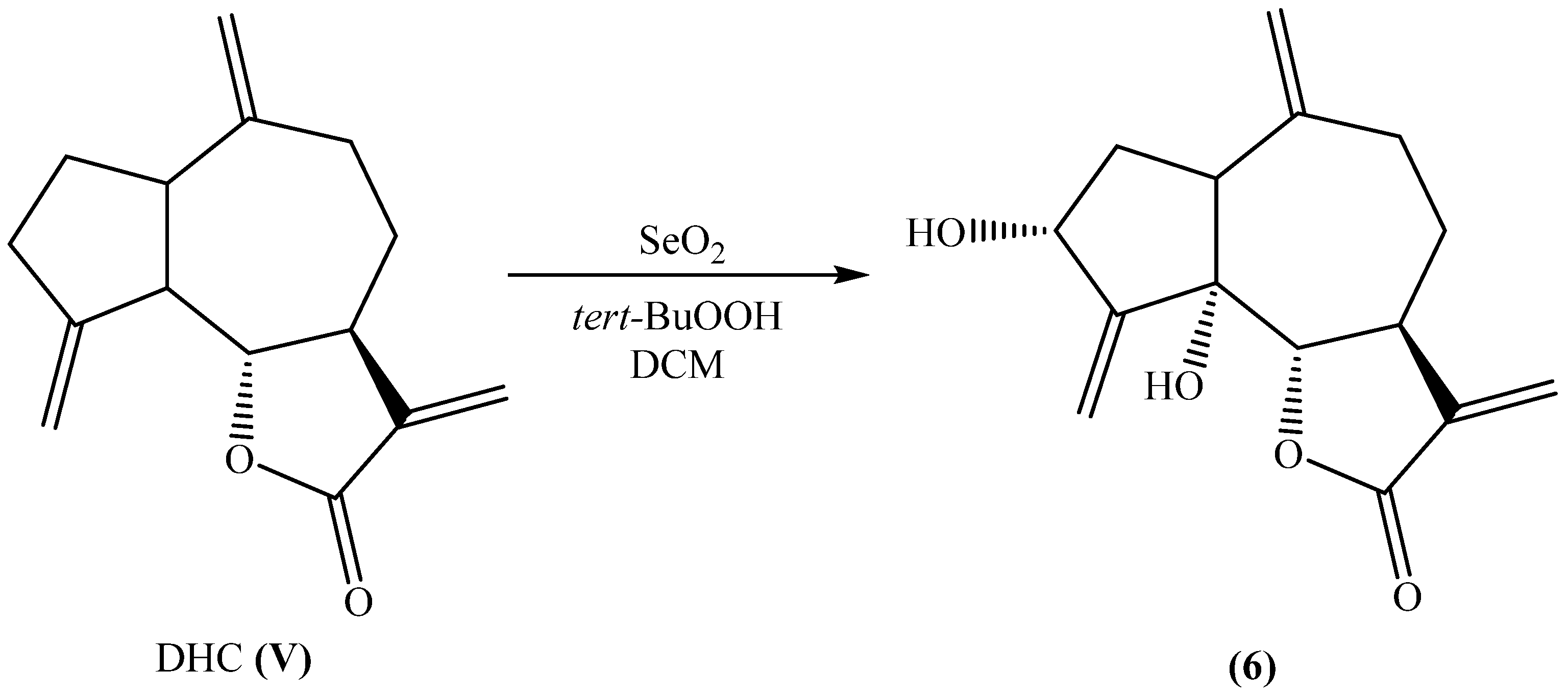
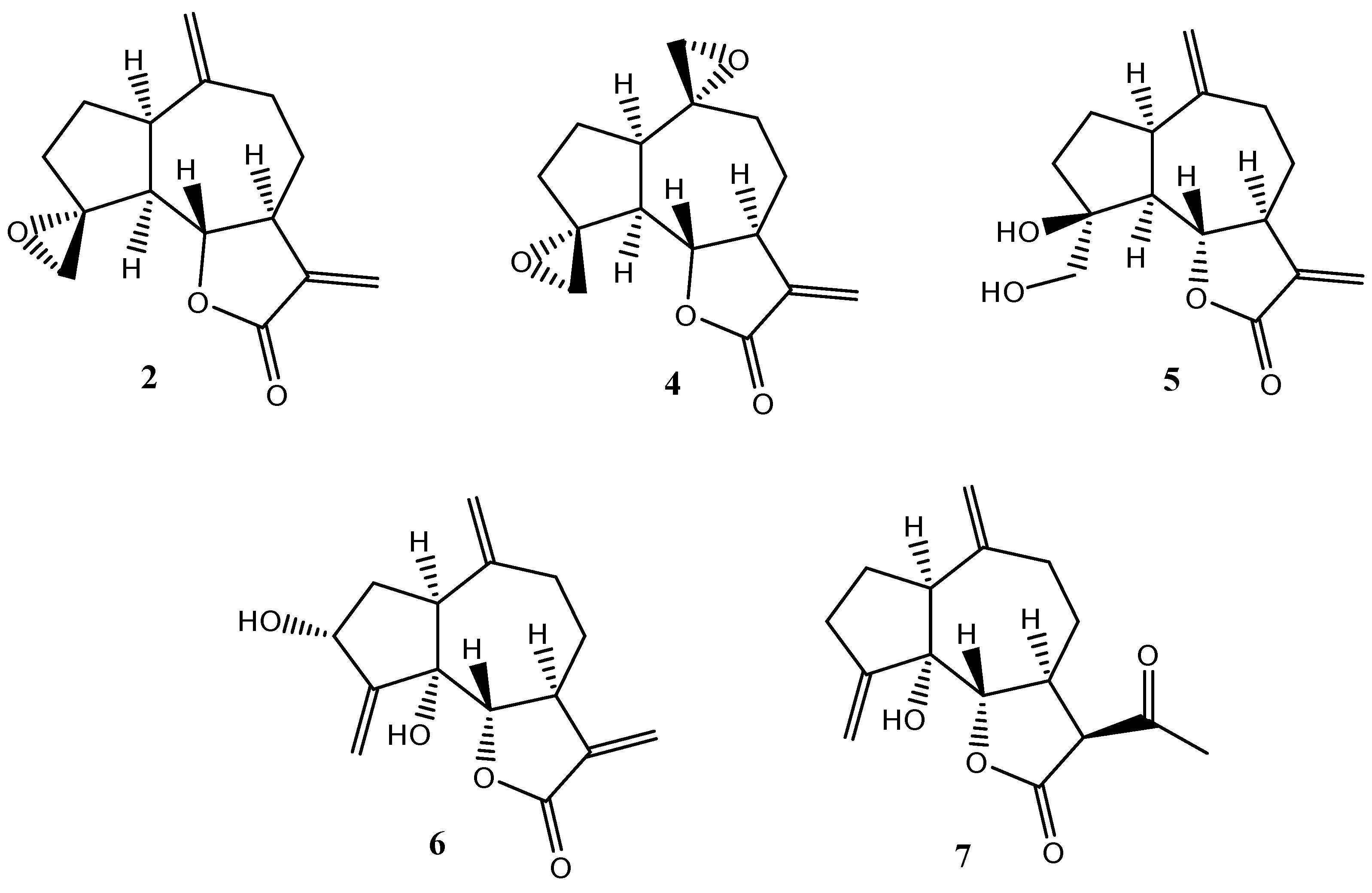
2.1. Synthesis
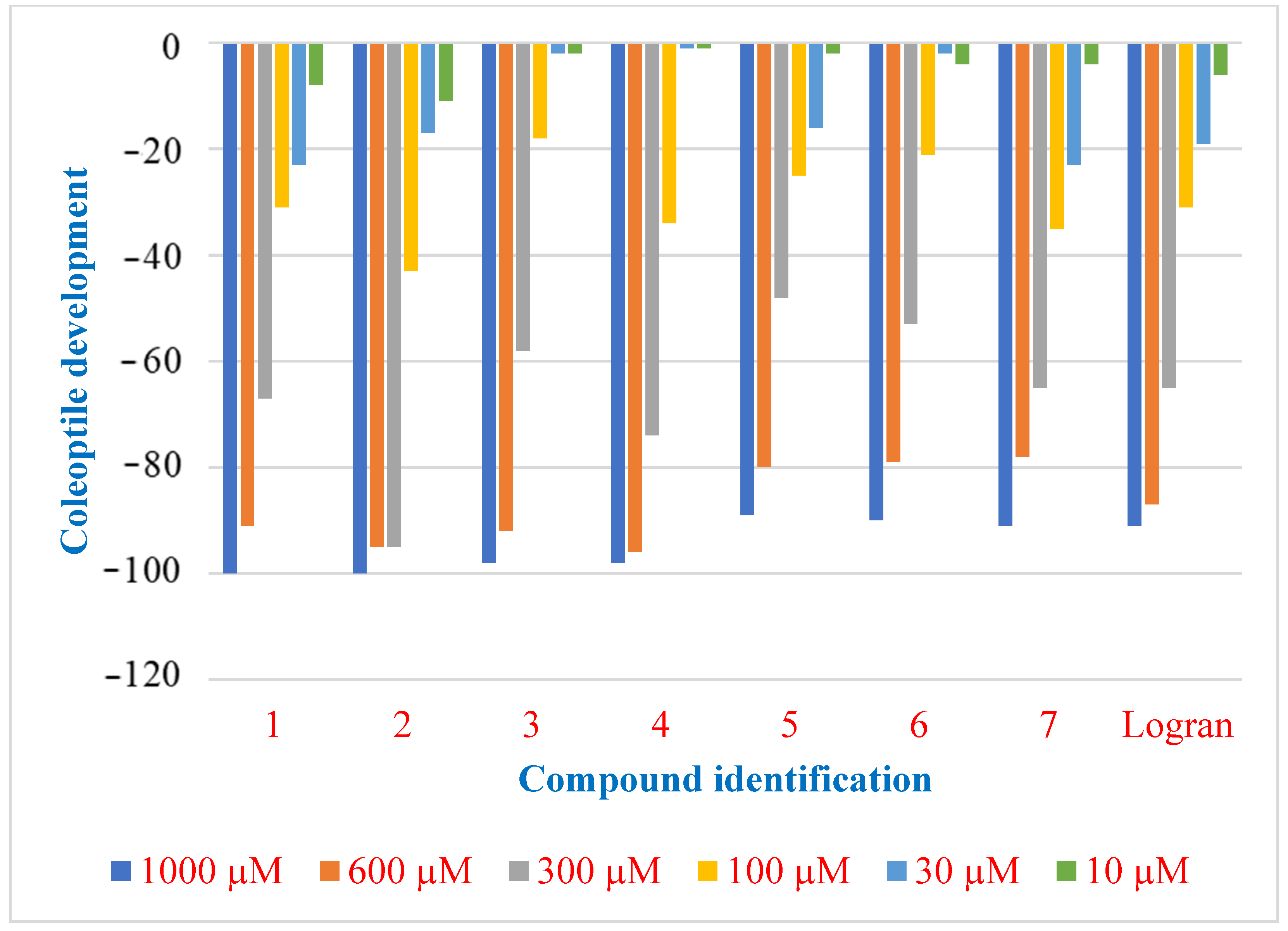
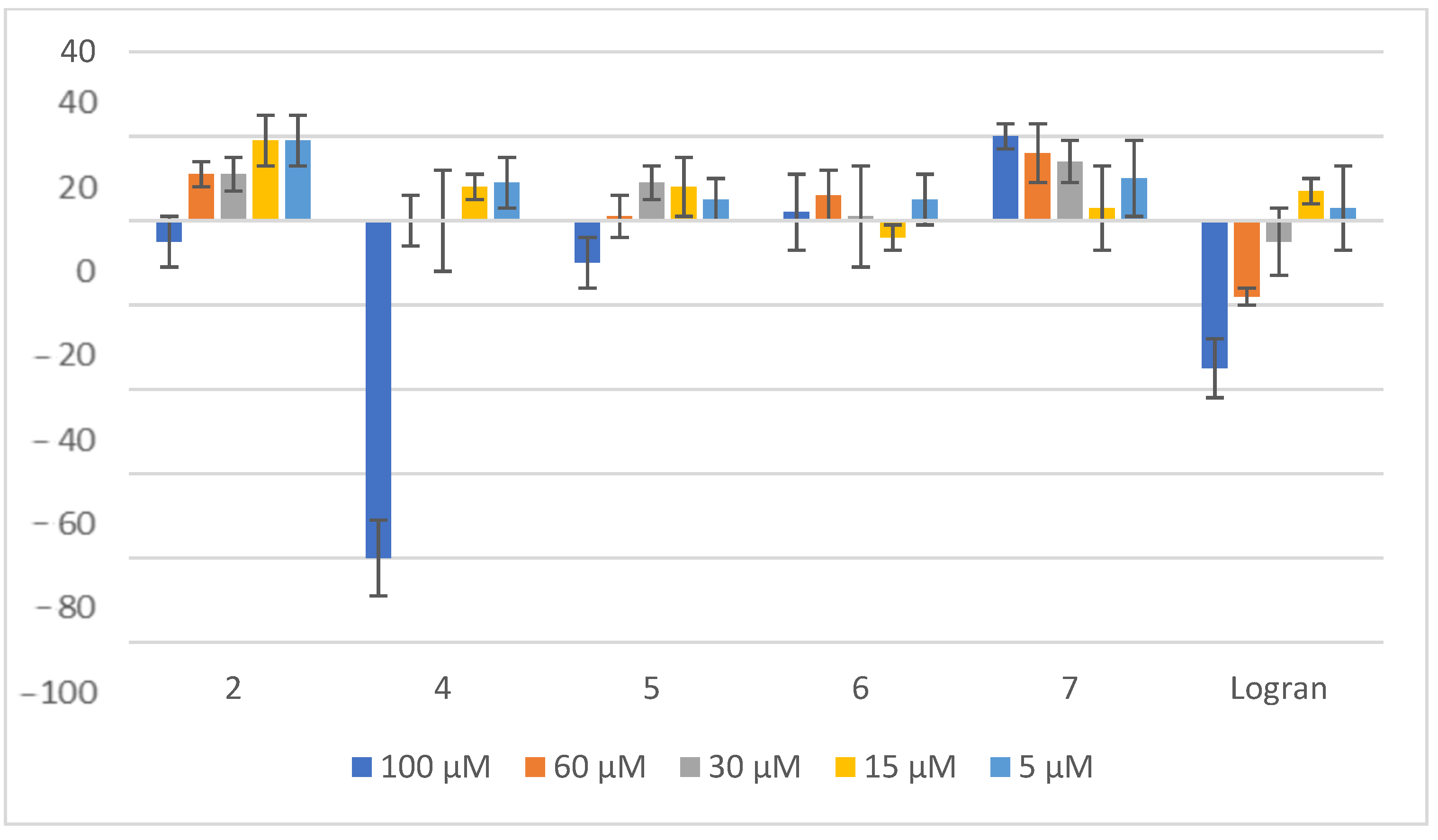
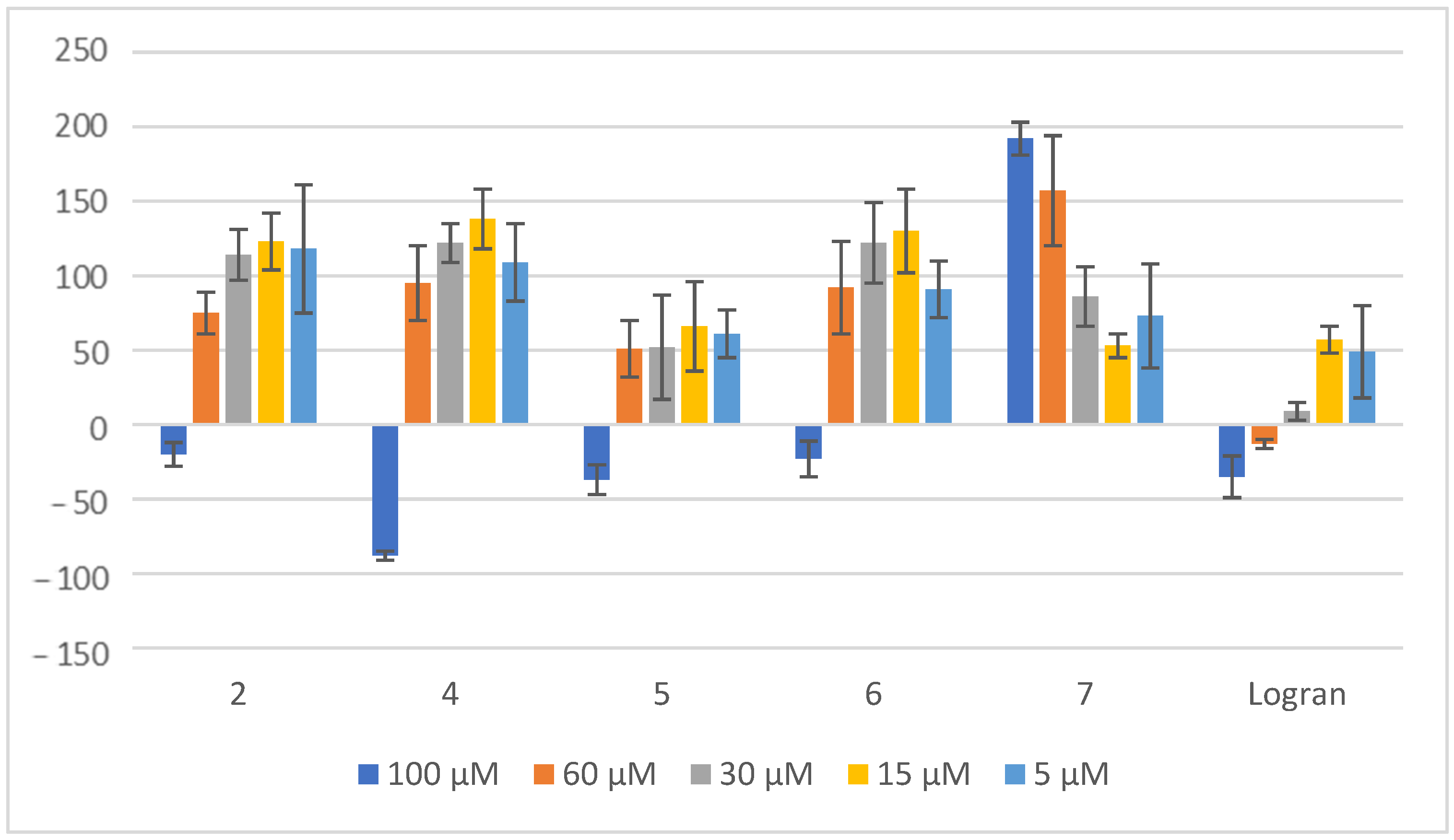
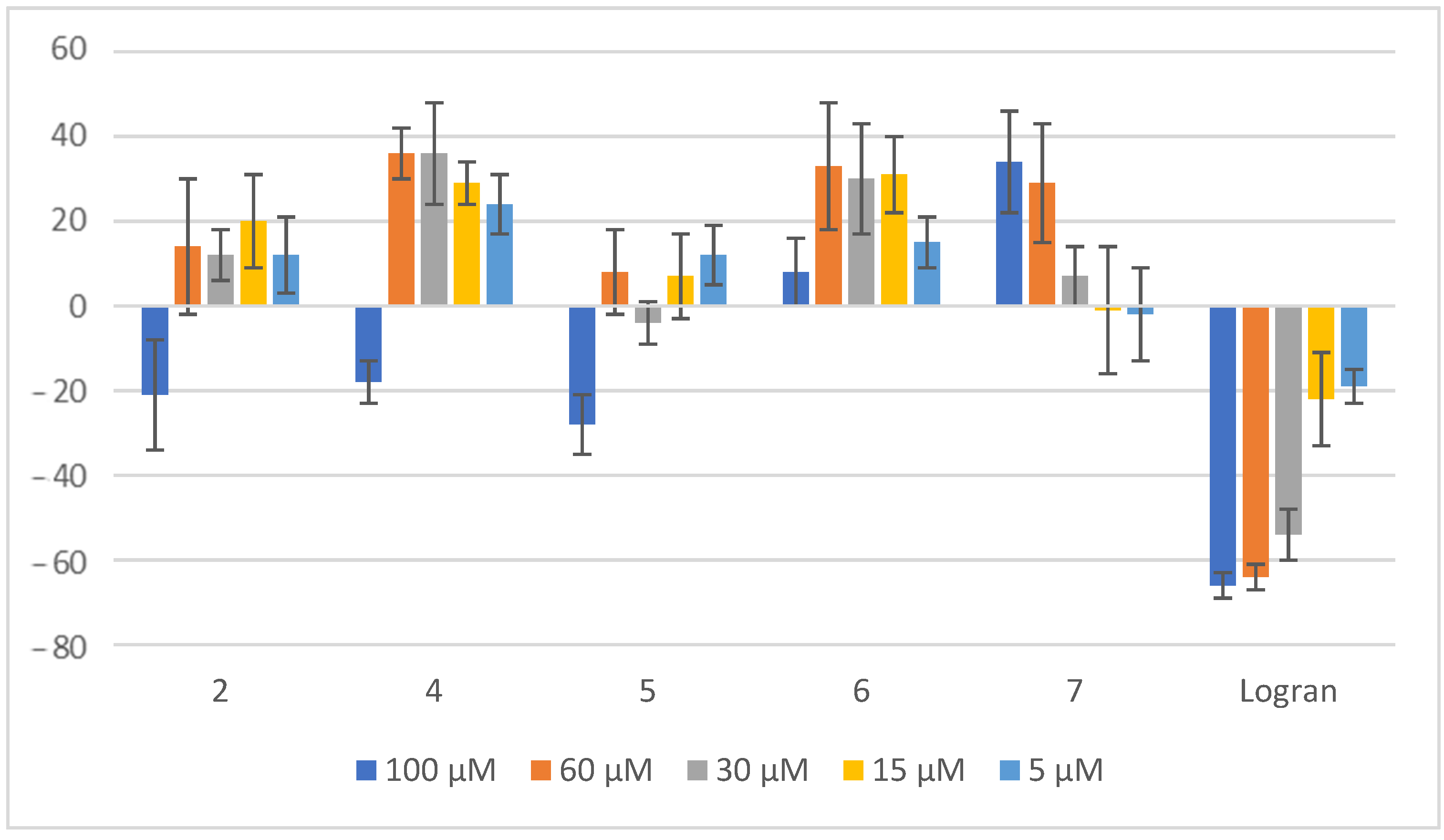
2.2. Coleoptile Bioactivity
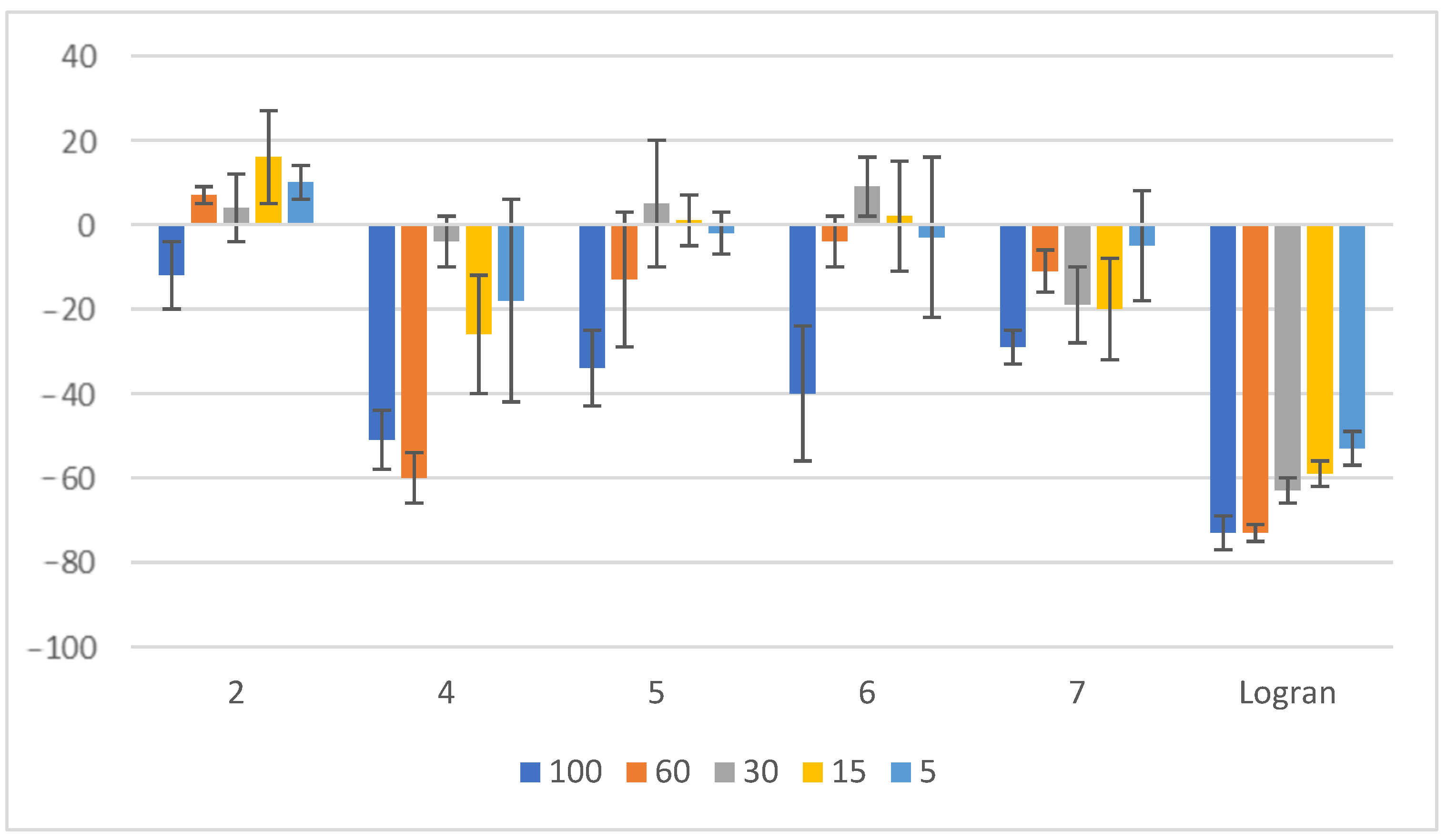
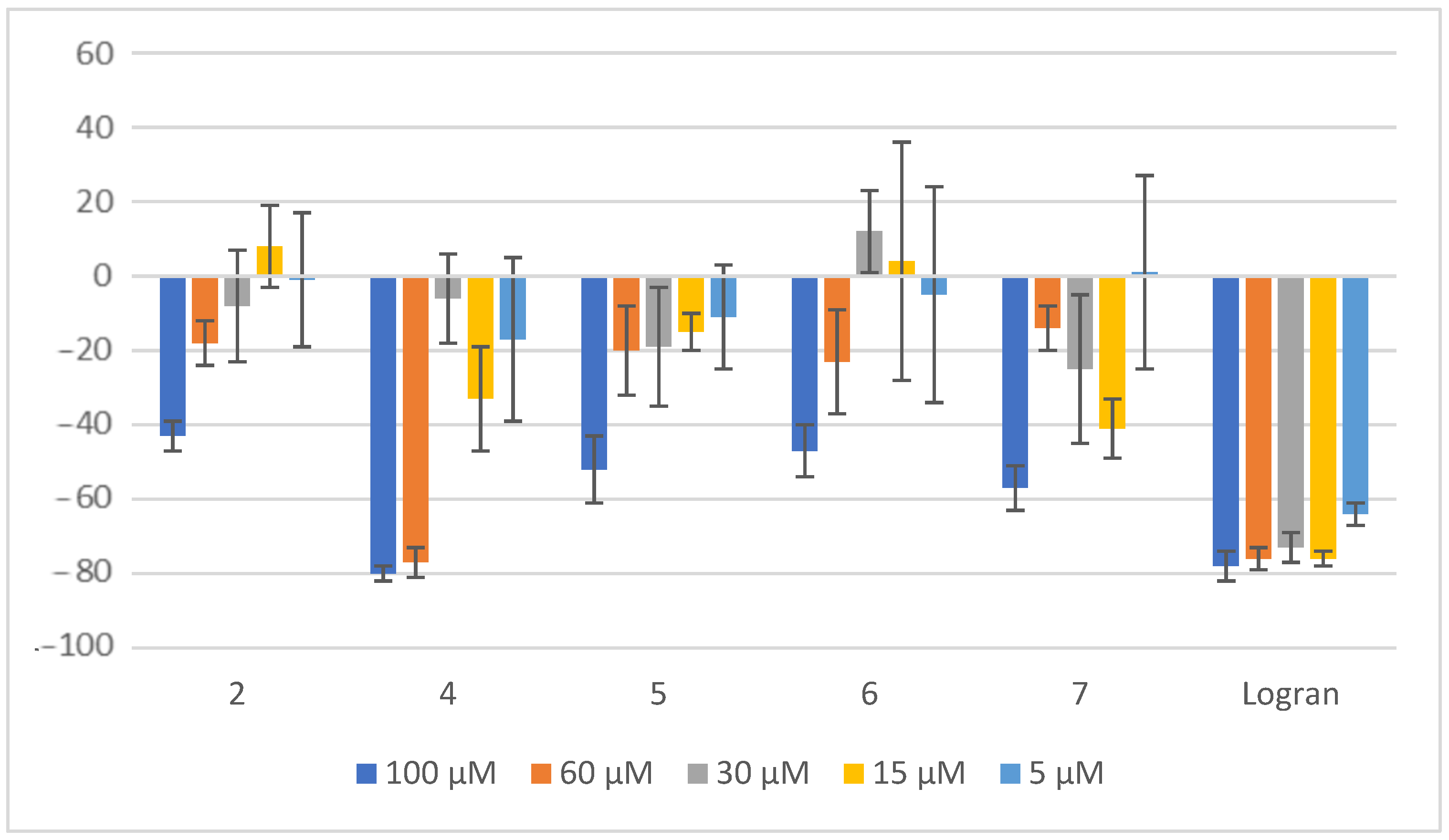
2.3. Herbicide Activity
3. Materials and Methods
3.1. Synthetic Procedures
3.2. Coleoptile Bioassay
3.3. Seed Germination Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cárdenas, D.M.; Bajsa-Hirschel, J.; Cantrell, C.L.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Evaluation of the Phytotoxic and Antifungal Activity of C17-Sesquiterpenoids as Potential Biopesticides. Pest Manag. Sci. 2022, 78, 4240–4251. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.A.; Santos, R.T.D.S.; Bacha, A.L.; Rodrigues, J.D.S.; Alves, P.L.d.C.A.; Kuva, M.A. Estudo de Seleção Da Comunidade Infestante Por Herbicidas Utilizando Técnicas de Análise Multivariada. Rev. Bras. De Herbic. 2020, 19, 1–13. [Google Scholar] [CrossRef]
- He, B.; Hu, Y.; Wang, W.; Yan, W.; Ye, Y. The Progress towards Novel Herbicide Modes of Action and Targeted Herbicide Development. Agronomy 2022, 12, 2792. [Google Scholar] [CrossRef]
- Qu, R.; He, B.; Yang, J.; Lin, H.; Yang, W.; Wu, Q.; Li, Q.X.; Yang, G. Where Are the New Herbicides? Pest Manag. Sci. 2021, 77, 2620–2625. [Google Scholar] [CrossRef]
- Fraga, B.M.; Díaz, C.E.; Bailén, M.; González-Coloma, A. Sesquiterpene Lactones from Artemisia Absinthium. Biotransformation and Rearrangement of the Insect Antifeedant 3α-Hydroxypelenolide. Plants 2021, 10, 891. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Impact of Natural Products on Discovery of, and Innovation in, Crop Protection Compounds. Pest Manag. Sci. 2022, 78, 399–408. [Google Scholar] [CrossRef]
- Cárdenas, D.; Rial, C.; Varela, R.; Molinillo, J.; Macías, F. Synthesis of Pertyolides A, B, and C: A Synthetic Procedure to C 17 -Sesquiterpenoids and a Study of Their Phytotoxic Activity. J. Nat. Prod. 2021, 84, 2295–2302. [Google Scholar] [CrossRef]
- Zhou, C.; Luo, X.; Chen, N.; Zhang, L.; Gao, J. C–P Natural Products as Next-Generation Herbicides: Chemistry and Biology of Glufosinate. J. Agric. Food Chem. 2020, 68, 3344–3353. [Google Scholar] [CrossRef]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef]
- Brás, T.; Neves, L.A.; Crespo, J.G.; Duarte, M.F. Advances in Sesquiterpene Lactones Extraction. TrAC Trends Anal. Chem. 2023, 158, 116838. [Google Scholar] [CrossRef]
- Seregheti, T.M.Q.; Pinto, A.P.R.; Gonçalves, M.d.C.; Antunes, A.S.; Almeida, W.A.d.S.; Machado, R.S.; Silva, J.N.; Ferreira, P.M.P.; Pessoa, C.; Dos Santos, V.M.R.; et al. Antiproliferative and Photoprotective Activities of the Extracts and Compounds from Calea Fruticosa. Braz. J. Med. Biol. Res. 2020, 53, 1–8. [Google Scholar] [CrossRef]
- Neverova, N.A.; Zhabayeva, A.N.; Levchuk, A.A.; Babkin, V.A.; Beisenbaev, A.R.; Larina, L.I.; Sapozhnikov, A.N.; Adekenov, C.M. A Study of the Physicochemical Properties of Mechanically Treated Arglabin and Its Mechanocomposites Based on Arabinogalactan. Russ. J. Bioorg. Chem. 2020, 46, 1358–1363. [Google Scholar] [CrossRef]
- Colasurdo, D.D.; Arancibia, L.A.; Naspi, M.L.; Laurella, S.L. Using DP4+ Probability for Structure Elucidation of Sesquiterpenic Lactones: The Case of (−)-Istanbulin A. J. Phys. Org. Chem. 2022, 35, e4282. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Q.; Wang, H.; Shi, L.; Wang, G.; Zhao, Y.; Fan, C.; Si, J. Sesquiterpenes and Sesquiterpene Derivatives from Ferula: Their Chemical Structures, Biosynthetic Pathways, and Biological Properties. Antioxidants 2024, 13, 7. [Google Scholar]
- Moraes, F.C.; Alvarenga, E.S.; Amorim, K.B.; Demuner, A.J.; Pereira-Flores, M.E. Novel Platensimycin Derivatives with Herbicidal Activity. Pest Manag. Sci. 2016, 72, 580–584. [Google Scholar] [CrossRef]
- Kadhila, N.P.; Sekhoacha, M.; Tselanyane, M.; Chinsembu, K.C.; Molefe-Khamanga, D.M. Determination of the Antiplasmodial Activity, Cytotoxicity and Active Compound of Pechuel-Loeschea Leubnitziae O. Hoffm. (Asteraceae) of Namibia. SN Appl. Sci. 2020, 2, 1328. [Google Scholar] [CrossRef]
- Agatha, O.; Mutwil-Anderwald, D.; Tan, J.Y.; Mutwil, M. Plant Sesquiterpene Lactones. Phil. Trans. R. Soc. B 2024, 379, 20230350. [Google Scholar] [CrossRef]
- Kovács, B.; Hohmann, J.; Csupor-Löffler, B.; Kiss, T.; Csupor, D. A Comprehensive Phytochemical and Pharmacological Review on Sesquiterpenes from the Genus Ambrosia. Heliyon 2022, 8, e09884. [Google Scholar]
- Wu, W.; Huang, H.; Su, J.; Yun, X.; Zhang, Y.; Wei, S.; Huang, Z.; Zhang, C.; Bai, Q. Dynamics of Germination Stimulants Dehydrocostus Lactone and Costunolide in the Root Exudates and Extracts of Sunflower. Plant Signal. Behav. 2022, 17, 2025669. [Google Scholar] [CrossRef]
- Laber, L.; Eichberg, C.; Zimmerbeutel, A.; Düring, R.A.; Donath, T.W. Effects of Macrocyclic Lactone Anthelmintics on Seed Germination of Temperate Grassland Species. Plant Biol. 2023, 25, 1046–1057. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Cárdenas, D.M.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Masi, M.; Macías, F.A. Bioprospection of Phytotoxic Plant-Derived Eudesmanolides and Guaianolides for the Control of Amaranthus Viridis, Echinochloa Crus-Galli, and Lolium Perenne Weeds. J. Agric. Food Chem. 2024, 72, 1797–1810. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy Is Pervasive in Invasive Plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- McCoy, R.M.; Widhalm, J.R.; McNickle, G.G. Allelopathy as an Evolutionary Game. Plant Direct 2022, 6, e382. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Mitchell, P.T.; Kishkentaeva, A.S.; Shaimerdenova, Z.R.; Atazhanova, G.A.; Adekenov, S.M.; Quinn, M.T. The Natural Sesquiterpene Lactones Arglabin, Grosheimin, Agracin, Parthenolide, and Estafiatin Inhibit T Cell Receptor (TCR) Activation. Phytochemistry 2018, 146, 36–46. [Google Scholar] [CrossRef]
- Csuk, R.; Heinold, A.; Siewert, B.; Schwarz, S.; Barthel, A.; Kluge, R.; Ströhl, D. Synthesis and Biological Evaluation of Antitumor-Active Arglabin Derivatives. Arch. Pharm. 2012, 345, 215–222. [Google Scholar] [CrossRef]
- Salin, A.V.; Shabanov, A.A.; Khayarov, K.R.; Islamov, D.R.; Voloshina, A.D.; Amerhanova, S.K.; Lyubina, A.P. Phosphine-Catalyzed Synthesis and Cytotoxic Evaluation of Michael Adducts of the Sesquiterpene Lactone Arglabin. ChemMedChem 2024, 19, e202400045. [Google Scholar] [CrossRef]
- Huo, J.; Yang, S.P.; Ding, J.; Yue, J.M. Cytotoxic Sesquiterpene Lactones from Eupatorium Lindleyanum. J. Nat. Prod. 2004, 67, 1470–1475. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Bryce, D.J. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; ISBN 9780470616376. [Google Scholar]
- Hernández-Guadarrama, A.; Díaz-Román, M.A.; Linzaga-Elizalde, I.; Domínguez-Mendoza, B.E.; Aguilar-Guadarrama, A.B. In Silico Analysis: Anti-Inflammatory and α-Glucosidase Inhibitory Activity of New α-Methylene-γ-Lactams. Molecules 2024, 29, 1973. [Google Scholar] [CrossRef]
- Van Vu, Q.; Sayama, S.; Ando, M.; Kataoka, T. Sesquiterpene Lactones Containing an α-Methylene-γ-Lactone Moiety Selectively Down-Regulate the Expression of Tumor Necrosis Factor Receptor 1 by Promoting Its Ectodomain Shedding in Human Lung Adenocarcinoma A549 Cells. Molecules 2024, 29, 1866. [Google Scholar] [CrossRef]
- Cala, A.; Zorrilla, J.G.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; MacÍas, F.A. Easy Access to Alkoxy, Amino, Carbamoyl, Hydroxy, and Thiol Derivatives of Sesquiterpene Lactones and Evaluation of Their Bioactivity on Parasitic Weeds. J. Agric. Food Chem. 2019, 67, 10764–10773. [Google Scholar] [CrossRef]
- Bruno, M.; Rosselli, S.; Maggio, A.; Raccuglia, R.A.; Bastow, K.F.; Lee, K.H. Cytotoxic Activity of Some Natural and Synthetic Guaianolides. J. Nat. Prod. 2005, 68, 1042–1046. [Google Scholar] [CrossRef]
- Wu, F.; Gao, Y.; Yang, W.; Sui, N.; Zhu, J. Biological Functions of Strigolactones and Their Crosstalk With Other Phytohormones. Front. Plant Sci. 2022, 13, 821563. [Google Scholar]
- Mansoor, S.; Mir, M.A.; Karunathilake, E.M.B.M.; Rasool, A.; Ştefănescu, D.M.; Chung, Y.S.; Sun, H.J. Strigolactones as Promising Biomolecule for Oxidative Stress Management: A Comprehensive Review. Plant Physiol. Biochem. 2024, 206, 108282. [Google Scholar]
- Kapoor, R.T.; Alam, P.; Chen, Y.; Ahmad, P. Strigolactones in Plants: From Development to Abiotic Stress Management. J. Plant Growth Regul. 2024, 43, 903–919. [Google Scholar] [CrossRef]
- Jamil, M.; Alagoz, Y.; Wang, J.Y.; Chen, G.T.E.; Berqdar, L.; Kharbatia, N.M.; Moreno, J.C.; Kuijer, H.N.J.; Al-Babili, S. Abscisic Acid Inhibits Germination of Striga Seeds and Is Released by Them Likely as a Rhizospheric Signal Supporting Host Infestation. Plant J. 2024, 117, 1305–1316. [Google Scholar] [CrossRef]
- Cao, K.; Qian, W.; Xu, Y.; Zhou, Z. Purification of Sesquiterpenes from Saussurea Lappa Roots by High Speed Counter Current Chromatography. Iran. J. Pharm. Res. 2019, 18, 1499–1507. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Li, Y.; Han, X.; Gao, K.; Fang, J. Bioassay-Guided Isolation of Dehydrocostus Lactone from Saussurea Lappa: A New Targeted Cytosolic Thioredoxin Reductase Anticancer Agent. Arch. Biochem. Biophys. 2016, 607, 20–26. [Google Scholar] [CrossRef]
- Mori, K.; Matsui, M.; Sumiki, Y. Biochemical Studies on “Bakanae” Fungus. Part 55 Synthesis of Gibberellins: Part II. Infrared Spectra of the Lactones of Cyclohexane Series. Agric. Biol. Chem. 1961, 25, 205–222. [Google Scholar] [CrossRef]
- Blank, D.E.; Demuner, A.J.; Baía, V.C.; Alvarenga, E.S.; Cerceau, C.I.; de Moura, N.F. Structural Elucidation of a Lactam Isolated from Bunchosia Glandulifera and Antioxidant Activity. Peer Rev. 2023, 5, 1–14. [Google Scholar] [CrossRef]
- Kolmer, A.; Edwards, L.J.; Kuprov, I.; Thiele, C.M. Conformational Analysis of Small Organic Molecules Using NOE and RDC Data: A Discussion of Strychnine and α-Methylene-γ-Butyrolactone. J. Magn. Reson. 2015, 261, 101–109. [Google Scholar] [CrossRef]
- Macías, F.A.; Alvarenga, E.S.; Galindo, J.C.G.; Molinillo, J.M.G.; Cerceau, C.I. Microwave-Assisted Rearrangement of Costunolide Catalyzed by Palladium(II). J. Braz. Chem. Soc. 2024, 35. [Google Scholar] [CrossRef]
- Cala, A.; Salcedo, J.R.; Torres, A.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. A Study on the Phytotoxic Potential of the Seasoning Herb Marjoram (Origanum majorana L.) Leaves. Molecules 2021, 26, 3356. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.G.; Calle, J.M.; Butrón, D.; Pérez, A.J.; Macías, F.A.; Simonet, A.M. Steroidal Saponins with Plant Growth Stimulation Effects; Yucca Schidigera as a Commercial Source. Plants 2022, 11, 3378. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mejías, F.J.; Mottaghipisheh, J.; Schwaiger, S.; Kiss, T.; Csupor, D.; Varela, R.M.; Macías, F.A. Allelopathic Studies with Furanocoumarins Isolated from Ducrosia Anethifolia. In Vitro and in Silico Investigations to Protect Legumes, Rice and Grain Crops. Phytochemistry 2023, 215, 113838. [Google Scholar] [CrossRef]
- Sangi, D.P.; Meira, Y.G.; Moreira, N.M.; Lopes, T.A.; Leite, M.P.; Pereira-Flores, M.E.; Alvarenga, E.S. Benzoxazoles as Novel Herbicidal Agents. Pest Manag. Sci. 2019, 75, 262–269. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Alvarenga, E.S.; Lopes, D.T.; Oliveira, D.F. Herbicidal Activity of Isobenzofuranones and in Silico Identification of Their Enzyme Target. Pest Manag. Sci. 2019, 75, 3331–3339. [Google Scholar] [CrossRef]
- Macías, F.A.; Galindo, J.C.G.; Massanet, G.M. Potential Allelopathic Activity of Several Sesquiterpene Lactone Models. Phytochemistry 1992, 31, 1969–1977. [Google Scholar] [CrossRef]
- Macías, F.A.; García-Díaz, M.D.; Pérez-de-Luque, A.; Rubiales, D.; Galindo, J.C.G. New Chemical Clues for Broomrape-Sunflower Host−Parasite Interactions: Synthesis of Guaianestrigolactones. J. Agric. Food Chem. 2009, 57, 5853–5864. [Google Scholar] [CrossRef]
- Elakbawy, W.M.; Shanab, S.M.M.; Shalaby, E.A. Enhancement of Plant Growth Regulators Production from Microalgae Cultivated in Treated Sewage Wastewater (TSW). BMC Plant Biol. 2022, 22, 377. [Google Scholar] [CrossRef]
- Macías, F.A.; Simonet, A.M.; Galindo, J.C.G. Bioactive Steroids and Triterpenes from Melilotus Messanensis and Their Allelopathic Potential. J. Chem. Ecol. 1997, 23, 1781–1803. [Google Scholar] [CrossRef]

| δH | Hydrogen | COSY | δC | Carbon |
|---|---|---|---|---|
| 0.82–0.72 | 8′ | 8 × 8′, 9 × 8′,7 × 8′ | 30.7 | 8 |
| 1.60–1.46 | 2,8,9′ | 9′ × 14, 7 × 8, 8′ × 8, 9′ × 9, 1 × 2 | 30.4 | 2 |
| 1.98 | 9 | 8′, 9′, 7, 14 | 32.8 | 3 |
| 2.11 | 7 | 6 × 7,7 × 8, 7 × 8′, 7 × 13, 7 × 13′ | 36.4 | 9 |
| 2.41–2.20 | 1,3,3′,5 (m) | 1 × 2, 1 × 2′, 3 × 2, 3 × 2′, 1 × 14 | 44.7 | 7 |
| 3.48 | 6 (t, J = 8 Hz) | 6 × 7, 5 × 6 | 47.5 | 5 |
| 4.60 | 14,14′ (m) | 1 × 14, 9 × 14, 9′ × 14 | 52.1 | 1 |
| 4.87 | 13′ (d, J = 4 Hz) | 13′ × 7, 13 × 13′ | 84.4 | 6 |
| 5.07 | 15′ (m) | 15′ × 5, 15′ × 3, 15′ × 3′ | 109.7 | 15 |
| 5.46 | 15 (m) | 15 × 5, 15 × 3, 15 × 3′ | 112.0 | 14 |
| 6.11 | 13 (d, J = 4 Hz) | 13 × 7, 13 × 13′ | 118.8 | 13 |
| 140.7 | 4 | |||
| 149.6 | 10 | |||
| 151.4 | 11 | |||
| 169.2 | C=O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarenga, E.S.; Macías, F.A.; Ferreira, S.S.; Galindo, J.C.G.; Molinillo, J.M.G. Exploring Sesquiterpene Lactones from Saussurea lappa: Isolation, Structural Modifications, and Herbicide Bioassay Evaluation. Plants 2025, 14, 1111. https://doi.org/10.3390/plants14071111
Alvarenga ES, Macías FA, Ferreira SS, Galindo JCG, Molinillo JMG. Exploring Sesquiterpene Lactones from Saussurea lappa: Isolation, Structural Modifications, and Herbicide Bioassay Evaluation. Plants. 2025; 14(7):1111. https://doi.org/10.3390/plants14071111
Chicago/Turabian StyleAlvarenga, Elson S., Francisco A. Macías, Stephani S. Ferreira, Juan C. G. Galindo, and José M. G. Molinillo. 2025. "Exploring Sesquiterpene Lactones from Saussurea lappa: Isolation, Structural Modifications, and Herbicide Bioassay Evaluation" Plants 14, no. 7: 1111. https://doi.org/10.3390/plants14071111
APA StyleAlvarenga, E. S., Macías, F. A., Ferreira, S. S., Galindo, J. C. G., & Molinillo, J. M. G. (2025). Exploring Sesquiterpene Lactones from Saussurea lappa: Isolation, Structural Modifications, and Herbicide Bioassay Evaluation. Plants, 14(7), 1111. https://doi.org/10.3390/plants14071111








