Abstract
In Santiago, Chile, urban plants are highly vulnerable to drought or climate change. We hypothesize that would find high growth and survival rates in conditions of water scarcity among native species of central Chile. The goal was to determine the effect of the year season and an irrigation gradient on the survival and growth of native plant, in order to evaluate potential plant for use in urban green areas of central Chile. Four plots of 20 m2 were located in the Santiago center. In June 2024 twelve species were planted and from November 2024 to March 2025 were irrigated with 13.3, 10.1, 1.7 and 1.4 L/m2/day. The GLM and Kaplan–Meier survival analyses were used. Shoot growth rate was highly variable among species, among irrigation treatments applied, and among year seasons. Eight species showed water-related growth and shoot growth during the winter was very small and higher in spring. Two species showed evidence of water-related survival; in the other 10 species, no significant differences were found between irrigation treatments. Winter was the season with the highest survival rates for eleven species. In conclusion, the results suggest that native plants can achieve high survival rates with limited irrigation. This highlights their potential for use in the urban area in Mediterranean-type climates where rainfall is expected to be low due to climate change.
1. Introduction
In Santiago, Chile, urban plants are highly vulnerable to drought, as they are mainly exotic from temperate origins. However, experimental studies seeking evidence of potential native species in central Chile that could replace and develop the current urban green infrastructure are limited in number.
Urban vegetation is known to improve air quality [1], reduce summer heat island temperatures, lower cooling costs [2], reduce storm-water runoff [3,4], reduce atmospheric carbon dioxide [5], contribute to the conservation of biodiversity [6], and improve various social and individual development indicators among residents [7,8,9,10,11]. The average opinion of urban populations on urban vegetation is very positive, with particular value placed on shade, aesthetics, air quality and noise reduction [12].
The lack of vegetation in central Chilean cities (for example, 5.7% of Santiago’s population has access to >9 m2 green space/inhabitant) is accentuated by the unsustainable design of urban areas [13]. This is characterized by high maintenance and irrigation requirements and is driven by the selection of plant species that are not adapted to the urban conditions of the region (e.g., soil, atmosphere, water and nutrients). Urban vegetation in cities in central Chile is also deteriorated and stressed by climate change, along with adverse growing conditions including compacted soil, extreme heat, lack of nutrients, drought, damage from cars, pruning, and vandalism [14]. Therefore, any plan for the development or replacement of urban vegetation in the region should thus be based on experimental evidence in real urban areas, at least considering the survival and growth of potential urban plants. Unfortunately, experimental studies with native species are not common in urban areas located in the Mediterranean-type climate region of central Chile.
Vegetation survival and growth in an urban setting depends on planting location, installation, and post-planting care, between others [15,16,17,18,19]. For example, the most common environmental conditions influencing urban tree mortality are related to water stress, nutrient deficiency, and soil compaction [20,21]. Plant species that successfully colonize and persist in public areas would likely possess traits for stress-tolerance or avoidance. In seasonally dry ecosystems, such as those of central Chile, water is the limiting factor for survival and vegetation growth [22]. In order to design urban areas that support urban biodiversity for future generations, it is important to understand which species persist and how plant communities change over time.
Because urban tree care can be inconsistent during and after planting, it is important to choose plant species that are likely to survive and grow well with minimal additional care [23]. Published studies have primarily focused on the survival and growth of urban trees, and most research on urban tree success originates from experiments conducted in relatively controlled nursery settings rather than in environments exposed to urban conditions. However, urban herbs and shrubs can contribute to the diversity and functionality of urban ecosystems. Their survival and growth are influenced by the urban habitat.
A study of this characteristic has important implications for urban species selection in a changing climate. Cities located in Mediterranean-type climates are particularly vulnerable to climate change [24]. Mainly are predicted to face reduced annual precipitation, temperature increase, and intensified rainfall events [25]. In Santiago, Chile, urban trees are highly vulnerable to drought because they are mainly exotic species that are not adapted to water scarcity [26]. This forces municipalities to use potable water for irrigation [24]. Assessing the tolerance or resistance of potential urban plants to climate change helps architects and urban landscape designers select plants suited to cities facing water scarcity [27].
In this study, we estimated the survival and growth rates of seedlings over 10 consecutive months through seasonal monitoring of 12 potential native species of central Chile for use in urban green infrastructure in Santiago. The analyses used the cumulative survival rate and growth index of the shoots per season for each species tested. These indicators are widely used in demography and population ecology.
Although species generally differ in their response to water restriction, we hypothesize that would find high survival rates among native species in central Chile during the first year of the trial in an urban area. The main objective of the study was to determine the effect of the year season and an irrigation gradient on the survival and growth of native species in order to evaluate potential plant for use in urban green infrastructure in Mediterranean-type climate cities in central Chile.
2. Results
2.1. Shoot Growth
Shoot growth varied among species (Figure 1a). The species with the highest average growth during all the study periods were the shrubs Baccharis linearis (16.7 ± 1.8 cm), Andeimalva chilensis (14.3 ± 1.1 cm), Sphaeralcea obtusiloba (12.0 ± 1.6 cm), and the tree Vachelia caven (13.0 ± 1.8 cm). At the opposite end of the spectrum, the woody Cistanthe laxiflora (0.6 ± 1.3) and the succulent herbs Puya alpestri (2.9 ± 1.8 cm) and P. coerulea (2.0 ± 1.9 cm) showed the lowest average growth of the species studied during the study period. The average growth of woody species (9.5 ± 0.6 cm) during the study period was higher than that of non-woody species (3.3 ± 0.8 cm), with a significant difference between both (F = 37.98; p < 0.001).
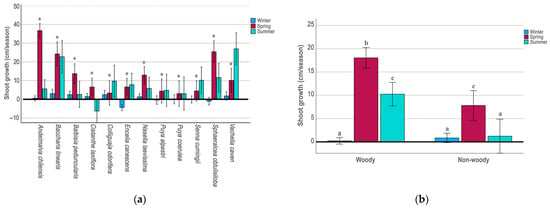
Figure 1.
(a) Shoot yield index for winter, spring, and summer seasons in 12 native plant species from central Chile. Bars represent ±1 E.D. * indicate statistically significant differences among seasonal for each species assessed according to Bonferroni test (p ≤ 0.05); (b) shoot yield index for winter, spring, and summer seasons for woody and non-woody species combined. Bars represent ±1 E.D. Different letters (a–c) above the bars indicate statistically significant differences among year seasonal according to Bonferroni test (p ≤ 0.05).
Eight species showed evidence of water-sensitive growth (Table 1). In all this species, individuals subjected to a higher irrigation volume tended to show greater shoot average growth. Overall, the combined water-sensitive growth species in the treatment with higher irrigation (average 9.9 cm throughout the test) was >50% higher than the combined water-sensitive growth species in the treatment with lower irrigation (average 3.3 cm throughout the test).

Table 1.
Shoot growth index (±1 E.D.) and survival rate (final %) for each irrigation treatment applied to the 12 species of native plants from central Chile tested in the experimental plots. N = replicates per experimental plot. Responses: Highly sensitive = Growth and survival with significant p-Value; Sensitive = Growth or survival with significant p-Value. Non-sensitive = Growth and survival with non-significant p-Value. Different letters indicate significant differences between treatments (p < 0.05) Note: * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; n.s. = p > 0.05.
In particular, the woody Cistanthe laxiflora (−3.2 ± 1.4 cm), the herb Nasella laevissima (0.9 ± 1.2 cm), the succulents P. alpestri (1.3 ± 1.1 cm) and P. coerulea (0.02 ± 1.1 cm) showed very low average growth in the lowest irrigation volume treatment (Table 1). In fact, C. laxiflora and S. cumingii stand out because they were the species that showed negative growth in response to the lowest water treatments of 1.4 L/m2/day and 1.7 L/m2/day, respectively (Table 1).
In the other 4 tested, Baccharis linearis, Balbisia peduncularis, Encelia canescens, and Sphaeralcea obtusiloba, did not exhibit water-sensitive growth (Table 1).
In a seasonal analysis, shoot growth was highly variable among year seasons in all the species tested (Table 2). In the 12 species, shoot growth during the winter season was very small, it was even negative in E. canescens (F = 12.25; p ≤ 0.001), and in S. obtusiloba (F = 19.65; p ≤ 0.001) although without differences among treatments in both species (Table 2). However, spring was the most productive season, except for the tree V. caven (Figure 1a), which grew faster in summer (F = 20.31; p < 0.001). In contrast, growth in C. laxiflora decreased significantly and was negative during the summer (F = 10.09; p < 0.01).

Table 2.
Generalized linear model of repeated measures results showing the effect Intra-subject, contrast Intra-subject and of the four irrigation treatments on the growth index by performing Inter-subject tests. N = replicates per experimental plot. TIME = Season. TREAT = Treatment. Note: * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; n.s. = p > 0.05.
2.2. Species Survival
The final cumulative survival rate for the 12 species combined averaged at 85.9%, and 10 showed a cumulative final survival rate of at least 85.4%. At one extreme, B. linearis, P. alpestri, and V. caven had a final cumulative survival rate of 100% (Figure 2a). At the other extreme, the species A. chilensis and E. canescens achieved a final cumulative survival of 75.0% and 59.6%, respectively. Even E. canescens achieved a statistical survival rate lower than A. chilensis (χ2 = 4.56; p = 0.033).
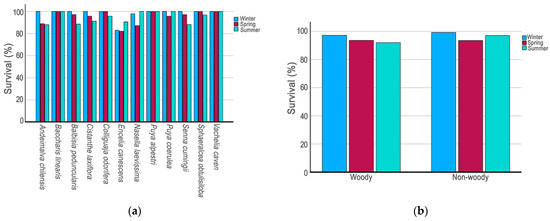
Figure 2.
(a) Survival percentage during for winter, spring, and summer seasons in 12 native plant species from central Chile; (b) survival percentage for winter, spring, and summer seasons for woody and non-woody species combined.
In a seasonal analysis, the 12 species showed survival rates above 80% in each season monitored (Figure 2a). Winter was the season with the highest survival rates for all species except E. canescens. Ten species showed 100% survival during winter (Figure 2a). The species C. odorífera, P. coerulea, and S. obtusiloba achieved a final survival rate of 100% in at least two seasons (Figure 2a). Four species had a final survival rate of 100% during the summer: B. linearis, P. alpestri, P. coerulea, and V. caven.
Globally, the combined non-woody species demonstrated higher survival rates during both the winter and summer months, while the combined woody species exhibited higher survival rates exclusively during the winter period (Figure 2b).
In conclusion, only the species C. laxiflora and N. laevisima showed evidence of water-sensitive survival (Table 1). In the other 10 species tested, no significant differences were found between irrigation treatments.
3. Discussion
Vegetation in public areas of cities has emerged as a key component of nature-based solutions in socio-ecologically stressed urban environments, although the biogeographical origin of its components has not been sufficiently emphasized in central Chile [28,29]. Several studies of Mediterranean-type climate regions around the globe suggest that native species should be preferred because they contribute more to ecosystem functioning and the ecological integrity of urban environments, and provide a greater number of ecosystem services [30,31,32,33,34]. Additionally, it could be crucial to select native species not only for their ecological features but also having evolved in particular habitats characterized by summer drought stress similar to those present in the urban area of central Chile [35]. This further inclusion could greatly expand the number of plants potentially suitable for growing in cities with Mediterranean-type climates. The present study fits within this conceptual framework.
In regions with a Mediterranean-type climate, methodologies have been designed to identify potential native species that are not used in various urban vegetation contexts. These methodologies use bibliographic information on the species ecology, distribution, and performance in their natural conditions [36]. However, it should not be forgotten that experimental evidence should also be gathered in the contexts of diverse urban habitats [37].
One set of evidence comes from assessing the condition and health of historic vegetation in different areas of the city [38]. A second set comes from managing and monitoring vegetation from the moment an urban green area is implemented, even if it was not considered an experimental design from the outset [39]. This study is closer to the second group, as it sought potential native species to be used in public areas in experimental urban contexts in a central Chile city. Although published information on native species from central Chile was used for an initial selection of potential species (see Table 3), the plants were then subjected to an experimental procedure to evaluate their performance in real urban conditions.

Table 3.
Plant species from central Chile selected for the study, indicating family affiliation, life form, and environmental, aesthetic, cultural, and management criteria considered in the potential landscape value of the species for use in urban vegetation in central Chile. Information obtained in [40,41,42,43].
The results of this study showed that the irrigation gradient tested did not affect survival in 83% of the potential native species selected for use in urban areas of central Chile. The result is consistent with studies of Mediterranean species in natural conditions, where survival differences due to irrigation are not always observed, probably because these species have low water requirements [44,45,46]. In fact, the results show that overall final cumulative survival is greater than 80%, with the exception of the E. canescens shrub. It should be noted that the first year is a bottleneck for urban plant survival, due to the stress suffered by individuals during their transfer and transplantation, including the effects of environmental pollution [18,47]. Even, the results even showed that survival was high after summer water stress.
On the other hand, the results showed that the shoot growth of about 66% of the studied species was significantly affected by the irrigation gradient, mainly in the summer. However, only two species had their survival rates affected by the applied irrigation gradient. Furthermore, overall species growth practically stopped in winter, although survival rates were high. On the other hand, the highest shoot growth in woody and non-woody species was in the spring season. Consequently, the research shows that the species studied exhibit temporal variation in their water behavior patterns consistent with species from Mediterranean-type climates, characterized in central Chile by a winter influenced by the polar jet stream, numerous frosts with snow at higher elevations, an extended period of summer drought, and high interannual variability and temperatures [48]. However, survival was not significantly affected in both seasons. According to the literature, cold ocean currents on the west coast of Mediterranean-type climate regions moderate temperatures, allowing survival and plant growth in the late winter and early spring [49].
The results of this study showed that only the herb N. laevissima and the shrub C. laxiflora presented highly water-sensitive responses, as both growth and survival were significantly affected (Table 1). In fact, growth was negative in C. laxiflora and zero in N. laevissima, and in both species, survival was lower in the treatment with less water availability (Table 1 and Table 2). Both species concentrated their shoot growth during the spring, and the results even show that C. laxiflora suffered significant self-thinning during the summer. ROS (Reactive Oxygen Species) analyses, which were carried out in parallel with this study, indicate potential oxidative stress in the herb N. laevissima in midsummer for low irrigation treatments [50]. The environmental stress suffered by this species could explain its low abundance in natural conditions in the Metropolitan Region of central Chile [51] and its displacement by exotic annual plants that use water more efficiently [52]. On the contrary, the same ROS analysis shows the absence of oxidative stress in the C. laxiflora shrub in any of the treatments applied during the same period of the year, probably due to the decrease in both the transpiration surface area and water loss suffered by the species during this period of greater water stress [53]. However, C. laxiflora survival was negatively affected (Figure 2a), probably due to a decrease in the photosynthetic area caused by self-thinning during the summer.
The succulent herbs P. alpestri and P. coerulea stand out among the eight species of water-sensitive growth, as they have adapted their photosynthesis to avoid summer water stress [54]. Although the results showed that the species were water-sensitive growth, however survival was 100% in all treatments (Table 1). This pattern demonstrates the ability of both succulent species to colonize sites exposed to solar radiation (Table 3) and to avoid water stress in the summer [54]. Even during this season, H2O2 levels associated with water stress are normal in both succulents of this study [50]. However, during the winter, growth was zero in both species, probably indicating stress levels due to cold weather events and good resistance to the same phenomenon, as the average survival rate was close to 100%. A pattern very similar to that found in P. alpestri and P. coerulea can be seen in the shrubs S. cumingii, B. linearis, and in the tree V. caven. The plants of S. cumingii have deep roots that perform hydraulic lifting, allowing them to reach more humid soil layers and move water to dry surface layers, thus avoiding water stress during the summer [55,56]. However, we can also see that summer is the season with the highest mortality rate and the season with the highest growth rate in urban areas as well. With regard to V. caven, a dominant species of thorny shrubland [57] and represented in the native urban flora of central Chile [26], research shows high survival rates throughout the year, although growth has been limited under severe water restrictions. On the other hand, unlike S. cumingii, in the tree V. caven there is no differentiated use of water within the root zone, but rather constant activity to capture the water available at a depth of 100 cm [58]. The last representative of the species water-sensitive is B. linearis, which develops shallow roots. This shrub is suitable for colonizing barren soils and remediating soils contaminated by mining [59], making it an attractive candidate for rehabilitating urban soils with abundant fill.
Analysis of the results also detected a group of four potential species that were not water-sensitive to the treatments applied and showed high survival rates, with the exception of E. canescens (Table 1). This shrub from arid regions showed a very low survival rate during winter and spring (Figure 2a), which could not even be reverted with the increased water availability in the irrigation gradient applied (Table 1). Interestingly, the literature shows that E. canescens is a common species that is resistant to low water availability [60]. It is suggested that plants from more arid areas with similar behavior could benefit from the establishment of seedlings in shaded environments, although this hypothesis would need to be evaluated more precisely [23,61]. The ROS analysis for E. canescens was consistent with the results for survival and shoot growth in the water gradient (Table 1) because it showed potential oxidative stress during the summer in all treatments, and was even reinforced in the plot with low water availability. The low survival rate of E. canescens during winter is probably due to frost stress, which cannot be reversed and recovered from before the onset of summer water stress in plots that are completely exposed to solar radiation. In contrast, although the S. obtusiloba shrub also showed oxidative stress during the summer, which was intensified in the low irrigation treatment [50], its survival rate was high with growth not stopping under any water conditions. In this species, there is probably a mechanism associated with secondary metabolites such as proline, sugars, or antioxidants to prevent water stress, which should be evaluated in further studies.
The last water-insensitive species, the shrub B. peduncularis, reduces its water deficit after the application of more irrigation in the summer season. The reviewed literature estimates that B. peduncularis responds efficiently to increases in surface water availability and is therefore dependent on rainfall and surface soil moisture [62].
Our study evaluated both plant survival and growth, although the indices used incorporate the shoot and exclude root condition and growth. Regarding survival, the monitoring of woody species did not record the regrowth of shoots that were considered dead during previous monitoring. Although regrowth was observed in perennial herbaceous, it occurred only occasionally. Furthermore, given the high survival rate of the studied species, our results suggest that the used index has not underestimated plant survival. Unfortunately, we could not incorporate root growth into the study. This would have required disruptive testing incompatible with the nature of our bio-urban shelter project and the available resources. Further studies will be needed to assess how roots compensate for losses and gains in the shoot biomass during the plant growth period to ensure the long-term sustainability of urban species used by landscape architects.
On the other hand, these survival and growth patterns may not persist in the long term. For example, there is evidence that the competitive species sown on the roof gradually gave way to stress-tolerant species [16]. However, it should be noted that our species list mainly includes long-lived woody plants that will be planted in areas with intensive urban use. These areas often prevent the spontaneous recruitment of new plants. In contrast, extensive green roofs are installed on vacant unmanaged green roofs that require low maintenance and spontaneous colonization is accepted as design criteria [16,19].
These types of studies must consider the importance of both current environmental conditions and future climate scenarios in areas undergoing urbanization in central Chile. We selected a group of species that are not only found in the central Chilean Mediterranean-type climate region, but also in more arid areas in northern Chile. Biodiversity in Mediterranean climate areas is particularly susceptible to global change [58]. Urban vegetation proposals should not only improve the adaptability of urban green infrastructure in a changing environment but also contribute to the broader goals of biodiversity conservation in a particularly vulnerable and endangered Mediterranean-type region [63,64,65]. This study is of significant practical value due to its implications for urban planning in Chile.
4. Materials and Methods
4.1. Study Site
Santiago (33° S; 70° W; 550 m.a.s.l.), the Chilean capital, has a Mediterranean climate type, characterized by mild wet winters and warm dry summers (Figure 3) [45,66]. The Santiago metropolitan area currently has about 6.3 million people, with a population density of roughly 8497 inhabitants per Km2 [67]. Since the late 20th and early 21st centuries, urban growth has also spread to surrounding areas, mostly consisting of agricultural lands and smaller remnants of semi-natural vegetation [68,69,70]. Urban vegetation of Santiago is dominated by exotic flora, which represents more than 80% of the total urban flora [26]. Water scarcity in Santiago metropolitan area has been increasing in recent years, associated with a sustained decrease in rainfall and the effects of climate change [24]. We chose Santiago as a study site in central Chile due to the Bio-Urban Shelter project designed for public area in Santiago [71].
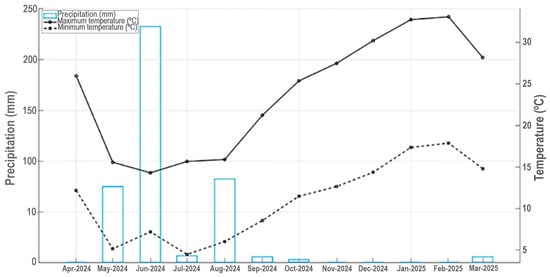
Figure 3.
Mean monthly maximum and minimum temperature and precipitation for Universidad Central de Chile, Santiago, for April 2024 to March 2025.
4.2. Species Selection
We used the landscape value method to select 12 potential native plant species from central Chile for use in urban vegetation in the same region (Figure 4) [36]. The method consists of four selection criteria: (a) environmental criteria, which are native species adapted to the environment where they will be used; (b) aesthetic criteria, morphological features that have aesthetic value for the landscape use of the species; (c) cultural criteria, which refer to the meanings and values of the use of the flora; and (d) management criteria, which is the ability of the species to respond to management and conditions in public area. Consideration was given to ensuring that the selected species were available in nurseries in the region as well, due to the potential demand for Bio-Urban Shelter in public areas of Santiago by local governments.

Figure 4.
The twelve native plant species from central Chile that were selected for the experimental study. 1. Andeimalva chilensis, 2. Baccharis linearis, 3. Balbisia peduncularis, 4. Cistanthe laxiflora, 5. Colliguaja odorífera, 6. Encelia canescens, 7. Nasella laevissima, 8. Puya alpestri, 9. Puya coerulea, 10. Senna cumingii, 11. Sphaeralcea obtusiloba and 12. Vachellia caven.
Consequently, the selected native plants are represented by trees, shrubs, and perennial herbs (Table 3), which are mostly common species of the thorny shrublands of central Chile and dominant species of a potential community within the urban perimeter of Santiago [72].
4.3. Experimental Design
The experimental plots are located in the historic center of the city of Santiago, Chile. To consider the environmental variation in Santiago, two university campus in the historic center were selected. Two experimental plots were set up on the campus of the Universidad Tecnológica Metropolitana (UTEM, 33.451071° S, 70.656622° W) and two similar plots at the Universidad Central de Chile (UCEN; 33.451360° S, 70.653433° W).
Each plot had an area of 20 m2. The soil in the plots was homogenized with a structure suitable for plant growth. Consequently, 60% of the existing soil in the top 150 cm of the plots was cleaned and loosened for recovery. The remaining 50% of the planting substrate consisted of 20% coarse sand, 15% gravel, and 5% compost, which increased the organic matter in the soil. A 5 cm layer of organic mulch was also added to the surface to prevent weed growth and excessive water loss from the soil. On average, soil pH = 7.8, nitrogen = 32 mg/kg, phosphorus = 67 mg/kg and potassium = 374 mg/kg. The 12 native species selected from central Chile, including woody and herbaceous perennials, were planted uniformly within each plot to reduce competition, cover spatial variation and avoid pseudo replicates (Figure 5a). The replicates per species are reported in Table 1. The spatial location and arrangement of the species was the same in the four experimental plots and responded to landscape and monitoring criteria, as the experiment is part of a project on bio-urban shelters in public area in Santiago city.
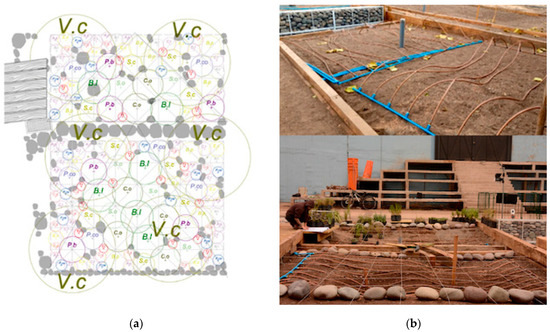
Figure 5.
(a) Planting plan for the four experimental plots. Two plots were located Universidad Central de Chile (UCEN) and another two Universidad Tecnológica Metropolitana (UTEM), in the historic center of the city of Santiago, central Chile. Vc = Vachelia caven, cl = Cistanthe laxiflora, nl = Nasella laevissima, Ac = Andeimalva chilensis, Co = Colliguaja odorífera, Pa = Puya alpestri, Pco = Puya coerulea, Senna cumingii, Bl = Baccharis linearis, Bp = Balbisia peduncularis, Ec = Encelia canescens, So = Sphaeralcea obtusiloba; (b) long-lasting, low-yield automated underground drip system with a pipe with a built-in emitter and Copper Shield technology.
Juvenile plants under two years old were obtained from certified nurseries and, after being purchased, were immediately planted in the plots. The soil was saturated with water to prevent stress and mortality when planting. The plants at UTEM were planted between 29 and 31 May 2024. At UCEN, they were planted between 18 and 20 June 2024. The experiment lasted from 29 June 2024 (the initial time) to 3 March 2025 (the final time). The irrigation system installed was a long-lasting, low-yield automated underground drip system with a pipe with a built-in emitter and Copper Shield technology (Figure 5b), which protects the dripper from root intrusion [73]. The hose has a diameter of 16 mm and a flow rate of 2.3 L/h (ESP, Rain Bird, Azusa, CA, USA). Irrigation is controlled automatically (X-Core, 601I, 230 VCA, Hunter, New York, NY, USA). The system was fed by the drinking water system. In each plot, the irrigation lines were buried at a depth of 30 cm and spaced 30.5 cm apart. In the center of the plots were installed soil humid and temperature sensors (Vantage Pro2 and Environmonitor, Sku 6440, Davis Instruments, Hayward, CA, USA). The soil water conditions and temperature for plots were studied (Figure 6a–c). Records demonstrated that water gradient provided by irrigation remained in the soil during the summer or February 2025 (Figure 6b).
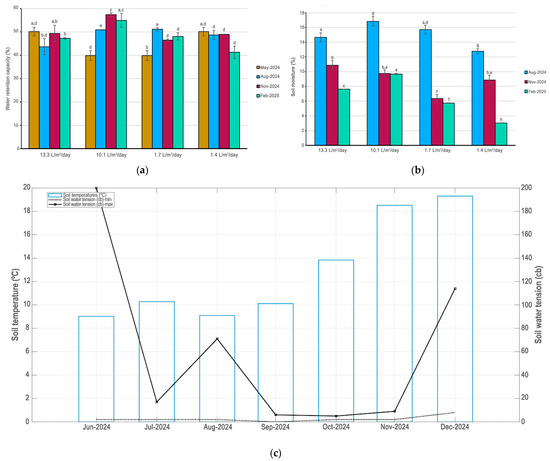
Figure 6.
(a) Water retention capacity of the soil. Bars represent ±1 E.D. Different letters indicate statistically significant differences among WRC assessed according to Bonferroni test (p ≤ 0.05); (b) soil moisture. Bars represent ±1 E.D. Different letters indicate statistically significant differences among soil moisture assessed according to Bonferroni test (p ≤ 0.05); (c) Mean monthly maximum and minimum soil water tension and temperature for Universidad Central de Chile campus, Santiago, for June 2024 to December 2024.
Each campus had one plot with high water availability for plants and another with low water availability with the aim of generating a water gradient in the experimental plots (Table 4). The 2 plots located at UCEN had an average daily irrigation regime of 13.3 L/m2/day and 1.4 L/m2/day. Meanwhile, the 2 plots located at UTEM were irrigated daily during the irrigation season with an average of 10.1 L/m2/day and 1.7 L/m2/day. Irrigation was maintained throughout the summer months on all four experimental plots, even after the experiment ended. Therefore, the plots were not watered between 29 June and 23 November 2024. The result was that each plot was subject to a different water regime during the irrigation period. Finally, the water gradient applied represents the recommended irrigation for public green areas in the Santiago Metropolitan Region at the upper end, and conditions considered to be water stress for urban plants at the lower end [74].

Table 4.
Initial and final irrigation dates for the experimental plots set up on the UCEN and UTEM campus. The plots are labeled according to the average daily amount of irrigation applied throughout the season. Irrigation began on 23 November 2024, when the soil reached 100 cb for the first time during the experiment. On 2 December 2024, the soil reached 200 cb for the first time. UCEN = Universidad Central de Chile; UTEM = Universidad Tecnológica Metropolitana.
4.4. Statistical Design
To evaluate survival in woody and herbaceous perennial species, plant shoots were visually monitored at the end of winter (13 September 2024), the end of spring (17 December 2024), and the end of summer (3 March 2024). If the plant shoot was completely dry, the individual was considered dead, considering that it could subsequently regrow at the base of the shoot and its condition could change to alive. To determine the effect of water treatment on species survival, Kaplan–Meier survival analyses were performed. Similarly, to determine the effect of water treatment on the survival of woody versus non-woody species in combination. This analysis is based on estimating the conditional probabilities of survival at each monitoring event or season (winter, spring, and summer) and considering the limit of the product of those probabilities to estimate the survival rate at each event.
The growth of each individual will be estimated using the following Growth Index:
GI = (A + B + C)/3
A = plant height; B = canopy length; C = canopy width.
The growth per individual for each season of the year was estimated by:
GIt1 − GIt0
t1 = end of the current year season,
t0 = immediately preceding year season.
The GI of each individual was monitored at the end of winter (14 September 2024), the end of spring (18 December 2024), and the end of summer (4 March 2025).
The GI was analyzed using a GLM of repeated measures. This analysis determines the effect of the four irrigation treatments on the GI by performing inter-subject tests. Bonferroni was used for comparisons between pairs of treatments. The data were transformed to log10 if they did not meet normality and homoscedasticity. The effect of the year season and its interaction with the treatments was determined using intra-subject effect tests. When the sphericity condition was not met according to Mauchly’s test, the Greenhouse-Geisser correction or, failing that, the Huynh-Feldt correction was performed in the intra-subject effect tests. Similarly, water retention capacity and moisture of the soil was analyzed using a GLM. A significance level of p < 0.05 and confidence interval = 95% were considered in the analyses. Statistical analyses were performed using the IBM SPSS Statistics for PC, version 30.0 (IBM SPSS, Armonk, NY, USA: IBM Corp. 2024).
For the purposes of this study: we defined a species as being highly water-sensitive if significant differences in plant growth and survival were observed between the different water treatments applied during the trial. A species is water-sensitive if significant differences in plant growth or survival is observed between the different water treatments applied during the trial. Finally, a species is considered non-sensitive if there are no significant differences in plant growth and survival between water treatments.
5. Conclusions
Survival results show that almost 80% of the native central Chileans species selected for this study were not affected by the water treatments applied and even reached high percentages in the first year of establishment, which is considered a survival bottleneck for urban plants. However, when water resources were severely limited, shoot growth can be slowed significantly, because they are water-sensitive species. The study also showed that the main growing season for both woody and non-woody species is spring. Growth is then slowed during the summer, with even biomass being lost. Finally, growth is stopped during the winter. Nevertheless, the survival rates may be high in each season.
In concluding, the evidence obtained in this study suggests that native species in central Chile can achieve high survival rates with limited irrigation. This highlights their potential for use in the sustainable urban green infrastructure of cities located in Mediterranean-type climates, which are expected to experience low rainfall due to climate change. Using native plants in urban green areas helps create more resilient plant communities and generate a broader array of ecological benefits.
Nevertheless, certain limitations persist. Resources should be focused on increasing the number of experimental plots and considering a randomized design from the outset. This calls for additional investigation into the potential use of native plants in a landscape that varies in both space and time.
Author Contributions
Conceptualization, J.A.F., F.F.C. and S.C.M.; methodology, J.A.F. and F.F.C.; software, J.A.F.; validation, J.A.F., R.C.-J., A.C.-C., F.F.C. and S.C.M.; formal analysis, J.A.F.; investigation, J.A.F., R.C.-J., A.C.-C., F.F.C. and S.C.M.; data curation, J.A.F. and F.F.C.; writing—original draft preparation, J.A.F.; writing—review and editing, J.A.F. and F.F.C.; visualization, J.A.F. and F.F.C.; supervision, J.A.F.; project administration, R.C.-J. and A.C.-C.; funding acquisition, R.C.-J., A.C.-C., F.F.C. and J.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID/IDEA ID23I10043, Universidad Tecnológica Metropolitana y Universidad Central de Chile.
Data Availability Statement
The data presented in this study are available on request from the corresponding author J.F.
Acknowledgments
We would like to thank Carlos Bustamante-Oleart, Carlos Bustamante Espina, Juan Jorquera-Hernández, Carlos Aracena Rivera, M. Villagrán-Escobar, Javier Ríos Vilche, José Cerón Córdova, Consuelo Chaparro Gómez and Margarita Reyes for maintaining the databases and providing technical support at various stages of the project. We would like to express our sincere gratitude to Claudia Ortiz-Calderón for her valuable contributions, which have enabled us to improve our analysis of the results.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, X.; Li, C.; Zhao, X.; Zhu, T. Arid Urban Green Areas Reimagined: Transforming Landscapes with Native Plants for a Sustainable Future in Aksu, Northwest China. Sustainability 2024, 16, 1546. [Google Scholar] [CrossRef]
- McPherson, E.G. Cooling urban heat islands with sustainable landscapes. In The Ecological City: Preserving and Restoring Urban Biodiversity; Platt, R.H., Rowntree, R.A., Muick, P.C., Eds.; University of Massachusetts Press: Amherst, MA, USA, 1994; pp. 151–171. [Google Scholar]
- Richter, M.; Heinemann, K.; Meiser, N.; Dickhaut, W. Trees in sponge cities—A systematic review of trees as a component of blue-green infrastructure, vegetation engineering principles, and Stormwater management. Water 2024, 16, 655. [Google Scholar] [CrossRef]
- Catalano, C.; Laudicina, V.A.; Badalucco, L.; Guarino, R. Some European green roof norms and guidelines through the lens of biodiversity: Do ecoregions and plant traits also matter? Ecol. Eng. 2018, 115, 15–26. [Google Scholar] [CrossRef]
- Rasoolzadeh, R.; Mobarghaee, N.; Esmaeilzadeh, H.; Rashidi, Y.; Marcu, M.V.; Sadeghi, S.M.M. Carbon sequestration and storage of urban trees in a polluted semiarid city. Forests 2024, 15, 1488. [Google Scholar] [CrossRef]
- Varela-Stasinopoulou, D.S.; Nektarios, P.A.; Ntoulas, N.; Trigas, P.; Roukounakis, G.I. Sustainable Growth of Medicinal and Aromatic Mediterranean Plants Growing as Communities in Shallow Substrate Urban Green Roof Systems. Sustainability 2023, 15, 5940. [Google Scholar] [CrossRef]
- Burch, W.R., Jr.; Grove, J.M. People, trees and participation on the urban frontier. Unasylva 1993, 44, 19–27. [Google Scholar]
- Dwyer, J.F.; McPherson, E.G.; Schroeder, H.W.; Rowntree, R.A. Assessing the benefits and costs of the urban forest. J. Arboric. 1992, 18, 227–234. [Google Scholar] [CrossRef]
- Simpson, J.R.; McPherson, E.G. San Francisco Bay Area State of the Urban Forest Final Report; Center for Urban Forest Research USDA Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2007; p. 81. [Google Scholar]
- Faber Taylor, A.; Kuo, F.E. Could exposure to everyday green spaces help treat ADHD? Evidence from children’s play settings. Appl. Psychol. Health Well-Being 2011, 3, 281–303. [Google Scholar] [CrossRef]
- Bele, A.; Chakradeo, U. Public perception of biodiversity: A literature review of its role in urban green spaces. J. Land. Ecol. 2021, 14, 1–28. [Google Scholar] [CrossRef]
- Lohr, V.I.; Pearson-Mims, C.H.; Tarnai, J.; Dillman, D.A. How urban resident rate and rank the benefits and problems associated with trees in cities. Arboric. Urban For. 2004, 30, 28–35. [Google Scholar] [CrossRef]
- Irarrázaval, F. El imaginario” verde” y el verde urbano como instrumento de consumo inmobiliario: Configurando las condiciones ambientales del área Metropolitana de Santiago. Rev. INVI 2012, 27, 73–103. [Google Scholar] [CrossRef]
- Uribe, S.V.; Villaseñor, N.R. Inequities in urban tree care based on socioeconomic status. Urban For. Urban Green. 2024, 96, 128363. [Google Scholar] [CrossRef]
- Kornienko, V.; Reuckaya, V.; Shkirenko, A.; Meskhi, B.; Olshevskaya, A.; Odabashyan, M.; Shevchenko, V.; Teplyakova, S. Silvicultural and Ecological Characteristics of Populus bolleana Lauche as a Key Introduced Species in the Urban Dendroflora of Industrial Cities. Plants 2025, 14, 2052. [Google Scholar] [CrossRef]
- Catalano, C.; Marcenò, C.; Laudicina, V.A.; Guarino, R. Thirty years unmanaged green roofs: Ecological research and design implications. Landsc. Urban Plan. 2016, 149, 11–19. [Google Scholar] [CrossRef]
- Allen, K.S.; Harper, R.W.; Bayer, A.; Brazee, N.J. A review of nursery production systems and their influence on urban tree survival. Urban For. Urban Green. 2017, 21, 183–191. [Google Scholar] [CrossRef]
- de la Fuente, L.M.; Ovalle, J.F.; Arellano, E.C.; Ginocchio, R. Does woody species with contrasting root architecture require different container size in nursery? Madera Bosques 2018, 24, e2421419. [Google Scholar] [CrossRef]
- Aloisio, J.M.; Palmer, M.I.; Tuininga, A.R.; Lewis, J.D. Introduced and native plant species composition of vacant unmanaged green roofs in New York City. Urban Ecosyst. 2020, 23, 1227–1238. [Google Scholar] [CrossRef]
- Esperon-Rodriguez, M.; Rymer, P.D.; Power, S.A.; Barton, D.N.; Cariñanos, P.; Dobbs, C.; Eleuterio, A.A.; Escobedo, F.J.; Hauer, R.; Hermy, M.; et al. Assessing climate risk to support urban forests in a changing climate. Plants People Planet 2022, 4, 201–213. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoo, G. Suggested key variables for assessment of soil quality in urban roadside tree systems. J. Soils Sediments 2021, 21, 2130–2140. [Google Scholar] [CrossRef]
- Vico, G.; Thompson, S.E.; Manzoni, S.; Molini, A.; Albertson, J.D.; Almeida-Cortez, J.S.; Fay, P.A.; Feng, X.; Guswa, A.J.; Liu, H.; et al. Climatic, ecophysiological, and phenological control son plant ecohydrological strategies in seasonally dry ecosystems. Ecohydrology 2015, 8, 660–681. [Google Scholar] [CrossRef]
- Yáñez, M.A.; Espinoza, S.E.; Magni, C.R.; Martínez-Herrera, E. Early Growth and physiological acclimation to shade and water restriction of seven sclerophyllous species of the Mediterranean forests of central Chile. Plants 2024, 13, 2410. [Google Scholar] [CrossRef]
- Rodríguez, C.; Serrano, J.; Sánchez, R.; Leiva, E. The Hydrosocial Cycle and the Inequalities in Access to Water in Rural Areas of Metropolitan Region of Santiago, Chile. Water 2024, 16, 2811. [Google Scholar] [CrossRef]
- Fuentes, I.; Fuster, R.; Avilés, D.; Vervoort, W. Water scarcity in central Chile: The effect of climate and land cover changes on hydrologic resources. Hydrol. Sci. J. 2021, 66, 1028–1044. [Google Scholar] [CrossRef]
- Figueroa, J.A.; Teillier, S.; Guerrero-Leiva, N.; Ray-Bobadilla, C.; Rivano, S.; Saavedra, D.; Castro, S.A. Flora vascular en el espacio público de Santiago, Chile. Gayana Bot. 2016, 73, 85–103. [Google Scholar] [CrossRef]
- Ekren, E.; Çorbacı, Ö.L.; Kordon, S. Evaluation of plants based on ecological tolerance criteria: A case study of urban open green spaces in Rize, Türkiye. Turk. J. For. Sci. 2024, 8, 107–131. [Google Scholar] [CrossRef]
- Figueroa, J.A.; Castro, S.A.; Reyes, M.; Teillier, S. Urban park area and age determine the richness of native and exotic plants in parks of a Latin American city: Santiago as a case study. Urban Ecosyst. 2018, 21, 645–655. [Google Scholar] [CrossRef]
- Guevara, B.R.; Uribe, S.V.; de la Maza, C.L.; Villaseñor, N.R. Socioeconomic disparities in urban forest diversity and structure in green areas of Santiago de Chile. Plants 2024, 13, 1841. [Google Scholar] [CrossRef] [PubMed]
- Blasi, C.; Biondi, E.; Izco, J. 100 years of plant sociology: A celebration. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2011, 145, 1–3. [Google Scholar] [CrossRef]
- Säumel, I.; Weber, F.; Kowarik, I. Toward livable and healthy urban streets: Roadside vegetation provides ecosystem services where people live and move. Environ. Sci. Policy 2016, 62, 24–33. [Google Scholar] [CrossRef]
- Conway, T.M.; Almas, A.D.; Coore, D. Ecosystem services, ecological integrity, and native species planting: How to balance these ideas in urban forest management? Urban For. Urban Green. 2019, 41, 1–5. [Google Scholar] [CrossRef]
- Arcos-LeBert, G.; Aravena-Hidalgo, T.; Figueroa, J.A.; Jaksic, F.M.; Castro, S.A. Native trees provide more benefits than exotic trees when ecosystem services are weighted in Santiago, Chile. Trees 2021, 35, 1663–1672. [Google Scholar] [CrossRef]
- D’Amato, L.; Bartoli, F.; Savo, V.; Caneva, G. Promoting native biodiversity: An evaluation of multifactorial and bioclimatic selection criteria for street trees in Italian cities. Urban For. Urban Green. 2025, 107, 128784. [Google Scholar] [CrossRef]
- Rundel, P.W. Landscape disturbance in Mediterranean-type ecosystems: An overview. In Landscape Disturbance and Biodiversity in Mediterranean-Type Ecosystems; Rundel, P.W., Montenegro, G., Jaksic, F.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 3–22. [Google Scholar]
- Fernández, F.; Delaunoy, J.; Chiang, L.; Reyes, M. Metodología de valor paisajístico: Selección de plantas nativas para la infraestructura verde pública de la Región Metropolitana. In Biodiversidad Urbana en Chile: Estado del Arte y los Desafíos Futuros, 2nd ed.; Figueroa, J.A., Lazzoni, I., Eds.; Ediciones Universidad Central de Chile: Santiago, Chile, 2025; in press. [Google Scholar]
- Grimm, N.B.; Pickett, S.T.; Hale, R.L.; Cadenasso, M.L. Does the ecological concept of disturbance have utility in urban social–ecological–technological systems? Ecosyst. Health Sustain. 2017, 3, e01255. [Google Scholar] [CrossRef]
- Degerickx, J.; Roberts, D.A.; McFadden, J.P.; Hermy, M.; Somers, B. Urban tree health assessment using airborne hyperspectral and LiDAR imagery. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 26–38. [Google Scholar] [CrossRef]
- Ward, E.B.; Doroski, D.A.; Felson, A.J.; Hallett, R.A.; Oldfield, E.E.; Kuebbing, S.E.; Bradford, M.A. Positive long-term impacts of restoration on soils in an experimental urban forest. Ecol. Appl. 2021, 31, e02336. [Google Scholar] [CrossRef]
- Agrupación de Especies Nativas Según Condiciones Agroecológicas Aptas para su Cultivo. Available online: https://www.pumahuida.cl/wp-content/uploads/2022/03/AGRUPACIÓN-DE-ESPECIES-NATIVAS-SEGÚN-CONDICIONES-AGROECOLÓGICAS-APTAS-PARA-SU-CULTIVO-M.-MUSALEM_VI-CONGRESO-DE-FLORA-NATIVA-2019_opt.pdf (accessed on 29 September 2025).
- Listas de Especies Nativas para Distintas Situaciones de Paisaje. Available online: https://www.pumahuida.cl/informacion-tecnica/ (accessed on 28 July 2025).
- Rodríguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catálogo de las plantas vasculares de Chile. Gayana Bot. 2018, 75, 1–430. [Google Scholar] [CrossRef]
- Hoffmann, J.A. Flora Silvestre de Chile. Zona Central. Una Guía para la Identificación de las Especies Vegetales Más Frecuentes, 5th ed.; Fundación Claudio Gay: Santiago, Chile, 2012; p. 250. [Google Scholar]
- Armesto, J.J.; Pickett, S.T.A. Experiments on disturbance in old-field plant communities: Impact on species richness and abundance. Ecology 1985, 66, 230–240. [Google Scholar] [CrossRef]
- Becerra, P.I.; Cruz, G.; Ríos, S.; Castelli, G. Importance of irrigation and plant size in the establishment success of different native species in a degraded ecosystem of central Chile. Bosque 2013, 34, 23–24. [Google Scholar] [CrossRef][Green Version]
- Becerra, P.I.; Arellano, E.C.; Vilagrosa, A.; Hernández, G.; Figueroa, C. The provision of water and shade but not soil amendments in degraded habitats increases the seedling survival of woody species in restoration processes of the Chilean sclerophyllous forest. Trees 2024, 38, 523–535. [Google Scholar] [CrossRef]
- Razzaghmanesh, M.; Beecham, S.; Kazemi, F. The growth and survival of plants in urban green roofs in a dry climate. Sci. Total Environ. 2014, 476, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Armesto, J.J.; Arroyo, M.T.K.; Hinojosa, F.L. The Mediterranean environment of central PM Chile. In The Physical Geography of South America; Veblen, T.T., Young, K.R., Orme, A.R., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 184–199. [Google Scholar]
- Rundel, P.W.; Arroyo, M.T.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Vargas, P. Mediterranean biomes: Evolution of their vegetation, floras, and climate. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 383–407. [Google Scholar] [CrossRef]
- Chandía-Jaure, R.; Cataldo-Cunich, A.; Fernández-Cano, F.; Figueroa, J.A.; Godoy-Donoso, D. Refugios biourbanos: Guía para evaluar la efectividad de soluciones basadas en la naturaleza para el diseño urbano sensible al agua, como estrategia de adaptación al cambio climático; Universidad Tecnológica Metropolitana: Santiago, Chile, 2025; in press. [Google Scholar]
- Teillier, S.; Macaya-Berti, J.; García, N.; Marticorena, A.; Rojas, G.; Niemeyer, H.M. Flora de la Región Metropolitana de Santiago. Guía para la Identificación de las Especies; Asia Pacific Offset: Hong-Kong, China, 2022; 671p. [Google Scholar]
- Everard, K.; Seabloom, E.W.; Harpole, W.S.; De Mazancourt, C. Plant water use affects competition for nitrogen: Why drought favors invasive species in California. Am. Nat. 2010, 175, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Burlett, R.; Trueba, S.; Bouteiller, X.P.; Forget, G.; Torres-Ruiz, J.M.; Martin-StPaul, N.K.; Parise, C.; Cochard, H.; Delzon, S. Minimum leaf conductance during drought: Unravelling its variability and impact on plant survival. New Phytol. 2025, 246, 1001–1014. [Google Scholar] [CrossRef]
- Ortuño, M.A.; Machtig, A.E.; Chacón, M.A.; Cuzmar, J.; Fontúrbel, F.E. Spatial distribution of Puya coerulea Lindl. in response to abiotic factors and accompanying species in the Río Clarillo National Reserve. Gayana Bot. 2019, 76, 115–118. [Google Scholar] [CrossRef]
- Prieto, I.; Martínez-Tillería, K.; Martínez-Manchego, L.; Montecinos, S.; Pugnaire, F.I.; Squeo, F.A. Hydraulic lift through transpiration suppression in shrubs from two arid ecosystems: Patterns and control mechanisms. Oecologia 2010, 163, 855–865. [Google Scholar] [CrossRef]
- León, M.F.; Squeo, F.A.; Gutiérrez, J.R.; Holmgren, M. Rapid root extension during water pulses enhances establishment of shrub seedlings in the Atacama Desert. J. Veg. Sci. 2011, 22, 120–129. [Google Scholar] [CrossRef]
- Gerstmann, C.; Miranda, M.; Condal, A. Description of space-time variability of the potential productivity of Acacia caven espinales based on MODIS images and the Enhanced Vegetation Index (EVI). Int. J. Agric. Nat. Res. 2010, 37, 63–73. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Bown, H.E.; Fernandez, B. Stomatal conductance responses of Acacia caven to seasonal patterns of water availability at different soil depths in a Mediterranean savanna. Water 2018, 10, 1534. [Google Scholar] [CrossRef]
- Ginocchio, R.; de la Fuente, L.M.; Orrego, F.; Díaz, M.J.; Báez, J.; Ovalle, J.F. A novel fast-vegetative propagation technique of the pioneer shrub Baccharis linearis on mine tailings by adding compost. Int. J. Phytoremediat. 2021, 23, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Tillería, K.; Loayza, A.P.; Sandquist, D.R.; Squeo, F.A.; Ward, D. No evidence of a trade-off between drought and shade tolerance in seedlings of six coastal desert shrub species in north-central Chile. J. Veg. Sci. 2012, 23, 1051–1061. [Google Scholar] [CrossRef]
- Carvajal, D.E.; Loayza, A.P.; López, R.P.; Toro, P.J.; Squeo, F.A. Growth and early seedling survival of four Atacama Desert shrub species under experimental light and water availability regimes. Rev. Chil. Hist. Nat. 2014, 87, 28. [Google Scholar] [CrossRef]
- Torres, R.; Squeo, F.A.; Jorquera, C.; Aguirre, E.; Ehleringer, J.R. Evaluación de la capacidad estacional de utilizar eventos de precipitación en tres especies de arbustos nativos de Chile con distintos sistemas radiculares. Rev. Chil. Hist. Nat. 2002, 75, 737–749. [Google Scholar] [CrossRef][Green Version]
- Newbold, T.; Oppenheimer, P.; Etard, A.; Williams, J.J. Tropical and Mediterranean biodiversity is disproportionately sensitive to land-use and climate change. Nat. Ecol. Evol. 2020, 4, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G.; Kumbaric, A.; Savo, V.; Casalini, R. Ecological approach in selecting extensive green roof plants: A data-set of Mediterranean plants. Plant Biosyst. 2015, 149, 374–383. [Google Scholar] [CrossRef]
- Bambach, N.; Meza, F.J.; Gilabert, H.; Miranda, M. Impacts of climate change on the distribution of species and communities in the Chilean Mediterranean ecosystem. Reg. Environ. Change 2013, 13, 1245–1257. [Google Scholar] [CrossRef]
- McPhee, J.; Cortés, G.; Rojas, M.; García, L.; Descalzi, A.; Vargas, L. Downscaling climate changes for Santiago: What effects can be expected? In Climate Adaptation Santiago; Krellenberg, K., Hansjürgens, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 19–41. [Google Scholar]
- Densidad de Población y Vivienda. Censo. 2017. Available online: https://storymaps.arcgis.com/stories/fc03ad1481f44b6299b81c22c91497fe (accessed on 28 July 2025).
- De Mattos, C. Globalización y transformación metropolitana en el caso de Santiago. In Los Nuevos Modos de Gestión de la Metropolización; Hidalgo, R., Arenas, F., Coll, J.L., Eds.; Pontificia Universidad Católica de Chile: Santiago, Chile, 2003; pp. 27–55. [Google Scholar]
- Romero, H.; Vásquez, A. Evaluación ambiental del proceso de urbanización de las cuencas del piedemonte andino de Santiago de Chile. Eur. Secur. 2005, 94, 97–118. [Google Scholar] [CrossRef]
- Romero, H.; Molina, M.; Moscoso, C.; Sarricolea, P.; Smith, P.; Vásquez, A. Caracterización de los cambios de usos y coberturas de suelo causados por la expansión urbana de Santiago, análisis de sus factores explicativos e inferencias ambientales. In Movilidad Espacial y Reconfiguración Metropolitana; de Mattos, C., Hidalgo, R., Eds.; Pontificia Universidad Católica de Chile: Santiago, Chile, 2007; pp. 251–270. [Google Scholar]
- Chandia-Jaure, R.; Cataldo-Cunich, A.; Bustamante-Oleart, C.; Fernandez Cano, F.; Figueroa, J.A.; Villagrán-Escobar, M. Bio-Urban Shelters. Neighborhood-scale intervention model for water sensitive urban design. In Proceedings of the 14° Encuentro Diseño Urbano, Readu, Punta Arenas, Chile, 6 November 2024. [Google Scholar]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile; Editorial Universitaria: Santiago, Chile, 2017; p. 316. [Google Scholar]
- Ivelic-Sáez, J.; Reckmann, O.; López, R.; Uribe, H.; Valenzuela, J.; Ibarra, D. Bases para el riego en Magallanes. Boletín INIA 2021, 459, 92. [Google Scholar]
- Fernández, F.; Chiang, L.; Figueroa, J.A. Guía de Recomendaciones para Jardines Eficientes en el Espacio Público en la Región Metropolitana; Universidad Central de Chile: Santiago, Chile, 2025; p. 63. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).