TALEN-Interceded Genome Editing in Plants: Unveiling New Frontiers in Secondary Metabolite Improvement and Genetic Diversity
Abstract
1. Introduction
2. Mechanisms and Recent Advancements in Transcription Activator-like Effector Nuclease Technology
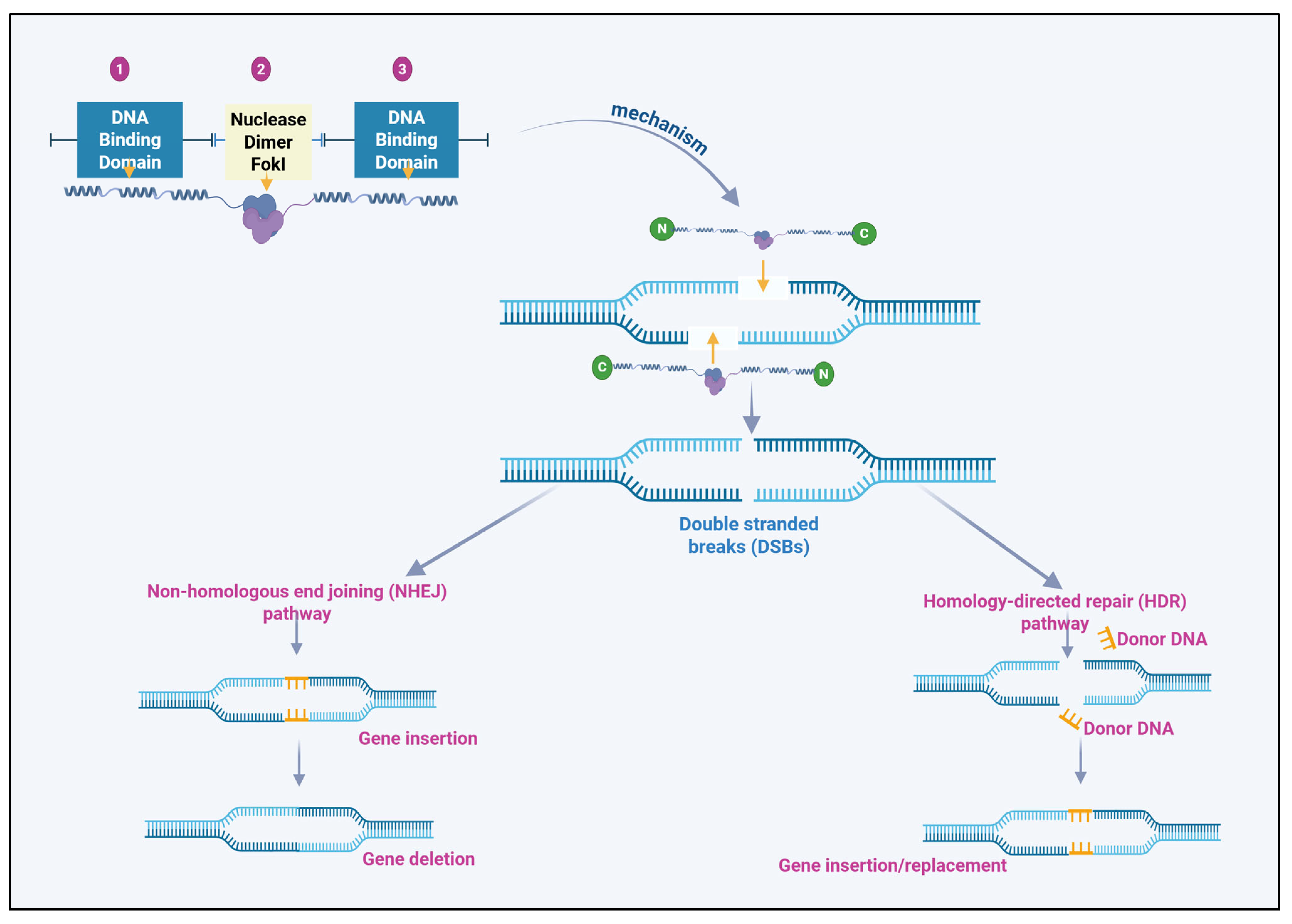
2.1. Structural Composition and Mechanism of Transcription Activator-like Effector Nuclease
2.2. Transcription Activator-like Effector Nuclease-Mediated Gene Editing Process
2.3. Comparisons of Transcription Activator-like Effector Nucleases with Other Genome Editing Tools
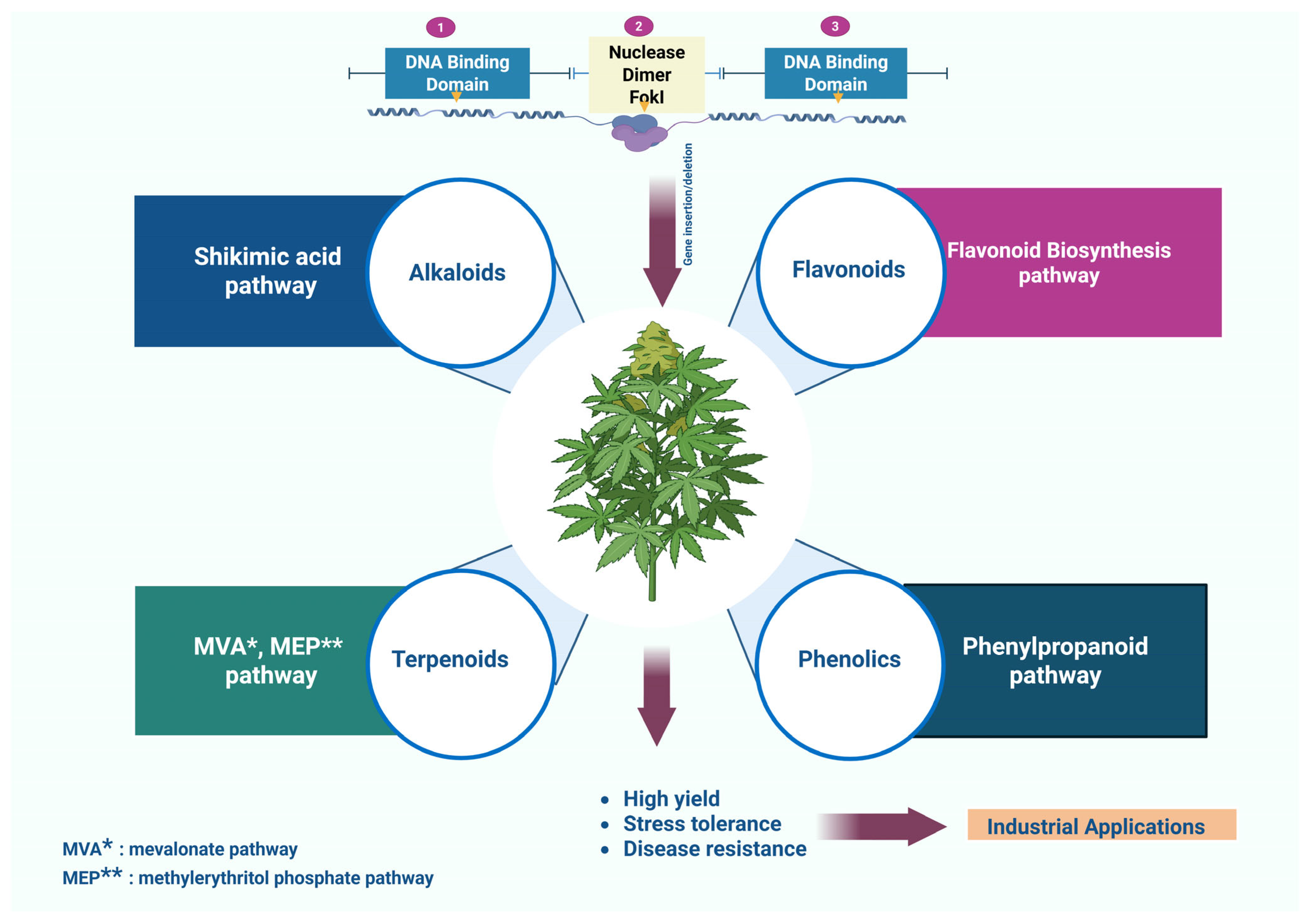
3. TALENs and Secondary Metabolite Synthesis
3.1. Biosynthesis of Secondary Metabolites
3.1.1. Terpenoid Pathways
3.1.2. Alkaloid Biosynthesis
3.1.3. Flavonoid and Phenolic Acid Pathways
3.2. Targeting Key Genes in Secondary Metabolite Pathways
3.2.1. Engineering Transcription Factors
3.2.2. Modulating Enzyme Activity
3.3. Case Studies of Transcription Activator-like Effector Nucleases in Secondary Metabolite Synthesis
4. TALENs and Genetic Diversity
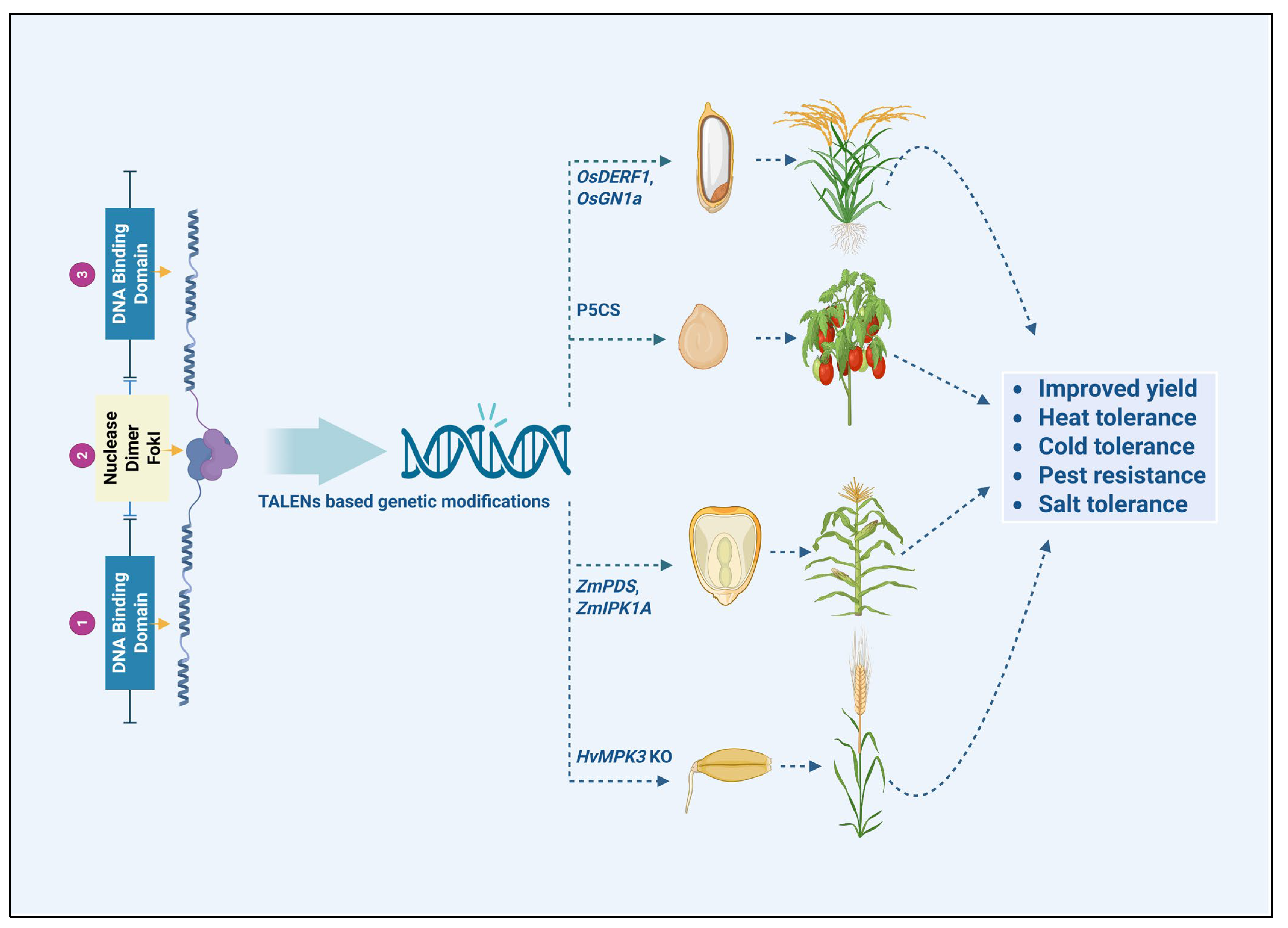
4.1. Enhancing Genetic Diversity with Transcription Activator-like Effector Nucleases
4.2. Case Studies in Crop Improvement
5. TALENs and Plant Stress Tolerance Mechanisms
5.1. Introduction to Plant Stress Tolerance
5.2. Transcription Activator-like Effector Nuclease-Mediated Modifications for Stress Tolerance
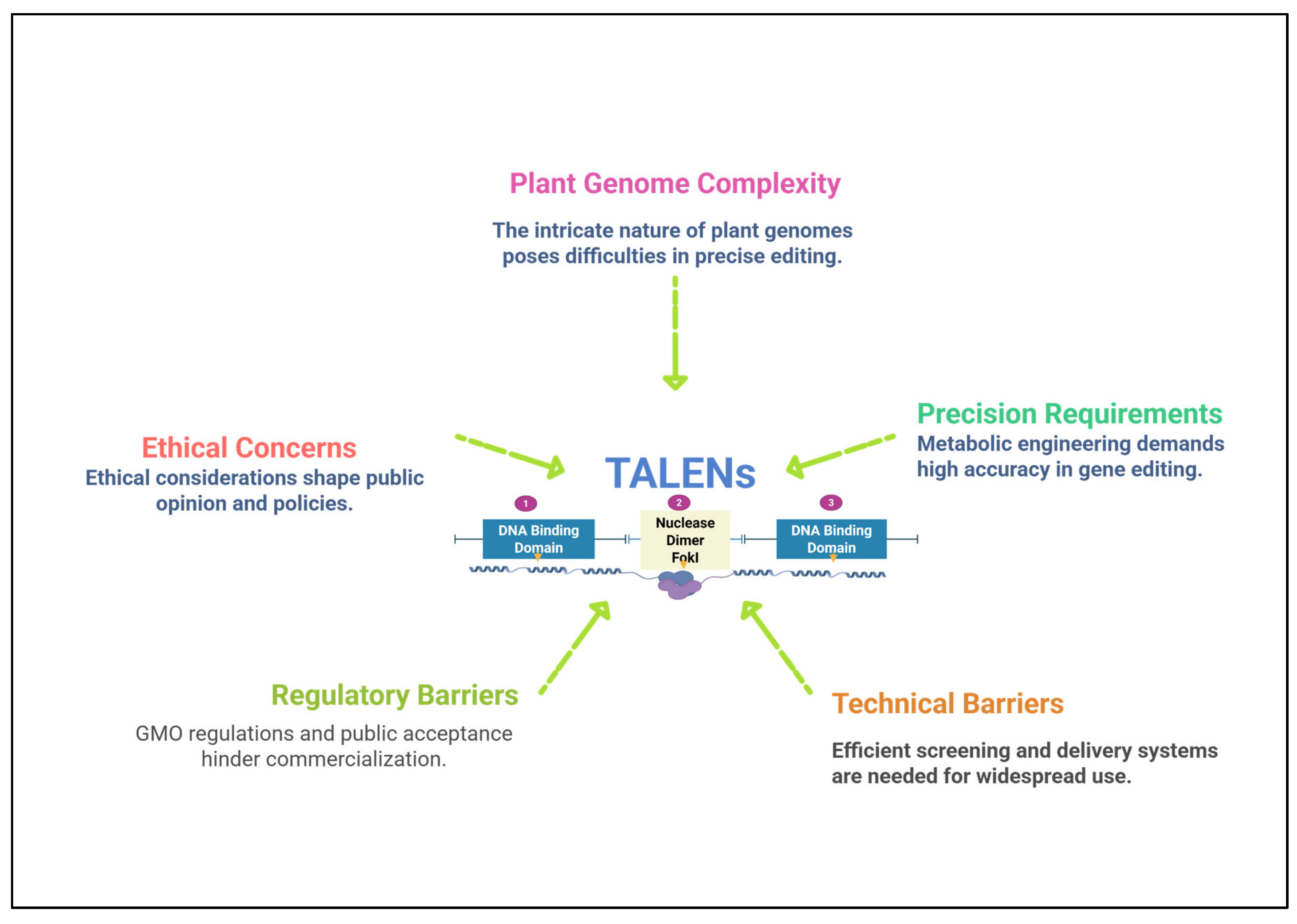
6. Challenges and Limitations of TALENs
6.1. Off-Target Effects
6.2. Regulatory and Ethical Concerns
6.3. Technical Barriers in High Throughput Screening
6.4. Editing Inefficiencies in Certain Crops or Genome Regions
7. Future Directions and Prospects
7.1. Next-Generation TALENs: Improved Specificity and Efficiency
7.2. Integration with Other Genetic Engineering Tools
7.3. Integration with Omics Data, Machine Learning and AI-Assisted Genome Design
7.4. Industrial Applications of Enhanced Secondary Metabolites
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salgotra, R.K.; Chauhan, B.S. Genetic diversity, conservation, and utilization of plant genetic resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Padulosi, S.; Mal, B.; Ravi, S.B.; Gowda, J.; Gowda, K.T.K.; Shanthakumar, G.; Yenagi, N.; Dutta, M. Food security and climate change: Role of plant genetic resources of minor millets. Indian J. Plant Genet. Resour. 2009, 22, 1–16. [Google Scholar]
- de Almeida Cançado, G.M. The importance of genetic diversity to manage abiotic stress. In Abiotic Stress in Plants–Mechanisms and Adaptations; InTechOpen: Rijeka, Croatia, 2011; pp. 351–366. [Google Scholar]
- Mackay, G.R. Propagation by traditional breeding methods. In Genetic Improvement of Solanaceous Crops; CRC Press: Boca Raton, FL, USA, 2005; Volume 1, pp. 65–81. [Google Scholar]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Gupta, R.M.; Musunuru, K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Investig. 2014, 124, 4154–4161. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Nain, V. TALENs—An indispensable tool in the era of CRISPR: A mini review. J. Genet. Eng. Biotechnol. 2021, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.F.; Fahrenkrug, S.C.; Hackett, P.B. Targeting DNA with fingers and TALENs. Mol. Ther. Nucleic Acids 2012, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.A.; Li, T.; Yang, B.; Spalding, M.H. TALEN-mediated genome editing: Prospects and perspectives. Biochem. J. 2014, 462, 15–24. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, S.H.; Mubarik, M.S.; Sadia, B.; Ahmad, A. Use of TALEs and TALEN technology for genetic improvement of plants. Plant Mol. Biol. Report. 2017, 35, 1–19. [Google Scholar] [CrossRef]
- Mishra, R.; Agarwal, P.; Mohanty, A. Applications of genome editing techniques for the improvement of medicinal plants. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 545–569. [Google Scholar]
- Harale, G.; Pardeshi, S.; Majumdar, P.; Ganger, S. Transcription activator-like effector nucleases (TALENs): Genome editing tool to explore enhanced activity of antidiabetic plants. In Antidiabetic Potential of Plants in the Era of Omics; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 403–428. [Google Scholar]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Saenz de la O, D.; Alvarado-Mariana, A.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of stress and defense in plant secondary metabolites production. Bioact. Nat. Prod. Pharm. Appl. 2021, 140, 151–195. [Google Scholar]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, R.; Bharti, R. Pharmacological activities and toxicities of alkaloids on human health. Mater. Today Proc. 2022, 48, 1407–1415. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res 2019, 10, 1567–1574. [Google Scholar]
- Pichersky, E.; Gang, D.R. Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci. 2000, 5, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Al Aboud, N.M. Unlocking the genetic potential: Strategies for enhancing secondary metabolite biosynthesis in plants. J. Saudi Soc. Agric. Sci. 2024, 23, 542–554. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Ghorai, M.; Kant, N.; Kumar, M.; Radha; Jha, N.K.; Swamy, M.K.; Proćków, J.; de la Lastra, J.M.P. Genome editing technologies, mechanisms and improved production of therapeutic phytochemicals: Opportunities and prospects. Biotechnol. Bioeng. 2023, 120, 82–94. [Google Scholar] [CrossRef]
- Guilinger, J.P.; Pattanayak, V.; Reyon, D.; Tsai, S.Q.; Sander, J.D.; Joung, J.K.; Liu, D.R. Broad specificity profiling of TALENs results in engineered nucleases with improved DNA-cleavage specificity. Nat. Methods 2014, 11, 429–435. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, F.; Li, S.; Wang, Y.; Bai, Y.; Xu, X. TALE: A tale of genome editing. Prog. Biophys. Mol. Biol. 2014, 114, 25–32. [Google Scholar] [CrossRef]
- Nemudryi, A.A.; Valetdinova, K.R.; Medvedev, S.P.; Zakian, S.á. TALEN and CRISPR/Cas genome editing systems: Tools of discovery. Acta Naturae 2014, 6, 19–40. [Google Scholar] [CrossRef]
- Li, T.; Huang, S.; Jiang, W.Z.; Wright, D.; Spalding, M.H.; Weeks, D.P.; Yang, B. TAL nucleases (TALNs): Hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain. Nucleic Acids Res. 2011, 39, 359–372. [Google Scholar] [CrossRef]
- Zaboikin, M.; Zaboikina, T.; Freter, C.; Srinivasakumar, N. Non-homologous end joining and homology directed DNA repair frequency of double-stranded breaks introduced by genome editing reagents. PLoS ONE 2017, 12, e0169931. [Google Scholar] [CrossRef]
- Rodgers, K.; McVey, M. Error-prone repair of DNA double-strand breaks. J. Cell. Physiol. 2016, 231, 15–24. [Google Scholar] [CrossRef]
- Haider, S.; Mussolino, C. Fine-Tuning Homology-Directed Repair (HDR) for Precision Genome Editing: Current Strategies and Future Directions. Int. J. Mol. Sci. 2025, 26, 4067. [Google Scholar] [CrossRef] [PubMed]
- Shamshirgaran, Y.; Liu, J.; Sumer, H.; Verma, P.J.; Taheri-Ghahfarokhi, A. Tools for efficient genome editing; ZFN, TALEN, and CRISPR. Appl. Genome Modul. Ed. 2022, 2495, 29–46. [Google Scholar]
- Waryah, C.B.; Moses, C.; Arooj, M.; Blancafort, P. Zinc fingers, TALEs, and CRISPR systems: A comparison of tools for epigenome editing. Epigenome Ed. Methods Protoc. 2018, 1767, 19–63. [Google Scholar]
- Deng, D.; Yan, C.; Wu, J.; Pan, X.; Yan, N. Revisiting the TALE repeat. Protein Cell 2014, 5, 297–306. [Google Scholar] [CrossRef][Green Version]
- Vanamee, É.S.; Santagata, S.; Aggarwal, A.K. FokI requires two specific DNA sites for cleavage. J. Mol. Biol. 2001, 309, 69–78. [Google Scholar] [CrossRef]
- Ochiai, H.; Yamamoto, T. Genome editing using zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). In Targeted Genome Editing Using Site-Specific Nucleases: ZFNs, TALENs, and the CRISPR/Cas9 System; Springer: Tokyo, Japan, 2015; pp. 3–24. [Google Scholar]
- Miller, J.C.; Patil, D.P.; Xia, D.F.; Paine, C.B.; Fauser, F.; Richards, H.W.; Shivak, D.A.; Bendaña, Y.R.; Hinkley, S.J.; Scarlott, N.A. Enhancing gene editing specificity by attenuating DNA cleavage kinetics. Nat. Biotechnol. 2019, 37, 945–952. [Google Scholar] [CrossRef]
- Filippova, J.; Matveeva, A.; Zhuravlev, E.; Stepanov, G. Guide RNA modification as a way to improve CRISPR/Cas9-based genome-editing systems. Biochimie 2019, 167, 49–60. [Google Scholar] [CrossRef]
- Gai, C.; Yu, J.; Xie, C.; Han, Y.; Song, Y.; Huang, F.; He, S.; Liu, C.; Lin, L.; Chen, D.; et al. A simple and efficient TALEN system for genome editing in plants. Plant Mol. Biol. 2025, 115, 25. [Google Scholar] [CrossRef] [PubMed]
- Kusano, H.; Onodera, H.; Kihira, M.; Aoki, H.; Matsuzaki, H.; Shimada, H. A simple Gateway-assisted construction system of TALEN genes for plant genome editing. Sci. Rep. 2016, 6, 30234. [Google Scholar] [CrossRef]
- Modrzejewski, D.; Hartung, F.; Sprink, T.; Krause, D.; Kohl, C.; Wilhelm, R. What is the available evidence for the range of applications of genome-editing as a new tool for plant trait modification and the potential occurrence of associated off-target effects: A systematic map. Environ. Evid. 2019, 8, 1–33. [Google Scholar] [CrossRef]
- Shiva Krishna, G.; Suma, K. Gene editing nucleases-ZFNs, TALENS and CRISPR: A review. Chettinad Health City Med. J. 2019, 8, 130–135. [Google Scholar]
- Karvelis, T.; Gasiunas, G.; Young, J.; Bigelyte, G.; Silanskas, A.; Cigan, M.; Siksnys, V. Rapid characterization of CRISPR-Cas9 protospacer adjacent motif sequence elements. Genome Biol. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Sprink, T.; Metje, J.; Hartung, F. Plant genome editing by novel tools: TALEN and other sequence specific nucleases. Curr. Opin. Biotechnol. 2015, 32, 47–53. [Google Scholar] [CrossRef]
- Zhong, Z.; Sretenovic, S.; Ren, Q.; Yang, L.; Bao, Y.; Qi, C.; Yuan, M.; He, Y.; Liu, S.; Liu, X. Improving plant genome editing with high-fidelity xCas9 and non-canonical PAM-targeting Cas9-NG. Mol. Plant 2019, 12, 1027–1036. [Google Scholar] [CrossRef]
- Cermak, T.; Starker, C.G.; Voytas, D.F. Efficient design and assembly of custom TALENs using the Golden Gate platform. In Chromosomal Mutagenesis; Springer: Berlin/Heidelberg, Germany, 2014; pp. 133–159. [Google Scholar]
- Franic, D.; Dobrinic, P.; Korac, P. Key achievements in gene therapy development and its promising progress with gene editing tools (ZFN, TALEN, CRISPR/CAS9). Mol. Exp. Biol. Med. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Kaya, H.; Rai, R.; Bogdanove, A. Using TALENs for genome editing in plants. In Genome Editing for Precision Crop Breeding; Burleigh Dodds Science Publishing Ltd.: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Chen, L.-L.; Xie, K. Recent advances in genome editing using CRISPR/Cas9. Front. Plant Sci. 2016, 7, 703. [Google Scholar] [CrossRef]
- Becker, S.; Boch, J. TALE and TALEN genome editing technologies. Gene Genome Ed. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Yasumoto, S.; Sawai, S.; Lee, H.; Mizutani, M.; Saito, K.; Umemoto, N.; Muranaka, T. Targeted genome editing in tetraploid potato through transient TALEN expression by Agrobacterium infection. Plant Biotechnol. 2020, 37, 205–211. [Google Scholar] [CrossRef]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.; Chen, L.; Sun, N.; Zeina, C.M.; Gao, X.; Rees, H.A.; Lin, Z. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Rana, C.S. Plant secondary metabolites: A review. Int. J. Eng. Res. Gen. Sci. 2015, 3, 661–670. [Google Scholar]
- Crozier, A.; Clifford, M.N.; Ashihara, H. Plant secondary metabolites. In Occurrence, Structure and Role in the Human Diet; Blackwell-Publishers: Hoboken, NJ, USA, 2006. [Google Scholar]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res 2019, 10, 494–504. [Google Scholar]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Biotechnol. Isoprenoids 2015, 148, 63–106. [Google Scholar]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H. Therapeutic and Biomedical Potentialities of Terpenoids—A Review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Kasahara, H.; Hanada, A.; Kuzuyama, T.; Takagi, M.; Kamiya, Y.; Yamaguchi, S. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J. Biol. Chem. 2002, 277, 45188–45194. [Google Scholar] [CrossRef]
- Rohmer, M. Mevalonate-independent methylerythritol phosphate pathway for isoprenoid biosynthesis. Elucidation and distribution. Pure Appl. Chem. 2003, 75, 375–388. [Google Scholar] [CrossRef]
- Opitz, S.; Nes, W.D.; Gershenzon, J. Both methylerythritol phosphate and mevalonate pathways contribute to biosynthesis of each of the major isoprenoid classes in young cotton seedlings. Phytochemistry 2014, 98, 110–119. [Google Scholar] [CrossRef]
- Van Schie, C.C.N.; Ament, K.; Schmidt, A.; Lange, T.; Haring, M.A.; Schuurink, R.C. Geranyl diphosphate synthase is required for biosynthesis of gibberellins. Plant J. 2007, 52, 752–762. [Google Scholar] [CrossRef]
- Bribi, N. Pharmacological activity of alkaloids: A review. Asian J. Bot. 2018, 1, 1–6. [Google Scholar]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamada, Y. Alkaloid biogenesis: Molecular aspects. Annu. Rev. Plant Biol. 1994, 45, 257–285. [Google Scholar] [CrossRef]
- Yamada, Y.; Sato, F. Transcription factors in alkaloid engineering. Biomolecules 2021, 11, 1719. [Google Scholar] [CrossRef]
- Pireyre, M.; Burow, M. Regulation of MYB and bHLH transcription factors: A glance at the protein level. Mol. Plant 2015, 8, 378–388. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Koes, R.E.; Quattrocchio, F.; Mol, J.N.M. The flavonoid biosynthetic pathway in plants: Function and evolution. BioEssays 1994, 16, 123–132. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Meldgaard, M. Expression of chalcone synthase, dihydroflavonol reductase, and flavanone-3-hydroxylase in mutants of barley deficient in anthocyanin and proanthocyanidin biosynthesis. Theor. Appl. Genet. 1992, 83, 695–706. [Google Scholar] [CrossRef]
- Vom Endt, D.; Kijne, J.W.; Memelink, J. Transcription factors controlling plant secondary metabolism: What regulates the regulators? Phytochemistry 2002, 61, 107–114. [Google Scholar] [CrossRef]
- Rabeh, K.; Hnini, M.; Oubohssaine, M. A comprehensive review of transcription factor-mediated regulation of secondary metabolites in plants under environmental stress. Stress Biol. 2025, 5, 15. [Google Scholar] [CrossRef]
- Sung, Y.H.; Baek, I.-J.; Kim, D.H.; Jeon, J.; Lee, J.; Lee, K.; Jeong, D.; Kim, J.-S.; Lee, H.-W. Knockout mice created by TALEN-mediated gene targeting. Nat. Biotechnol. 2013, 31, 23–24. [Google Scholar] [CrossRef]
- Li, S. Transcriptional control of flavonoid biosynthesis: Fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal Behav. 2014, 9, e27522. [Google Scholar] [CrossRef]
- Gnanaraj, M.; Samuel, P.; Jebastin, T.; Rajadurai, M.; Rajasudhakar, D.; Manikandan, R. Strategies for Precision Breeding of Medicinal Plants for Enhanced Secondary Metabolite Production. In Biotechnology, Multiple Omics, and Precision Breeding in Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2025; pp. 35–45. [Google Scholar]
- Kajla, M.; Roy, A.; Singh, I.K.; Singh, A. Regulation of the regulators: Transcription factors controlling biosynthesis of plant secondary metabolites during biotic stresses and their regulation by miRNAs. Front. Plant Sci. 2023, 14, 1126567. [Google Scholar] [CrossRef]
- Kang, J.-H.; McRoberts, J.; Shi, F.; Moreno, J.E.; Jones, A.D.; Howe, G.A. The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 2014, 164, 1161–1174. [Google Scholar] [CrossRef]
- Hong, K.; Wang, L.; Johnpaul, A.; Lv, C.; Ma, C. Key enzymes involved in the synthesis of hops phytochemical compounds: From structure, functions to applications. Int. J. Mol. Sci. 2021, 22, 9373. [Google Scholar] [CrossRef]
- Yee, D.; Kakule, T.; Cheng, W.; Chen, M.; Chong, C.; Hai, Y.; Hang, L.; Hung, Y.-S.; Liu, N.; Ohashi, M.; et al. Genome Mining of Alkaloidal Terpenoids from a Hybrid Terpene and Nonribosomal Peptide Biosynthetic Pathway. J. Am. Chem. Soc. 2019, 142, 710–714. [Google Scholar] [CrossRef]
- Aouida, M.; Li, L.; Mahjoub, A.; Alshareef, S.; Ali, Z.; Piatek, A.; Mahfouz, M.M. Transcription activator-like effector nucleases mediated metabolic engineering for enhanced fatty acids production in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2015, 120, 364–371. [Google Scholar] [CrossRef]
- Wang, X.; Xia, X.; Huang, F.; Zhang, S. Genetic modification of secondary metabolite biosynthesis in higher plants: A review. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2012, 28, 1151–1163. [Google Scholar]
- Patra, B.; Schluttenhofer, C.; Wu, Y.; Pattanaik, S.; Yuan, L. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochim. Biophys. Acta 2013, 1829, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-F.; Zhu, Y.; Yu, Y.; Zhao, Q.; Wang, S.; Wang, X.; Yao, M.; Luo, D.; Li, X.; Chen, L.; et al. Global transcriptome and gene regulation network for secondary metabolite biosynthesis of tea plant (Camellia sinensis). BMC Genom. 2015, 16, 560. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Liu, Q.; Zha, R.; Xiong, A.; Wei, Q. Genome-Wide Identification, Expansion, and Evolution Analysis of Homeobox Gene Family Reveals TALE Genes Important for Secondary Cell Wall Biosynthesis in Moso Bamboo (Phyllostachys edulis). Int. J. Mol. Sci. 2022, 23, 4112. [Google Scholar] [CrossRef]
- Wen, S.; Liu, H.; Li, X.; Chen, X.; Hong, Y.; Li, H.; Lu, Q.; Liang, X. TALEN-mediated targeted mutagenesis of fatty acid desaturase 2 (FAD2) in peanut (Arachis hypogaea L.) promotes the accumulation of oleic acid. Plant Mol. Biol. 2018, 97, 177–185. [Google Scholar] [CrossRef]
- Curtin, S.J.; Xiong, Y.; Michno, J.M.; Campbell, B.W.; Stec, A.O.; Čermák, T.; Starker, C.; Voytas, D.F.; Eamens, A.L.; Stupar, R.M. Crispr/cas9 and talen s generate heritable mutations for genes involved in small rna processing of glycine max and medicago truncatula. Plant Biotechnol. J. 2018, 16, 1125–1137. [Google Scholar] [CrossRef]
- Jung, J.H.; Altpeter, F. TALEN mediated targeted mutagenesis of the caffeic acid O-methyltransferase in highly polyploid sugarcane improves cell wall composition for production of bioethanol. Plant Mol. Biol. 2016, 92, 131–142. [Google Scholar] [CrossRef]
- Haun, W.; Coffman, A.; Clasen, B.M.; Demorest, Z.L.; Lowy, A.; Ray, E.; Retterath, A.; Stoddard, T.; Juillerat, A.; Cedrone, F.; et al. Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 2014, 12, 934–940. [Google Scholar] [CrossRef]
- Li, J.; Stoddard, T.J.; Demorest, Z.L.; Lavoie, P.O.; Luo, S.; Clasen, B.M.; Cedrone, F.; Ray, E.E.; Coffman, A.P.; Daulhac, A.; et al. Multiplexed, targeted gene editing in Nicotiana benthamiana for glyco-engineering and monoclonal antibody production. Plant Biotechnol. J. 2016, 14, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Zhang, Y.; Chen, K.; Zhang, K.; Gao, C. Creation of fragrant rice by targeted knockout of the OsBADH2 gene using TALEN technology. Plant Biotechnol. J. 2015, 13, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of genetic diversity assessment in crop plants and its recent advances: An overview of its analytical perspectives. Genet. Res. Int. 2015, 2015, 431487. [Google Scholar] [CrossRef]
- Zhang, H.; Gou, F.; Zhang, J.; Liu, W.; Li, Q.; Mao, Y.; Botella, J.R.; Zhu, J.K. TALEN-mediated targeted mutagenesis produces a large variety of heritable mutations in rice. Plant Biotechnol. J. 2016, 14, 186–194. [Google Scholar]
- Acquaah, G. Conventional plant breeding principles and techniques. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Springer: Cham, Switzerland, 2015; pp. 115–158. [Google Scholar]
- Van de Wiel, C.C.M.; Schaart, J.G.; Lotz, L.A.P.; Smulders, M.J.M. New traits in crops produced by genome editing techniques based on deletions. Plant Biotechnol. Rep. 2017, 11, 1–8. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef]
- Schaart, J.G.; van de Wiel, C.C.M.; Smulders, M.J.M. Genome editing of polyploid crops: Prospects, achievements and bottlenecks. Transgenic Res. 2021, 30, 337–351. [Google Scholar] [CrossRef]
- Gan, Y.; Lin, Y.; Guo, Y.; Qi, X.; Wang, Q. Metabolic and genomic characterisation of stress-tolerant industrial Saccharomyces cerevisiae strains from TALENs-assisted multiplex editing. FEMS Yeast Res. 2018, 18, foy045. [Google Scholar] [CrossRef]
- Rosero, A.; Granda, L.; Berdugo-Cely, J.A.; Šamajová, O.; Šamaj, J.; Cerkal, R. A dual strategy of breeding for drought tolerance and introducing drought-tolerant, underutilized crops into production systems to enhance their resilience to water deficiency. Plants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Wang, L.-R.; Xu, Y.-S.; Jiang, W.-T.; Shi, T.-Q.; Sun, X.-M.; Huang, H. Recent advances in the application of multiplex genome editing in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 3873–3882. [Google Scholar] [CrossRef]
- Uauy, C.; Wulff, B.B.H.; Dubcovsky, J. Combining traditional mutagenesis with new high-throughput sequencing and genome editing to reveal hidden variation in polyploid wheat. Annu. Rev. Genet. 2017, 51, 435–454. [Google Scholar] [CrossRef]
- Holme, I.B.; Wendt, T.; Gil-Humanes, J.; Deleuran, L.C.; Starker, C.G.; Voytas, D.F.; Brinch-Pedersen, H. Evaluation of the mature grain phytase candidate HvPAPhy_a gene in barley (Hordeum vulgare L.) using CRISPR/Cas9 and TALENs. Plant Mol. Biol. 2017, 95, 111–121. [Google Scholar] [CrossRef]
- Römer, P.; Recht, S.; Strauß, T.; Elsaesser, J.; Schornack, S.; Boch, J.; Wang, S.; Lahaye, T. Promoter elements of rice susceptibility genes are bound and activated by specific TAL effectors from the bacterial blight pathogen, Xanthomonas oryzae pv. oryzae. New Phytol. 2010, 187, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Lu, K.-K.; Li, T.; Zhang, Y.; Guo, J.-X.; Song, R.; Liu, W.-C. Maize PHYTOMELATONIN RECEPTOR1 functions in plant osmotic and drought stress tolerance. J. Exp. Bot. 2021, 73, 5961–5973. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B.; Ibrahim, A.S.; Shawky, B.T. Enhancement of the expression of ZmBZR1 and ZmBES1 regulatory genes and antioxidant defense genes triggers water stress mitigation in maize (Zea mays L.) plants treated with 24-epibrassinolide in combination with spermine. Agronomy 2022, 12, 2517. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, G.; Yue, G.; Li, Z.; Qu, X.; Hou, G.; Zhu, Y.; Zhang, J. Effects of water-deficit stress on the transcriptomes of developing immature ear and tassel in maize. Plant Cell Rep. 2007, 26, 2137–2147. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, K.; Chen, K.; Gao, C. Targeted Mutagenesis in Zea mays Using TALENs and the CRISPR/Cas System. J. Genet. Genom. 2014, 41, 63–68. [Google Scholar] [CrossRef]
- Luo, M.; Li, H.; Chakraborty, S.; Morbitzer, R.; Rinaldo, A.; Upadhyaya, N.; Bhatt, D.; Louis, S.; Richardson, T.; Lahaye, T. Efficient TALEN-mediated gene editing in wheat. Plant Biotechnol. J. 2019, 17, 2026. [Google Scholar] [CrossRef]
- Xiao, X.; Ohm, H.W.; Hunt, G.J.; Poland, J.A.; Kong, L.; Nemacheck, J.A.; Williams, C.E. Genotyping-by-sequencing to remap QTL for type II Fusarium head blight and leaf rust resistance in a wheat–tall wheatgrass introgression recombinant inbred population. Mol. Breed. 2016, 36, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Zong, Y.; Gao, C. Targeted Mutagenesis in Hexaploid Bread Wheat Using the TALEN and CRISPR/Cas Systems. Methods Mol. Biol. 2017, 1679, 169–185. [Google Scholar] [CrossRef]
- Rehman, S.; Ul Rehman, I.; Jan, B.; Rashid, I.; Ah Reshi, Z.; Ganie, A.H. Chapter 6—Genome editing: Applications for medicinal and aromatic plants. In Medicinal and Aromatic Plants; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 119–144. [Google Scholar]
- Raza, A.; Ashraf, F.; Zou, X.; Zhang, X.; Tosif, H. Plant adaptation and tolerance to environmental stresses: Mechanisms and perspectives. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Springer: Singapore, 2020; pp. 117–145. [Google Scholar]
- Bohnert, H.J.; Nelson, D.E.; Jensen, R.G. Adaptations to environmental stresses. Plant Cell 1995, 7, 1099. [Google Scholar] [CrossRef]
- Solbrig, O.T. Plant traits and adaptive strategies: Their role in ecosystem function. In Biodiversity and Ecosystem Function; Springer: Berlin/Heidelberg, Germany, 1994; pp. 97–116. [Google Scholar]
- Mareri, L.; Parrotta, L.; Cai, G. Environmental stress and plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2009; pp. 153–188. [Google Scholar]
- Carillo, P.; Annunziata, M.G.; Pontecorvo, G.; Fuggi, A.; Woodrow, P. Salinity stress and salt tolerance. Abiotic Stress Plants-Mech. Adapt. 2011, 1, 21–38. [Google Scholar]
- Bhatla, S.C.; Lal, M.A.; Kathpalia, R.; Sisodia, R.; Shakya, R. Biotic stress. In Plant Physiology, Development and Metabolism; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1029–1095. [Google Scholar]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of plant responses to water stress and related genes: A review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Duque, A.S.; de Almeida, A.M.; da Silva, A.B.; da Silva, J.M.; Farinha, A.P.; Santos, D.; Fevereiro, P.; de Sousa Araújo, S. Abiotic stress responses in plants: Unraveling the complexity of genes and networks to survive. In Abiotic Stress-Plant Responses and Applications in Agriculture; IntechOpen: London, UK, 2013. [Google Scholar]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Ma, F.; Wei, J.; Li, H.; Ma, H.; Sun, P. Physiological functions of heat shock proteins. Curr. Protein Pept. Sci. 2020, 21, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Nürnberger, T.; Kemmerling, B. Pathogen-associated molecular patterns (PAMP) and PAMP-triggered immunity. Annu. Plant Rev. 2009, 34. [Google Scholar]
- Jwa, N.-S.; Agrawal, G.K.; Tamogami, S.; Yonekura, M.; Han, O.; Iwahashi, H.; Rakwal, R. Role of defense/stress-related marker genes, proteins and secondary metabolites in defining rice self-defense mechanisms. Plant Physiol. Biochem. 2006, 44, 261–273. [Google Scholar] [CrossRef]
- Wang, Y.; Pruitt, R.N.; Nürnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef]
- Ayan, A.L.P.; Meriç, S.; Atak, Ç. Molecular abiotic stress tolerans strategies: From genetic engineering to genome editing era. In Abiotic Stress in Plants; IntechOpen: London, UK, 2020. [Google Scholar]
- Mishra, U.N.; Saha, D.; Chauhan, J.; Kumar, V.; Jatav, H.S.; Lal, D.; Kumari, A.; Singhal, R.K.; Chandra, K. Emerging roles of Osmoprotectants in response to multiple abiotic stress tolerance in plants. In Omics Analysis of Plants Under Abiotic Stress; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 179–206. [Google Scholar]
- Shahwar, D.; Ahn, N.; Kim, D.; Ahn, W.; Park, Y. Mutagenesis-based plant breeding approaches and genome engineering: A review focused on tomato. Mutat. Res./Rev. Mutat. Res. 2023, 792, 108473. [Google Scholar] [CrossRef]
- Takáč, T.; Křenek, P.; Komis, G.; Vadovič, P.; Ovečka, M.; Ohnoutková, L.; Pechan, T.; Kašpárek, P.; Tichá, T.; Basheer, J. TALEN-based HvMPK3 knock-Out attenuates proteome and root hair phenotypic responses to flg22 in barley. Front. Plant Sci. 2021, 12, 666229. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, F.; Li, Z.; Zhang, J.; Li, X.; Dong, J.; Wang, T. TALEN-based mutagenesis of lipoxygenase LOX3 enhances the storage tolerance of rice (Oryza sativa) seeds. PLoS ONE 2015, 10, e0143877. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Chen, C.Y.; Yang, B. TALEN-Mediated Homologous Recombination Produces Site-Directed DNA Base Change and Herbicide-Resistant Rice. J. Genet. Genom. 2016, 43, 297–305. [Google Scholar] [CrossRef]
- Li, J.; Guo, X.; Zhang, M.; Wang, X.; Zhao, Y.; Yin, Z.; Zhang, Z.; Wang, Y.; Xiong, H.; Zhang, H.; et al. OsERF71 confers drought tolerance via modulating ABA signaling and proline biosynthesis. Plant Sci. 2018, 270, 131–139. [Google Scholar] [CrossRef]
- Singha, D.; Sarma, S.; Singh, S. Understanding the mode of regulation of proline biosynthesis for drought tolerance in transgenic rice overexpressing PDH47 gene. Indian. J. Biotechnol. 2020, 19, 73–81. [Google Scholar]
- Li, Y.; Qin, L.; Zhao, J.; Muhammad, T.; Cao, H.-H.; Li, H.; Zhang, Y.; Liang, Y. SlMAPK3 enhances tolerance to tomato yellow leaf curl virus (TYLCV) by regulating salicylic acid and jasmonic acid signaling in tomato (Solanum lycopersicum). PLoS ONE 2017, 12, e0172466. [Google Scholar] [CrossRef]
- Dolatabadi, B.; Ranjbar, G.; Tohidfar, M.; Dehestani, A. Genetic transformation of Tomato with three pathogenesis-related protein genes for increased resistance to Fusarium oxysporum f.sp. lycopersici. J. Plant Mol. Breedin 2014, 2, 1–11. [Google Scholar] [CrossRef]
- Schwessinger, B.; Bahar, O.; Thomas, N.; Holton, N.; Nekrasov, V.; Ruan, D.; Canlas, P.; Daudi, A.; Petzold, C.; Singan, V.; et al. Transgenic expression of the dicotyledonous pattern recognition receptor EFR in rice leads to ligand-dependent activation of defense responses. bioRxiv 2014. [Google Scholar] [CrossRef]
- Bergmann, T.; Schulz, E.; Ehrhardt, A. Progress and problems with viral vectors for delivery of talens. J. Mol. Genet. Med. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Mahfouz, M.M.; Piatek, A.; Stewart, C.N., Jr. Genome engineering via TALENs and CRISPR/Cas9 systems: Challenges and perspectives. Plant Biotechnol. J. 2014, 12, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, M.Y. Genome editing: Development and regulatory challenges. Cell. Ther. Transplant. 2017, 6, 60–63. [Google Scholar] [CrossRef]
- Zhao, H.; Wolt, J.D. Risk associated with off-target plant genome editing and methods for its limitation. Emerg. Top. Life Sci. 2017, 1, 231–240. [Google Scholar] [CrossRef]
- Zheng, N.; Li, L.; Wang, X. Molecular mechanisms, off-target activities, and clinical potentials of genome editing systems. Clin. Transl. Med. 2020, 10, 412–426. [Google Scholar] [CrossRef]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas systems in genome editing: Methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Hendel, A.; Fine, E.J.; Bao, G.; Porteus, M.H. Quantifying on-and off-target genome editing. Trends Biotechnol. 2015, 33, 132–140. [Google Scholar] [CrossRef]
- Bachtarzi, H.; Farries, T. The genetically modified organism medicinal framework in Europe, United States, and Japan: Underlying scientific principles and considerations toward the development of gene therapy and genetically modified cell-based products. Hum. Gene Ther. Clin. Dev. 2019, 30, 114–128. [Google Scholar] [CrossRef]
- Alexandrova, N.; Georgieva, K.; Atanassov, A. Biosafety regulations of GMOs: National and international aspects and regional cooperation. Biotechnol. Biotechnol. Equip. 2005, 19, 153–172. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R. Genetically Modified Organisms in Food: Production, Safety, Regulation and Public Health; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Wunderlich, S.; Gatto, K.A. Consumer perception of genetically modified organisms and sources of information. Adv. Nutr. 2015, 6, 842–851. [Google Scholar] [CrossRef]
- Yeh, S.-C.; Chen, P.-X.; Lin, C.-C.; Chiang, I.C. Genetically modified organisms and sustainable development goals: A survey of Taiwanese public opinion. PLoS ONE 2025, 20, e0325790. [Google Scholar] [CrossRef] [PubMed]
- González Castro, N.; Bjelic, J.; Malhotra, G.; Huang, C.; Alsaffar, S.H. Comparison of the feasibility, efficiency, and safety of genome editing technologies. Int. J. Mol. Sci. 2021, 22, 10355. [Google Scholar] [CrossRef]
- Hussain, A.; Imran, Q.M.; Yun, B.-W. CRISPR/Cas9-mediated gene editing in grain crops. In Recent Advances in Grain Crops Research; IntechOpen: London, UK, 2019. [Google Scholar]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Advances in cereal crop genomics for resilience under climate change. Life 2021, 11, 502. [Google Scholar] [CrossRef]
- Frank, S.; Skryabin, B.V.; Greber, B. A modified TALEN-based system for robust generation of knock-out human pluripotent stem cell lines and disease models. Bmc Genom. 2013, 14, 773. [Google Scholar] [CrossRef]
- Reyon, D.; Tsai, S.Q.; Khayter, C.; Foden, J.A.; Sander, J.D.; Joung, J.K. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar] [CrossRef]
- Wu, T.; Hu, Y.; Tang, L.V. Gene therapy for polygenic or complex diseases. Biomark. Res. 2024, 12, 99. [Google Scholar] [CrossRef]
- Arya, A.; Gautam, S.; Goel, S.; Grewal, S.; Bhattacharyya, M. Metabolic engineering for high-value bioactive compounds from medicinal plants. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 521–544. [Google Scholar]
- Kanchiswamy, C.N.; Maffei, M.; Malnoy, M.; Velasco, R.; Kim, J.-S. Fine-tuning next-generation genome editing tools. Trends Biotechnol. 2016, 34, 562–574. [Google Scholar] [CrossRef]
- Mahfouz, M.M.; Li, L. TALE nucleases and next generation GM crops. GM Crops 2011, 2, 99–103. [Google Scholar] [CrossRef]
- Beurdeley, M.; Bietz, F.; Li, J.; Thomas, S.; Stoddard, T.; Juillerat, A.; Zhang, F.; Voytas, D.F.; Duchateau, P.; Silva, G.H. Compact designer TALENs for efficient genome engineering. Nat. Commun. 2013, 4, 1762. [Google Scholar] [CrossRef]
- Llewellyn, N.; Miller, J.C.; Campo, J.; Exline, C.; Mulhern, O.; Henley, J.; Wang, J.; Guschin, D.; Xia, D.; Ankoudinova, I. 184. Next Generation TALENs Mediate Efficient Disruption of the CCR5 Gene in Human HSCs. Mol. Ther. 2013, 21, 1. [Google Scholar] [CrossRef]
- Ain, Q.U.; Chung, J.Y.; Kim, Y.-H. Current and future delivery systems for engineered nucleases: ZFN, TALEN and RGEN. J. Control. Release 2015, 205, 120–127. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 structures and mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Singh, R.; Prakash, A.; Raza, S.H.A.; Cavalu, S.; Chopra, C.; Madkour, M.; Elolimy, A.; Hashem, N.M. Genome centric engineering using ZFNs, TALENs and CRISPR-Cas9 systems for trait improvement and disease control in Animals. Vet. Res. Commun. 2023, 47, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, L.; Rossi, J.J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar] [CrossRef]
- Ali, H. Artificial intelligence in multi-omics data integration: Advancing precision medicine, biomarker discovery and genomic-driven disease interventions. Int. J. Sci. Res. Arch. 2023, 8, 1012–1030. [Google Scholar]
- Luo, M. AI-Assisted Genomic Prediction Models in Cotton Breeding. Cotton Genom. Genet. 2025, 16, 137–147. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gao, S.; Hassan, M.A.; Huang, Z.; Rasheed, A.; Hearne, S.; Prasanna, B.; Li, X.; Li, H. Artificial intelligence in plant breeding. Trends Genet. 2024, 40, 891–908. [Google Scholar] [CrossRef]
- Pandey, A.K. Genome editing for value addition in medicinal plants. In Medicinal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2025; pp. 465–478. [Google Scholar]
- Balažová, A.; Balažová, E. Biotechnological techniques in the production of plant-made pharmaceuticals. De Remediis 2024, 1, 11. [Google Scholar] [CrossRef]
- Hammouti, Y.; Marhri, A.; Addi, M. Integrated Molecular Strategies for Enhanced Production of Terpenes, Terpenoids, and Essential Oils from Medicinal Plants. In Biotechnology, Multiple Omics, and Precision Breeding in Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2025; pp. 333–340. [Google Scholar]
- Balasubramaniam, S.; Eswaran, S.P.; Sakthivel, A.; Subramani, M.; Gnanajothi, K. Genetic Engineering and Genome Remodeling for Enhanced Production of Therapeutic Terpenes and Essential Oils of Lamiaceae. In Biotechnology, Multiple Omics, and Precision Breeding in Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2025; pp. 366–388. [Google Scholar]
| Feature | TALENs | CRISPR-Cas9 | ZFNs | Citations |
|---|---|---|---|---|
| Specificity | High specificity due to protein-DNA interactions, reducing off-target effects | Can have off-target effects due to reliance on RNA sequences (guide RNAs) | Similar to TALENs in specificity but less precise in some contexts | [36,45] |
| Target Range | Can target a broader range of DNA sequences without sequence motif restrictions | Limited by PAM (Protospacer Adjacent Motif) sequence availability | Limited by target DNA sequence and zinc-finger binding specificity | [45] |
| Suitability for Complex Genomes | Ideal for editing complex genomes where off-target mutations are a concern | Less suitable for complex genomes due to potential off-target effects | Can be used for complex genomes, but less flexible than TALENs | [41,46] |
| Risk of Genomic Rearrangements | Lower risk of genomic rearrangements or insertions at off-target sites | Higher risk of off-target genomic rearrangements | Higher risk of off-target genomic rearrangements | [47,48] |
| Flexibility in Genome Editing | High flexibility, can target nearly any sequence in the genome | Limited by PAM sequences, reducing flexibility in certain regions | Can target a variety of sequences, but less flexible than TALENs | [10,12] |
| Cost and Construction | Expensive and time-consuming to design and construct | Easier and more cost-effective to design and implement | Expensive and time-consuming to design and construct | [36,37] |
| Ease of Use | Labor-intensive, requires iterative design and validation | Simpler design, widely accessible and easier to use | Requires extensive optimization, less user-friendly than CRISPR-Cas9 | [37,49] |
| Application in Plant Species | Effective in both dicots and monocots, even with complex or poorly characterized genomes | Variable success in different plant species due to PAM sequence availability | Effective, but less flexible than TALENs for targeting specific plant genomes | [41] |
| Strategy/Approach | Target Genes/Enzymes | Examples of Secondary Metabolites | Impact of TALEN Modification | Reference |
|---|---|---|---|---|
| Enhancing Production by Targeting Key Enzymes | Terpene Synthases (e.g., TPS1, TPS2), Flavonoid Synthases (e.g., CHS, F3H), Cytochrome P450s (e.g., CYPs) | Terpenoids (e.g., limonene, artemisinin), Flavonoids, Phenylpropanoids | TALENs can directly target key genes in the biosynthesis of terpenoids and flavonoids, enhancing the production of valuable secondary metabolites for medicinal, fragrance, and flavor uses | [45] |
| Manipulating Precursor Pathways | Amino Acid Synthesis Genes (e.g., TRP1, TAT1), DAHPS (Shikimate Pathway), AroB (Aromatic Pathway) | Alkaloids (e.g., nicotine, morphine), Amino Acid-Derived Compounds (e.g., tryptophan derivatives) | TALENs can modify precursor pathways, enhancing the availability of amino acids and redirecting metabolic flow to increase alkaloid and other valuable secondary metabolite production | [82] |
| Targeting Regulatory Proteins | MYB Transcription Factors (e.g., MYB46, MYB75), bHLH Factors, WRKY Transcription Factors | Various secondary metabolites (e.g., terpenoids, flavonoids, alkaloids) | TALENs can be used to activate or suppress transcription factors involved in regulating secondary metabolic pathways, enabling increased production of targeted metabolites | [83] |
| Multiplex Editing for Enhanced Production | Multiple Genes in the Flavonoid Pathway (e.g., CHS, F3H, F3′H, DFR, ANS) | Flavonoids, Lignans, Alkaloids | TALENs can be used to target multiple genes within the same pathway, leading to an enhanced overall yield of secondary metabolites while improving plant traits | [84,85] |
| Combination with Metabolic Engineering | Shikimate Pathway Enzymes (e.g., DAHPS, AroB), Precursor Enzymes (e.g., Tyrosine Decarboxylase, Tryptophan Synthase) | Aromatic compounds (e.g., flavonoids, lignans), Alkaloids | TALENs can be combined with metabolic engineering to optimize precursor production, enhancing the efficiency of secondary metabolite biosynthesis |
| Stress Type | Targeted Mechanism/Genes | Example of TALEN-Mediated Modification | Impact of Modification | References |
|---|---|---|---|---|
| Abiotic Stress | Water Retention/Osmotic Regulation Genes encoding osmoprotectants (e.g., Proline Synthesis Genes, P5CS gene) | TALENs used to modify genes involved in proline biosynthesis in rice and tomato to enhance drought tolerance | Increased proline production | [135] |
| Abiotic Stress | Ion Transport Genes encoding Sodium-Potassium Transporters (e.g., HKT1 for sodium uptake, NHX1 for vacuolar Na+/H+ exchange) | TALENs targeting genes for improved salt tolerance in rice and tomato, specifically targeting HKT1 and NHX1 genes | Enhanced salt tolerance | [136] |
| Abiotic Stress | Temperature Stress (Heat and Cold Tolerance) Genes encoding Heat Shock Proteins (HSPs) and Antifreeze Proteins (e.g., HSP70, COR15a) | TALENs used to modify HSP70 and COR15a genes in tobacco and Arabidopsis to enhance heat and cold tolerance | Increased expression of heat shock proteins (HSPs) | |
| Biotic Stress | Plant Immunity Genes, Genes encoding Antimicrobial Peptides (AMPs), Defensin-like Proteins, Defense Signaling Pathways (e.g., SA and JA pathways) | TALENs used to enhance resistance to Fusarium wilt and bacterial blight in tomato by targeting defense-related genes like PR1 (pathogenesis-related protein) and PR2 (glucanase) | TALEN-induced overexpression of defense-related genes like PR1 and PR2 | [137,138] |
| Biotic Stress | Pathogen Recognition Genes involved in Pattern Recognition Receptors (PRRs) (e.g., FLS2, EFR) for pathogen detection. | TALENs employed to enhance recognition of PAMPs (Pathogen-Associated Molecular Patterns) in Arabidopsis and rice by targeting FLS2 (flagellin receptor) and EFR (elongation factor receptor) genes. | Enhanced pathogen recognition | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, W.; Khalil, A.A.K.; Amin, A. TALEN-Interceded Genome Editing in Plants: Unveiling New Frontiers in Secondary Metabolite Improvement and Genetic Diversity. Plants 2025, 14, 3024. https://doi.org/10.3390/plants14193024
Zaman W, Khalil AAK, Amin A. TALEN-Interceded Genome Editing in Plants: Unveiling New Frontiers in Secondary Metabolite Improvement and Genetic Diversity. Plants. 2025; 14(19):3024. https://doi.org/10.3390/plants14193024
Chicago/Turabian StyleZaman, Wajid, Atif Ali Khan Khalil, and Adnan Amin. 2025. "TALEN-Interceded Genome Editing in Plants: Unveiling New Frontiers in Secondary Metabolite Improvement and Genetic Diversity" Plants 14, no. 19: 3024. https://doi.org/10.3390/plants14193024
APA StyleZaman, W., Khalil, A. A. K., & Amin, A. (2025). TALEN-Interceded Genome Editing in Plants: Unveiling New Frontiers in Secondary Metabolite Improvement and Genetic Diversity. Plants, 14(19), 3024. https://doi.org/10.3390/plants14193024









