Drainage Recycling Ratio Influences Yield, Fruit Quality, and Antioxidant Properties of Korean Strawberry ‘Seolhyang’
Abstract
1. Introduction
2. Results and Discussion
2.1. Inorganic Ionic Contents in Supplied Nutrient Solution
2.2. Strawberry Productivity
2.3. Antioxidant Contents in Strawberry Fruits
2.4. Principal Component Analysis
3. Materials and Methods
3.1. Plant Cultivation and Greenhouse Environment
3.2. Experimental Drainage Recycling Ratio Design
3.3. Ionic Concentration Measurement in Nutrient Solutions
3.4. Yield, Fruit Quality, and Antioxidant Concentration Investigation
3.4.1. Yield and Fruit Quality Investigation
3.4.2. Determination of Fruit Development Period
3.4.3. Antioxidant Concentration Measurement
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Electrical conductivity |
| TSS | Total soluble solids content |
| TA | Titratable acidity |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| FRAP | Ferric reducing antioxidant power |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| PCA | Principal component analysis |
References
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/metadata (accessed on 1 August 2025).
- KOSIS. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1AG20409&conn_path=I2 (accessed on 1 August 2025).
- Poling, E.B. Strawberry Plant Structure and Growth Habit. New York State Berry Growers Association, Berry EXPO. 2012. Available online: http://www.hort.cornell.edu/expo/proceedings/2012/Berries/Berry%20Plant%20Structure%20Poling.pdf (accessed on 1 August 2025).
- Oğyz, İ.; Oğyz, H.İ.; Kafkas, N.E. Strawberry cultivation techniques. In Recent Studies on Strawberries; IntechOpen: London, UK, 2022; pp. 3–23. [Google Scholar]
- Asaduzzaman, M.; Asao, T. Autotoxicity in strawberry under recycled hydroponics and its mitigation methods. Hortic. J. 2020, 89, 124–137. [Google Scholar] [CrossRef]
- Jang, D.C.; Choi, K.Y.; Kim, I.S. Effect of drainage reusing ratio on growth and yield of summer-cultivated paprika in recycling hydroponic cultivation. Prot. Hortic. Plant Fact. 2017, 26, 7–12. [Google Scholar] [CrossRef]
- Ahn, T.I.; Shin, J.W.; Son, J.E. Analysis of changes in ion concentration with time and drainage ratio under EC-based nutrient control in closed-loop soilless culture for sweet pepper plants (Capsicum annum L. ‘Boogie’). J. Bio-Environ. Control. 2010, 19, 298–304. [Google Scholar]
- Lim, M.Y.; Kim, S.H.; Roh, M.Y.; Choi, G.L.; Kim, D.I. Nutrient dynamics and resource-use efficiency in greenhouse strawberries: Effects of control variables in closed-loop hydroponics. Horticulturae 2024, 10, 851. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Soil solution. In Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; pp. 33–52. [Google Scholar]
- Carillo, P.; Rouphael, Y. Nitrate uptake and use efficiency: Pros and cons of chloride interference in the vegetable crops. Front. Plant Sci. 2022, 13, 899522. [Google Scholar] [CrossRef] [PubMed]
- Adak, N. Effect of different K+/Ca2+ ratios on yield, quality and physiological disorder in soilless strawberry cultivation. Acta Sci. Pol. Hortorum Cultus 2019, 18, 229–236. [Google Scholar] [CrossRef]
- Yeo, K.H.; Choi, G.L.; Lee, J.H.; Park, K.S.; Choi, K.Y. Development of nutrient solution compositions for paprika cultivation in a closed coir substrate hydroponic system in Republic of Korea’s winter cropping season. Horticulturae 2023, 9, 412. [Google Scholar] [CrossRef]
- Galli, V.; Messias, R.S.; Perin, E.C.; Borowski, J.M.; Bamberg, A.L.; Rombaldi, C.V. Mild salt stress improves strawberry fruit quality. LWT 2016, 73, 693–699. [Google Scholar] [CrossRef]
- Alnayef, M.; Hartley, J.; Orsini, F.; Silvestro, R.D.; Sanoubar, R.; Marotti, I.; Gianquinto, G.; Dinelli, G.; Puniran-Hartley, N. Using salinity to improve nutritional and market value of strawberries. Aust. J. Crop Sci. 2022, 16, 7–17. [Google Scholar] [CrossRef]
- Bugbee, B. Nutrient management in recirculating hydroponic culture. In Proceedings of the South Pacific Soilless Culture Conference-SPSCC, Palmerston North, New Zealand, 10–13 February 2003. [Google Scholar]
- Savvas, D.; Prosdocimi Gianquinto, G.; Tuzel, Y.; Gruda, N. Soilless culture. In Good Agricultural Practices for Greenhouse Vegetable Crops Principles for Mediterranean Climate Agreas; Food and Agriculture Organization of the United Nations (FAO-UN): Rome, Italy, 2013; pp. 303–354. [Google Scholar]
- Daugovish, O.; Larson, K.D. Strawberry production with protected culture in southern California. Acta Hortic. 2009, 842, 163–166. [Google Scholar] [CrossRef]
- Menzel, C. Higher temperature decrease fruit size in strawberry growing in the subtropics. Horticulturae 2021, 7, 34. [Google Scholar] [CrossRef]
- Simkova, K.; Veberic, R.; Hudina, M.; Crohar, M.C.; Ivancic, T.; Smrke, T.; Pelacc, M.; Jakopic, J. Variability in ‘Capri’ ever-bearing strawberry quality during a harvest seasons. Foods 2023, 12, 1349. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Egea, I.; Massaretto, I.L.; Morales, B.; Purgatto, E.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Traditional Tomato Varieties Improve Fruit Quality Without Affecting Fruit Yield Under Moderate Salt Stress. Front. Plant Sci. 2020, 11, 587754. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Nutrient management in strawberry: Effects of yield, quality and plant health. In Strawberries; Malone, N., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2014; pp. 239–267. [Google Scholar]
- Choi, J.M.; Latigui, A.; Yoon, M.K. Growth and nutrient uptake of ‘Seolhyang’ strawberry (Fragaria × ananassa Duch) responded to elevated nitrogen concentrations in nutrient solution. Korean J. Hortic. Sci. Technol. 2010, 28, 777–782. [Google Scholar]
- Jiang, W.; Zhang, J.; Jia, Z.H.; Zhang, T.; Zhang, W.J.; Wei, M. Physiological and nutrient responses to nitrogen, phosphorus, or potassium deficiency of hydroponically grown strawberry. HortScience 2023, 58, 628–634. [Google Scholar] [CrossRef]
- Mackenzie, S.J.; Chandler, C.K.; Hasing, T.; Whitaker, V.M. The role of temperature in the late-season decline in soluble solids content of strawberry fruit in a sub-tropical production system. HortScience 2011, 46, 1562–1566. [Google Scholar] [CrossRef]
- Agehara, S.; Nunes, M.C.d.N. Season and Nitrogen Fertilization Effects on Yield and Physicochemical Attributes of Strawberry under Subtropical Climate Conditions. Agronomy 2021, 11, 1391. [Google Scholar] [CrossRef]
- Cardeñosa, V.; Medrano, E.; Lorenzo, P.; Sánchez-Guerrero, M.C.; Cuevas, F.; Pradas, I.; Moreno-Rojas, J.M. Effects of salinity and nitrogen supply on the quality and health-related compounds of strawberry fruits (Fragaria × ananassa cv. Primoris). J. Sci. Food Agric. 2015, 95, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Agüero, J.J.; Salazar, S.M.; Kirschbaum, D.S.; Jerez, E.F. Factors affecting fruit quality in strawberries grown in a subtropical environment. Int. J. Fruit Sci. 2015, 15, 223–234. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Yao, T.; Wang, K.; Xu, J.; Zhang, H.; Qi, S.; Ao, H.; Qin, B.; Zhang, H. The homeostasis of ions and reactive oxygen species in root and shoot play crucial roles in the tolerance of alfalfa to salt alkali stress. Plant Physiol. Biochem. 2024, 216, 109175. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Keutgen, A.J.; Pawelzik, E. Modifications of strawberry fruit antioxidant pools and fruit quality under NaCl stress. J. Agric. Food Chem. 2007, 55, 4066–4072. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plants growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Zebro, M.; Heo, J.Y. Salicylic acid treatment improves the shelf life and quality of ‘Cheonghyang’ grapes during cold storage. S. Afr. J. Enol. Vitic. 2024, 45, 58–65. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]

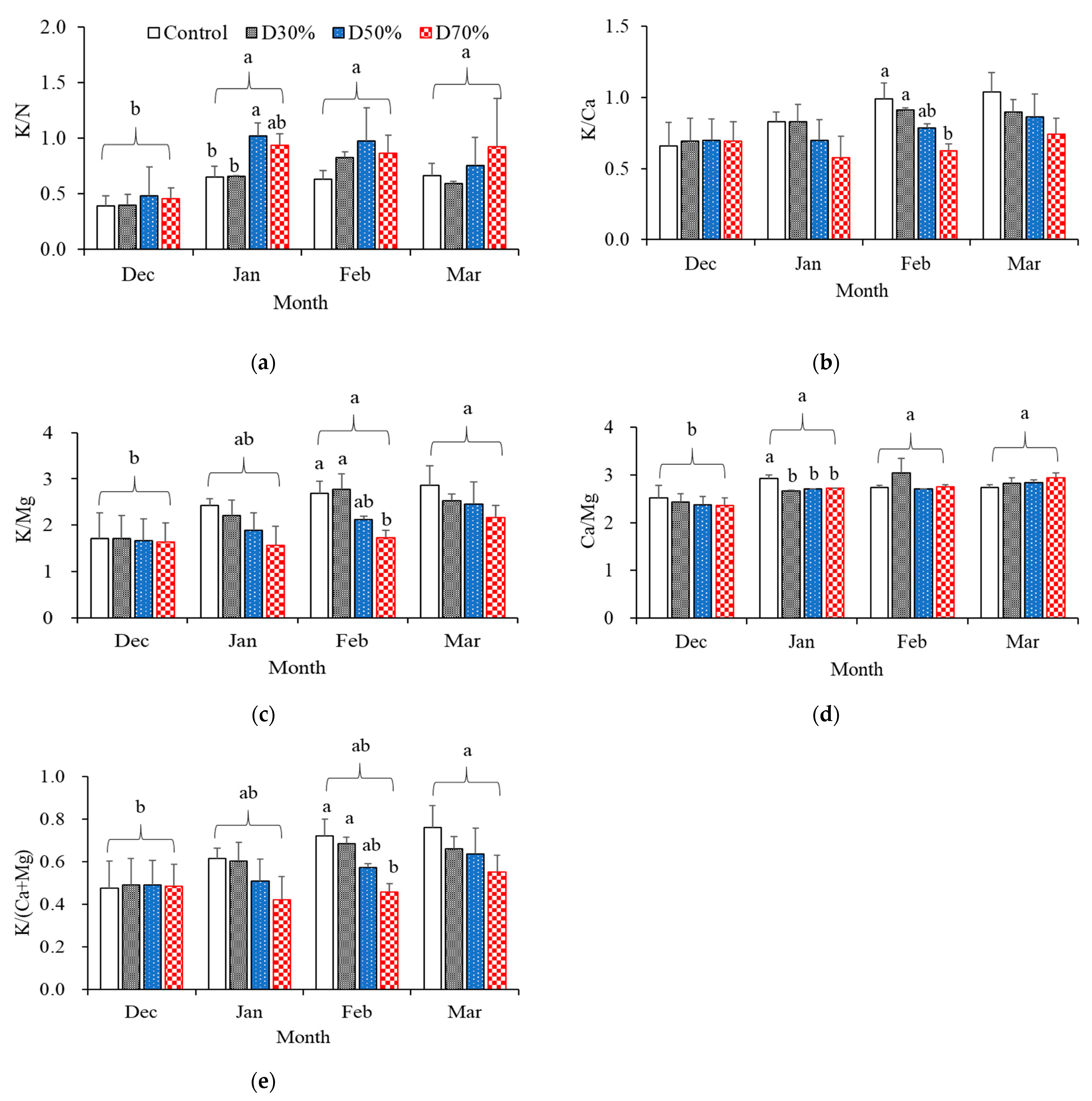

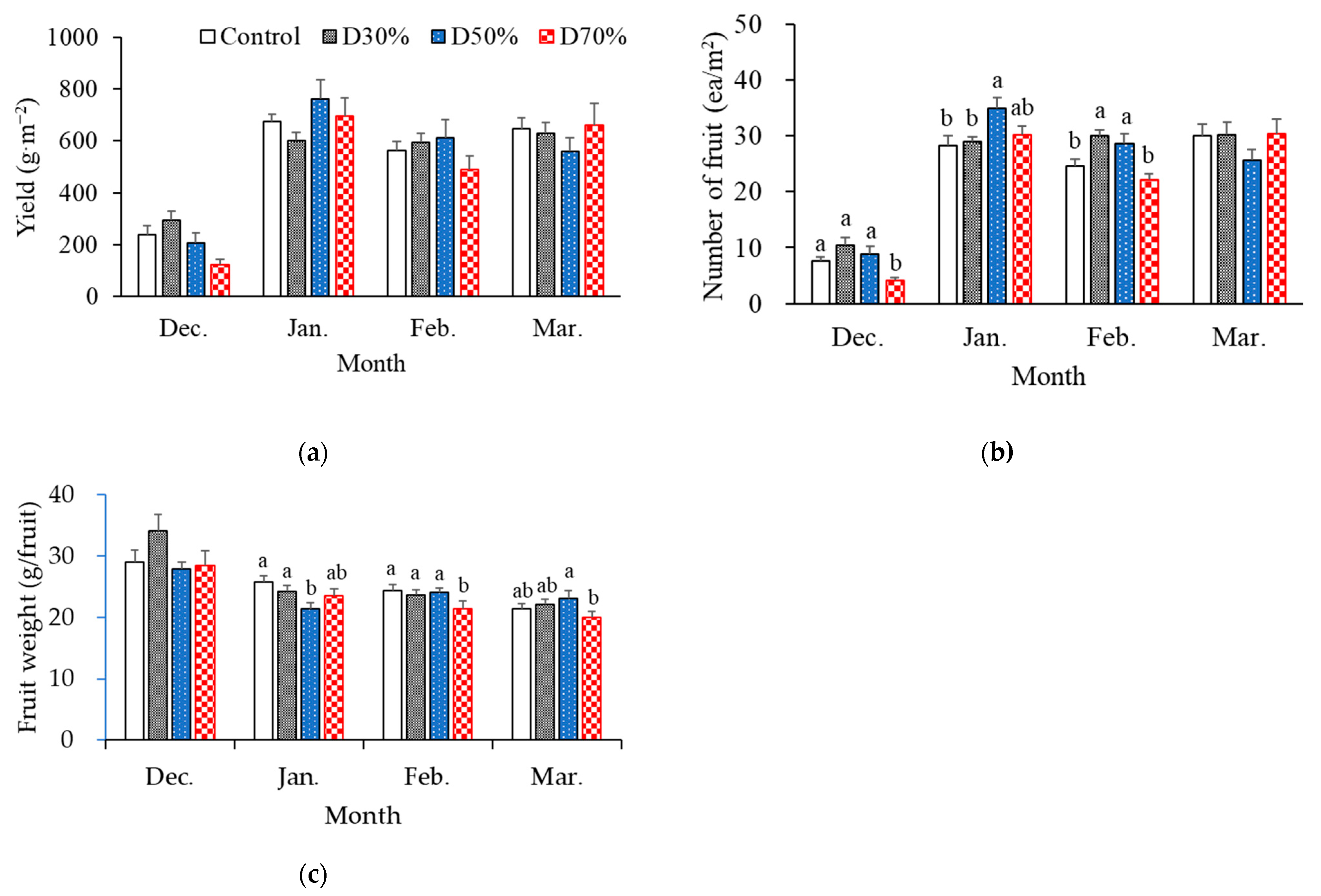



| Treatment | NO3− | NH4+ | PO43− | K+ | Ca2+ | Mg2+ | SO42− | Total | Na+ | Cl− | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cation | Anion | C/A | ||||||||||
| --------------------------------------------------------(mEq·L−1) -------------------------------------------------------- | ||||||||||||
| December | ||||||||||||
| Control | 7.1 a 2 | 0.6 | 1.6 | 3.0 | 4.6 | 1.9 | 2.2 | 9.4 | 10.9 ab | 0.87 | 1.5 c | 1.5 b |
| D30% | 6.9 a | 0.5 | 1.6 | 3.0 | 4.4 | 1.8 | 2.6 | 9.2 | 11.2 a | 0.82 | 1.8 bc | 1.8 a |
| D50% | 5.6 b | 0.5 | 1.3 | 2.9 | 4.3 | 1.8 | 2.4 | 9.1 | 9.4 b | 1.08 | 2.0 ab | 1.6 a |
| D70% | 6.0 c | 0.5 | 1.4 | 3.0 | 4.3 | 1.8 | 2.9 | 9.1 | 10.3 ab | 0.89 | 2.2 a | 1.9 a |
| January | ||||||||||||
| Control | 5.5 a | 0.7 a | 2.8 | 4.0 a | 4.9 a | 1.7 a | 1.6 c | 10.5 a | 9.9 a | 1.07 bc | 1.6 c | 1.3 b |
| D30% | 5.1 a | 0.3 b | 2.3 | 3.6 ab | 4.3 b | 1.6 b | 2.2 a | 9.4 ab | 9.6 a | 1.03 c | 2.3 bc | 1.7 a |
| D50% | 2.8 b | 0.3 b | 1.3 | 3.0 ab | 4.3 b | 1.6 b | 1.8 bc | 9.0 b | 5.7 b | 1.75 a | 2.8 ab | 1.3 b |
| D70% | 2.6 b | 0.3 b | 1.2 | 2.6 b | 4.6 ab | 1.7 a | 2.0 ab | 8.9 b | 5.8 b | 1.57 ab | 3.3 a | 1.6 ab |
| February | ||||||||||||
| Control | 6.4 a | 0.5 a | 3.8 | 4.4 a | 4.5 | 1.6 | 1.8 | 10.5 a | 12.0 a | 0.90 b | 1.6 d | 1.5 |
| D30% | 4.7 ab | 0.3 b | 2.7 | 4.1 ab | 4.6 | 1.5 | 1.7 | 10.2 ab | 9.1 ab | 1.13 ab | 2.0 c | 1.5 |
| D50% | 3.5 b | 0.3 bc | 2.1 | 3.7 ab | 4.7 | 1.7 | 1.7 | 10.1 ab | 7.3 b | 1.53 a | 2.7 b | 1.5 |
| D70% | 3.3 b | 0.1 c | 2.1 | 2.9 b | 4.7 | 1.7 | 2.3 | 9.3 b | 7.7 ab | 1.29 ab | 3.5 a | 2.1 |
| March | ||||||||||||
| Control | 6.7 a | 0.6 a | 4.1 | 4.8 | 4.7 | 1.7 | 1.9 c | 11.2 | 12.7 | 0.89 | 1.7 b | 1.6 c |
| D30% | 6.9 a | 0.6 a | 4.0 | 4.4 | 4.9 | 1.7 | 2.1 bc | 11.0 | 13.0 | 0.84 | 1.8 b | 1.8 c |
| D50% | 5.3 b | 0.3 ab | 3.5 | 4.2 | 5.0 | 1.7 | 2.5 ab | 10.9 | 11.2 | 0.99 | 3.2 ab | 2.3 b |
| D70% | 4.2 b | 0.1 b | 3.2 | 4.0 | 5.3 | 1.8 | 3.0 a | 11.1 | 10.5 | 1.11 | 4.9 a | 2.8 a |
| Overall mean | ||||||||||||
| Control | 6.5 ± 0.3 | 0.6 ± 0.0 | 3.0 ± 0.5 | 4.0 ± 0.3 | 4.6 ± 0.1 | 1.7 ± 0.0 | 1.9 ± 0.1 | 10.4 ± 0.3 | 11.5 ± 0.5 | 0.9 ± 0.0 | 1.6 ± 0.0 | 1.5 ± 0.1 |
| D30% | 6.1 ± 0.5 | 0.4 ± 0.1 | 2.7 ± 0.4 | 3.7 ± 0.3 | 4.6 ± 0.1 | 1.7 ± 0.1 | 2.2 ± 0.2 | 10.0 ± 0.4 | 11.0 ± 0.8 | 0.9 ± 0.1 | 2.0 ± 0.1 | 1.7 ± 0.1 |
| D50% | 4.5 ± 0.6 | 0.3 ± 0.1 | 2.1 ± 0.4 | 3.5 ± 0.3 | 4.6 ± 0.1 | 1.7 ± 0.0 | 2.2 ± 0.2 | 9.8 ± 0.4 | 8.8 ± 1.0 | 1.3 ± 0.2 | 2.7 ± 0.2 | 1.7 ± 0.2 |
| D70% | 4.3 ± 0.6 | 0.2 ± 0.1 | 2.1 ± 0.4 | 3.2 ± 0.3 | 4.8 ± 0.2 | 1.8 ± 0.0 | 2.6 ± 0.2 | 9.7 ± 0.4 | 9.0 ± 0.1 | 1.2 ± 0.1 | 3.5 ± 0.5 | 2.2 ± 0.2 |
| Significance 1 | ||||||||||||
| Month (A) | *** | *** | *** | *** | n.s. | n.s. | n.s. | *** | ** | * | n.s. | n.s. |
| Treatment (B) | ** | n.s. | ** | ** | *** | *** | ** | n.s. | * | * | ** | ** |
| (A) × (B) | *** | *** | *** | *** | *** | *** | *** | *** | * | * | *** | *** |
| Treatment | Fruit Size | TSS | TA | TSS/TA | |
|---|---|---|---|---|---|
| Length | Width | ||||
| (mm) | (mm) | (°Brix) | (%) | ||
| December | |||||
| Control | 49.5 2 | 39.3 | 12.8 | 0.64 | 21.6 |
| D30% | 51.3 | 42.2 | 12.7 | 0.61 | 21.5 |
| D50% | 46.6 | 38.7 | 12.6 | 0.55 | 22.4 |
| D70% | 45.6 | 39.1 | 12.3 | 0.56 | 22.8 |
| January | |||||
| Control | 44.9 a | 37.7 ab | 13.4 | 0.57 | 24.8 |
| D30% | 43.1 ab | 38.2 a | 13.1 | 0.52 | 26.2 |
| D50% | 41.4 bc | 36.1 b | 13.3 | 0.55 | 25.3 |
| D70% | 40.4 c | 36.1 b | 12.8 | 0.54 | 24.9 |
| February | |||||
| Control | 46.7 a | 36.9 | 13.3 | 0.61 a | 22.6 |
| D30% | 45.4 ab | 36.8 | 12.7 | 0.56 b | 23.0 |
| D50% | 46.0 a | 36.6 | 12.6 | 0.54 b | 23.8 |
| D70% | 43.4 b | 35.6 | 12.9 | 0.56 b | 23.5 |
| March | |||||
| Control | 45.4 ab | 33.7 | 10.5 | 0.50 b | 21.6 |
| D30% | 45.7 ab | 34.6 | 11.3 | 0.65 a | 17.9 |
| D50% | 46.8 a | 34.8 | 10.5 | 0.51 b | 20.7 |
| D70% | 43.5 b | 33.3 | 10.7 | 0.50 b | 21.9 |
| Overall average | |||||
| Control | 45.9 | 35.8 | 12.7 | 0.57 | 22.8 |
| D30% | 45.1 | 36.2 | 12.8 | 0.54 | 24.0 |
| D50% | 44.6 | 35.7 | 12.7 | 0.57 | 23.2 |
| D70% | 43.2 | 35.6 | 12.2 | 0.58 | 23.3 |
| Significance 1 | |||||
| Month (A) | ** | n.s. | *** | *** | * |
| Treatment (B) | n.s. | n.s. | n.s. | n.s. | n.s. |
| (A) × (B) | ** | n.s. | n.s. | * | n.s. |
| Treatment | Total Phenolic Contents | Total Flavonoid Contents | FRAP | DPPH |
|---|---|---|---|---|
| (mg GAE/g DW) | (μg QE/g DW) | (mM TE/100 g DW) | (%) | |
| December | ||||
| Control | 25.5 2 | 33.9 a | 1.12 b | 79.5 b |
| D30% | 24.0 | 23.0 bc | 1.02 b | 75.8 b |
| D50% | 23.9 | 17.8 c | 0.98 b | 75.8 b |
| D70% | 28.2 | 24.4 b | 1.28 a | 88.3 a |
| January | ||||
| Control | 26.3 a | 23.3 | 1.17 a | 86.4 a |
| D30% | 21.3 b | 22.0 | 1.00 b | 77.0 b |
| D50% | 26.0 a | 27.2 | 1.13 a | 84.4 a |
| D70% | 25.7 a | 28.7 | 1.18 a | 85.1 a |
| February | ||||
| Control | 26.7 | 14.6 b | 1.22 | 88.7 |
| D30% | 26.2 | 25.9 a | 1.19 | 85.9 |
| D50% | 25.8 | 15.2 b | 1.16 | 85.0 |
| D70% | 25.1 | 20.1 ab | 1.18 | 84.2 |
| March | ||||
| Control | 27.6 b | 20.1 | 1.20 b | 48.0 b |
| D30% | 32.5 a | 17.6 | 1.35 ab | 57.2 a |
| D50% | 31.5 ab | 20.2 | 1.47 a | 55.0 ab |
| D70% | 29.0 ab | 15.5 | 1.32 ab | 52.5 ab |
| Overall average | ||||
| Control | 26.4 | 23.2 | 1.17 | 77.5 |
| D30% | 25.5 | 22.4 | 1.13 | 75.6 |
| D50% | 26.5 | 20.1 | 1.16 | 76.4 |
| D70% | 26.8 | 22.6 | 1.24 | 79.2 |
| Significance 1 | ||||
| Month (A) | *** | ** | *** | *** |
| Treatment (B) | n.s. | n.s. | n.s. | n.s. |
| (A) × (B) | *** | *** | *** | *** |
| Month 1 | ISR | Temperature (°C) | Relative Humidity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h-Mean | Daytime | Nighttime | 24 h-Mean | Daytime | Nighttime | ||||||
| (J·cm−2/Day) | Ave. | Max. | Ave. | Min. | Ave. | Max. | Ave. | Min. | |||
| October | 983 ± 153 2 | 19.6 ± 0.3 | 23.1 ± 0.8 | 27.7 ± 1.2 | 16.8 ± 0.7 | 14.6 ± 0.8 | 83.1 ± 0.9 | 76.2 ± 2.7 | 64.5 ± 3.1 | 88.6 ± 0.4 | 92.0 ± 0.0 |
| November | 872 ± 100 | 17.5 ± 1.1 | 21.5 ± 1.6 | 24.3 ± 1.3 | 14.8 ± 0.7 | 12.4 ± 0.9 | 80.4 ± 0.5 | 72.8 ± 0.9 | 58.7 ± 4.0 | 85.6 ± 1.1 | 86.6 ± 5.8 |
| December | 848 ± 31 | 15.9 ± 0.2 | 19.3 ± 0.3 | 22.8 ± 1.4 | 13.7 ± 0.1 | 11.6 ± 0.7 | 78.9 ± 1.8 | 71.8 ± 3.1 | 57.7 ± 6.7 | 83.4 ± 1.0 | 85.4 ± 6.4 |
| January | 885 ± 214 | 13.7 ± 1.4 | 17.6 ± 1.3 | 21.3 ± 1.3 | 11.0 ± 1.7 | 8.5 ± 1.9 | 77.2 ± 4.5 | 72.9 ± 3.1 | 57.6 ± 2.9 | 80.1 ± 5.4 | 84.4 ± 6.3 |
| February | 1241 ± 280 | 13.2 ± 0.3 | 17.4 ± 1.0 | 19.7 ± 2.9 | 9.7 ± 0.3 | 6.9 ± 1.1 | 73.5 ± 3.5 | 71.9 ± 2.5 | 50.0 ± 5.6 | 74.9 ± 4.4 | 77.9 ± 9.3 |
| March | 1636 ± 226 | 16.2 ± 1.0 | 20.4 ± 1.1 | 25.1 ± 3.6 | 12.1 ± 1.1 | 9.3 ± 1.7 | 85.4 ± 8.7 | 83.0 ± 10.1 | 66.2 ± 17.0 | 87.7 ± 7.3 | 87.6 ± 12.6 |
| Mean | 1082 ± 336 | 16.0 ± 2.4 | 20.1 ± 2.3 | 23.1 ± 3.6 | 13.1 ± 2.6 | 10.3 ± 2.9 | 79.4 ± 6.3 | 74.2 ± 7.2 | 84.2 ± 10.1 | 83.1 ± 6.3 | 57.2 ± 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Rabbani, M.G.; Jeong, Y.; Zebro, M.; Baek, J.; Choi, K.-Y. Drainage Recycling Ratio Influences Yield, Fruit Quality, and Antioxidant Properties of Korean Strawberry ‘Seolhyang’. Plants 2025, 14, 2984. https://doi.org/10.3390/plants14192984
Kim M, Rabbani MG, Jeong Y, Zebro M, Baek J, Choi K-Y. Drainage Recycling Ratio Influences Yield, Fruit Quality, and Antioxidant Properties of Korean Strawberry ‘Seolhyang’. Plants. 2025; 14(19):2984. https://doi.org/10.3390/plants14192984
Chicago/Turabian StyleKim, Minkyung, M. G. Rabbani, Youngae Jeong, Mewuleddeg Zebro, Jeonghyeon Baek, and Ki-Young Choi. 2025. "Drainage Recycling Ratio Influences Yield, Fruit Quality, and Antioxidant Properties of Korean Strawberry ‘Seolhyang’" Plants 14, no. 19: 2984. https://doi.org/10.3390/plants14192984
APA StyleKim, M., Rabbani, M. G., Jeong, Y., Zebro, M., Baek, J., & Choi, K.-Y. (2025). Drainage Recycling Ratio Influences Yield, Fruit Quality, and Antioxidant Properties of Korean Strawberry ‘Seolhyang’. Plants, 14(19), 2984. https://doi.org/10.3390/plants14192984






