Implementation of a Tunnel System for Scaling-Out High-Quality Cassava Planting Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Locations
2.2. Origin of Plant Material and Selection of Mini-Cuttings
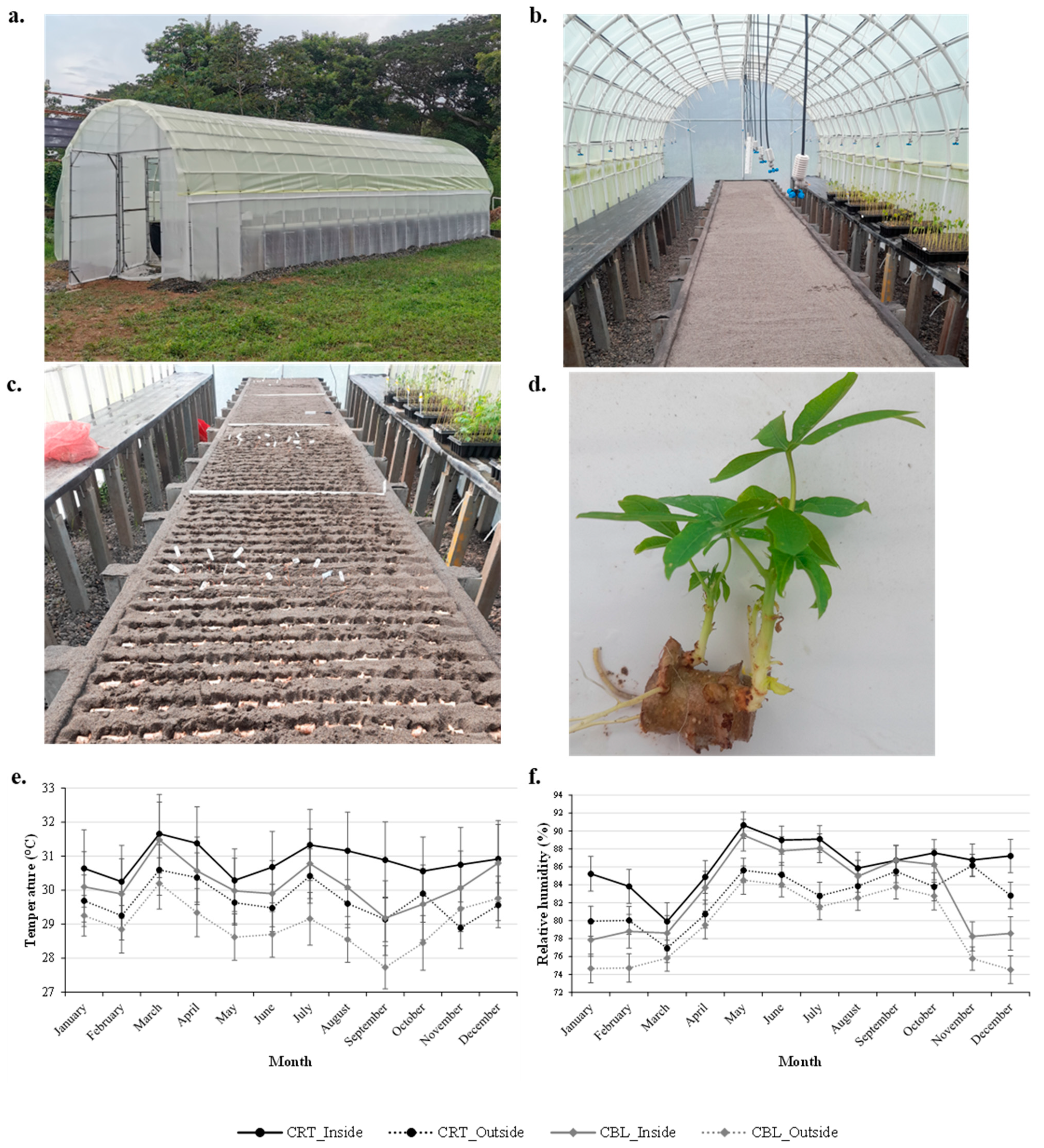
2.3. Description of the Multiplication Tunnels
2.3.1. Structure of the Tunnels for Rapid Propagation
2.3.2. Central Bed and Content
2.3.3. Irrigation System and Frequency
2.4. Establishment of Mini-Cuttings and Management
2.5. Cuttings’ Harvest for Multiplication and Evaluation
2.6. Planting and Monitoring Cuttings in Germination Trays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Internal Temperature Conditions of Multiplication Tunnels
3.2. Characteristics of the Sand Used for the Establishment of the Multiplication Tunnels
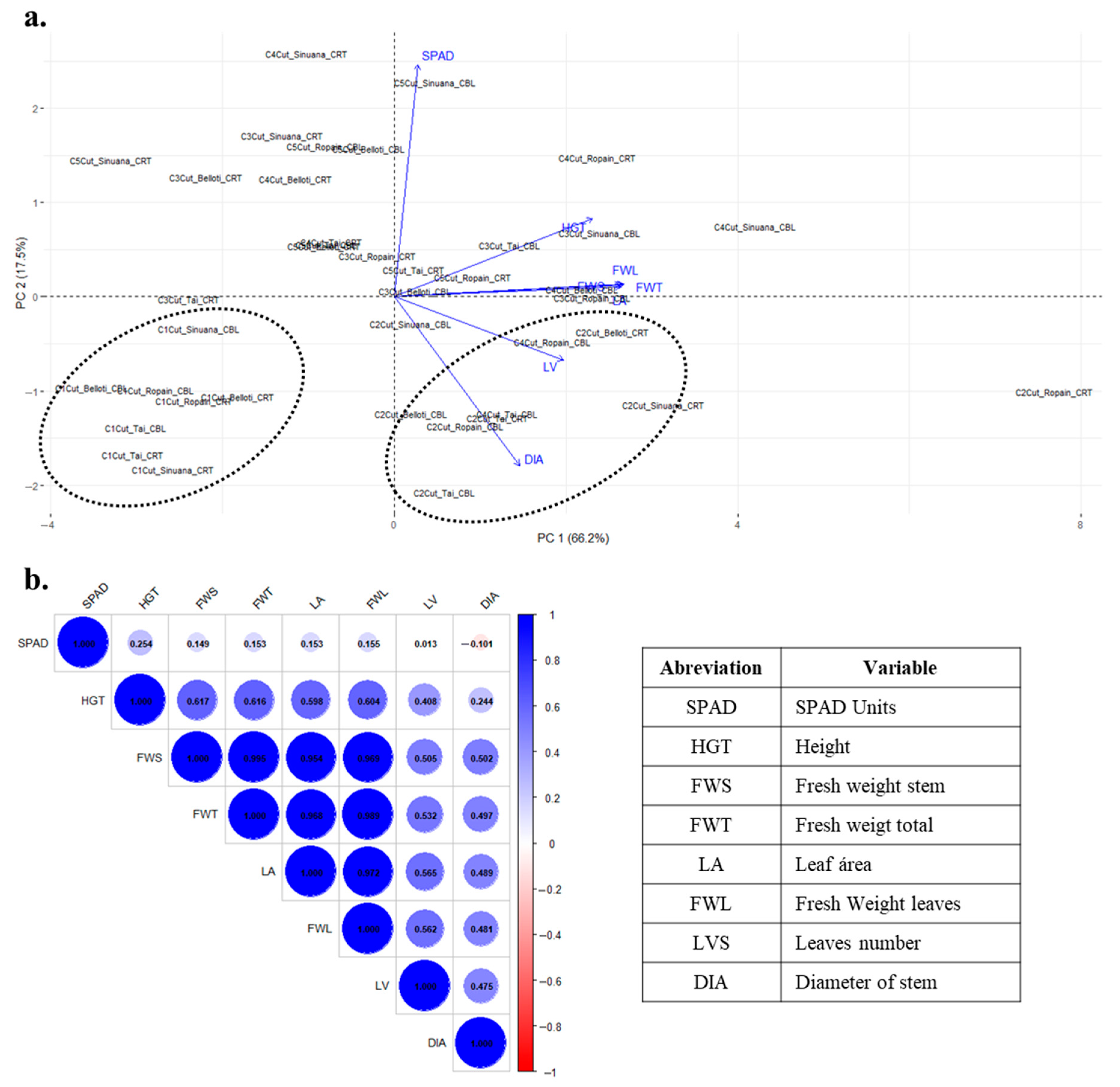
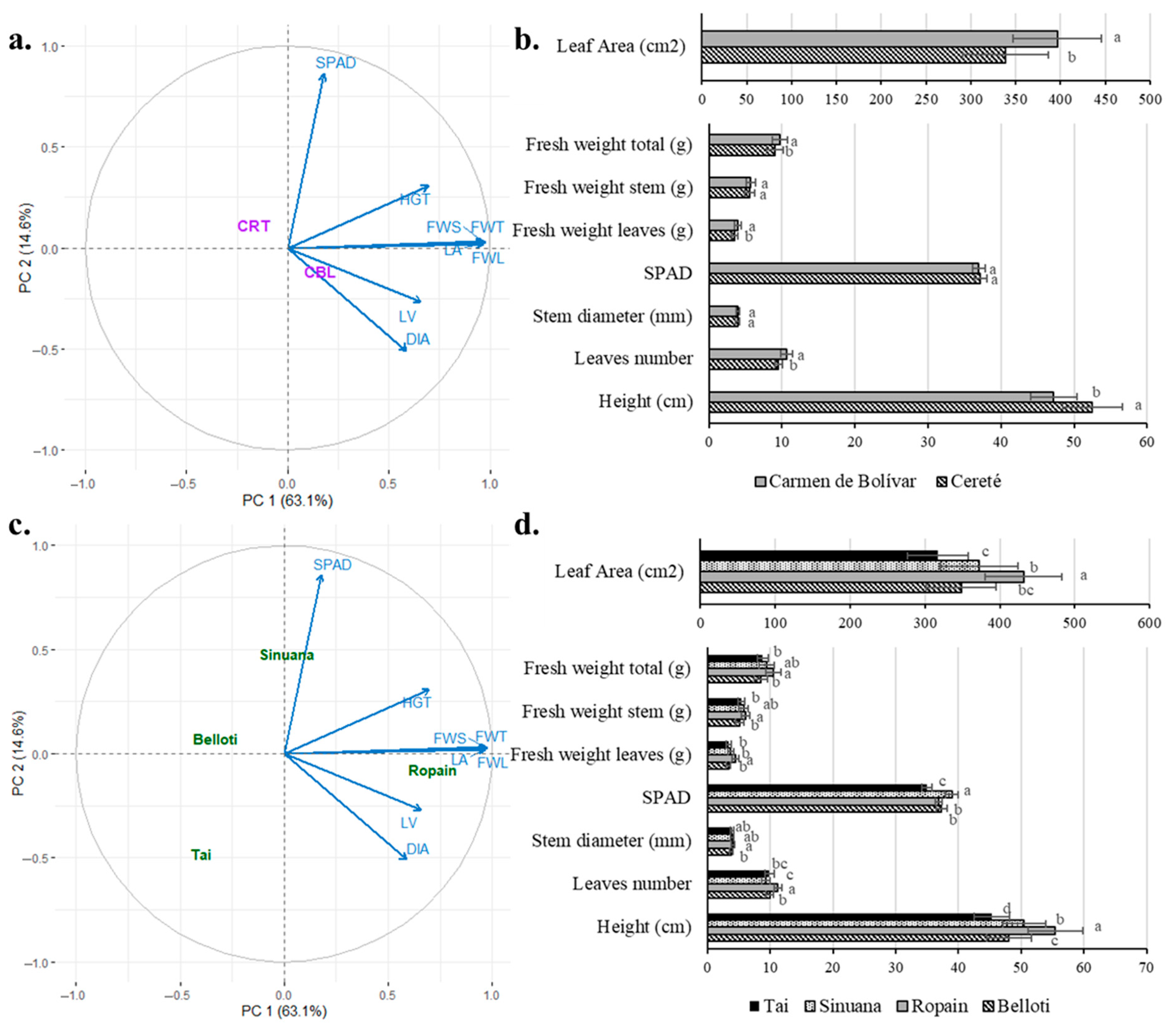
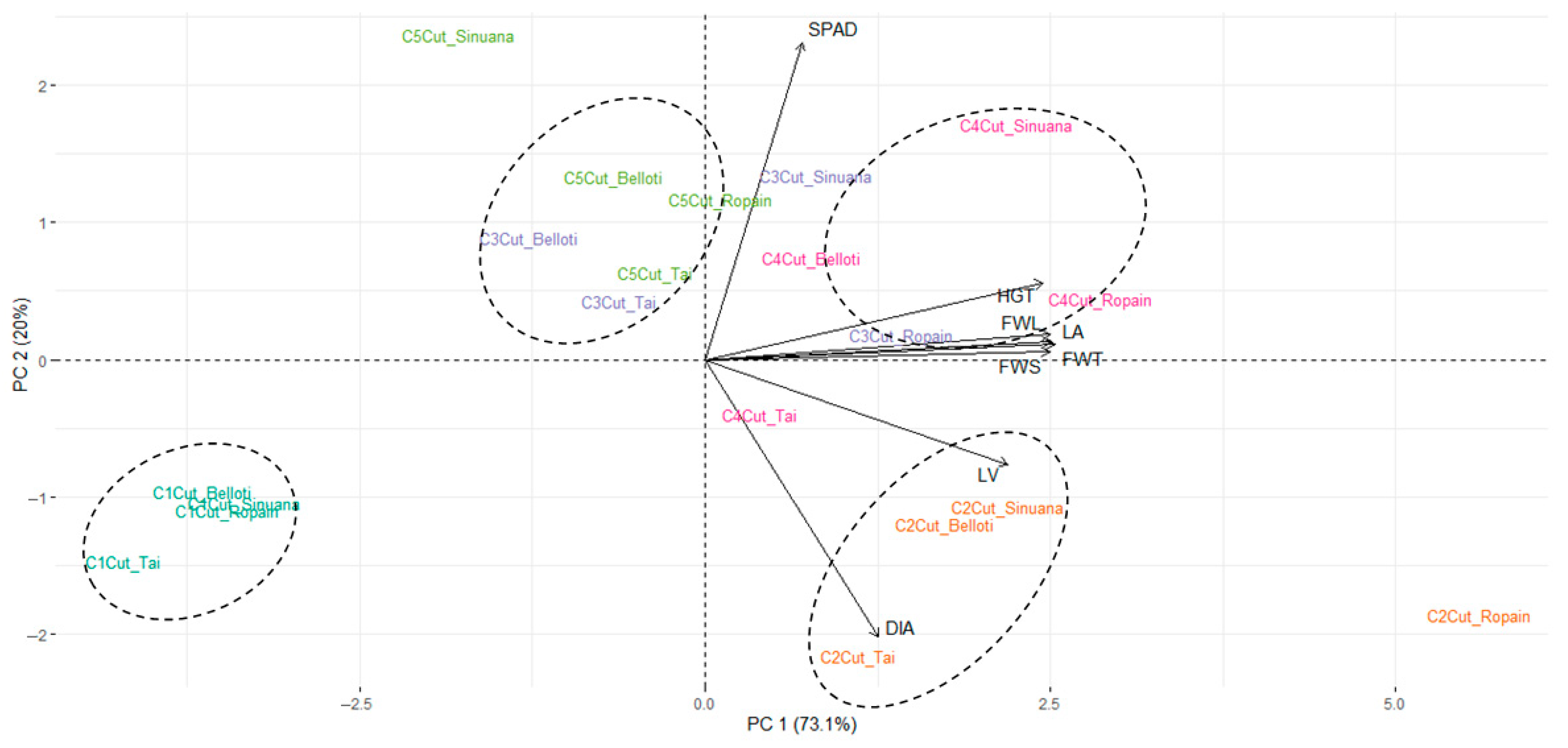
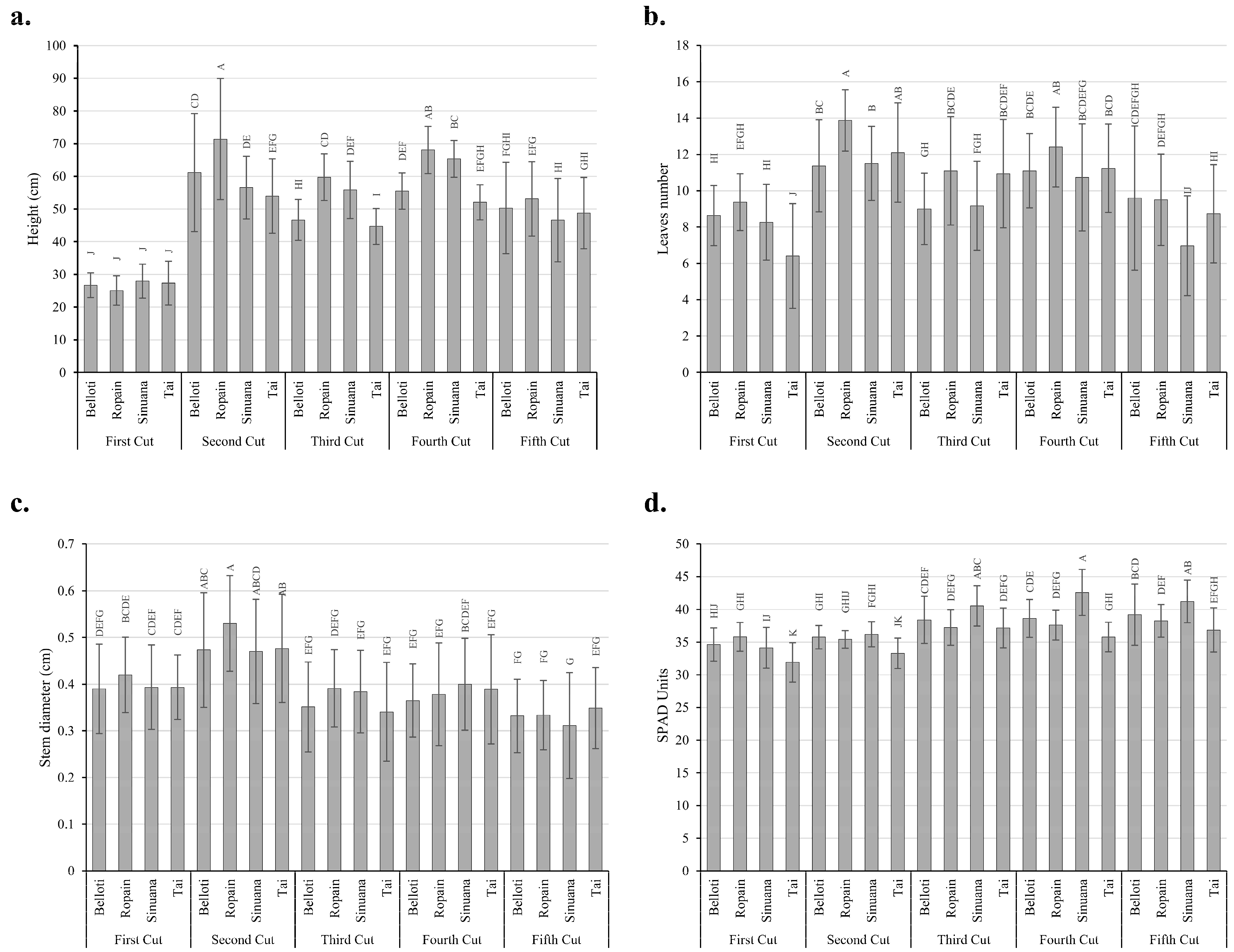
3.3. Multifactorial Effect of Location, Cut, and Variety on Growth Variables Under TxRPs
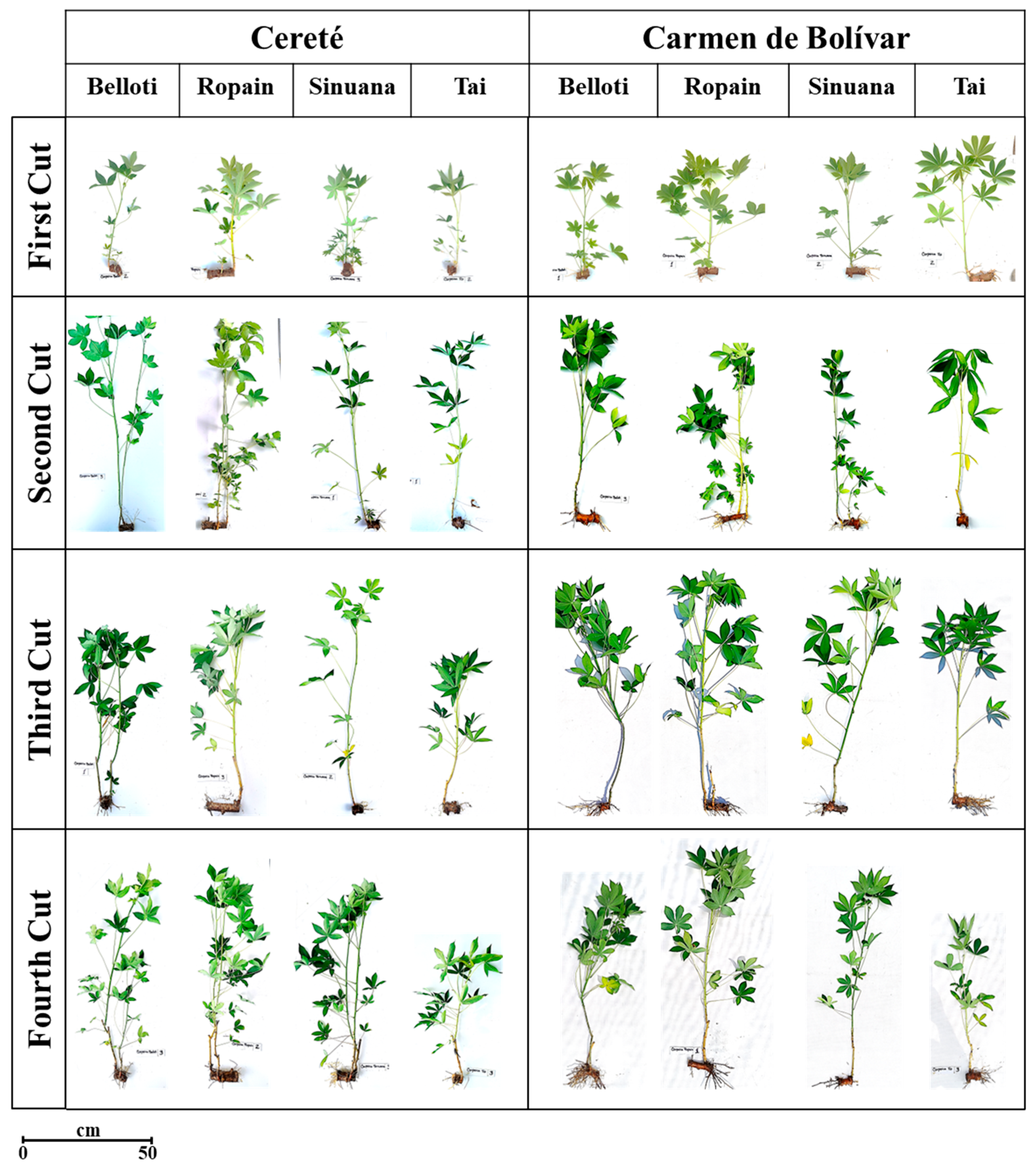
3.4. Growth Behavior of Cassava Variety Under TxRPs in Two Locations
3.5. Growth Behavior of Cassava Variety and Cuts Under TxRPs
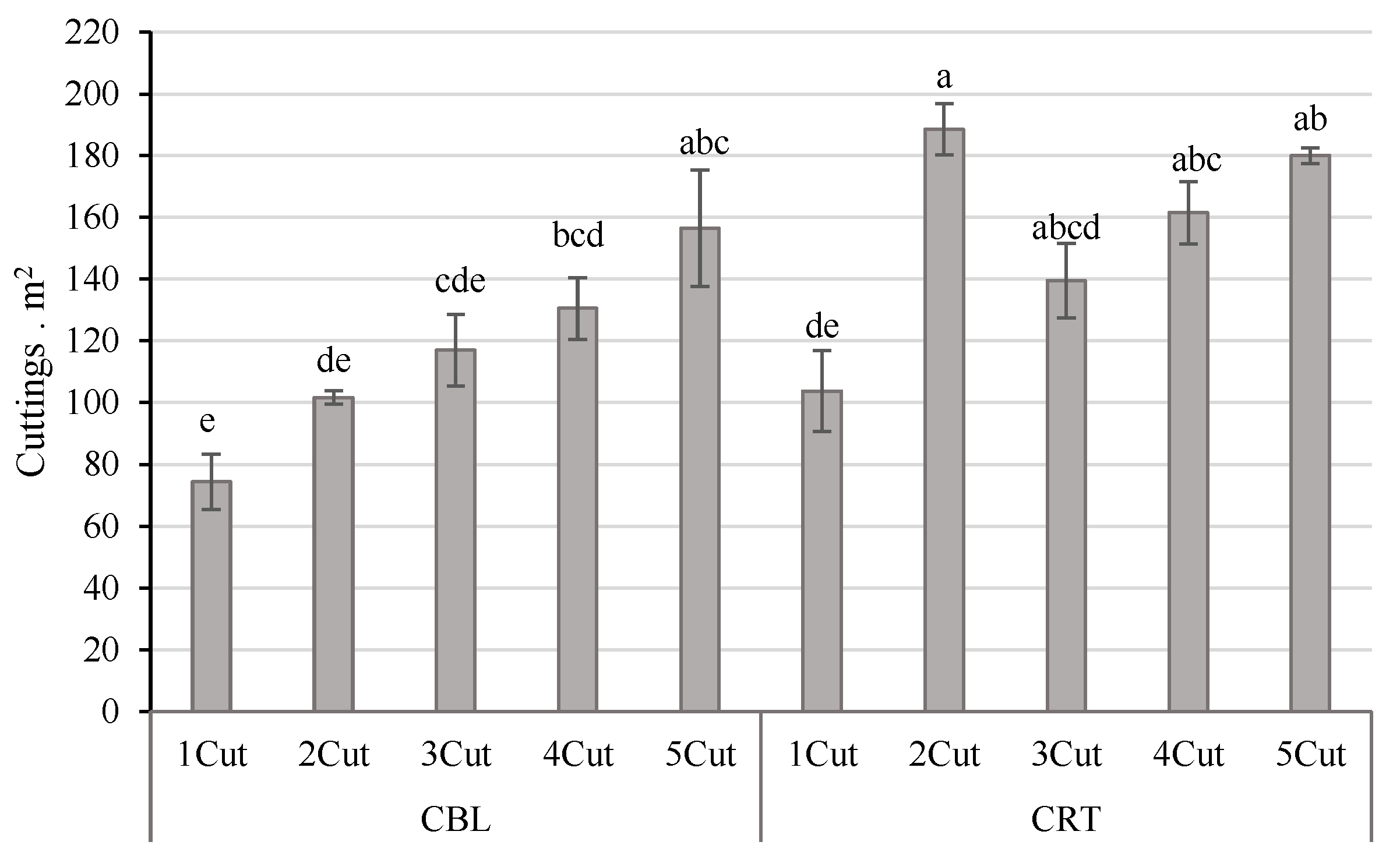
3.6. Cutting Yield Estimation and Survival
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borku, A.W. Cassava (Manihot esculenta Crantz): Its Nutritional Composition Insights for Future Research and Development in Ethiopia. Discov. Sustain. 2025, 6, 404. [Google Scholar] [CrossRef]
- Fathima, A.A.; Sanitha, M.; Tripathi, L.; Muiruri, S. Cassava (Manihot esculenta) Dual Use for Food and Bioenergy: A Review. Food Energy Secur. 2023, 12, e380. [Google Scholar] [CrossRef]
- Scaria, S.S.; Balasubramanian, B.; Meyyazhagan, A.; Gangwar, J.; Jaison, J.P.; Kurian, J.T.; Pushparaj, K.; Pappuswamy, M.; Park, S.; Joseph, K.S. Cassava (Manihot esculenta Crantz)—A Potential Source of Phytochemicals, Food, and Nutrition—An Updated Review. eFood 2024, 5, e127. [Google Scholar] [CrossRef]
- Pardo, J.M.; Gil-Ordóñez, A.; Leiva, A.M.; Enjelvin, L.; Chourrot, A.; Kime, S.C.K.; Demade-Pellorce, L.; Marchand, M.; Wilson, V.; Jeandel, C.; et al. First Report of Cassava Witches’ Broom Disease and Ceratobasidium Theobromae in the Americas. New Dis. Rep. 2024, 50, e70002. [Google Scholar] [CrossRef]
- Pardo, J.M.; Alvarez, E.; Becerra Lopez-Lavalle, L.A.; Olaya, C.; Leiva, A.M.; Cuellar, W.J. Cassava Frogskin Disease: Current Knowledge on a Re-Emerging Disease in the Americas. Plants 2022, 11, 1841. [Google Scholar] [CrossRef]
- Hareesh, P.S.; Resmi, T.R.; Sheela, M.N.; Makeshkumar, T. Cassava Mosaic Disease in South and Southeast Asia: Current Status and Prospects. Front. Sustain. Food Syst. 2023, 7, 1086660. [Google Scholar] [CrossRef]
- Ano, C.U.; Ochwo-Ssemakula, M.; Ibanda, A.; Ozimati, A.; Gibson, P.; Onyeka, J.; Njoku, D.; Egesi, C.; S. Kawuki, R. Cassava Brown Streak Disease Response and Association with Agronomic Traits in Elite Nigerian Cassava Cultivars. Front. Plant Sci. 2021, 12, 720532. [Google Scholar] [CrossRef]
- Zárate-Chaves, C.A.; Moufid, Y.; López, C.E.; Bernal, A.; Szurek, B.; Yánez, J.M. First Report of Cassava Bacterial Blight Caused by Xanthomonas phaseoli Pv. manihotis in the Amazonian Forest of Ecuador. Plant Dis. 2024, 108, 1879. [Google Scholar] [CrossRef]
- Legg, J.P.; Jeremiah, S.C.; Obiero, H.M.; Maruthi, M.N.; Ndyetabula, I.; Okao-Okuja, G.; Bouwmeester, H.; Bigirimana, S.; Tata-Hangy, W.; Gashaka, G.; et al. Comparing the Regional Epidemiology of the Cassava Mosaic and Cassava Brown Streak Virus Pandemics in Africa. Virus Res. 2011, 159, 161–170. [Google Scholar] [CrossRef]
- Wydra, K.; Verdier, V. Occurrence of Cassava Diseases in Relation to Environmental, Agronomic and Plant Characteristics. Agric. Ecosyst. Environ. 2002, 93, 211–226. [Google Scholar] [CrossRef]
- Sheat, S.; Mushi, E.; Gwandu, F.; Sikirou, M.; Baleke, P.; Kayondo, S.I.; Kulembeka, H.; Adetoro, N.; Winter, S. Cut, Root, and Grow: Simplifying Cassava Propagation to Scale. Plants 2024, 13, 471. [Google Scholar] [CrossRef]
- Mbise, V.E.; Sibuga, K.P.; Mtui, H.D.; Ibrahim, A. Impact of Planting Techniques and Cutting Sizes on Cassava (Manihot esculenta Crantz) Sprouting and Subsequent Vegetative Growth in Various Nursery Environments. J. Curr. Opin. Crop Sci. 2024, 5, 113–124. [Google Scholar] [CrossRef]
- Jimenez, J.; Caicedo, S.; Pardo, J.M.; Gil-Ordóñez, A.; Alvarez-Quinto, R.; Mollov, D.; Cuellar, W.J. Single Torradovirus Infections Explain the Mysterious Cassava Frogskin Disease in the Americas. Sci. Rep. 2024, 14, 29648. [Google Scholar] [CrossRef]
- Patil, B.L.; Fauquet, C.M. Cassava Mosaic Geminiviruses: Actual Knowledge and Perspectives. Mol. Plant Pathol. 2009, 10, 685–701. [Google Scholar] [CrossRef]
- Gatto, M.; Le, P.D.; Pacillo, G.; Maredia, M.; Labarta, R.; Hareau, G.; Spielman, D.J. Policy Options for Advancing Seed Systems for Vegetatively Propagated Crops in Vietnam. J. Crop Improv. 2021, 35, 763–789. [Google Scholar] [CrossRef]
- Delaquis, E.; Almekinders, C.J.M.; de Haan, S.; Newby, J.C.; Le Thuy, C.T.; Srean, P.; Wannarat, W.; Aiemnaka, P.; Rojanaridpiched, C.; Nhan, P.T.; et al. Public and Private Institutional Arrangements for Early Generation Seed Production: Cassava Seed Value Chains in Southeast Asia. Agric. Syst. 2024, 221, 104131. [Google Scholar] [CrossRef]
- Yabeja, J.W.; Manoko, M.L.K.; Legg, J.P. Comparing Fresh Root Yield and Quality of Certified and Farmer-Saved Cassava Seed. Crop Prot. 2025, 187, 106932. [Google Scholar] [CrossRef]
- Kilwinger, F.; Mugambi, S.; Manners, R.; Schut, M.; Tumwegamire, S.; Nduwumuremyi, A.; Bambara, S.; Paauwe, M.; Almekinders, C. Characterizing Cassava Farmer Typologies and Their Seed Sourcing Practices to Explore Opportunities for Economically Sustainable Seed Business Models in Rwanda. Outlook Agric. 2021, 50, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Espitia, A.A.; Pérez, S.P.; Regino, S.M.; Támara, R.E.; García, J.L.; Martínez, R.R.; García, J.A. Manual Para La Producción y Escalamiento de Semilla de Yuca (Manihot esculenta) de Calidad, 1st ed.; Editorial AGROSAVIA: Monteria, Colombia, 2022; Volume 1, ISBN 978-958-740-555-2. [Google Scholar]
- Pérez, D.; Mora, R.; López-Carrascal, C.; De Revisión, A.; Article, R. Conservación de La Diversidad de Yuca En Los Sistemas Tradicionales de Cultivo de La Amazonía. Acta Biolo Colomb. 2019, 24, 202–212. [Google Scholar] [CrossRef]
- Sánchez-Cevallos, E.S.; Cobeña Ruiz, G.A.; Mendoza García, A.A.; Mendoza García, V. Comportamiento de Genotipos de Yuca En Sustratos y Soluciones Nutritivas. Espamciencia 2019, 10, 37–45. [Google Scholar]
- Feyisa, A.S. Micropropagation of Cassava (Manihot esculenta Crantz): Review. Extensive Rev. 2021, 1, 49–57. [Google Scholar] [CrossRef]
- Escobar, R.H.; Rosero, E.A.; López, L.A.; Dorado, C.; Montejo, L.G.M.; Espitia, A.A.; Bohórquez, A.; Regino, S.M.; Pérez-Pazos, J.V.; Fuentes, D.A.; et al. Técnicas de Multiplicación Rápida de Semilla de Yuca de Alta Calidad. Editor. Agrosavia 2024, 1, 109. [Google Scholar] [CrossRef]
- NTC-ISO-IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ICONTEC: Bogotá, Colombia, 2017.
- Le Bissonnais, Y. Aggregate Stability and Assessment of Soil Crustability and Erodibility: I. Theory and Methodology. Eur. J. Soil. Sci. 2016, 67, 11–21. [Google Scholar] [CrossRef]
- Pérez-Pazos, J.V.; Rosero, A.; Vergara, E.; Gámez, R. Response of Sweet Potato to Substrates and Acclimatization Conditions in the Greenhouse to Produce High-Quality Planting Material. Hortic. J. 2023, 92, 451–463. [Google Scholar] [CrossRef]
- RTeam R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 13 February 2022).
- Dag, O.; Dolgun, A.; Konar, N.M. Onewaytests: An R Package for One-Way Tests in Independent Groups Designs. R J. 2018, 10, 175–199. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Exploratory Multivariate Analysis. In Modern Applied Statistics with S; Venables, W.N., Ripley, B.D., Eds.; Springer: New York, NY, USA, 2002; pp. 301–330. ISBN 978-0-387-21706-2. [Google Scholar]
- Taiyun, W.; Viliam, S. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.92). Available online: https://github.com/taiyun/corrplot/blob/master/corrplot.Rproj (accessed on 13 February 2022).
- Omid, M.; Shafaei, A. Temperature and Relative Humidity Changes Inside Greenhouse. Int. Agrophys. 2005, 19, 153–158. [Google Scholar]
- Vanegas-Ayala, S.C.; Barón-Velandia, J.; Leal-Lara, D.D. A Systematic Review of Greenhouse Humidity Prediction and Control Models Using Fuzzy Inference Systems. Adv. Hum.-Comput. Interact. 2022, 2022, 8483003. [Google Scholar] [CrossRef]
- Phanthanong, P.; Promnikorn, K.; Kongsil, P.; Kraichak, E.; Jenweerawat, S.; Vuttipongchaikij, S.; Kittipadakul, P. Variety-Specific Responses to Climatic and Edaphic Factors in Cassava Productivity. Front. Agron. 2025, 7, 1476033. [Google Scholar] [CrossRef]
- Keating, B.A.; Evenson, J.P. Effect of Soil Temperature on Sprouting and Sprout Elongation of Stem Cuttings of Cassava (Manihot esculenta Crantz.). Field Crops Res. 1979, 2, 241–251. [Google Scholar] [CrossRef]
- Vongcharoen, K.; Santanoo, S.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P. Seasonal Variation in Photosynthesis Performance of Cassava at Two Different Growth Stages under Irrigated and Rain-Fed Conditions in a Tropical Savanna Climate. Photosynthetica 2018, 56, 1398–1413. [Google Scholar] [CrossRef]
- Sandoval-Estrada, M.; Celis Hidalgo, J.; Stolpe Lau, N.; Capulín Grande, J. Effect of Sewage Sludge and Salmon Wastes Amendments on the Structure of an Entisol and Alfisol in Chile. Agrociencia 2010, 44, 503–515. [Google Scholar]
- Cardona, W.A.; Benavides, M.M.B.; Montoya, W.C. Effect of Chemical and Organic Fertilizers on the Aggregation of a Soil Cultivated with Musa acuminata AA. Acta Agron. 2016, 65, 144–148. [Google Scholar] [CrossRef]
- Manrique-Cantillo, A.P.; Morales-Acuña, E.D.J.; Linero-Cueto, J.R. Variability, Cycles, and Trends of Mean Air Temperature North of Colombia. Atmósfera 2024, 38, 327–350. [Google Scholar] [CrossRef]
- Schoffel, A.; Lopes, S.J.; Koefender, J.; Lúcio, A.D.; Camera, J.N.; Golle, D.P. Adaptation of the Rapid Multiplication Method: Selecting Stem Cuttings Based on Their Number of Leaves for Cassava Seedling Production. Acta Sci. Agron. 2021, 43, e50289. [Google Scholar] [CrossRef]
- Huang, W.; Su, X.; Ratkowsky, D.A.; Niklas, K.J.; Gielis, J.; Shi, P. The Scaling Relationships of Leaf Biomass vs. Leaf Surface Area of 12 Bamboo Species. Glob. Ecol. Conserv. 2019, 20, e00793. [Google Scholar] [CrossRef]
- Liu, M.; Niklas, K.J.; Niinemets, Ü.; Hölscher, D.; Chen, L.; Shi, P. Comparison of the Scaling Relationships of Leaf Biomass versus Surface Area between Spring and Summer for Two Deciduous Tree Species. Forests 2020, 11, 1010. [Google Scholar] [CrossRef]
- Kandel, B.P. Spad Value Varies with Age and Leaf of Maize Plant and Its Relationship with Grain Yield. BMC Res. Notes 2020, 13, 475. [Google Scholar] [CrossRef]
- Eke-Okoro, O.N.; Okereke, O.U.; Okeke, J.E. Effect of Stake Sizes on Some Growth Indices and Yield of Three Cassava Cultivars (Manihot esculenta). J. Agric. Sci. 2001, 137, 419–426. [Google Scholar] [CrossRef]
- CIAT.; Ospina Patiño, B.; Ceballos, H.; Álvarez, E.; Bellotti, A.C.; Calvert, L.A.; Arias, V.B.; Cadavid López, L.F.; Pineda López, B.; Llano Rodríguez, G.A.; et al. La Yuca En El Tercer Milenio: Sistemas Modernos de Producción, Procesamiento, Utilización y Comercialización; International Center for Tropical Agriculture: Cali, Colombia, 2002; ISBN 958-694-043-8. [Google Scholar]
- Meibuko, N.M.; Mtui, H.D.; Baltazari, A. Effect of Cassava (Manihot esculenta Crantz) Varieties on Leaf Bud Sprouting for Rapid Multiplication of Planting Materials. Front. Plant Sci. 2024, 15, 1453538. [Google Scholar] [CrossRef]
- Edet, M.A.; Tijani-Eniola, H.; Lagoke, S.T.O.; Tarawali, G. Relationship of Cassava Growth Parameters with Yield, Yield Related Components and Harvest Time in Ibadan, Southwestern Nigeria. J. Nat. Sci. Res. 2015, 5, 87–92. [Google Scholar]
- Masisila, F.F. Cassava Production Improvement through Staggered Planting for Industrial Processing and Utilization in Eastern and Southern Zone of Tanzania. Master’s Thesis, Sokoine Universitu of Agriculture, Morogoro, Tanzania, 2020. [Google Scholar]
- Varshini, S.V.; Jayanthi, C. Planting Methods and Number of Buds of Setts on Sprouting, Growth and Productivity of Vegetative Propagated Crops: A Review. Agric. Rev. 2021, 42, 413–419. [Google Scholar] [CrossRef]
- Dey, A.K.; Sharma, M.; Meshram, M.R. An Analysis of Leaf Chlorophyll Measurement Method Using Chlorophyll Meter and Image Processing Technique. Procedia Comput. Sci. 2016, 85, 286–292. [Google Scholar] [CrossRef]

| Location | Landscape | Climate | Geographic Location | Mean Temperature (°C) | Accumulated Precipitation (mm/Year) |
|---|---|---|---|---|---|
| CRT | Plain | Warm humid | 8°50′27.47″ N, 75°48′27.56″ W | 31.3 | 1244 |
| CBL | Mountains and Piedmont | Warm dry | 9°42′50.8″ N, 75°6′26.9″ W | 27.5 | 1150 |
| Variety | Internode Length (cm) | Cutting Length (cm) | Cutting Diameter (cm) |
|---|---|---|---|
| Belloti | 0.89 ± 0.30 | 3.48 ± 0.58 | 2.19 ± 0.13 |
| Ropain | 1.19 ± 0.50 | 4.39 ± 1.28 | 2.06 ± 0.21 |
| Sinuana | 1.08 ± 0.45 | 4.11 ± 1.06 | 2.22 ± 0.35 |
| Tai | 0.70 ± 0.31 | 3.22 ± 0.82 | 2.21 ± 0.27 |
| Parameter | Units | CBL Origin | CRT Origin | ||
|---|---|---|---|---|---|
| Value | Interpretation | Value | Interpretation | ||
| pH | 7.99 | Alkaline | 7.07 | Near neutral or neutral | |
| Electrical Conductivity | dS·m−1 | 0.68 | Non-saline | 0.07 | Non-saline |
| Organic Carbon | g·100 g−1 | <0.22 | Not applicable | <0.22 | Not applicable |
| Organic Matter | g·100 g−1 | <0.38 | Low | <0.38 | Low |
| Available Phosphorus | mg·kg−1 | 30.46 | Medium | 3.39 | Low |
| Available Sulfur | mg·kg−1 | 19 | Medium | 4.25 | Low |
| ECEC * | cmol(+)·kg−1 | 6.07 | Low | 3.09 | Low |
| Available Boron | mg·kg−1 | 0.1 | Low | 0.1 | Low |
| Acidity | cmol(+)·kg−1 | ND | Not indicated | ND | Not indicated |
| Exchangeable Aluminum | cmol(+)·kg−1 | ND | No restriction | ND | No restriction |
| Available Calcium | cmol(+)·kg−1 | 4.15 | Medium | 2.2 | Low |
| Available Magnesium | cmol(+)·kg−1 | 1.73 | Medium | 0.77 | Low |
| Available Potassium | cmol(+)·kg−1 | 0.12 | Low | <0.09 | Low |
| Available Sodium | cmol(+)·kg−1 | <0.14 | Normal | <0.14 | Normal |
| Available Iron | mg·kg−1 | 69.13 | High | 5.34 | Low |
| Available Cupper | mg·kg−1 | <1 | Low | <1 | Low |
| Available Manganese | mg·kg−1 | 1.66 | Low | 2.85 | Low |
| Available Zinc | mg·kg−1 | 2.86 | Medium | <1 | Low |
| Calcium Saturation | % | 68 | High | 71 | High |
| Magnesium Saturation | % | 29 | High | 25 | Medium |
| Potassium Saturation | % | 2 | Medium | 2 | Medium |
| Sodium Saturation | % | 1 | Normal | 2 | Normal |
| Aluminum Saturation | % | 0 | Normal | 0 | Normal |
| Parameter | CBL | CRT |
|---|---|---|
| Mean weight diameter (mm) | 0.306 | 0.268 |
| Fine aggregates < 0.5 mm (%) | 86.49 | 94.1 |
| Medium aggregates 0.5 mm–2 mm (%) | 13.47 | 5.9 |
| Extreme aggregates > 2 mm (%) | 0.04 | 0 |
| SV | df | Height | Leaf Number | Stem Diameter | Leaf Area | SPAD | FWL | FWS | FWT |
|---|---|---|---|---|---|---|---|---|---|
| Model | 39 | 3650.27 *** | 86.43 *** | 0.08 *** | 335,254.68 *** | 138.43 *** | 31.28 *** | 67.05 *** | 184.97 *** |
| Locality | 1 | 4197.61 *** | 191.54 *** | 0.0017 | 503,107.37 *** | 6.94 | 37.97 *** | 6.43 | 75.65 * |
| Cut | 4 | 22,911.61 *** | 352.49 *** | 0.41 *** | 1,502,381.11 *** | 591.43 *** | 103.28 *** | 245.19 *** | 662.6 *** |
| Variety | 3 | 2765.31 *** | 99.34 *** | 0.02 * | 350,419.7 *** | 395.69 *** | 40.45 *** | 24.45 ** | 123.05 *** |
| Locality*Cut | 4 | 5467.37 *** | 190.72 *** | 0.17 *** | 682,176.21 *** | 238.03 *** | 77.33 *** | 232.61 *** | 574.21 *** |
| Locality*Variety | 3 | 1443.72 *** | 16.4 ** | 0.03 * | 308,907.85 *** | 38.58 *** | 45.22 *** | 71.44 *** | 228.3 *** |
| Cut*Variety | 12 | 650.46 *** | 21.51 *** | 0.01 | 78,572.22 *** | 39.93 *** | 6.91 *** | 10.17 * | 32.4 ** |
| Locality*Cut*Variety | 12 | 351.2 *** | 33.43 *** | 0.03 *** | 76,062.2 ** | 24.32 *** | 9.98 *** | 23.97 *** | 62.35 *** |
| Error | 560 | 39.02 | 4.07 | 0.01 | 27,552.1 | 6.06 | 2.03 | 4.8 | 13.03 |
| Parameter | CRT | CBL | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | |||||||||
| Val | Analysis | Val | Analysis | Val | Analysis | Val | Analysis | Val | Analysis | Val | Analysis | Val | Analysis | Val | Analysis | |
| pH | 7.65 | Alk | 7.32 | Nt | 6.96 | Nt | 7.85 | Alk | 7.71 | Alk | 7.18 | Nt | 7.1 | N | 7.87 | Alk |
| Electrical Conductivity | 2.81 | SS | 6.55 | MS | 4.35 | MS | 1.99 | NS | 1.62 | NS | 5.93 | MS | 4.6 | MS | 0.59 | NS |
| Organic Carbon | 0.27 | NA | 0.55 | NA | 1.26 | NA | 0.25 | NA | 1.33 | NA | 2.37 | NA | 2.42 | NA | 1.11 | NA |
| Organic Matter | 0.47 | L | 0.95 | L | 2.17 | M | 0.43 | L | 2.29 | M | 4.09 | H | 4.17 | H | 1.91 | L |
| Available Phosphorus | 36.88 | M | 164.19 | H | 190.2 | H | 9.81 | L | 84.73 | H | 218.33 | H | 194.62 | H | 26.9 | M |
| Available Sulfur | 139.62 | H | 234.14 | H | 124.24 | H | 137.37 | H | 40.07 | H | 152.43 | H | 116.31 | H | 19.52 | M |
| ECEC | 17.8 | M | 26.85 | H | 13.83 | M | 17.1 | M | 41.39 | H | 44.19 | H | 23.54 | H | 43.37 | H |
| Available Boron | 0.3 | M | 0.69 | H | 0.65 | H | 0.19 | L | 0.76 | H | 1.07 | H | 0.76 | H | 0.58 | H |
| Available Calcium | 8.97 | H | 13.51 | H | 7.38 | H | 8.27 | H | 32.76 | H | 30.13 | H | 14.06 | H | 35.17 | H |
| Available Magnesium | 7.14 | H | 9.22 | H | 3.81 | H | 7.34 | H | 6.25 | H | 8.37 | H | 5.45 | H | 6.61 | H |
| Available Potassium | 0.34 | M | 2.35 | H | 1.89 | H | 0.12 | L | 1.81 | H | 4.61 | H | 3.2 | H | 1.1 | H |
| Available Sodium | 1.35 | H | 1.77 | H | 0.75 | N | 1.37 | H | 0.57 | N | 1.08 | H | 0.83 | N | 0.49 | N |
| Available Iron | 18.37 | L | 65.88 | H | 58.38 | H | 8.21 | L | 19.39 | L | 38.94 | M | 39.08 | M | 10.53 | L |
| Available Cupper | <1 | L | <1 | L | <1 | L | <1 | L | 3.31 | H | 2.71 | M | <1 | L | 4.12 | H |
| Available Manganese | <1 | L | 2.34 | L | 6.05 | M | <1 | L | <1 | L | 1.54 | L | 2.86 | L | <1 | L |
| Available Zinc | <1 | L | 3.79 | H | 3.18 | H | <1 | L | <1 | L | 3.09 | H | 2.47 | M | <1 | L |
| Calcium Saturation | 50 | M | 50 | M | 53 | H | 48 | M | 79 | H | 68 | H | 60 | H | 81 | H |
| Magnesium Saturation | 40 | H | 34 | H | 28 | H | 43 | H | 15 | M | 19 | M | 23 | M | 15 | M |
| Potassium Saturation | 2 | M | 9 | H | 14 | H | 1 | L | 4 | H | 10 | H | 14 | H | 3 | M |
| Sodium Saturation | 8 | N | 7 | N | 5 | N | 8 | N | 1 | N | 2 | N | 4 | N | 1 | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Pazos, J.V.; Fuentes-Cassiani, D.; Regino, S.-M.; García, J.-L.; Osorio, N.; Espitia, A.; Araujo, H.; Escobar, R.H.; Rosero, A. Implementation of a Tunnel System for Scaling-Out High-Quality Cassava Planting Material. Plants 2025, 14, 2983. https://doi.org/10.3390/plants14192983
Pérez-Pazos JV, Fuentes-Cassiani D, Regino S-M, García J-L, Osorio N, Espitia A, Araujo H, Escobar RH, Rosero A. Implementation of a Tunnel System for Scaling-Out High-Quality Cassava Planting Material. Plants. 2025; 14(19):2983. https://doi.org/10.3390/plants14192983
Chicago/Turabian StylePérez-Pazos, Jazmín Vanessa, Deimer Fuentes-Cassiani, Sol-Mara Regino, Jorge-Luis García, Nilson Osorio, Amaury Espitia, Hernando Araujo, Roosevelt H. Escobar, and Amparo Rosero. 2025. "Implementation of a Tunnel System for Scaling-Out High-Quality Cassava Planting Material" Plants 14, no. 19: 2983. https://doi.org/10.3390/plants14192983
APA StylePérez-Pazos, J. V., Fuentes-Cassiani, D., Regino, S.-M., García, J.-L., Osorio, N., Espitia, A., Araujo, H., Escobar, R. H., & Rosero, A. (2025). Implementation of a Tunnel System for Scaling-Out High-Quality Cassava Planting Material. Plants, 14(19), 2983. https://doi.org/10.3390/plants14192983







