Tubulin Cytoskeleton Organization in Cells of Determinate Nodules in Vigna radiata, Vigna unguiculata, and Lotus corniculatus
Abstract
1. Introduction
2. Results
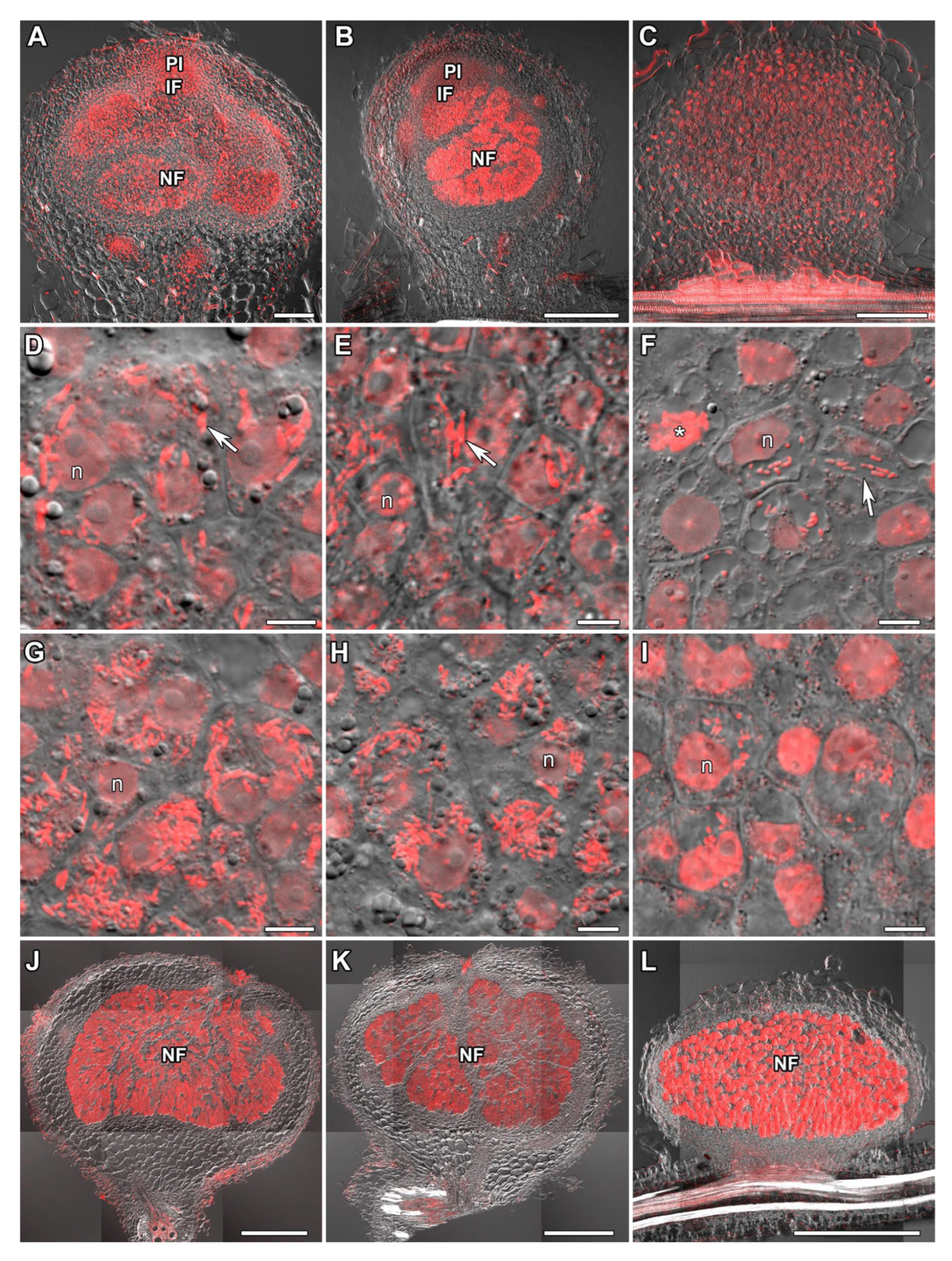
2.1. Histological Organization of Nodules
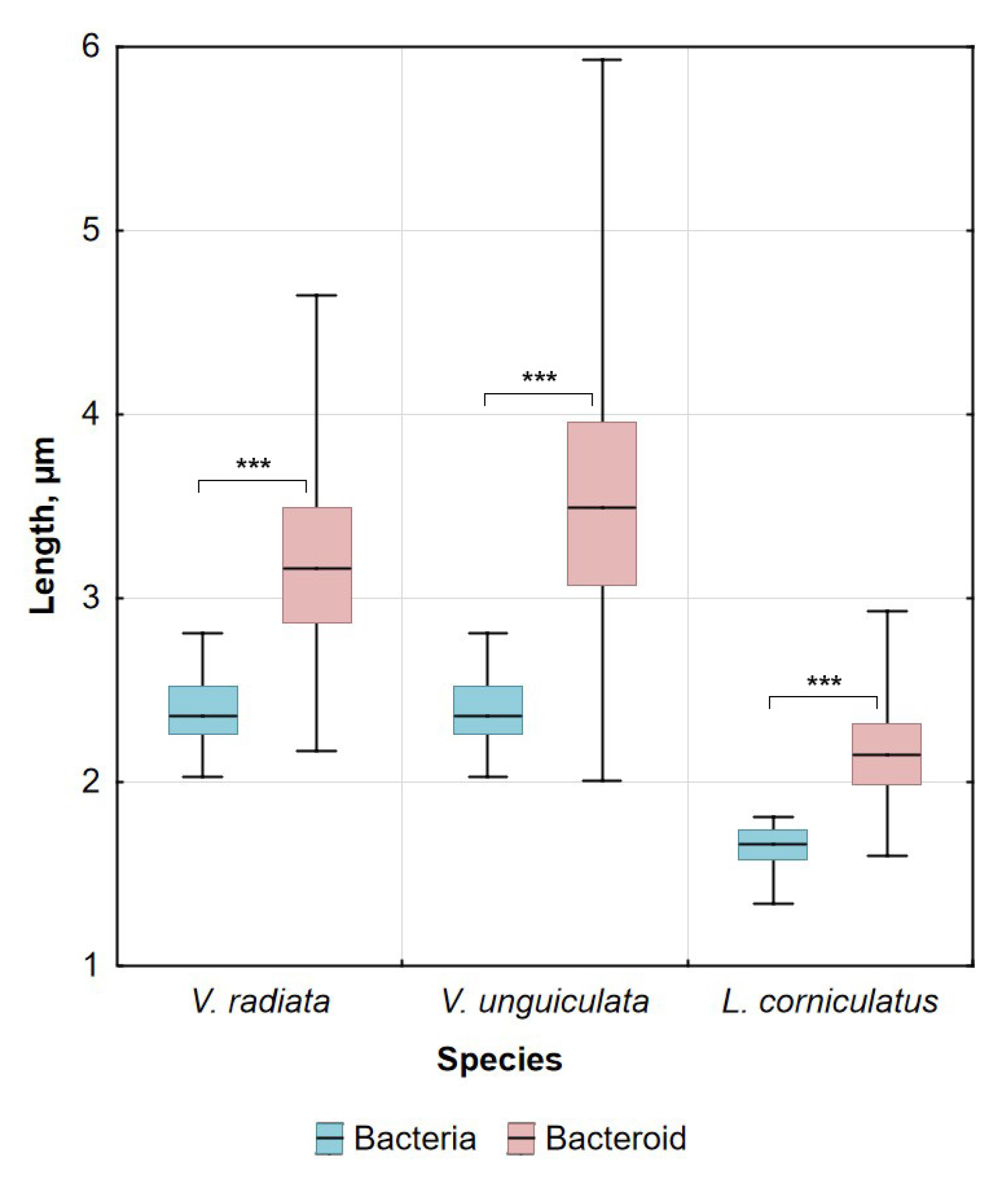
2.2. Morphology of Bacteria and Bacteroids
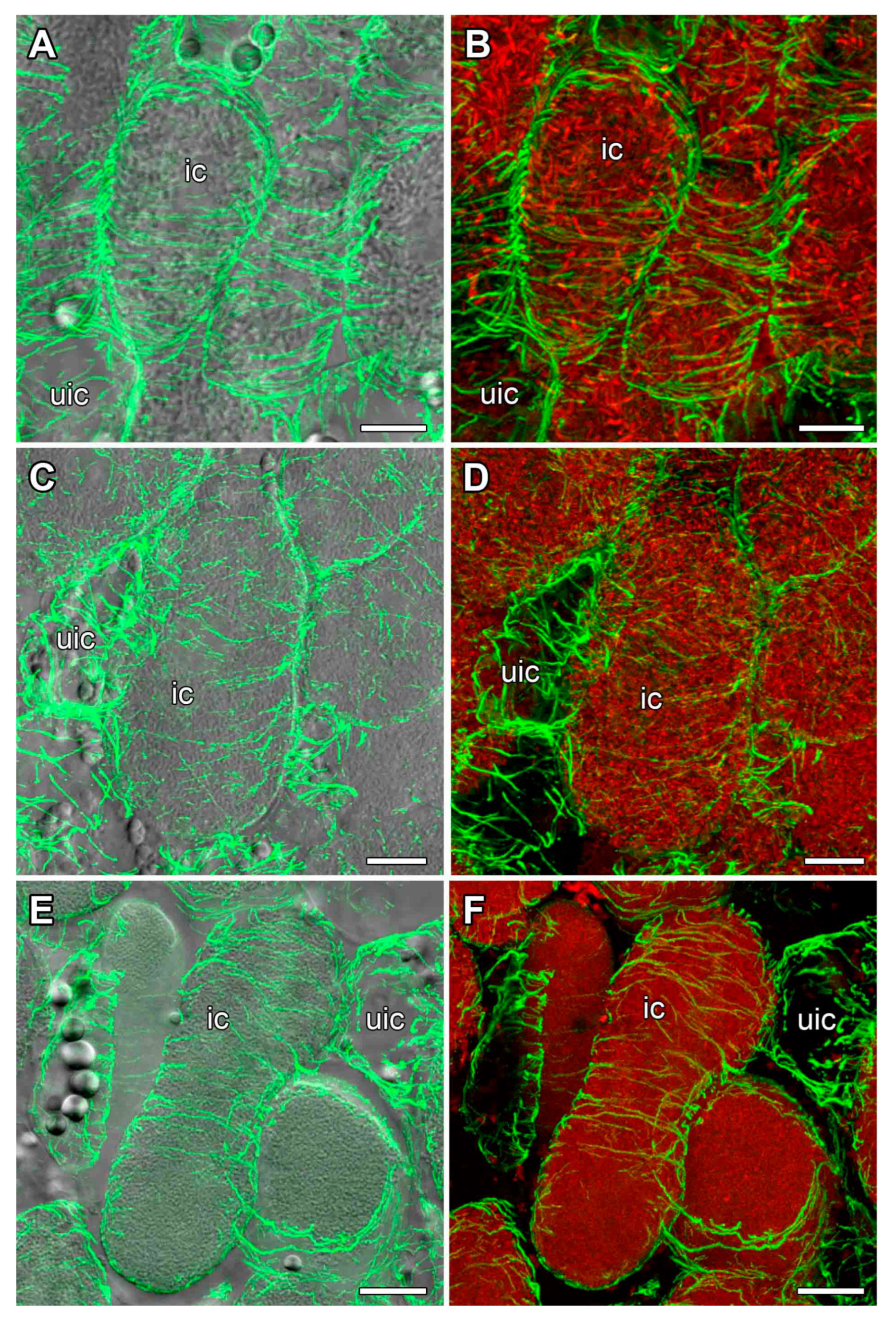
2.3. Microtubule Organization in Young Infected Cells
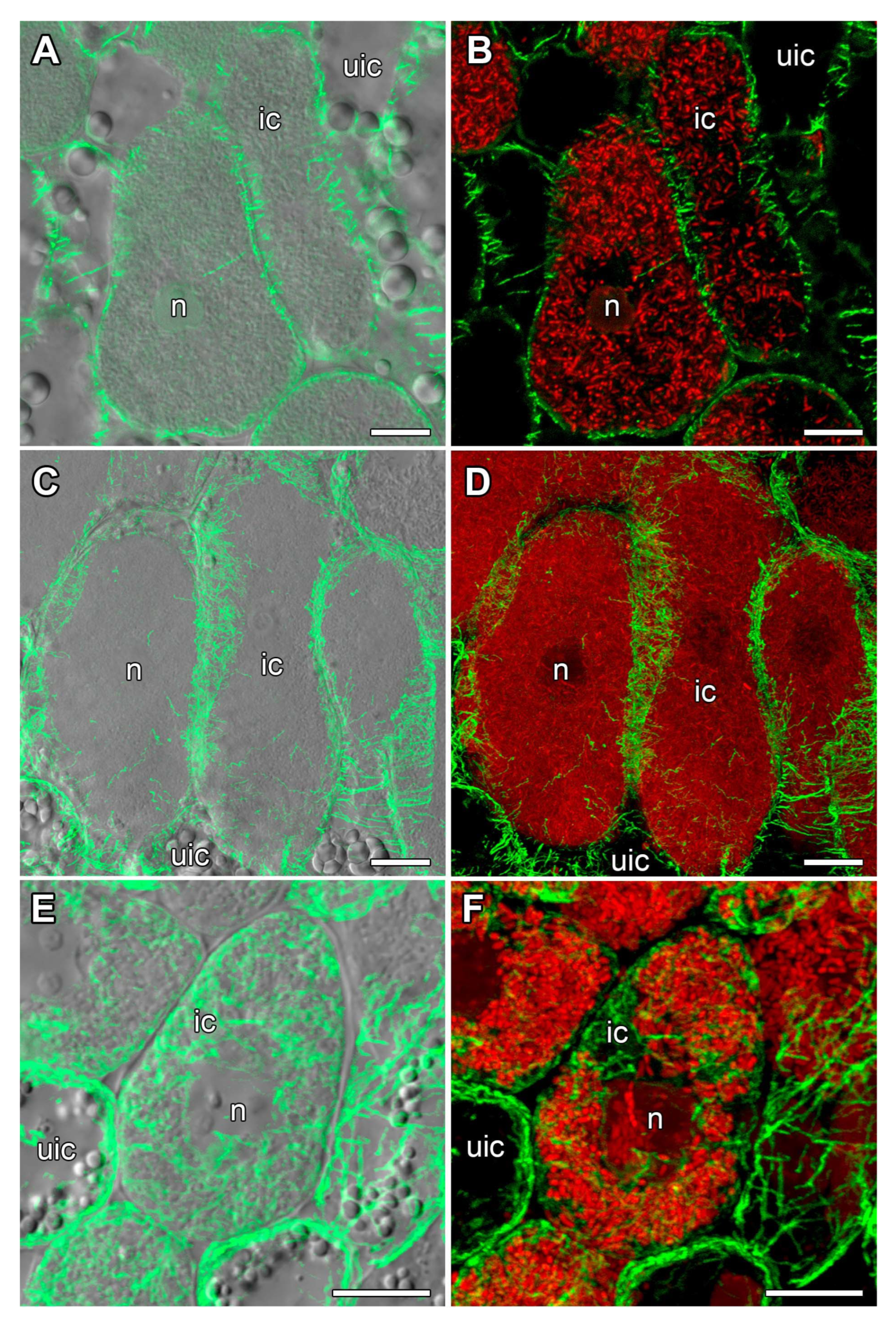
2.4. Microtubule Organization in Uninfected Cells
2.5. Organization of Cortical Microtubules in Nitrogen-Fixing Cells
2.6. Organization of Endoplasmic Microtubules in Nitrogen-Fixing Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material and Bacterial Strains
4.2. Microscopy
4.2.1. Electron Microscopy
4.2.2. Immunolocalization and Laser Scanning Confocal Microscopy
4.3. Visualization of Free-Living Bacteria and Bacteroids
4.4. Quantitative Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, R.; Schreinemachers, P. Global status and economic importance of mungbean. In The Mungbean Genome; Nair, R.M., Schafleitner, R., Lee, S.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–8. [Google Scholar] [CrossRef]
- Abebe, B.K.; Alemayehu, M.T. A review of the nutritional use of cowpea (Vigna unguiculata L. Walp) for human and animal diets. J. Agric. Food Res. 2022, 10, 100383. [Google Scholar] [CrossRef]
- Akpapunam, M. Mung bean (Vigna radiata (L.) Wilczek). In Food and Feed from Legumes and Oilseeds; Nwokolo, E., Smartt, J., Eds.; Springer: Boston, MA, USA, 1996; pp. 209–215. [Google Scholar] [CrossRef]
- Ranawake, A.L.; Dahanayaka, N.; Amarasingha, U.G.S.; Rodrigo, W.; Rodrigo, U.T.D. Effect of water stress on growth and yield of mung bean (Vigna radiata L). Trop. Agric. Res. Ext. 2012, 14. [Google Scholar] [CrossRef]
- Ayalew, T.; Yoseph, T. Cowpea (Vigna unguiculata L. Walp.): A choice crop for sustainability during the climate change periods. J. Appl. Biol. Biotechnol. 2022, 10, 154–162. [Google Scholar] [CrossRef]
- Minchin, F.; Summerfield, R. Symbiotic nitrogen fixation and vegetative growth of cowpea (Vigna unguiculata (L.) Walp.) in waterlogged conditions. Plant Soil 1976, 45, 113–127. [Google Scholar] [CrossRef]
- Xolocotzi-Acoltzi, S.; Pedroza-Sandoval, A.; García-De los Santos, G.; Álvarez-Vázquez, P.; Gramillo-Ávila, I. Growth, productivity, yield components and seasonality of different genotypes of forage clover Lotus corniculatus L. under varied soil moisture contents. Plants 2024, 13, 1407. [Google Scholar] [CrossRef]
- van Spronsen, P.C.; Grønlund, M.; Bras, C.P.; Spaink, H.P.; Kijne, J.W. Cell biological changes of outer cortical root cells in early determinate nodulation. Mol. Plant Microbe Interact. 2001, 14, 839–847. [Google Scholar] [CrossRef]
- Crespi, M.; Gálvez, S. Molecular mechanisms in root nodule development. J. Plant Growth Regul. 2000, 19, 155–166. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and development of the legume-rhizobial symbiotic interface in infection threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Oono, R.; Denison, R.F. Comparing symbiotic efficiency between swollen versus nonswollen rhizobial bacteroids. Plant Physiol. 2010, 154, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Kitaeva, A.B.; Gorshkov, A.P.; Kusakin, P.G.; Sadovskaya, A.R.; Tsyganova, A.V.; Tsyganov, V.E. Tubulin cytoskeleton organization in cells of determinate nodules. Front. Plant Sci. 2022, 13, 823183. [Google Scholar] [CrossRef]
- Pertsev, V.S.; Kitaeva, A.B.; Tsyganov, V.E. The effect of temperature on the size of bacteroids in nodules of Glycine max and Glycine soja plants inoculated with the Bradyrhizobium liaoningense strain RCAM 04656. Microbiology 2025, 94, 295–298. [Google Scholar] [CrossRef]
- Newcomb, W.; McIntyre, L. Development of root nodules of mung bean (Vigna radiata): A reinvestigation of endocytosis. Can. J. Bot. 1981, 59, 2478–2499. [Google Scholar] [CrossRef]
- Guinel, F.C. Getting around the legume nodule: I. The structure of the peripheral zone in four nodule types. Botany 2009, 87, 1117–1138. [Google Scholar] [CrossRef]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume–rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef]
- Maunoury, N.; Kondorosi, A.; Kondorosi, E.; Mergaert, P. Cell biology of nodule infection and development. In Nitrogen-Fixing Leguminous Symbioses; Dilworth, M.J., James, E.K., Sprent, J.I., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 153–189. [Google Scholar] [CrossRef]
- Timmers, A.C.; Auriac, M.C.; Truchet, G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 1999, 126, 3617–3628. [Google Scholar] [CrossRef]
- Xiao, T.T.; Schilderink, S.; Moling, S.; Deinum, E.E.; Kondorosi, E.; Franssen, H.; Kulikova, O.; Niebel, A.; Bisseling, T. Fate map of Medicago truncatula root nodules. Development 2014, 141, 3517–3528. [Google Scholar] [CrossRef]
- Maróti, G.; Kondorosi, É. Nitrogen-fixing Rhizobium-legume symbiosis: Are polyploidy and host peptide-governed symbiont differentiation general principles of endosymbiosis? Front. Microbiol. 2014, 5, 326. [Google Scholar] [CrossRef]
- Hlaváčková, K.; Šamaj, J.; Ovečka, M. Cytoskeleton as a roadmap navigating rhizobia to establish symbiotic root nodulation in legumes. Biotechnol. Adv. 2023, 69, 108263. [Google Scholar] [CrossRef]
- Hamada, T. Microtubule organization and microtubule-associated proteins in plant cells. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Boston, MA, USA, 2014; Volume 312, pp. 1–52. [Google Scholar] [CrossRef]
- Hsiao, A.-S.; Huang, J.-Y. Microtubule regulation in plants: From morphological development to stress adaptation. Biomolecules 2023, 13, 627. [Google Scholar] [CrossRef]
- Fournier, J.; Timmers, A.C.J.; Sieberer, B.J.; Jauneau, A.; Chabaud, M.; Barker, D.G. Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization. Plant Physiol. 2008, 148, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Perrine-Walker, F.M.; Lartaud, M.; Kouchi, H.; Ridge, R.W. Microtubule array formation during root hair infection thread initiation and elongation in the Mesorhizobium-Lotus symbiosis. Protoplasma 2014, 251, 1099–1111. [Google Scholar] [CrossRef]
- Whitehead, L.F.; Day, D.A.; Hardham, A.R. Cytoskeletal arrays in the cells of soybean root nodules: The role of actin microfilaments in the organisation of symbiosomes. Protoplasma 1998, 203, 194–205. [Google Scholar] [CrossRef]
- Kitaeva, A.B.; Demchenko, K.N.; Tikhonovich, I.A.; Timmers, A.C.J.; Tsyganov, V.E. Comparative analysis of the tubulin cytoskeleton organization in nodules of Medicago truncatula and Pisum sativum: Bacterial release and bacteroid positioning correlate with characteristic microtubule rearrangements. New Phytol. 2016, 210, 168–183. [Google Scholar] [CrossRef]
- Fedorova, E.E.; de Felipe, M.R.; Pueyo, J.J.; Lucas, M.M. Conformation of cytoskeletal elements during the division of infected Lupinus albus L. nodule cells. J. Exp. Bot. 2007, 58, 2225–2236. [Google Scholar] [CrossRef]
- Kitaeva, A.B.; Gorshkov, A.P.; Kirichek, E.A.; Kusakin, P.G.; Tsyganova, A.V.; Tsyganov, V.E. General patterns and species-specific differences in the organization of the tubulin cytoskeleton in indeterminate nodules of three legumes. Cells 2021, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Tsyganova, A.V.; Kitaeva, A.B.; Gorshkov, A.P.; Kusakin, P.G.; Sadovskaya, A.R.; Borisov, Y.G.; Tsyganov, V.E. Glycyrrhiza uralensis nodules: Histological and ultrastructural organization and tubulin cytoskeleton dynamics. Agronomy 2021, 11, 2508. [Google Scholar] [CrossRef]
- Tu, T.; Gao, Z.; Li, L.; Chen, J.; Ye, K.; Xu, T.; Mai, S.; Han, Q.; Chen, C.; Wu, S.; et al. Soybean symbiotic-nodule zonation and cell differentiation are defined by NIN2 signaling and GH3-dependent auxin homeostasis. Dev. Cell 2024, 59, 2254–2269.e2256. [Google Scholar] [CrossRef]
- Ardley, J.; Sprent, J. Evolution and biogeography of actinorhizal plants and legumes: A comparison. J. Ecol. 2021, 109, 1098–1121. [Google Scholar] [CrossRef]
- Selker, J.M.L.; Newcomb, E.H. Spatial relationships between uninfected and infected cells in root nodules of soybean. Planta 1985, 165, 446–454. [Google Scholar] [CrossRef]
- Newcomb, E.H.; Tandon, S.R. Uninfected cells of soybean root nodules: Ultrastructure suggests key role in ureide production. Science 1981, 212, 1394–1396. [Google Scholar] [CrossRef]
- Vance, C.; Johnson, L.; Stade, S.; Groat, R. Birdsfoot trefoil (Lotus corniculatus) root nodules: Morphogenesis and the effect of forage harvest on structure and function. Can. J. Bot. 1982, 60, 505–518. [Google Scholar] [CrossRef]
- Oono, R.; Schmitt, I.; Sprent, J.I.; Denison, R.F. Multiple evolutionary origins of legume traits leading to extreme rhizobial differentiation. New Phytol. 2010, 187, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, C.F.; Chen, W.F.; Wang, L.; Sui, X.H.; Chen, W.X. High-resolution transcriptomic analyses of Sinorhizobium sp. NGR234 bacteroids in determinate nodules of Vigna unguiculata and indeterminate nodules of Leucaena leucocephala. PLoS ONE 2013, 8, e70531. [Google Scholar] [CrossRef]
- Sen, D.; Weaver, R.; Bal, A.K. Structure and organization of effective peanut and cowpea root nodules induced by rhizobial strain 32H1. J. Exp. Bot. 1986, 37, 356–363. [Google Scholar] [CrossRef]
- Fåhraeus, G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J. Gen. Microbiol. 1957, 16, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Van Brussel, A.; Tak, T.; Wetselaar, A.; Pees, E.; Wijffelman, C. Small leguminosae as test plants for nodulation of Rhizobium leguminosarum and other rhizobia and agrobacteria harbouring a leguminosarum sym-plasmid. Plant Sci. Lett. 1982, 27, 317–325. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Jacques, E.; Buytaert, J.; Wells, D.M.; Lewandowski, M.; Bennett, M.J.; Dirckx, J.; Verbelen, J.P.; Vissenberg, K. MicroFilament Analyzer, an image analysis tool for quantifying fibrillar orientation, reveals changes in microtubule organization during gravitropism. Plant J. 2013, 74, 1045–1058. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitaeva, A.B.; Kusakin, P.G.; Gorshkov, A.P.; Tsyganova, A.V.; Tsyganov, V.E. Tubulin Cytoskeleton Organization in Cells of Determinate Nodules in Vigna radiata, Vigna unguiculata, and Lotus corniculatus. Plants 2025, 14, 2986. https://doi.org/10.3390/plants14192986
Kitaeva AB, Kusakin PG, Gorshkov AP, Tsyganova AV, Tsyganov VE. Tubulin Cytoskeleton Organization in Cells of Determinate Nodules in Vigna radiata, Vigna unguiculata, and Lotus corniculatus. Plants. 2025; 14(19):2986. https://doi.org/10.3390/plants14192986
Chicago/Turabian StyleKitaeva, Anna B., Pyotr G. Kusakin, Artemii P. Gorshkov, Anna V. Tsyganova, and Viktor E. Tsyganov. 2025. "Tubulin Cytoskeleton Organization in Cells of Determinate Nodules in Vigna radiata, Vigna unguiculata, and Lotus corniculatus" Plants 14, no. 19: 2986. https://doi.org/10.3390/plants14192986
APA StyleKitaeva, A. B., Kusakin, P. G., Gorshkov, A. P., Tsyganova, A. V., & Tsyganov, V. E. (2025). Tubulin Cytoskeleton Organization in Cells of Determinate Nodules in Vigna radiata, Vigna unguiculata, and Lotus corniculatus. Plants, 14(19), 2986. https://doi.org/10.3390/plants14192986







