Water-Holding Capacity, Ion Release, and Saturation Dynamics of Mosses as Micro-Scale Buffers Against Water Stress in Semi-Arid Ecosystems
Abstract
1. Introduction
2. Results
2.1. Descriptive Statistics
2.2. Saturation Kinetics
2.3. Species Differences in Water-Holding Capacity
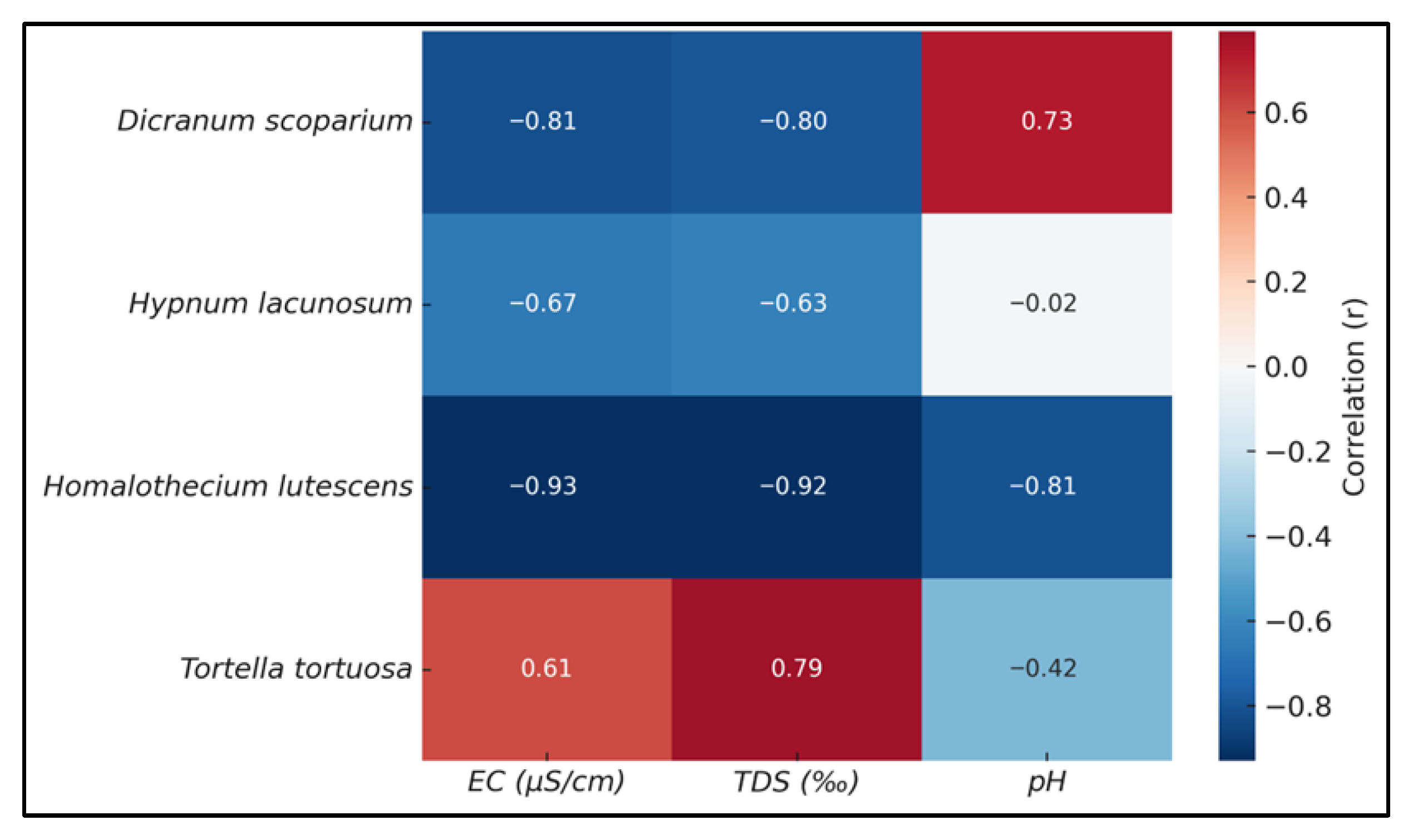
2.4. Physicochemical Properties (EC, TDS, and pH) of Retained Water
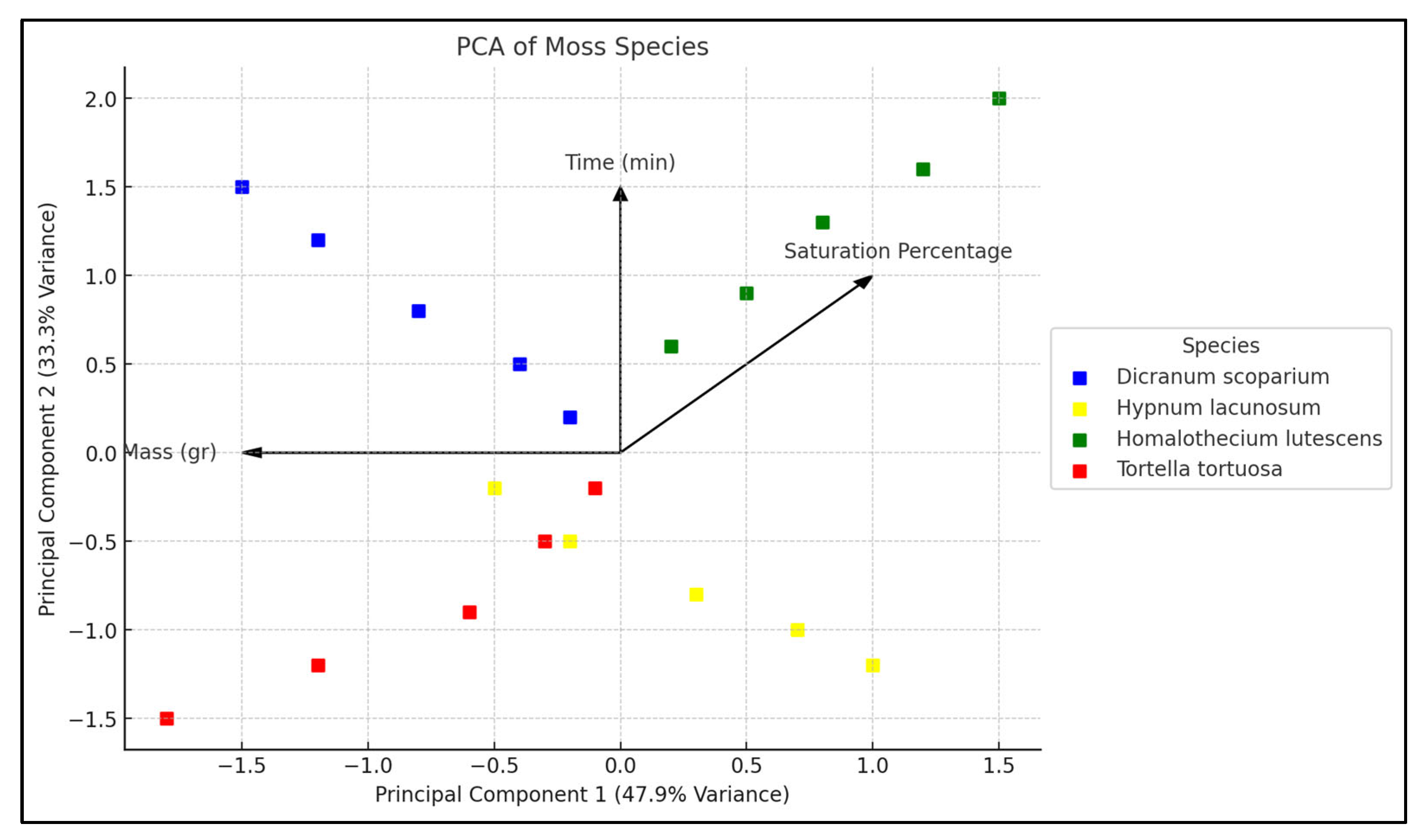
2.5. Multivariate Analyses
3. Discussion
3.1. Water-Holding Dynamics and Species Differences
3.2. Ion Release and Ecohydrological Implications
3.3. Methodological Considerations and Future Research
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Field Sampling
4.2.2. Sample Preparation
4.2.3. WHC Measurement
4.2.4. Ion Release Assay
4.2.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell-Butler, P.; Waka, T.H. Moss Matters: Unravelling the Intricate Web of Interactions Between Mosses, Microclimate, and Seed Germination in New Zealand’s Alpine Ecosystems. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2024. [Google Scholar]
- Ladrón De Guevara, M.; Maestre, F.T. Ecology and responses to climate change of biocrust-forming mosses in drylands. J. Exp. Bot. 2022, 73, 4380–4395. [Google Scholar] [CrossRef] [PubMed]
- Glime, J.M. Roles of Bryophytes in Forest Sustainability—Positive or Negative? Sustainability 2024, 16, 2359. [Google Scholar] [CrossRef]
- Wilke, C.M. Mosses Play Key Roles in Ecosystems from Tropics to Tundra. Eos 2023, 104. [Google Scholar] [CrossRef]
- Marschall, M. Ecophysiology of bryophytes in the changing environment. Acta Biol. Plant. Agriensis 2017, 5, 34. [Google Scholar] [CrossRef]
- Slate, M.L.; Antoninka, A.; Bailey, L.; Berdugo, M.B.; Callaghan, D.A.; Cárdenas, M.; Chmielewski, M.W.; Fenton, N.J.; Holland-Moritz, H.; Hopkins, S.; et al. Impact of changing climate on bryophyte contributions to terrestrial water, carbon, and nitrogen cycles. New Phytol. 2024, 242, 2411–2429. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Z.; Liu, X.; Sun, J.; Xu, C.; Xiong, D.; Lin, W.; Li, Y.; Guo, J.; Yang, Y. Moss regulates soil evaporation leading to decoupling of soil and near-surface air temperatures. J. Soils Sediments 2019, 19, 2903–2912. [Google Scholar] [CrossRef]
- Gall, C.; Nebel, M.; Scholten, T.; Thielen, S.M.; Seitz, S. Water’s path from moss to soil Vol. 2: How soil-moss combinations affect soil water fluxes and soil loss in a temperate forest. Biologia 2025, 80, 1101–1113. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Sardans, J.; Chevallier, F.; Ciais, P.; Obersteiner, M.; Vicca, S.; Canadell, J.G.; Bastos, A.; Friedlingstein, P.; Sitch, S.; et al. Global trends in carbon sinks and their relationships with CO2 and temperature. Nat. Clim. Change 2019, 9, 73–79. [Google Scholar] [CrossRef]
- Oliver, M.J.; Velten, J.; Mishler, B.D. Desiccation tolerance in bryophytes: A reflection of the primitive strategy for plant survival in dehydrating habitats? Integr. Comp. Biol. 2005, 45, 788–799. [Google Scholar] [CrossRef]
- Proctor, M.C.F. The bryophyte paradox: Tolerance of desiccation, evasion of drought. Plant Ecol. 2000, 151, 41–49. [Google Scholar] [CrossRef]
- Coe, K.K.; Sparks, J.P.; Belnap, J. Physiological Ecology of Dryland Biocrust Mosses. In Photosynthesis in Bryophytes and Early Land Plants. Advances in Photosynthesis and Respiration; Hanson, D., Rice, S., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 37, pp. 29–58. ISBN 978-94-007-6987-8. [Google Scholar]
- Kröpfl, A.I.; Distel, R.A.; Cecchi, G.A.; Villasuso, N.M. Functional Role of Moss Biocrust in Disturbed Semiarid Shrublands of North-Eastern Patagonia, Argentina. Appl. Ecol. Environ. Res. 2022, 20, 905–917. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Eldridge, D.J.; Bowker, M.A.; Jeffries, T.C.; Singh, B.K. Biocrust-forming mosses mitigate the impact of aridity on soil microbial communities in drylands: Observational evidence from three continents. New Phytol. 2018, 220, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Thielen, S.M.; Gall, C.; Nebel, M.; Scholten, T.; Seitz, S. Soil-Moss-Relations: The path of water from dripping to infiltration Soil-Moss-Relations: The path of water from dripping to infiltration. In Proceedings of the EGU General Assembly 2021, Online, 19–30 April 2021; p. 8390. [Google Scholar]
- Lee, S.Y.; Kim, E.G.; Park, J.R.; Ryu, Y.H.; Moon, W.; Park, G.H.; Ubaidillah, M.; Ryu, S.N.; Kim, K.M. Effect on chemical and physical properties of soil each peat moss, elemental sulfur, and sulfur-oxidizing bacteria. Plants 2021, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Hájek, M.; Plesková, Z.; Syrovátka, V.; Peterka, T.; Laburdová, J.; Kintrová, K.; Jiroušek, M.; Hájek, T. Patterns in moss element concentrations in fens across species, habitats, and regions. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 203–218. [Google Scholar] [CrossRef]
- Rasmussen, G.; Andersen, S. Episodic release of arsenic, copper and chromium from a wood preservation site monitored by transplanted aquatic moss. Water Air Soil Pollut. 1999, 103, 239–248. [Google Scholar] [CrossRef]
- Joshi, S.; Alam, A. Bryomonitoring of atmospheric elements in Sphagnum sp. commonly growing bryophyte in the Indian Himalayan region of Uttarakhand. Nova Geod. 2023, 3, 127. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Q.; Liu, C.; Fang, Y. Using moss to assess airborne heavy metal pollution in Taizhou, China. Int. J. Environ. Res. Public Health 2017, 14, 430. [Google Scholar] [CrossRef]
- Daniels, W.L.; Zipper, C.E.; Orndorff, Z.W. Predicting release and aquatic effects of total dissolved solids from Appalachian USA coal mines. Int. J. Coal Sci. Technol. 2014, 1, 152–162. [Google Scholar] [CrossRef]
- Kasimir, Å.; He, H.; Jansson, P.E.; Lohila, A.; Minkkinen, K. Mosses are Important for Soil Carbon Sequestration in Forested Peatlands. Front. Environ. Sci. 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Nijp, J.J.; Limpens, J.; Metselaar, K.; van der Zee, S.E.A.T.M.; Berendse, F.; Robroek, B.J.M. Can frequent precipitation moderate the impact of drought on peatmoss carbon uptake in northern peatlands? New Phytol. 2014, 203, 70–80. [Google Scholar] [CrossRef]
- Xiao, J.; Lin, Y.; He, X.; He, Z.; Kong, X. Drought Exerted a Stronger Controlling Effect on Soil Carbon Release than Moisturizing in a Global Meta-Analysis. Forests 2023, 14, 1957. [Google Scholar] [CrossRef]
- Zha, J.; Zhuang, Q. Quantifying the role of moss in terrestrial ecosystem carbon dynamics in northern high latitudes. Biogeosciences 2021, 18, 6245–6269. [Google Scholar] [CrossRef]
- Smith, A.P.; Bond-Lamberty, B.; Benscoter, B.W.; Tfaily, M.M.; Hinkle, C.R.; Liu, C.; Bailey, V.L. Shifts in pore connectivity from precipitation versus groundwater rewetting increases soil carbon loss after drought. Nat. Commun. 2017, 8, 1335. [Google Scholar] [CrossRef]
- Oishi, Y. Evaluation of the water-storage capacity of bryophytes along an altitudinal gradient from temperate forests to the alpine zone. Forests 2018, 9, 433. [Google Scholar] [CrossRef]
- Hashem, H.A.; Mohamed, A.H. Strategies for drought tolerance in xerophytes. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Springer: Sigapore, 2020; pp. 269–293. ISBN 9789811521560. [Google Scholar]
- Wainwright, C.E.; HilleRisLambers, J.; Lai, H.R.; Loy, X.; Mayfield, M.M. Distinct responses of niche and fitness differences to water availability underlie variable coexistence outcomes in semi-arid annual plant communities. J. Ecol. 2019, 107, 293–306. [Google Scholar] [CrossRef]
- Hájek, T.; Beckett, R.P. Effect of water content components on desiccation and recovery in Sphagnum mosses. Ann. Bot. 2008, 101, 165–173. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, W.; Tao, F.; Shi, X.; Fu, B. A Global Synthesis of Multi-Factors Affecting Water Storage Capacity in Forest Canopy, Litter and Soil Layers. Geophys. Res. Lett. 2023, 50, e2022GL099888. [Google Scholar] [CrossRef]
- Liu, D.; She, D. Combined effects of moss crusts and pine needles on evaporation of carbonate-derived laterite from karst mountainous lands. J. Hydrol. 2020, 586, 124859. [Google Scholar] [CrossRef]
- Rice, S.K.; Neal, N.; Mango, J.; Black, K. Relationships among shoot tissue, canopy and photosynthetic characteristics in the feathermoss Pleurozium schreberi. Bryologist 2011, 114, 367–378. [Google Scholar] [CrossRef]
- Schröder, W.; Nickel, S. Spatial structures of heavy metals and nitrogen accumulation in moss specimens sampled between 1990 and 2015 throughout Germany. Environ. Sci. Eur. 2019, 31, 33. [Google Scholar] [CrossRef]
- Lazo, P.; Kika, A.; Qarri, F.; Bekteshi, L.; Allajbeu, S.; Stafilov, T. Air Quality Assessment by Moss Biomonitoring and Trace Metals Atmospheric Deposition. Aerosol Air Qual. Res. 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Hui, R.; Li, X.; Zhao, R.; Tan, H.; Jia, R. Physiological response of moss/cyanobacteria crusts along a precipitation gradient from semi-arid to arid desert in China. Plant Soil 2021, 468, 97–113. [Google Scholar] [CrossRef]
- Volkova, I.I.; Volkov, I.V.; Morozova, Y.A.; Nikitkin, V.A.; Vishnyakova, E.K.; Mironycheva-Tokareva, N.P. Water Holding Capacity of Some Bryophyta Species from Tundra and North Taiga of the West Siberia. Water 2023, 15, 2626. [Google Scholar] [CrossRef]
- Thielen, S.M.; Gall, C.; Ebner, M.; Nebel, M.; Scholten, T.; Seitz, S. Water’s path from moss to soil: A multi-methodological study on water absorption and evaporation of soil-moss combinations. J. Hydrol. Hydromech. 2021, 69, 421–435. [Google Scholar] [CrossRef]
- Rydgren, K.; Økland, R.H.; Økland, T. Species response curves along environmental gradients. A case study from SE Norwegian swamp forests. J. Veg. Sci. 2003, 14, 869–880. [Google Scholar] [CrossRef]
- Coe, K.; Carter, B.; Slate, M.; Stanton, D. Moss functional trait ecology: Trends, gaps, and biases in the current literature. Am. J. Bot. 2024, 111, e16288. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.C.F.; Oliver, M.J.; Wood, A.J.; Alpert, P.; Stark, L.R.; Cleavitt, N.L.; Mishler, B.D. Desiccation-tolerance in bryophytes: A review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Slate, M.L.; Sullivan, B.W.; Callaway, R.M. Desiccation and rehydration of mosses greatly increases resource fluxes that alter soil carbon and nitrogen cycling. J. Ecol. 2019, 107, 1767–1778. [Google Scholar] [CrossRef]
- Sugden, A.M. Mosses in forest nutrient fluxes. Science 2019, 364, 448–449. [Google Scholar] [CrossRef]
- Gorham, E.; Janssens, J.A.; Wheeler, G.A.; Glaser, P.H. The natural and anthropogenic acidification of peatlands. In Effects of Atmospheric Pollutants on Forests, Wetlands and Agricultural Ecosystems; Springer: Berlin/Heidelberg, Germany, 1987; Volume 16, pp. 493–512. [Google Scholar]
- Maimer, N.; Horton, D.G.; Vitt, D.H. Element concentrations in mosses and surface waters of western Canadian mires relative to precipitation chemistry and hydrology. Ecography 1992, 15, 114–128. [Google Scholar] [CrossRef]
- Bland, S.N. Influence of Two Common Bryophytes on Acidity and Divalent Cation Concentrations in Standing Spring Water. Ph.D. Thesis, State University of New York, Brockport, NY, USA, 2002. [Google Scholar]
- Lobachevska, O.; Karpinets, L. Water Exchange of the Forest Ecosystems Epigeic Bryophytes Depending on Changes of the Structural and Functional Organization of Their Turfs and the Influence of the Local Growth Environmental Conditions. Biol. Stud. 2024, 18, 139–156. [Google Scholar] [CrossRef]
- Wang, Z.; Bader, M.Y. Associations between shoot-level water relations and photosynthetic responses to water and light in 12 moss species. AoB Plants 2018, 10, ply034. [Google Scholar] [CrossRef]
- van de Koot, W.Q.M.; Msonda, J.; Olver, O.P.; Doonan, J.H.; Nibau, C. Variation in Water-Holding Capacity in Sphagnum Species Depends on Both Plant and Colony Structure. Plants 2024, 13, 1061. [Google Scholar] [CrossRef]
- Wojtuń, B. Mineral content in Sphagnum mosses from ombrotrophic bogs of southwestern Poland: Pattern in species and elements. Acta Soc. Bot. Pol. 1994, 62, 203–208. [Google Scholar] [CrossRef]
- Gall, C.; Nebel, M.; Scholten, T.; Thielen, S.M.; Seitz, S. Water-related soil-moss interactions at different scales. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 23–28 April 2023; p. 6861. [Google Scholar]
- Tu, N.; Dai, Q.; Yan, Y.; Peng, X.; Meng, W.; Cen, L. Effects of Moss Overlay on Soil Patch Infiltration and Runoff in Karst Rocky Desertification Slope Land. Water 2022, 14, 3429. [Google Scholar] [CrossRef]
- Gao, L.; Sun, H.; Xu, M.; Zhao, Y. Biocrusts resist runoff erosion through direct physical protection and indirect modification of soil properties. J. Soils Sediments 2020, 20, 133–142. [Google Scholar] [CrossRef]
- Stanton, D.E.; Coe, K.K. 500 Million Years of Charted Territory: Functional Ecological Traits in Bryophytes. Bryophyt. Divers. Evol. 2021, 43, 234–252. [Google Scholar] [CrossRef]
- Price, J.S.; Whittington, P.N.; Elrick, D.E.; Strack, M.; Brunet, N.; Faux, E. A Method to Determine Unsaturated Hydraulic Conductivity in Living and Undecomposed Sphagnum Moss. Soil Sci. Soc. Am. J. 2008, 72, 487–491. [Google Scholar] [CrossRef]
- Voortman, B.R.; Bartholomeus, R.P.; van Bodegom, P.M.; Gooren, H.; van der Zee, S.E.A.T.M.; Witte, J.P.M. Unsaturated hydraulic properties of xerophilous mosses: Towards implementation of moss covered soils in hydrological models. Hydrol. Process. 2014, 28, 6251–6264. [Google Scholar] [CrossRef]
- Weber, T.K.D.; Iden, S.C.; Durner, W. Unsaturated hydraulic properties of Sphagnum moss and peat reveal trimodal pore-size distributions. Water Resour. Res. 2017, 53, 415–434. [Google Scholar] [CrossRef]
- Slate, M.L.; Stark, L.R.; Greenwood, J.L.; Clark, T.A.; Brinda, J.C. The role of prehydration in rescuing shoots of mosses damaged by extreme desiccation events: Syntrichia norvegica (Pottiaceae). Bryologist 2018, 121, 193–204. [Google Scholar] [CrossRef]
- Jauregui-Lazo, J.; Wilson, M.; Mishler, B.D. The dynamics of external water conduction in the dryland moss Syntrichia. AoB Plants 2023, 15, plad025. [Google Scholar] [CrossRef]
- Hu, X.; Gao, Z.; Li, X.Y.; Wang, R.Z.; Wang, Y.M. Structural characteristics of the moss (bryophyte) layer and its underlying soil structure and water retention characteristics. Plant Soil 2023, 490, 305–323. [Google Scholar] [CrossRef]
- Ediş, S.; Pehlivan, S. Comparison of climate classification methods and spatial distribution analysis in the Research Forest of Çankırı Faculty of Forestry. Anadolu J. For. Res. 2025, 11, 201–211. [Google Scholar] [CrossRef]
- Elumeeva, T.G.; Soudzilovskaia, N.A.; During, H.J.; Cornelissen, J.H.C. The importance of colony structure versus shoot morphology for the water balance of 22 subarctic bryophyte species. J. Veg. Sci. 2011, 22, 152–164. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Wang, Y.R.; Zhang, J.M. Is reduced seed germination due to water limitation a special survival strategy used by xerophytes in arid dunes? J. Arid Environ. 2010, 74, 508–511. [Google Scholar] [CrossRef]
- Dulai, S.; Horváth, F.; Orbán, S.; Darkó, É.; Csizi, K.; Molnár, I. Water deficit under continuous light enhances the thermal stability of photosystem II in Homalothecium lutescens moss. Acta Biol. Szeged. 2002, 46, 159–160. [Google Scholar]
- Lawton, E. Keys for the Identification of teh Mosses of the Pacific Northwest; Hattori Botanical Laboratory: Nichinan City, Japan, 1971; ISBN 0073-0912. [Google Scholar]
- Cortini Pedrotti, C.; Aleffi, M. Lista rossa delle Briofite del Trentino. Stud. Trent. Sci. Nat. 2011, 88, 5–27. [Google Scholar]
- Manz, B.; Müller, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol. 2005, 138, 1538–1551. [Google Scholar] [CrossRef]
- Clesceri, L.; Greenberg, A. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]



| Variables | Count | Mean | Std | Min | Max | Skewness | Kurtosis | CV (%) |
|---|---|---|---|---|---|---|---|---|
| Air dry weight (gr) | 80.00 | 7.43 | 2.47 | 4.52 | 10.78 | 0.03 | −1.97 | 33.32 |
| Water saturated weight (gr) | 80.00 | 79.67 | 25.81 | 22.70 | 126.65 | −0.07 | −0.88 | 32.40 |
| Electrical Conductivity (µs/cm) | 80.00 | 109.70 | 57.45 | 26.10 | 216.90 | 0.37 | −1.21 | 52.37 |
| Total dissolved solids (‰) | 80.00 | 0.06 | 0.03 | 0.01 | 0.11 | 0.22 | −1.29 | 54.83 |
| pH | 80.00 | 4.65 | 0.65 | 3.53 | 5.68 | −0.24 | −0.95 | 13.95 |
| Species | Mass (gr) | Min Saturation Time (min) |
|---|---|---|
| Dicranum scoparium | 5 | 30 |
| Dicranum scoparium | 10 | 30 |
| Hypnum lacunosum | 5 | 10 |
| Hypnum lacunosum | 10 | 10 |
| Homalothecium lutescens | 5 | 20 |
| Homalothecium lutescens | 10 | 30 |
| Tortella tortuosa | 5 | 20 |
| Tortella tortuosa | 10 | 20 |
| Species | %WHC 5 g (±SE) | %WHC 5 g (±SE) |
|---|---|---|
| Tortella tortuosa | 642 c | 688 c |
| Homalothecium lutescens | 1292 a | 915 b |
| Hypnum lacunosum | 1072 b | 979 b |
| Dicranum scoparium | 1326 a | 1085 a |
| Species | pH (±SE) | EC (µS/cm) (±SE) | TDS (‰) (±SE) |
|---|---|---|---|
| Tortella tortuosa | 4.9 b | 40.0 a | 0.020 a |
| Homalothecium lutescens | 5.4 c | 130.0 b | 0.070 b |
| Hypnum lacunosum | 5.2 b | 120.0 b | 0.065 b |
| Dicranum scoparium | 3.9 a | 110.0 b | 0.060 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ursavas, S.; Edis, S. Water-Holding Capacity, Ion Release, and Saturation Dynamics of Mosses as Micro-Scale Buffers Against Water Stress in Semi-Arid Ecosystems. Plants 2025, 14, 2728. https://doi.org/10.3390/plants14172728
Ursavas S, Edis S. Water-Holding Capacity, Ion Release, and Saturation Dynamics of Mosses as Micro-Scale Buffers Against Water Stress in Semi-Arid Ecosystems. Plants. 2025; 14(17):2728. https://doi.org/10.3390/plants14172728
Chicago/Turabian StyleUrsavas, Serhat, and Semih Edis. 2025. "Water-Holding Capacity, Ion Release, and Saturation Dynamics of Mosses as Micro-Scale Buffers Against Water Stress in Semi-Arid Ecosystems" Plants 14, no. 17: 2728. https://doi.org/10.3390/plants14172728
APA StyleUrsavas, S., & Edis, S. (2025). Water-Holding Capacity, Ion Release, and Saturation Dynamics of Mosses as Micro-Scale Buffers Against Water Stress in Semi-Arid Ecosystems. Plants, 14(17), 2728. https://doi.org/10.3390/plants14172728







