Modulation of Root Nitrogen Uptake Mechanisms Mediated by Beneficial Soil Microorganisms
Abstract
1. Introduction
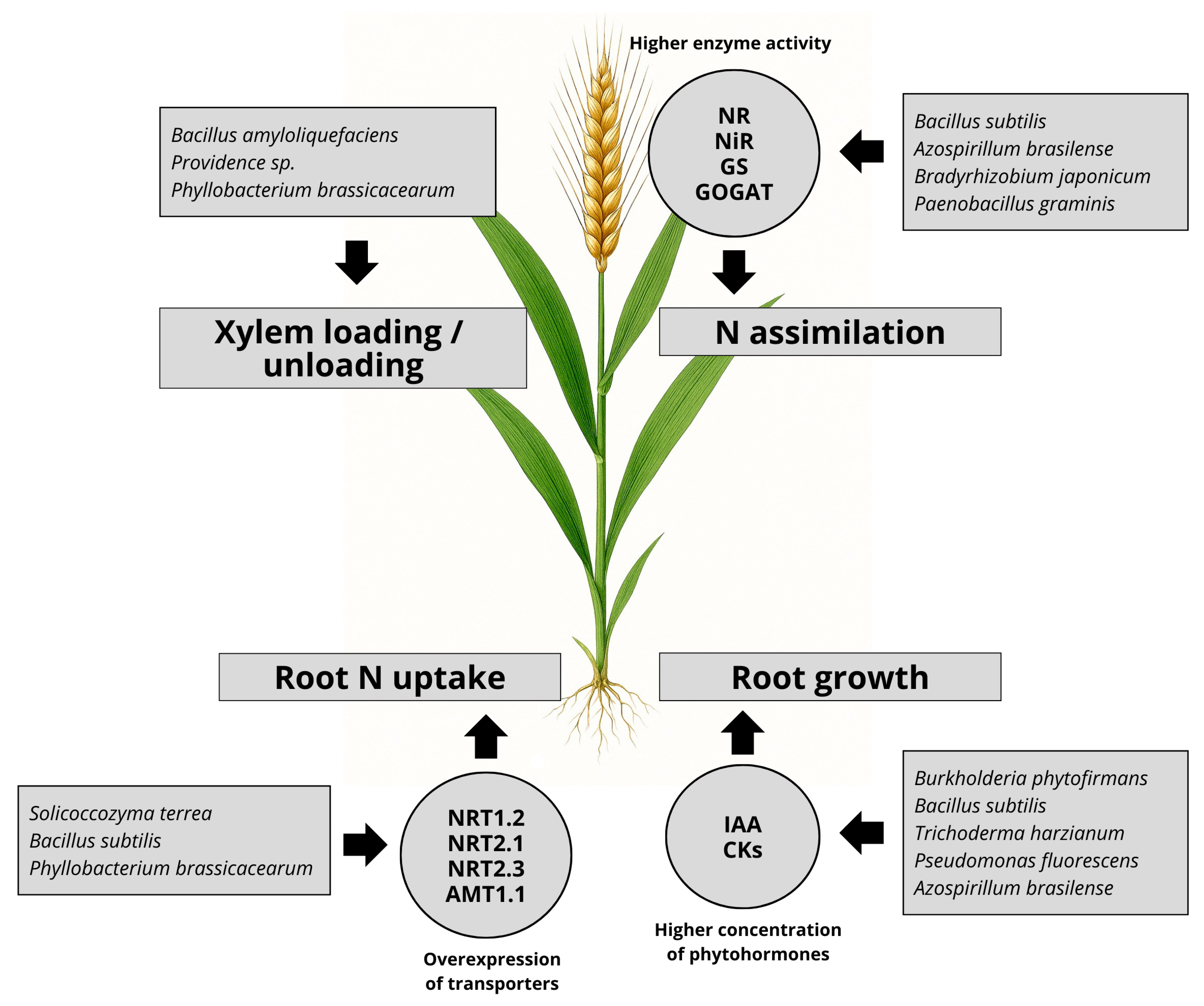
2. Promotion of Root Growth by PGP Microorganisms
3. Regulation of N Uptake Transporters by PGP Microorganisms
4. Regulation of N Assimilation by PGP Microorganisms
5. Increase in Xylem Transport of N Mediated by PGP Microorganisms
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GOGAT | Glutamine oxoglutarate amino transferase |
| GS | Glutamine synthetase |
| IAA | Indole-3-acetic acid |
| NiR | Nitrite reductase |
| NR | Nitrate reductase |
| PGP | Plant-growth promoting |
| VOCs | Volatile organic compounds |
References
- Han, Y.; Lv, M.; Liu, J.; He, S.; Shi, W.; Li, M.; Gao, Z. Agronomic practices-driven response of nitrogen-related microorganisms. Plant Soil 2025, 1–16. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2017, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Tharanath, A.C.; Upendra, R.S.; Rajendra, K. Soil symphony: A comprehensive overview of plant–microbe interactions in agricultural systems. Appl. Microbiol. 2024, 4, 1549–1567. [Google Scholar] [CrossRef]
- Raglin, S.S.; Kent, A.D. Navigating nitrogen sustainability with microbiome-associated phenotypes. Trends Plant Sci. 2025, 30, 471–483. [Google Scholar] [CrossRef]
- Bell, C.W.; Asao, S.; Calderon, F.; Wolk, B.; Wallenstein, M.D. Plant nitrogen uptake drives rhizosphere bacterial community assembly during plant growth. Soil Biol. Biochem. 2015, 85, 170–182. [Google Scholar] [CrossRef]
- Srivastava, A.; Singh, R.D.; Pandey, G.K.; Mukherjee, P.K.; Foyer, C.H. Unravelling the molecular dialogue of beneficial microbe-plant interactions. Plant Cell Environ. 2024, 48, 2534–2548. [Google Scholar] [CrossRef]
- Liu, M.; Adl, S.; Cui, X.; Tian, Y.; Xu, X.; Kuzyakov, Y. In situ methods of plant-microbial interactions for nitrogen in rhizosphere. Rhizosphere 2020, 13, 100186. [Google Scholar] [CrossRef]
- Sanow, S.; Kuang, W.; Schaaf, G.; Huesgen, P.; Schurr, U.; Roessner, U.; Watt, M.; Arsova, B. Molecular mechanisms of Pseudomonas-assisted plant nitrogen uptake: Opportunities for modern agriculture. Mol. Plant Microbe Interact. 2023, 36, 536–548. [Google Scholar] [CrossRef]
- Sah, S.; Krishnani, S.; Singh, R. Pseudomonas mediated nutritional and growth promotion activities for sustainable food security. Curr. Res. Microbial Sci. 2021, 2, 100084. [Google Scholar] [CrossRef]
- Mckinlay, J.B. Are bacteria leaky? Mechanisms of metabolite externalization in bacteria cross-feeding. Ann. Rev. Microbiol. 2023, 77, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yan, G.; Liu, G.; Xing, Y.; Wang, Q. Nitrogen deposition changes the root nutrient uptake strategies by affecting microbial diversity of the rhizosphere. Appl. Soil Ecol. 2025, 205, 105773. [Google Scholar] [CrossRef]
- Verbon, E.H.; Liberman, L.M. Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Mechanisms and impact of rhizosphere microbial metabolites on crop health, traits, functional components: A comprehensive review. Molecules 2024, 29, 5922. [Google Scholar] [CrossRef]
- Albornoz, F.; Carvajal, M.; Catrileo, D.; Gebauer, M.; Godoy, L. Volatile organic compounds produced after exposure of tomato roots to the soil yeast Solicoccozyma terrea modulate root nitrate transporters in tomato. Plant Soil 2025, 1–13. [Google Scholar] [CrossRef]
- Salunke, T.R.; Sontakke, O.P.; Chavan, S.C.; Bhosale, K.S.; Wayase, U.R.; Barmukh, R.B.; Ahire, M.L.; Shelar, P.V.; Nikalje, G.C.; Mankar, G.D. Microbial modulation of plant epigenetics: The role of miRNA and lncRNA in enhancing salt tolerance. Discov. Plants 2025, 2, 166. [Google Scholar] [CrossRef]
- Sharma, P.; Sangwan, S.; Kaur, H.; Patra, A.; Anamika; Mehta, S. Diversity and evolution of nitrogen fixing bacteria. In Sustainable Agriculture Reviews; Singh, N., Chattopadhyay, A., Lichtfouse, E., Eds.; Springer: New York, NY, USA, 2023; Volume 60, pp. 95–120. [Google Scholar]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Plant growth-promoting soil bacteria: Nitrogen fixation, phosphate solubilization, siderophore production, and other biological activities. Plants 2023, 12, 4074. [Google Scholar] [CrossRef]
- Goyal, R.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef]
- Desvoyes, B.; Echevarria, C.; Gutierrez, C. A perspective on cell proliferation kinetics in the root apical meristem. J. Exp. Bot. 2021, 72, 6708–6715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, J.; Huang, L.; Ding, Y.; Lin, X.; Sun, C. Auxin and root hair defective six-like 4 regulate Azospirillum brasilense-induced root hair development in Arabidopsis. Plant Cell Environ. 2025. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next generation plant growth additives: Functions, applications, challenges and circular bioeconomy based solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Guilfoyle, T.J. The PB1 domain in auxin response factor and Aux/IAA proteins: A versatile protein interaction module in the auxin response. Plant Cell 2015, 27, 33–43. [Google Scholar] [CrossRef]
- Yang, H.; Klopotek, Y.; Hajirezaei, M.R.; Zerche, S.; Franken, P.; Druege, U. Role of auxin homeostasis and response in nitrogen limitation and dark stimulation of adventitious root formation in petunia cuttings. Ann. Bot. 2019, 124, 1053–1066. [Google Scholar] [CrossRef]
- Song, X.; Yan, M.; Liang, Q.; Zhang, X.; Li, C.; Malviya, M.K.; Sharma, A.; Khan, Q.; Guo, D.; Li, Y.; et al. Recent advances in employing plant rhizobacteria for environmental stress mitigation in plants. Plant Stress 2025, 17, 100947. [Google Scholar] [CrossRef]
- Poprzen, T.; Nikolic, I.; Krstic-Milosevic, D.; Uzelac, B.; Trifunovic-Momcilov, M.; Markovic, M.; Radulovic, O. Characterization of the IAA-producing and -degrading Pseudomonas strains regulating growth of the common duckweed (Lemna minor L.). Int. J. Mol. Sci. 2023, 24, 17207. [Google Scholar] [CrossRef]
- Carvajal, M.; Godoy, L.; Gebauer, M.; Catrileo, D.; Albornoz, F. Screening for indole-3-acetic acid synthesis and 1-aminocyclopropane-carboxylate deaminase activity in soil yeasts from Chile uncovers Solicoccozyma terrea as an effective plant growth promoter. Plant Soil 2024, 496, 83–93. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; He, Y.; Yan, Y.; Yu, X.; Ali, M.; Pan, C.; Lu, G. Cytokinin-inducible response regulator SlRR6 controls plant height through gibberellin and auxin pathways in tomato. J. Exp. Bot. 2023, 74, 4471–4488. [Google Scholar] [CrossRef]
- Rivas, M.A.; Friero, I.; Alarcon, M.V.; Salguero, J. Auxin-cytokinin balance shapes maize root architecture by controlling primary root elongation and lateral root development. Front. Plant Sci. 2022, 13, 836592. [Google Scholar] [CrossRef] [PubMed]
- Mishra, C.N.; Pawar, S.K.; Sharma, S.; Thakur, A.; Sabhyata, S.; Mishra, S.; Kumar, S.; Gupta, O.P.; Joshi, A.K.; Tiwari, R. Transcriptomic analysis to understand the nitrogen stress response mechanisms in BNI-enabled wheat. Intl. J. Mol. Sci. 2025, 26, 4610. [Google Scholar] [CrossRef]
- Naveed, M.; Qureshi, M.A.; Zahir, Z.A.; Hussain, M.B.; Sessitsch, A.; Mitter, B. L-Tryptophan-dependent biosynthesis of indole-3-acetic acid (IAA) improves plant growth and colonization of maize by Burkholderia phytofirmans PsJN. Ann. Microbiol. 2015, 65, 1381–1389. [Google Scholar] [CrossRef]
- Jawad, N.; Kamal, J.A.K. Biochemical and molecular identification of Azospirillum brasilense bacteria and evaluation of their efficiency in producing hormones, dissolving phosphorus, and fixing nitrogen. J. Environ. Earth Sci. 2024, 6, 92–103. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.G.; Chiquito-Contreras, C.J.; Murillo-Amador, B.; Vidal-Hernández, L.; Quiñones-Aguilar, E.E.; Chiquito-Contreras, R.G. Efficiency of two inoculation methods of Pseudomonas putida on growth and yield of tomato plants. J. Soil Sci. Plant Nutr. 2017, 17, 1003–1012. [Google Scholar] [CrossRef]
- Sharma, P.; Verma, P.P.; Kaur, M. Phytohormones production and phosphate solubilization capacities of fluorescent Pseudomonas sp. Isolated from Shimla Dist. Of Himachal Pradesh. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2447–2454. [Google Scholar] [CrossRef][Green Version]
- Anguiano Cabello, J.C.; Flores Olivas, A.; Olalde Portugal, V.; Arredondo Valdés, R.; Laredo Alcalá, E.I. Evaluation of Bacillus subtilis as promoters of plant growth. Rev. Bio Cienc. 2019, 6, e418. [Google Scholar][Green Version]
- Saint-Pierre, G.; Henriquez, D.; Paredes, L.; Gaete, M. Bacillus amyloliquefaciens. Rev. Chil. Infectol. 2023, 40, 289–290. [Google Scholar] [CrossRef]
- Bean, K.M.; Kisiala, A.B.; Morrison, E.N.; Emery, R.J.E. Trichoderma synthesizes cytokinins and alters cytokinin dynamics of inoculated arabidopsis seedlings. J. Plant Growth Regul. 2022, 41, 2678–2694. [Google Scholar] [CrossRef]
- Derkach, S.М.; Volkohon, V.V.; Horban, V.P. Exogenous physiologically active substances of Trichoderma harzianum 128 and their synthesis while introduction of micromycetes into composted substrate. Agric. Microbiol. 2019, 29, 37–45. [Google Scholar] [CrossRef]
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How auxin and cytokinin phytohormones modulate root microbe interactions. Front. Plant Sci. 2016, 7, 1240. [Google Scholar] [CrossRef]
- Huang, J.; Yang, M.; Lu, L.; Zhang, X. Diverse functions of small RNAs in different plant-pathogen communications. Front. Microbiol. 2016, 7, 1552. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, X.; Zhang, Z.; Yuan, S. Synergistic effects of nitrogen metabolites on auxin regulating plant growth and development. Front. Plant Sci. 2022, 13, 1098787. [Google Scholar] [CrossRef]
- Abualia, R.; Riegler, S.; Benkova, E. Nitrate, Auxin and Cytokinin—A Trio to Tango. Cells 2023, 12, 1613. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, U.B.; Manzoor, N.; Wen, J.; Pandit, N.R. Integrating Agronomic and Molecular Advancements to Enhance Nitrogen Use Efficiency (NUE) and Promote Sustainable Rice Production. Nitrogen 2025, 6, 34. [Google Scholar] [CrossRef]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef]
- Lee, H.; Jun, Y.S.; Cha, O.; Sheen, J. Mitogen-activated protein kinases MPK3 and MPK6 are required for stem cell maintenance in the arabidopsis shoot apical meristem. Plant Cell Rep. 2018, 38, 311–319. [Google Scholar] [CrossRef]
- Dong, B.; Liu, Y.; Huang, G.; Song, A.; Chen, S.; Jiang, J.; Chen, F.; Fang, W. Plant NAC transcription factors in the battle against pathogens. BMC Plant Biol. 2024, 24, 958. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Osuna, A.; Calatrava, V.; Galvan, A.; Fernández, E.; Llamas, A. Identification of the MPK cascade and its relationship with nitrogen metabolism in the green alga Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2020, 21, 3417. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutar, D.M.K.; Noman, M.; Alzawar, N.S.; Azizullah; Li, D.; Song, F. Cyclic lipopetides of Bacillus amyloliquefaciens DHA6 are the determinants to suppress watermelon fusarium wilt by direct antifungal activity and host defense modulation. J. Fungi 2023, 9, 687. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Chen, X.; Liu, X.; Gu, Y.; Li, F. The silent conversation: How small RNAs shape plant-microbe relationships. Int. J. Mol. Sci. 2025, 26, 2631. [Google Scholar] [CrossRef]
- Aluko, O.O.; Kant, S.; Adedire, O.M.; Li, C.; Yuan, G.; Liu, H.; Wang, Q. Unlocking the potentials of nitrate transporters at improving plant nitrogen use efficiency. Front. Plant Sci. 2023, 14, 1074839. [Google Scholar] [CrossRef]
- Hao, D.-L.; Zhou, J.-Y.; Yang, S.-Y.; Qi, W.; Yang, K.-J.; Su, Y.-H. Function and regulation of ammonium transporters in plants. Int. J. Mol. Sci. 2020, 21, 3557. [Google Scholar] [CrossRef]
- Lee, S.; Trịnh, C.S.; Lee, W.J.; Jeong, C.Y.; Truong, H.A.; Chung, N.; Kang, C.; Lee, H. Bacillus subtilis strain L1 promotes nitrate reductase activity in Arabidopsis and elicits enhanced growth performance in Arabidopsis, lettuce, and wheat. J. Plant Res. 2020, 133, 231–244. [Google Scholar] [CrossRef]
- Kechid, M.; Desbrosses, G.; Gamet, L.; Castaings, L.; Varoquaux, F.; Djekoun, A.; Touraine, B. Arabidopsis growth-promotion and root architecture responses to the beneficial rhizobacterium Phyllobacterium brassicacearum strain STM196 are independent of the nitrate assimilatory pathway. Plants 2022, 11, 128. [Google Scholar] [CrossRef]
- Trinh, C.S.; Lee, H.; Lee, W.J.; Lee, S.K.; Chung, N.; Han, J.; Kim, J.; Hong, S.; Lee, H. Evaluation of the plant growth-promoting activity of Pseudomonas nitroreducens in Arabidopsis thaliana and Lactuca sativa. Plant Cell Rep. 2018, 37, 873–885. [Google Scholar] [CrossRef]
- Calvo, P.; Zebelo, S.; McNear, D.; Kloepper, J.; Fadamiro, H. Plant growth-promoting rhizobacteria induce changes in Arabidopsis thaliana gene expression of nitrate and ammonium. J. Plant Interact. 2019, 14, 224–231. [Google Scholar] [CrossRef]
- Wang, S.; Chen, A.; Xie, K.; Xu, G. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Plant Biol. 2020, 117, 16649–16659. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Padilla, V.; Molina-Henares, M.A.; Udaondo, Z.; Ramos-González, M.I.; Espinosa-Urgel, M. Genetic basis of biofilm formation and salt adaptation in the plant-beneficial strain Stutzerimonas stutzeri. Appl. Microbiol. Biotechnol. 2025, 109, 130. [Google Scholar] [CrossRef] [PubMed]
- Noar, J.D.; Bruno-Bárcena, J.M. Azotobacter vinelandii: The source of 100 years of discoveries and many more to come. Microbiology 2018, 164, 421–436. [Google Scholar] [CrossRef]
- Laishram, B.; Devi, O.R.; Dutta, R.; Senthilkumar, T.; Goyal, G.; Paliwal, D.K.; Panotra, N.; Rasool, A. Plant-microbe interactions: PGPM as microbial inoculants/biofertilizers for sustainable crop productivity and soil fertility. Curr. Res. Microbial Sci. 2025, 8, 100333. [Google Scholar] [CrossRef]
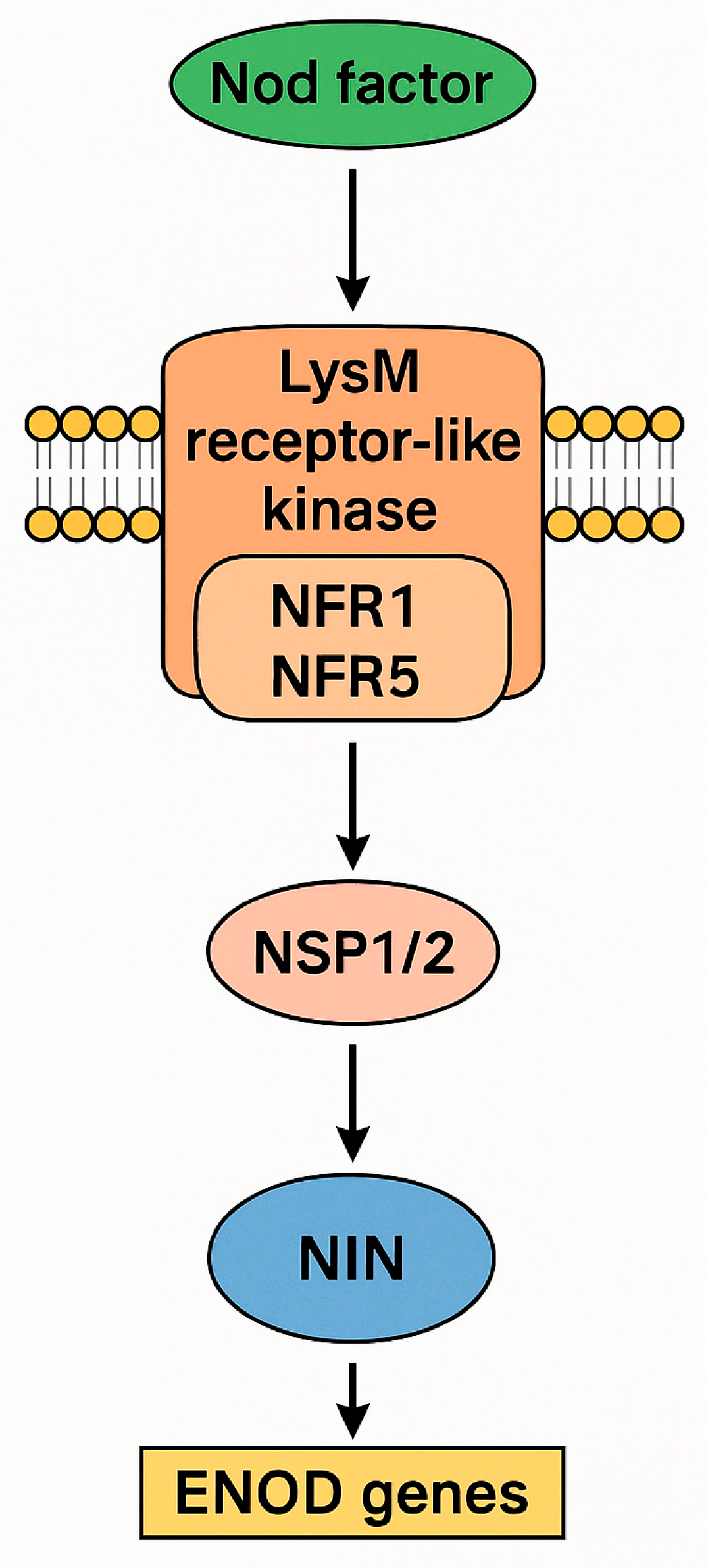
- Singh, S.; Katzer, K.; Lambert, J.; Cerri, M.; Parniske, M. CYCLOPS, a DNA-binding transcription activator, orchestrates symbiotic root nodule development. Cell Host Microbe 2014, 15, 139–152. [Google Scholar] [CrossRef]
- Shen, L.; Feng, J. NIN-at the heart of nitrogen-fixing nodule symbiosis. Fron. Plant Sci. 2024, 14, 1284720. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Bacterial indole-3-acetic acid: A key regulator for plant growth, plant-microbe interactions, and agricultural adaptive resilience. Microbiol. Res. 2024, 281, 127602. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Huang, K.; Wang, F.; Mei, Z. Molecular mechanism and agricultural application of the NifA-NifL system for nitrogen fixation. Int. J. Mol. Sci. 2023, 24, 907. [Google Scholar] [CrossRef] [PubMed]
- Baloch, F.B.; Zeng, N.; Gong, H.; Zhang, H.; Zhang, N.; Baloch, S.B.; Ali, S.; Li, B. Rhizobacterial volatile organic compounds: Implications for agricultural ecosystems’ nutrient cycling and soil health. Heliyon 2024, 10, e40522. [Google Scholar] [CrossRef] [PubMed]
- Montejano-Ramírez, V.; Ávila-Oviedo, J.L.; Campos-Mendoza, F.J.; Valencia-Cantero, E. Microbial volatile organic compounds: Insights into plant defense. Plants 2024, 13, 2013. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Tanarsuwongkul, S.; Fischer, K.W.; Mullis, B.T.; Negi, H.; Roberts, J.; Tomlin, F.; Wang, Q.; Stratmann, J.W. Green leaf volatiles co-opt proteins involved in molecular pattern signalling in plant cells. Plant Cell Environ. 2024, 47, 928–946. [Google Scholar] [CrossRef]
- Medina-Castellanos, E.; Esquivel-Naranjo, E.U.; Heil, M.; Herrera-Estrella, A. Extracellular ATP activates MAPK and ROS signaling during injury response in the fungus Trichoderma atroviride. Front. Plant Sci. 2014, 5, 659. [Google Scholar] [CrossRef]
- Chen, X.L.; Sun, M.C.; Chong, S.L.; Si, J.P.; Wu, L.S. Transcriptomic and metabolomic approaches deepen our knowledge of plant-endophyte interactions. Front. Plant Sci. 2022, 12, 700200. [Google Scholar] [CrossRef]
- Yusuf, A.; Li, M.; Zhang, S.; Odedishemi-Ajibade, F.; Luo, R.; Wu, Y.; Zhang, T.; Ugya, A.; Zhang, Y.; Duan, S. Harnessing plant–microbe interactions: Strategies for enhancing resilience and nutrient acquisition for sustainable agriculture. Front. Plant Sci. 2025, 16, 1503730. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A. Root or shoot nitrate assimilation in terrestrial vascular plants—Does it matter? Plant Soil 2022, 476, 31–62. [Google Scholar] [CrossRef]
- Di Benedetto, N.A.; Corbo, M.R.; Campaniello, D.; Cataldi, M.P.; Bevilacqua, A.; Sinigaglia, M.; Flagella, Z. The role of Plant Growth Promoting Bacteria in improving nitrogen use efficiency for sustainable crop production: A focus on wheat. AIMS Microbiol. 2017, 3, 413–434. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Yuan, H.; You, L.; Wei, Q.; Feng, R.; Jiang, S.; Zhao, X. Plant growth regulators improve nitrogen metabolism, yield, and quality of soybean–rhizobia symbiosis. Ann. Microbiol. 2023, 73, 15. [Google Scholar] [CrossRef]
- Santos, A.; Silveira, J.A.; Guilherme, E.; Bonifacio, A.; Rodrigues, A.C.; Figueiredo, M. Changes induced by co-inoculation in nitrogen-carbon metabolism in cowpea under salinity stress. Braz. J. Microbiol. 2018, 49, 685–694. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, H. Modification of rhizosphere microbial communities: A possible mechanism of plant growth promoting rhizobacteria enhancing plant growth and fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef] [PubMed]
- Mashabela, M.D.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Rhizosphere tripartite interactions and PGPR-mediated metabolic reprogramming towards ISR and plant priming: A metabolomics review. Biology 2022, 11, 346. [Google Scholar] [CrossRef]
- Tresas, T.; Isaioglou, I.; Roussis, A.; Haralampidis, K. A brief overview of the epigenetic regulatory mechanisms in plants. Int. J. Mol. Sci. 2025, 26, 4700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Xiao, J. Epigenetic regulation of nitrogen signaling and adaptation in plants. Plants 2023, 12, 2725. [Google Scholar] [CrossRef]
- Chopra, S.; Sharma, S.G.; Kaur, S.; Kumar, V.; Guleria, P. Understanding the microRNA-mediated regulation of plant-microbe interaction and scope for regulation of abiotic and biotic stress tolerance in plants. Physiol. Mol. Plant Pathol. 2025, 136, 102565. [Google Scholar] [CrossRef]
- Fal, A.; Berr, A.; Le Masson, M.; Faigenboim, A.; Pano, E.; Ishkhneli, N.; Moyal, N.; Villette, C.; Tomkova, D.; Chaboute, M.; et al. Lysine 27 of histone H3.3 is a fine modulator of developmental gene expression and stands as an epigenetic checkpoint for lignin biosynthesis in Arabidopsis. New Phytol. 2023, 238, 1085–1100. [Google Scholar] [CrossRef]
- Wang, Q.; Ou, E.; Wang, P.; Chen, Y.; Wang, Z.; Fang, X.; Zhang, J. Bacillus amyloliquefaciens GB03 augmented tall fescue growth by regulating phytohormone and nutrient homeostasis under nitrogen deficiency. Front. Plant Sci. 2022, 13, 979883. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.; Li, J.; Al-Huqail, A.A.; Du, D.; El-Khamisy, R.R.; El-Gamal, B.A. Sustainable microbial strategies for enhancing soil fertility and wheat (Triticum aestivum L.) production. J. Soil Sci. Plant Nutr. 2025, 25, 496–513. [Google Scholar] [CrossRef]
- Kechid, M.; Desbrosses, G.; Rokhsi, W.; Varoquaux, F.; Djekoun, A.; Touraine, B. The NRT2.5 and NRT2.6 genes are involved in growth promotion of Arabidopsis by the plant growth-promoting rhizobacterium (PGPR) strain Phyllobacterium brassicacearum STM196. New Phytol. 2013, 198, 514–524. [Google Scholar] [CrossRef]
- Malinowski, R.; Singh, D.; Kasprzewska, A.; Blicharz, S.; Basinska-Barczak, A. Vascular tissue—Boon or bane? How pathogens usurp long-distance transport in plants and the defence mechanisms deployed to counteract them. New Phytol. 2024, 243, 2075–2092. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.M.; Vega, D.; Correa, O.S. Azospirillum brasilense mitigates water stress imposed by a vascular disease by increasing xylem vessel area and stem hydraulic conductivity in tomato. Appl. Soil Ecol. 2014, 82, 38–43. [Google Scholar] [CrossRef]
- Tripathi, R.; Aravind, T.; Kumar, S.; Keswani, C.; Singh, S.P.; Tewari, R.; Tewari, A.K.; Singh, K.P.; Minkina, T. Microbial phytohormones: The potential orchestrators of plant growth and defense. Discov. Plants 2025, 2, 180. [Google Scholar] [CrossRef]
- Marasco, R.; Mosqueira, M.J.; Seferji, K.A.; Al Romaih, S.M.; Michoud, G.; Xu, J.; Bez, C.; Castillo Hernandez, T.; Venturi, V.; Bilou, I.; et al. Desert-adapted plant growth-promoting pseudomonads modulate plant auxin homeostasis and mitigate salinity stress. Microbial Biotechnol. 2024, 17, e70043. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Dellagi, A.; Quillere, I.; Hirel, B. Beneficial soil-borne bacteria and fungi: A promising way to improve plant nitrogen acquisition. J. Exp. Bot. 2020, 71, 4469–4479. [Google Scholar] [CrossRef]
| Microorganism | Auxin Production | Cytokinin Production | References |
|---|---|---|---|
| Azospirillum brasilense | 35 µg mL−1 | 30 µg mL−1 | [34] |
| Pseudomonas putida | 20–25 µg mL−1 | No information available | [35] |
| Pseudomonas fluorescens | 10–20 µg mL−1 | 20–30 µg mL−1 | [36] |
| Bacillus subtilis | 5–30 µg mL−1 | 5–25 µg mL−1 | [37] |
| Bacillus amyloliquefaciens | 5–35 µg mL−1 | 10–40 µg mL−1 | [38] |
| Trichoderma harzianum | 20 µg g−1 d.w. | 0.1–8.3 ng mL−1 | [39,40] |
| Rhizobium spp. | 5–20 µg mL−1 | 5–15 µg mL−1 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albornoz, F.; Godoy, L. Modulation of Root Nitrogen Uptake Mechanisms Mediated by Beneficial Soil Microorganisms. Plants 2025, 14, 2729. https://doi.org/10.3390/plants14172729
Albornoz F, Godoy L. Modulation of Root Nitrogen Uptake Mechanisms Mediated by Beneficial Soil Microorganisms. Plants. 2025; 14(17):2729. https://doi.org/10.3390/plants14172729
Chicago/Turabian StyleAlbornoz, Francisco, and Liliana Godoy. 2025. "Modulation of Root Nitrogen Uptake Mechanisms Mediated by Beneficial Soil Microorganisms" Plants 14, no. 17: 2729. https://doi.org/10.3390/plants14172729
APA StyleAlbornoz, F., & Godoy, L. (2025). Modulation of Root Nitrogen Uptake Mechanisms Mediated by Beneficial Soil Microorganisms. Plants, 14(17), 2729. https://doi.org/10.3390/plants14172729






