Functional Trait Variation and Reverse Phenology in the Tropical Dry Forest Species Bonellia nervosa
Abstract
1. Introduction
- Trait variation will correlate with plant size and reproductive status: smaller, non-reproductive plants maximize light capture and growth by having thinner, larger leaves and a more acquisitive physiology (i.e., higher SLA, greater photosynthetic efficiency). As they grow larger and become reproductive, their strategy shifts toward a conservative and stress-tolerant strategy, developing traits that help them survive in a more variable and harsher environment (i.e., lower SLA, relatively lower photosynthetic efficiency), consistent with DRH.
- Canopy openness will modulate trait expression (i.e., the light gradient hypothesis): in more open canopies, plants maximize light capture and show higher photochemical performance, while under more shaded canopies, traits become more conservative due to low light. This pattern would be especially pronounced in B. nervosa, whose inverted phenology and phreatophytic character mean light, not water, limits growth during the dry season. Therefore, individuals in more open canopies are expected to exhibit higher photochemical performance during this period.
2. Results
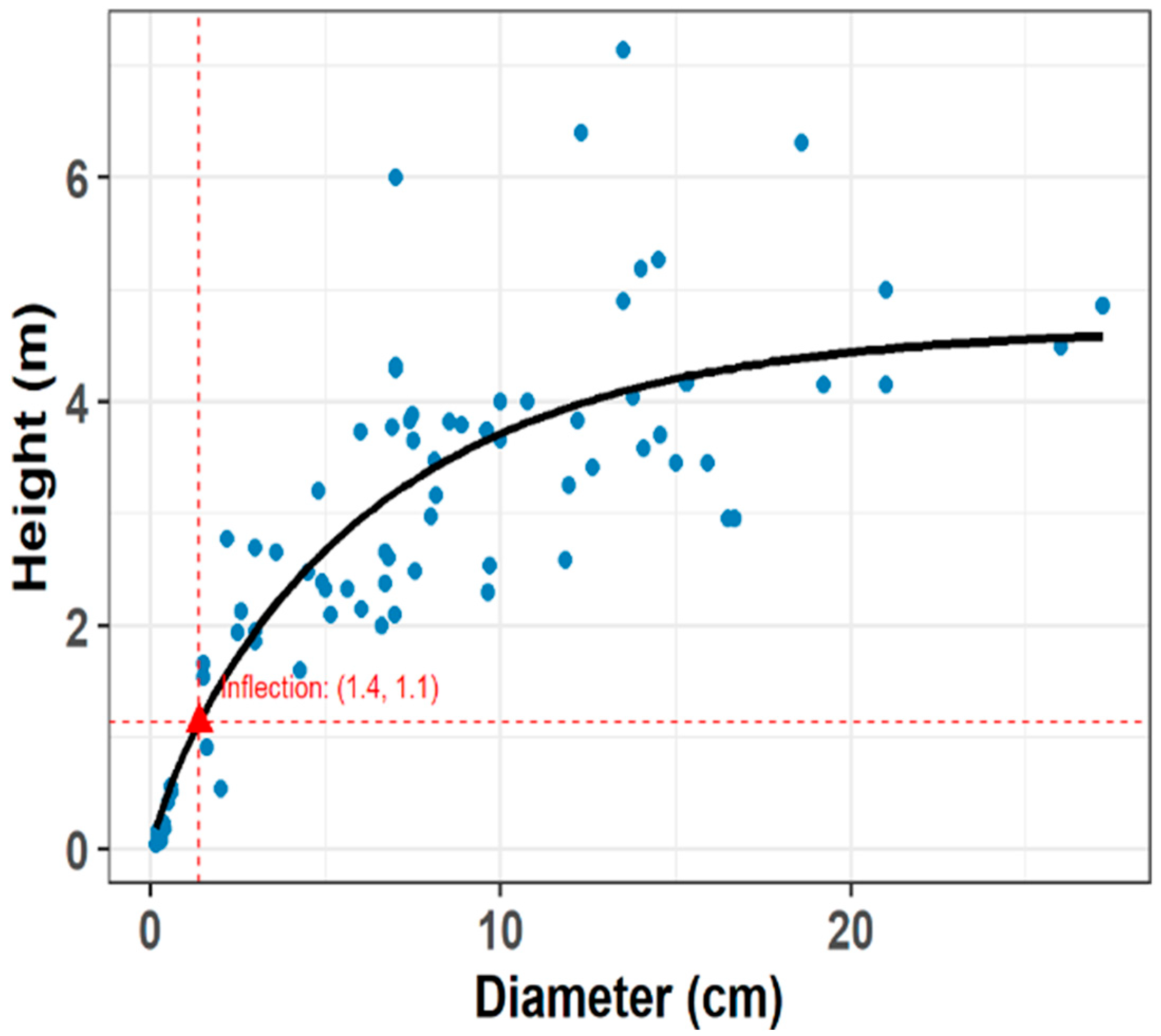
2.1. Definition of the Threshold for Reproductive Status
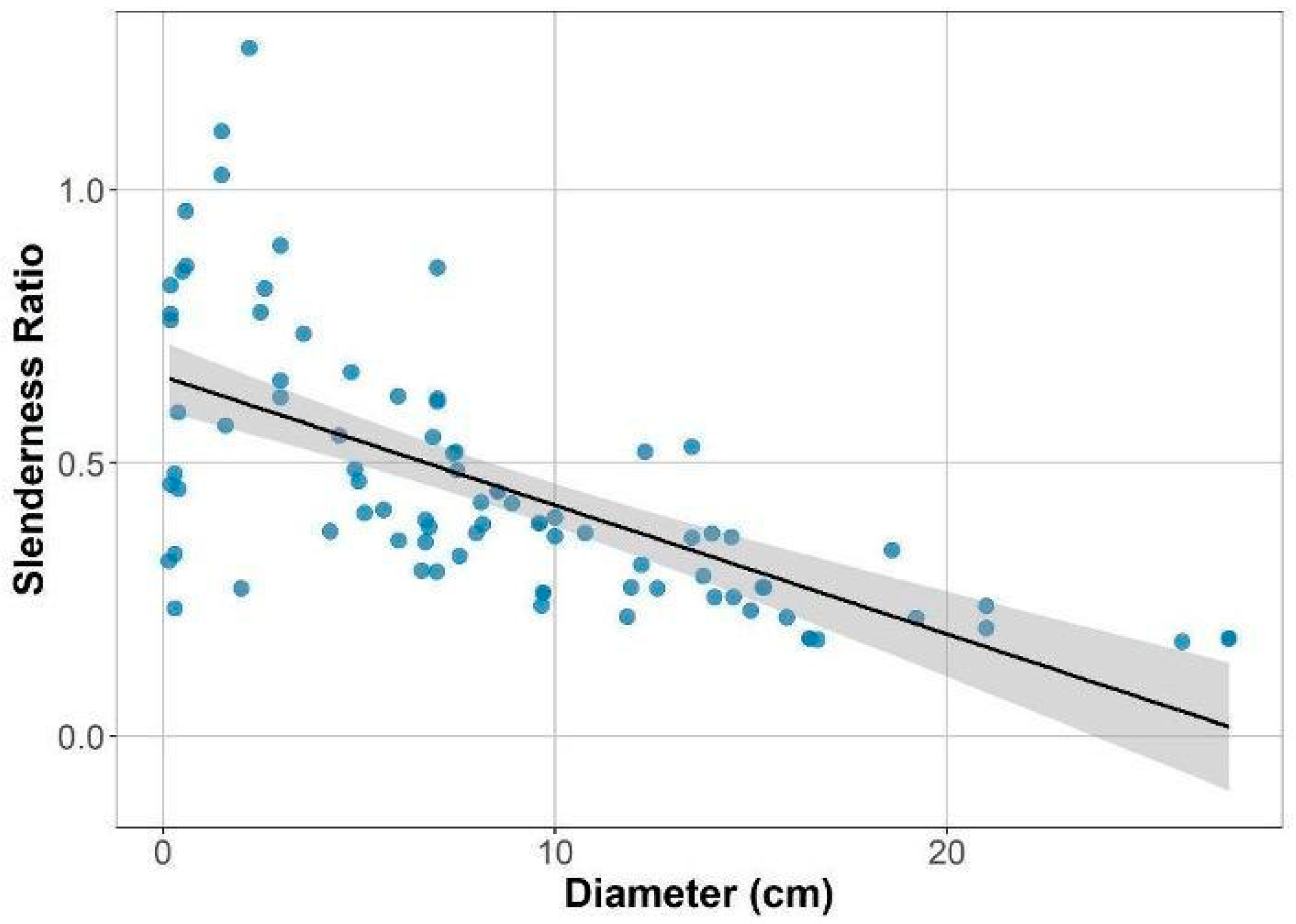
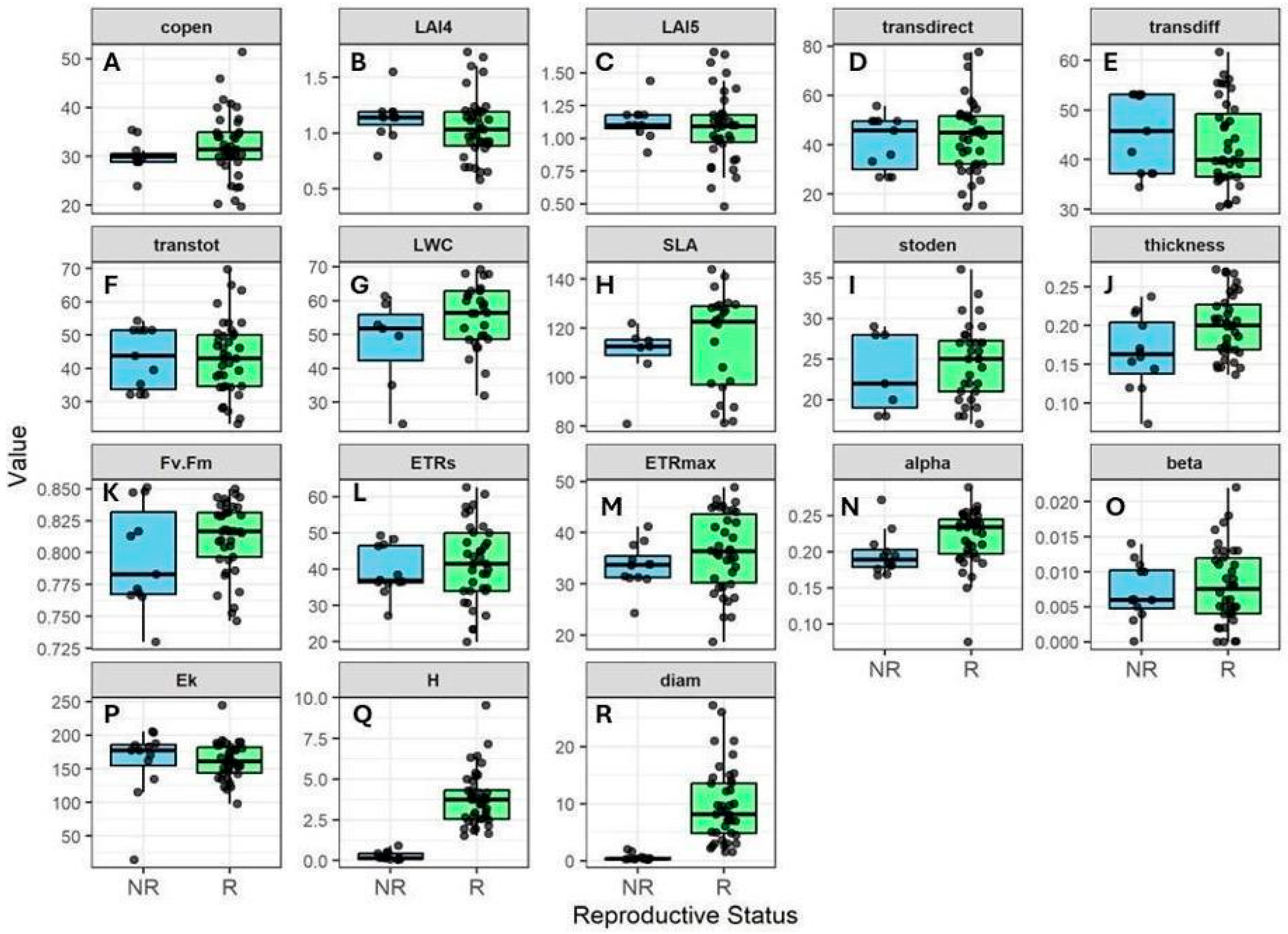
2.2. Variation in Canopy Structure and Functional Traits
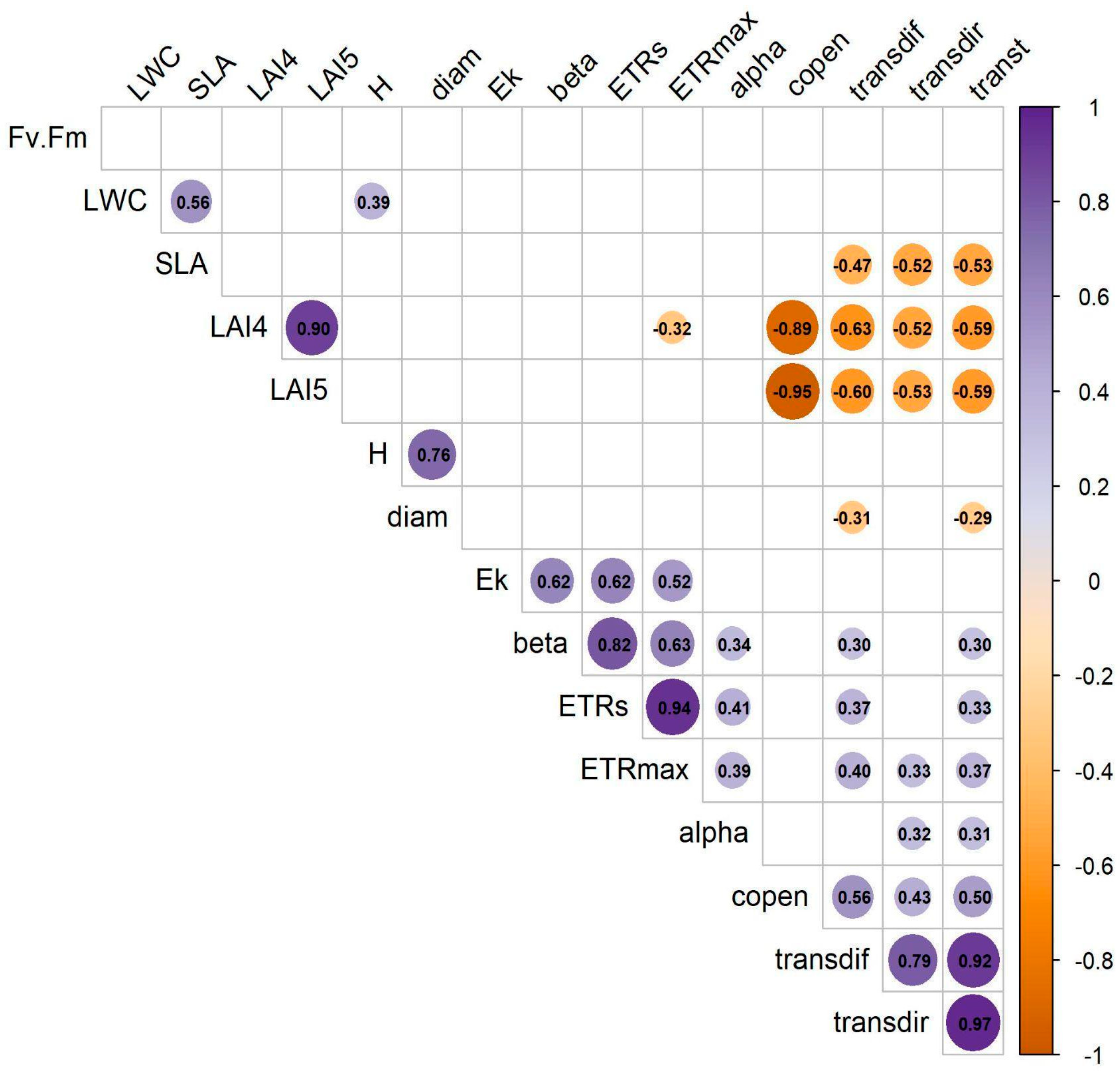
2.3. Correlation of Canopy Structure and Functional Traits
2.4. Principal Component Analysis
2.5. Relationships Between Canopy Structure, Plant Size, SLA, and Photosynthetic Performance
2.6. Photosynthetic Efficiency
3. Discussion
3.1. Canopy Structure Drives Leaf Trait Variation
3.2. SLA Was More Responsive to Light Variation than Physiological Traits
3.3. Weak Support for the Diminishing Returns Hypothesis
3.4. Adaptive Significance of Reverse Phenology and Trait Conservatism
3.5. Implications for Dry Forest Ecology and Conservation
4. Materials and Methods
4.1. Study Site
4.2. Study Species
4.3. Height-Diameter Relationship
4.4. Measurement of Leaf Structural Traits
4.5. Photosynthetic Efficiency Analysis
4.6. Canopy Structure Analysis
4.7. Principal Component Analysis
4.8. Regression Between Size and Canopy Structure (Predictor Variables) and Morphological and Fluorescence Variables (Response Variables)
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TDF | Tropical dry forest |
| SLA | Specific leaf area |
| LAI | Leaf area index |
| LES | Leaf economics spectrum |
| LL | Leaf lifespan |
| DRH | Diminishing returns hypothesis |
| ETR | Electron transport rate |
| RLC | Rapid light curve |
| PCA | Principal component analysis |
| PAR | Photosynthetically active radiation |
| SRNP | Santa Rosa National Park |
| DBH | Diameter at breast height |
References
- Janzen, D.H. Management of habitat fragments in a tropical dry forest: Growth. Ann. Mo. Bot. Gard. 1988, 75, 105–116. [Google Scholar] [CrossRef]
- Jiménez, Q.; Carrillo, E.; Kappelle, M. The Northern Pacific Lowland Seasonal Dry Forests of Guanacaste and the Nicoya Peninsula. In Costa Rican Ecosystems; Kappelle, M., Ed.; The University of Chicago Press: Chicago, IL, USA, 2016; pp. 247–289. [Google Scholar]
- Meinzer, F.C.; Andrade, J.L.; Goldstein, G.; Holbrook, N.M.; Cavelier, J.; Wright, S.J. Partitioning of soil water among canopy trees in a seasonally dry tropical forest. Oecologia 1999, 121, 293–301. [Google Scholar] [CrossRef]
- Borchert, R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 1994, 75, 1437–1449. [Google Scholar] [CrossRef]
- Sandoval-Granillo, V.; Meave, J.A. Leaf functional diversity and environmental filtering in a tropical dry forest: Comparison between two geological substrates. Ecol. Evol. 2023, 13, e10491. [Google Scholar] [CrossRef]
- Gutiérrez, G.V.; Pérez-Aviles, D.; Raczka, N.; Pereira-Arias, D.; Tijerín-Triviño, J.; Pereira-Arias, L.D.; Medvigy, D.; Waring, B.G.; Morrisey, E.; Brzostek, E.; et al. Throughfall exclusion and fertilization effects on tropical dry forest tree plantations, a large-scale experiment. Biogeosciences 2023, 20, 2143–2160. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Pandey, S.K.; Tripathi, A.; Goparaju, L.; Raghubanshi, A.S.; Singh, J.S. Variations in the plasticity of functional traits indicate the differential impacts of abiotic and biotic factors on the structure and growth of trees in tropical dry forest fragments. Front. Plant Sci. 2024, 14, 1181293. [Google Scholar] [CrossRef]
- Ribeiro, D.R.; Silva, J.L.A.; do Nascimento, M.T.; Vitória, A.P. Leaf habits and their relationship with leaf and wood traits in tropical dry forests. Trees 2022, 36, 7–24. [Google Scholar] [CrossRef]
- Vargas, G.G.; Brodribb, T.J.; Dupuy, J.M.; González-M, R.; Hulshof, C.M.; Medvigy, D.; Allerton, T.A.P.; Pizano, C.; Salgado-Negret, B.; Schwartz, N.B.; et al. Beyond leaf habit: Generalities in plant function across 97 tropical dry forest tree species. New Phytol. 2021, 232, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, M.V.; Terrazas, T.; Yang, Y. Tree species differ in plant economic spectrum traits in the tropical dry forest of Mexico. PLoS ONE 2023, 18, e0293430. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alarcón, S.; González-M, R.; Carmona, C.P.; Tordoni, E. Trait–growth relationships in Colombian tropical dry forests: Incorporating intraspecific variation and trait interactions. J. Veg. Sci. 2024, 35, e13233. [Google Scholar] [CrossRef]
- Borchert, R. Climatic periodicity, phenology, and cambium activity in tropical dry forest trees. IAWA J. 1999, 20, 239–247. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Poorter, L.; Bongers, F. Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 2011, 27, 477–489. [Google Scholar] [CrossRef]
- Derroire, G.; Powers, J.S.; Hulshof, C.M.; Varela, L.E.C.; Healey, J.R. Contrasting patterns of leaf trait variation among and within species during tropical dry forest succession in Costa Rica. Sci. Rep. 2018, 8, 285. [Google Scholar] [CrossRef]
- Werden, L.K.; Calderón-Morales, E.; Alvarado, J.P.; Gutiérrez, L.M.; Nedveck, D.A.; Powers, J.S. Using large-scale tropical dry forest restoration to test successional theory. Ecol. Appl. 2020, 30, e02116. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Bongers, F. Light-dependent leaf trait variation in 43 tropical dry forest tree species. Am. J. Bot. 2007, 94, 515–525. [Google Scholar] [CrossRef]
- Nascimento, A.R.T.; Fagg, J.M.F.; Fagg, C.W. Canopy openness and LAI estimates in two seasonally deciduous forests on limestone outcrops in Central Brazil using hemispherical photographs. Rev. Árvore 2007, 31, 167–176. [Google Scholar] [CrossRef]
- Avalos, G.; Castillo, E.M.; Fernández, V.A.; Villalobos, E.Z.; Bermúdez, T.A. Population structure, light preferences, and late-wet season pre-dispersal fruit predation in Bonellia nervosa (Primulaceae), a tropical dry forest understory tree with reverse phenology. Rev. Biol. Trop. 2025. [Google Scholar]
- Chaves, Ó.M.; Avalos, G. Is the inverse leafing phenology of the dry forest understory shrub Jacquinia nervosa (Theophrastaceae) a strategy to escape herbivory? Rev. Biol. Trop. 2006, 54, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H. Jacquinia pungens, a heliophile from the understory of tropical deciduous forest. Biotropica 1970, 2, 112–119. [Google Scholar] [CrossRef]
- Janzen, D.H.; Wilson, D.E. The cost of being dormant in the tropics. Biotropica 1974, 6, 260–262. [Google Scholar] [CrossRef]
- Chaves, Ó.M.; Avalos, G. Do seasonal changes in light availability influence the inverse leafing phenology of the neotropical dry forest understory shrub Bonellia nervosa (Theophrastaceae)? Rev. Biol. Trop. 2008, 56, 257–268. [Google Scholar] [CrossRef]
- Sánchez, O.; Quesada, M.; Dirzo, R.; Schlichting, C.D. A field experiment to determine the effect of dry-season irrigation on vegetative and reproductive traits in the wet-deciduous tree Bonellia nervosa. J. Trop. Ecol. 2020, 36, 29–35. [Google Scholar] [CrossRef]
- Pineda-García, F.; Paz, H.; Meinzer, F.C. Drought resistance in early and late secondary successional species from a tropical dry forest: The interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ. 2013, 36, 405–418. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Bongers, F.; Paz, H.; Sack, L. Hydraulics and life history of tropical dry forest tree species: Coordination of species’ drought and shade tolerance. New Phytol. 2011, 191, 480–495. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Swenson, N.G. Variation in leaf functional trait values within and across individuals and species: An example from a Costa Rican dry forest. Funct. Ecol. 2010, 24, 217–223. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Martínez-Yrízar, A.; Burquez, A.; Boyle, B.; Enquist, B.J. Plant functional trait variation in tropical dry forests: A review and synthesis. In Tropical Dry Forests in the Americas: Ecology, Conservation, and Management; Sanches-Azofeifa, A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 129–140. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Villar, R. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Sobrado, M.A. Cost-benefit relationships in deciduous and evergreen leaves of tropical dry forest species. Funct. Ecol. 1991, 5, 608–616. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Yang, J.; Cao, M.; Swenson, N.G. Why functional traits do not predict tree demographic rates. Trends Ecol. Evol. 2018, 33, 326–336. [Google Scholar] [CrossRef]
- Avalos, G. Specific leaf area (SLA) serves as a proxy to predict total carbon content in understory individuals of the neotropical canopy palm Socratea exorrhiza. Trees 2023, 37, 1831–1840. [Google Scholar] [CrossRef]
- de Freitas, G.V.; Silva, J.L.A.; Ribeiro, D.R.; Simioni, P.; Campbell, G.; Pireda, S.; Souza, A.F.; Nascimento, M.T.; Da Cunha, M.; Vitória, A.P. Functional trait patterns: Investigating variation-covariation relationships and the importance of intraspecific variability along distinct vegetation types. Community Ecol. 2024, 25, 221–236. [Google Scholar] [CrossRef]
- Avalos, G.; Frazer, K.; Le Gall, H. Plant size influences specific leaf area in palms: A case for the diminishing returns hypothesis. Oecologia 2025, 207, 56. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A multi-trait approach reveals the structure and the relative importance of intra- vs. interspecific variability in plant traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Fajardo, A.; Siefert, A. Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 2018, 99, 1024–1030. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Niklas, K.J.; Cobb, E.D. Evidence for “diminishing returns” from the scaling of stem diameter and specific leaf area. Am. J. Bot. 2008, 95, 549–557. [Google Scholar] [CrossRef]
- Falster, D.S.; Duursma, R.A.; FitzJohn, R.G. How functional traits influence plant growth and shade tolerance across the life cycle. Proc. Natl. Acad. Sci. USA 2018, 115, E6789–E6798. [Google Scholar] [CrossRef]
- Kitajima, K.; Poorter, L. Functional basis for resource niche partitioning by tropical trees. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 160–181. [Google Scholar]
- Dayrell, R.L.C.; Arruda, A.J.; Pierce, S.; Negreiros, D.; Meyer, P.B.; Lambers, H.; Silveira, F.A.O.; Godoy, O. Ontogenetic shifts in plant ecological strategies. Funct. Ecol. 2018, 32, 2730–2741. [Google Scholar] [CrossRef]
- Sendall, K.M.; Lusk, C.H.; Reich, P.B. Becoming less tolerant with age: Sugar maple, shade, and ontogeny. Oecologia 2015, 179, 1011–1021. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Mishra, A.N. Chlorophyll Fluorescence: A Practical Approach to Study Ecophysiology of Green Plants. In Advances in Plant Ecophysiology Techniques; Sánchez-Moreiras, A., Reigosa, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 77–97. [Google Scholar]
- Pleban, J.R.; Guadagno, C.R.; Mackay, D.S.; Weinig, C.; Ewers, B.E. Rapid chlorophyll a fluorescence light response curves mechanistically inform photosynthesis modeling. Plant Physiol. 2020, 183, 602–619. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.C.; de Souza, J.P.; Silva, L.C.; Franco, A.C. Leaf economic spectrum and photosynthetic performance in Neotropical savanna tree species. Funct. Plant Biol. 2021, 48, 133–143. [Google Scholar]
- Liu, Y.H.; Liu, F.L.; Long, B.; Zhou, X.L.; Zhang, X.; Zhang, Y.; Wang, W.L.; Shen, S.K. Chlorophyll fluorescence characteristics and rapid light response curves of alpine rhododendron species across elevation gradients. Hortic. Sci. Technol. 2019, 37, 463–472. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental stress-what can we learn from chlorophyll a fluorescence analysis in woody plants? A review. Front Plant Sci. 2022, 13, 1048582. [Google Scholar] [CrossRef]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- Opler, P.A.; Frankie, G.W.; Baker, H.G. Comparative phenological studies of treelet and shrub species in tropical wet and dry forests in the lowlands of Costa Rica. J. Ecol. 1980, 68, 167–188. [Google Scholar] [CrossRef]
- Tripathi, S.; Bhadouria, R.; Srivastava, P.; Devi, R.S.; Chaturvedi, R.; Raghubanshi, A.S. Effects of light availability on leaf attributes and seedling growth of four tree species in tropical dry forest. Ecol. Process. 2020, 9, 2. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Sterck, F.; Markesteijn, L.; Schieving, F.; Poorter, L. Functional traits determine trade-offs and niches in a tropical forest community. Proc. Natl. Acad. Sci. USA 2011, 108, 20627–20632. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function: Enhanced species-level trait dataset. Sci. Data 2022, 9, 755. [Google Scholar] [CrossRef]
- Santiago, L.S.; Kitajima, K.; Wright, S.J.; Mulkey, S.S. Coordinated changes in photosynthesis, water relations and leaf nutritional traits of canopy trees along a precipitation gradient in lowland tropical forest. Oecologia 2004, 139, 495–502. [Google Scholar] [CrossRef]
- Nathan, J.; Osem, Y.; Shachak, M.; Meron, E.; Salguero-Gómez, R. Linking functional diversity to resource availability and disturbance: A mechanistic approach for water-limited plant communities. J. Ecol. 2016, 2, 419–429. [Google Scholar] [CrossRef]
- Liu, Y.; Dawson, W.; Prati, D.; Haeuser, E.; Feng, Y.; van Kleunen, M. Does greater specific leaf area plasticity help plants to maintain a high performance when shaded? Ann. Bot. 2016, 118, 1329–1336. [Google Scholar] [CrossRef]
- Holdridge, L.R.; Grenke, W.C.; Hatheway, W.H.; Liang, T.; Tosi, J.A. Forest Environments in Tropical Life Zones: A Pilot Study; Pergamon Press: Oxford, UK, 1971. [Google Scholar]
- Ståhl, B. A synopsis of Central American Theophrastaceae. Nord. J. Bot. 1989, 9, 15–30. [Google Scholar] [CrossRef]
- Ståhl, B.; Källersjö, M. Reinstatement of Bonellia (Theophrastaceae). Novon 2004, 14, 115–118. [Google Scholar]
- Morales, J.F. Theophrastaceae. In Manual de Plantas de Costa Rica; Hammel, B.E., Grayum, M.H., Herrera, C., Zamora, N., Eds.; Dicotiledóneas (Sabiaceae–Zygophyllaceae); Monographs in Systematic Botany from the Missouri Botanical Garden: St. Louis, MO, USA, 2015; Volume 8, pp. 415–420. [Google Scholar]
- Domínguez-Calleros, P.A.; Rodríguez-Flores, F.D.J.; Lizárraga-Mendiola, L.; Jiménez-Gómez, M.A.; Navar, J. Aplicaciones y ejemplos de modelos de crecimiento diamétrico para árboles tropicales. Ecosist. Recur. Agropec. 2017, 4, 265–274. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 57, 341–345. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.; Bolker, B. minpack.lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds. R Package Version 1.2-4. 2023. Available online: https://CRAN.R-project.org/package=minpack.lm (accessed on 7 July 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 7 July 2025).
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data. R Package Version 1.3.1. 2024. Available online: https://github.com/tidyverse/tidyr (accessed on 7 July 2025).
- Stenberg, P.; Kangas, T.; Smolander, H.; Linder, S. Shoot structure, canopy openness, and light interception in Norway spruce. Plant Cell Environ. 1999, 22, 1133–1142. [Google Scholar] [CrossRef]
- JMP Statistical Discovery LLC. JMP®, Version 18.2.1; JMP Statistical Discovery LLC: Cary, NC, USA, 2025; Available online: https://www.jmp.com/ (accessed on 7 July 2025).
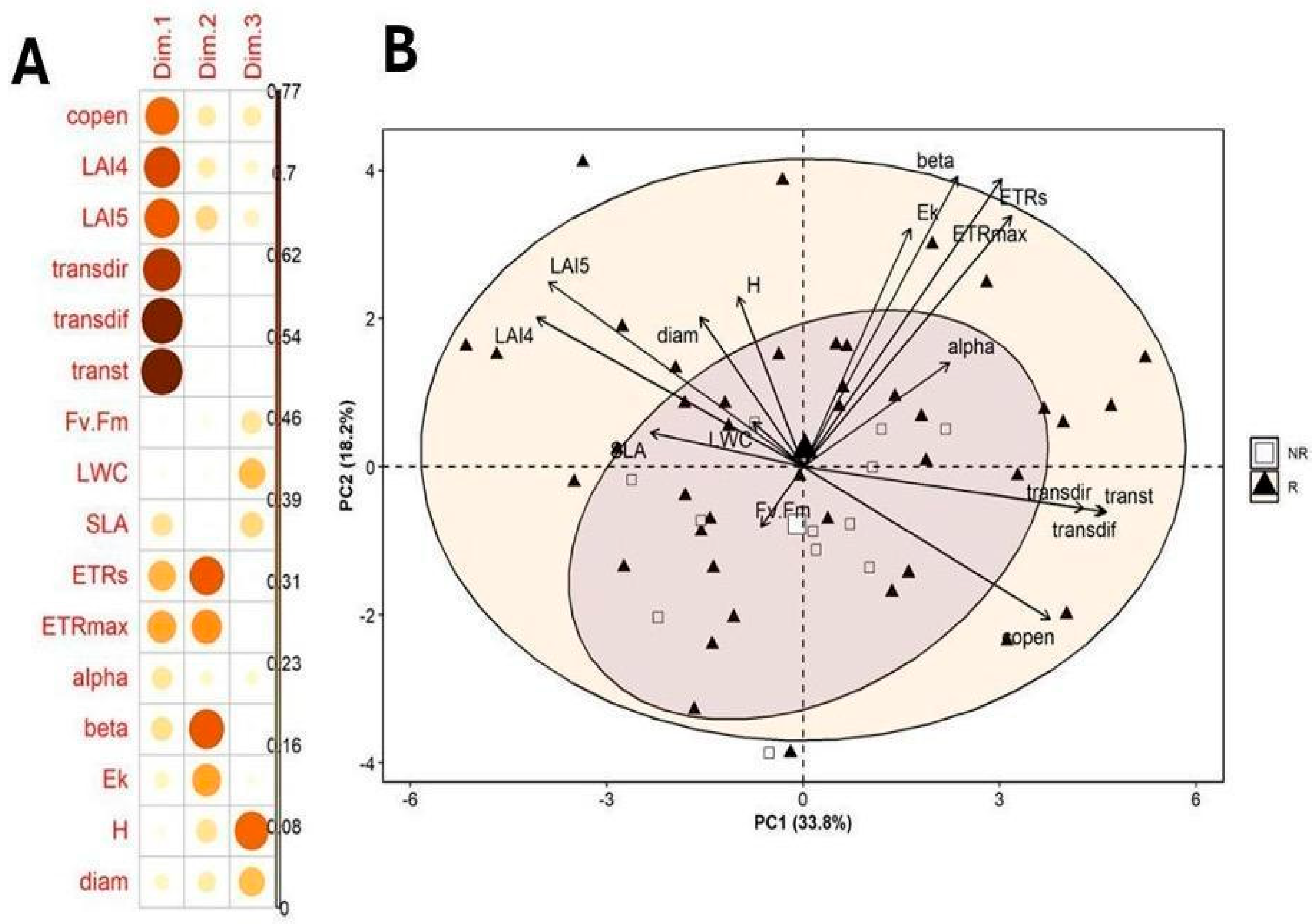
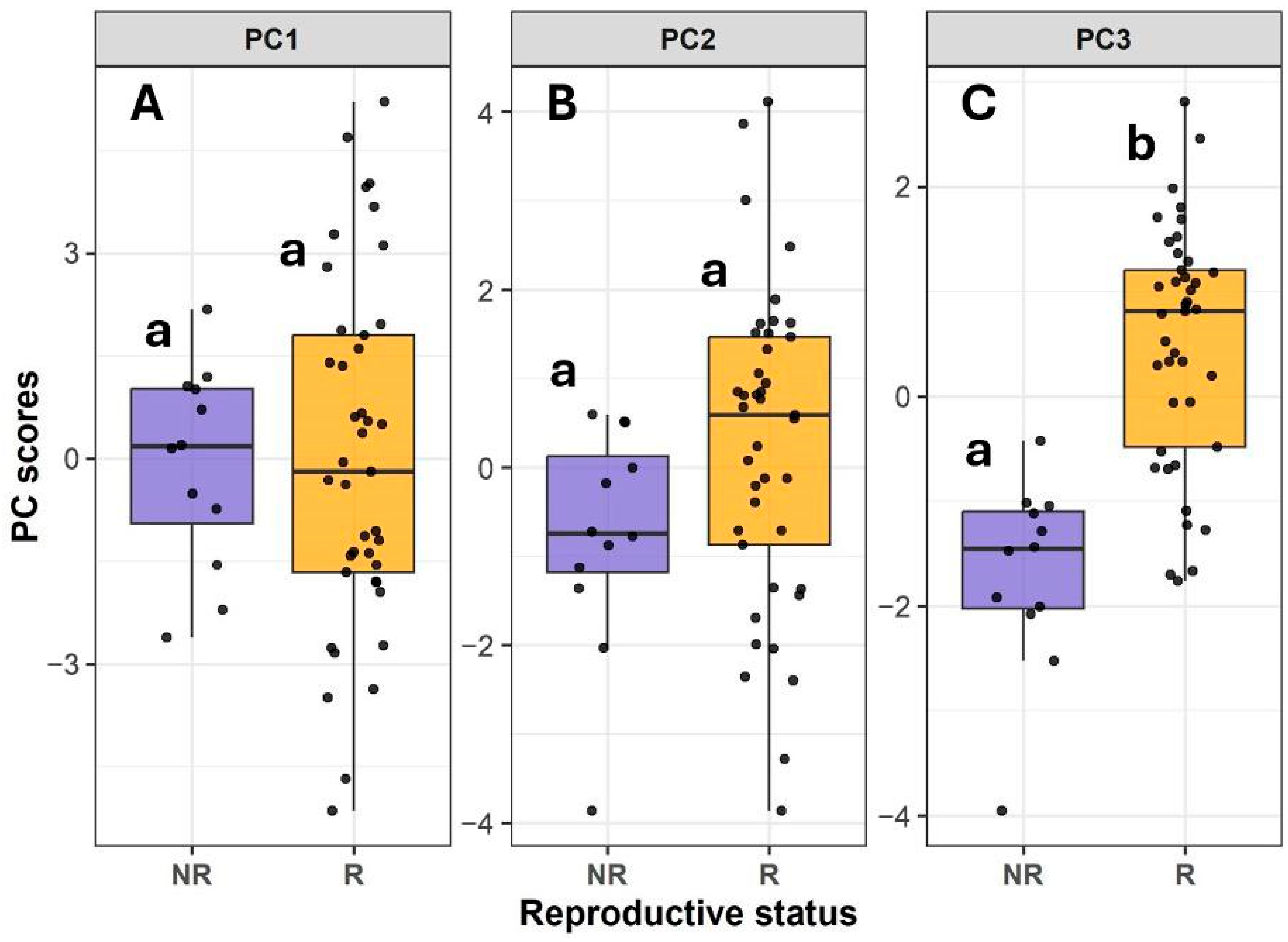
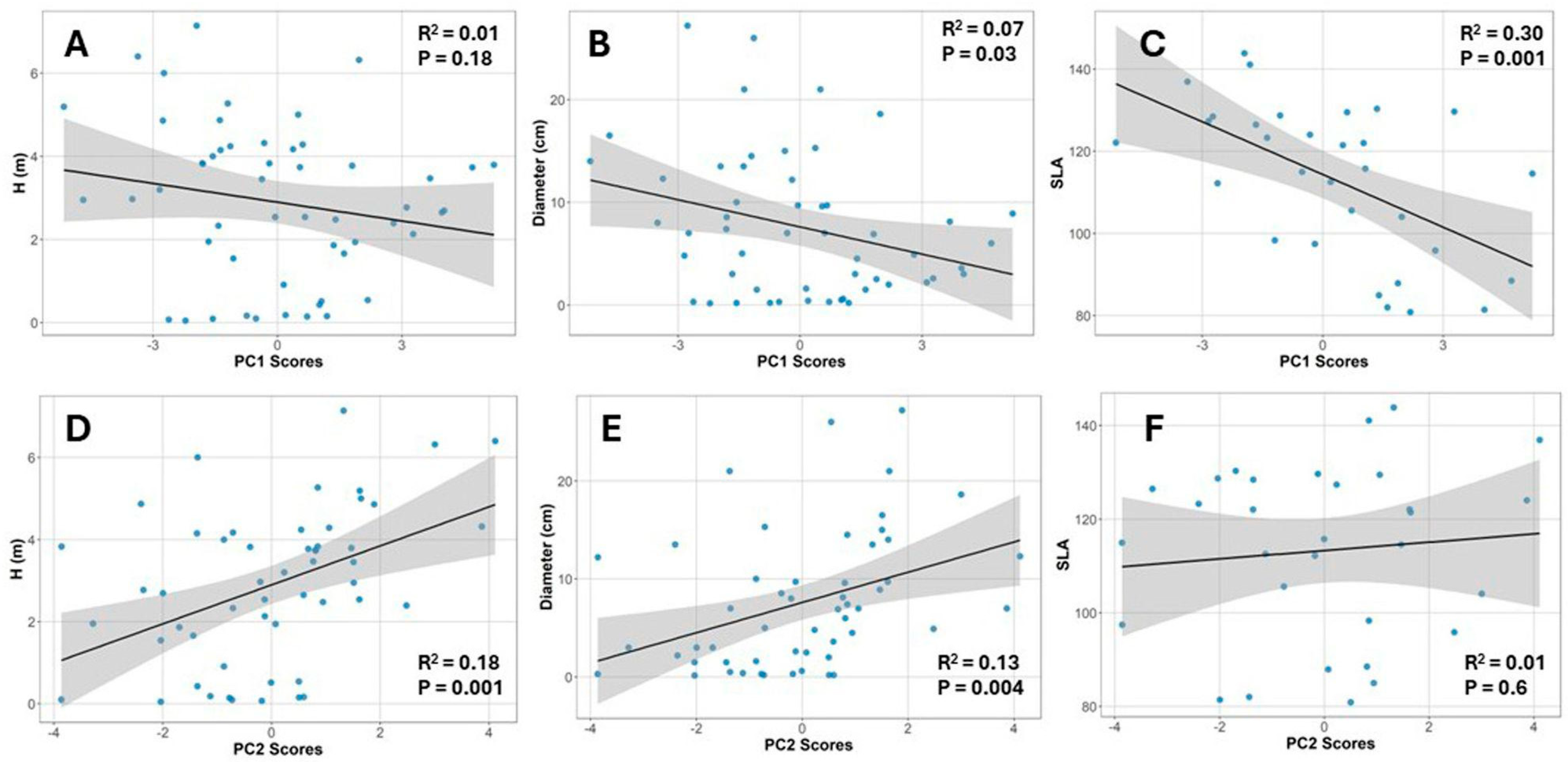
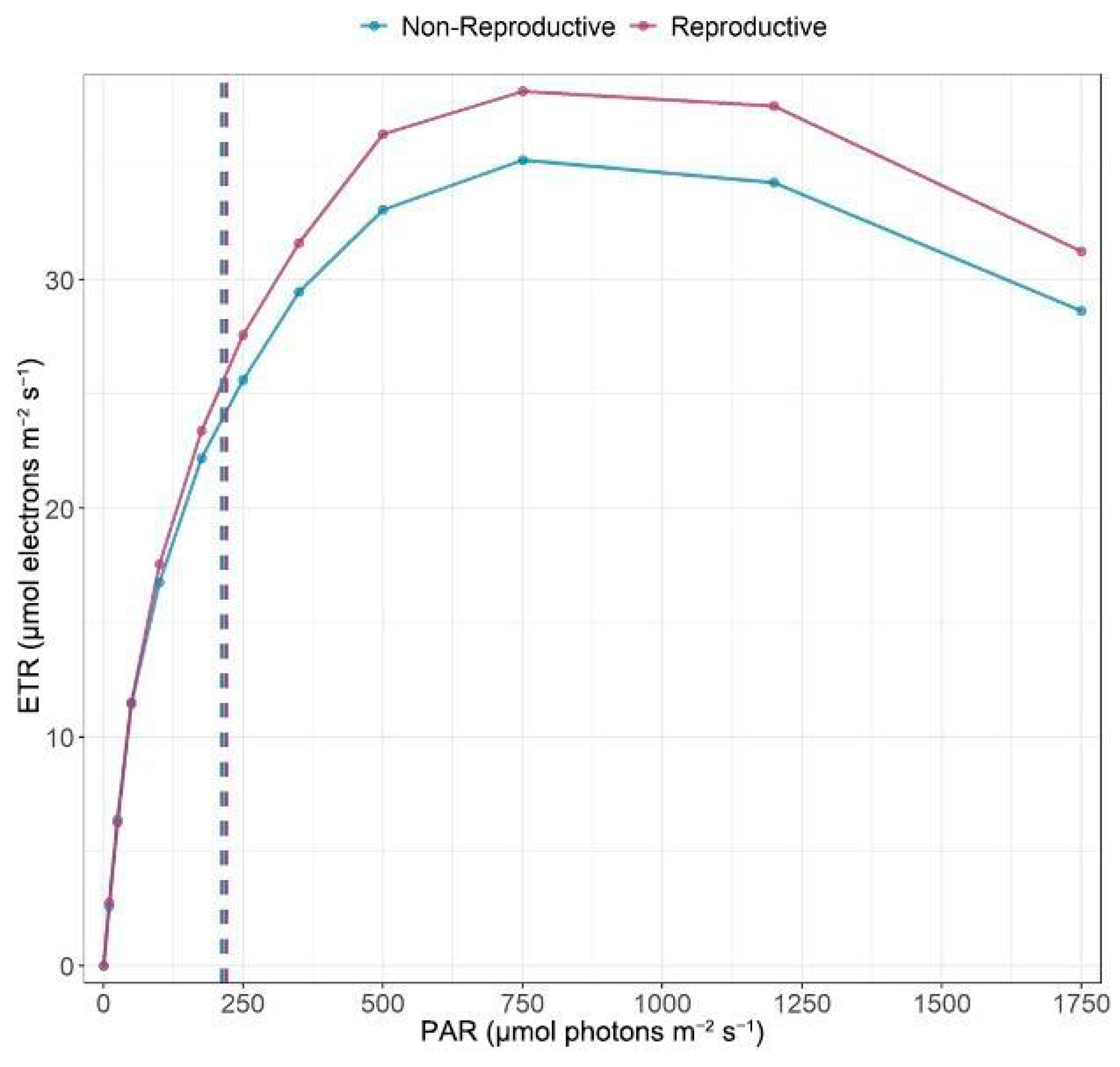
| Component | Eigenvalue | Percent | Cum Percent |
|---|---|---|---|
| 1 | 5.40 | 33.75 | 33.75 |
| 2 | 2.91 | 18.18 | 51.94 |
| 3 | 2.00 | 12.56 | 64.50 |
| Trait | PC1 | PC2 | PC3 |
| Canopy openness (copen, %) | 0.71 | −0.39 | 0.38 |
| LAI4 | −0.77 | 0.38 | −0.29 |
| LAI5 | −0.74 | 0.47 | −0.33 |
| Transmitted direct light (transdir, µmol·m−2·s−1) | 0.81 | −0.10 | −0.01 |
| Transmitted diffuse light (transdif, µmol·m−2·s−1) | 0.87 | −0.11 | −0.03 |
| Transmitted total light (transt, µmol·m−2·s−1) | 0.88 | −0.11 | −0.02 |
| Fv/Fm | −0.12 | −0.15 | 0.43 |
| Leaf water content (LWC) | −0.14 | 0.11 | 0.55 |
| Specific leaf area (SLA, cm2/g) | −0.44 | 0.08 | 0.48 |
| Electron transport rate (ETR, µmol electrons m−2 s−1) | 0.58 | 0.73 | −0.05 |
| Maximum electron transport rate (ETRmax, µmol electrons m−2 s−1) | 0.60 | 0.64 | −0.04 |
| alpha | 0.42 | 0.26 | 0.26 |
| beta | 0.45 | 0.74 | −0.08 |
| Ek (µmol photons m−2 s−1) | 0.31 | 0.61 | −0.17 |
| Height (H, m) | −0.18 | 0.43 | 0.72 |
| Diameter (diam, cm) | −0.30 | 0.38 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duff, C.; McBride, B.; Avalos, G. Functional Trait Variation and Reverse Phenology in the Tropical Dry Forest Species Bonellia nervosa. Plants 2025, 14, 2659. https://doi.org/10.3390/plants14172659
Duff C, McBride B, Avalos G. Functional Trait Variation and Reverse Phenology in the Tropical Dry Forest Species Bonellia nervosa. Plants. 2025; 14(17):2659. https://doi.org/10.3390/plants14172659
Chicago/Turabian StyleDuff, Ciara, Bridget McBride, and Gerardo Avalos. 2025. "Functional Trait Variation and Reverse Phenology in the Tropical Dry Forest Species Bonellia nervosa" Plants 14, no. 17: 2659. https://doi.org/10.3390/plants14172659
APA StyleDuff, C., McBride, B., & Avalos, G. (2025). Functional Trait Variation and Reverse Phenology in the Tropical Dry Forest Species Bonellia nervosa. Plants, 14(17), 2659. https://doi.org/10.3390/plants14172659









