Plant Architectural Structure and Leaf Trait Responses to Environmental Change: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
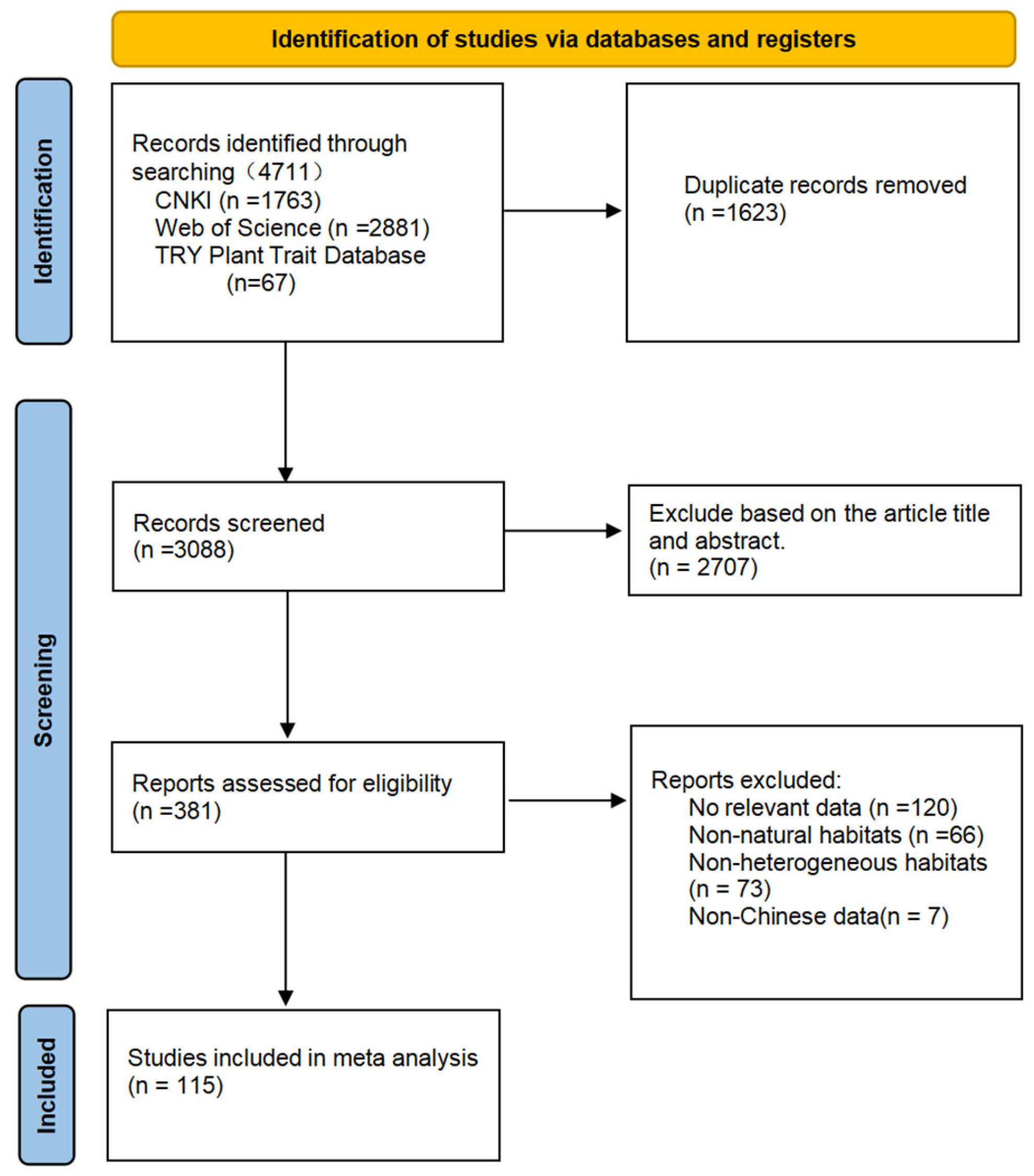
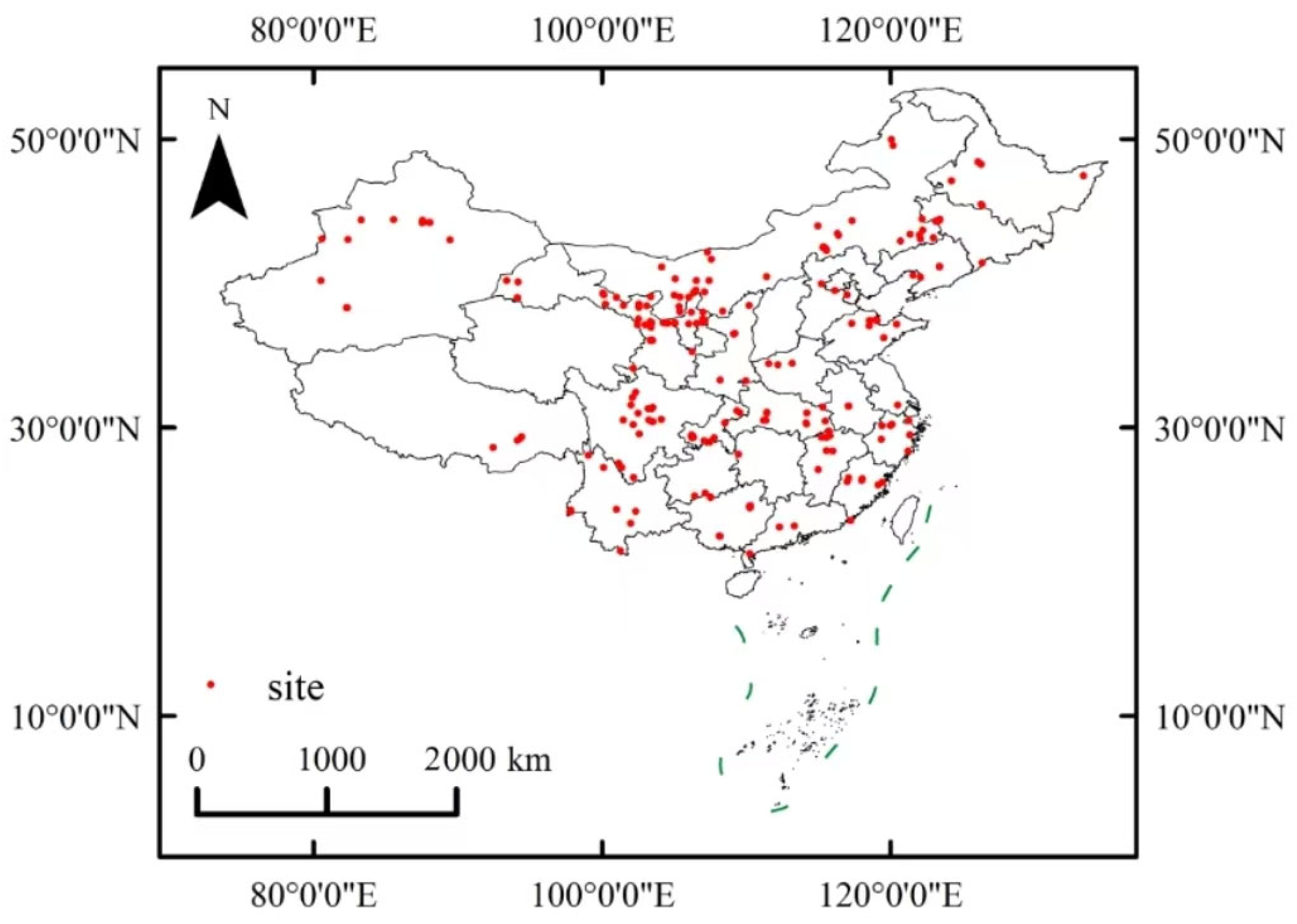
2.1. Data Sources
2.2. Data Categorization
2.3. Data Retrieval
2.4. Statistical Evaluation
3. Results
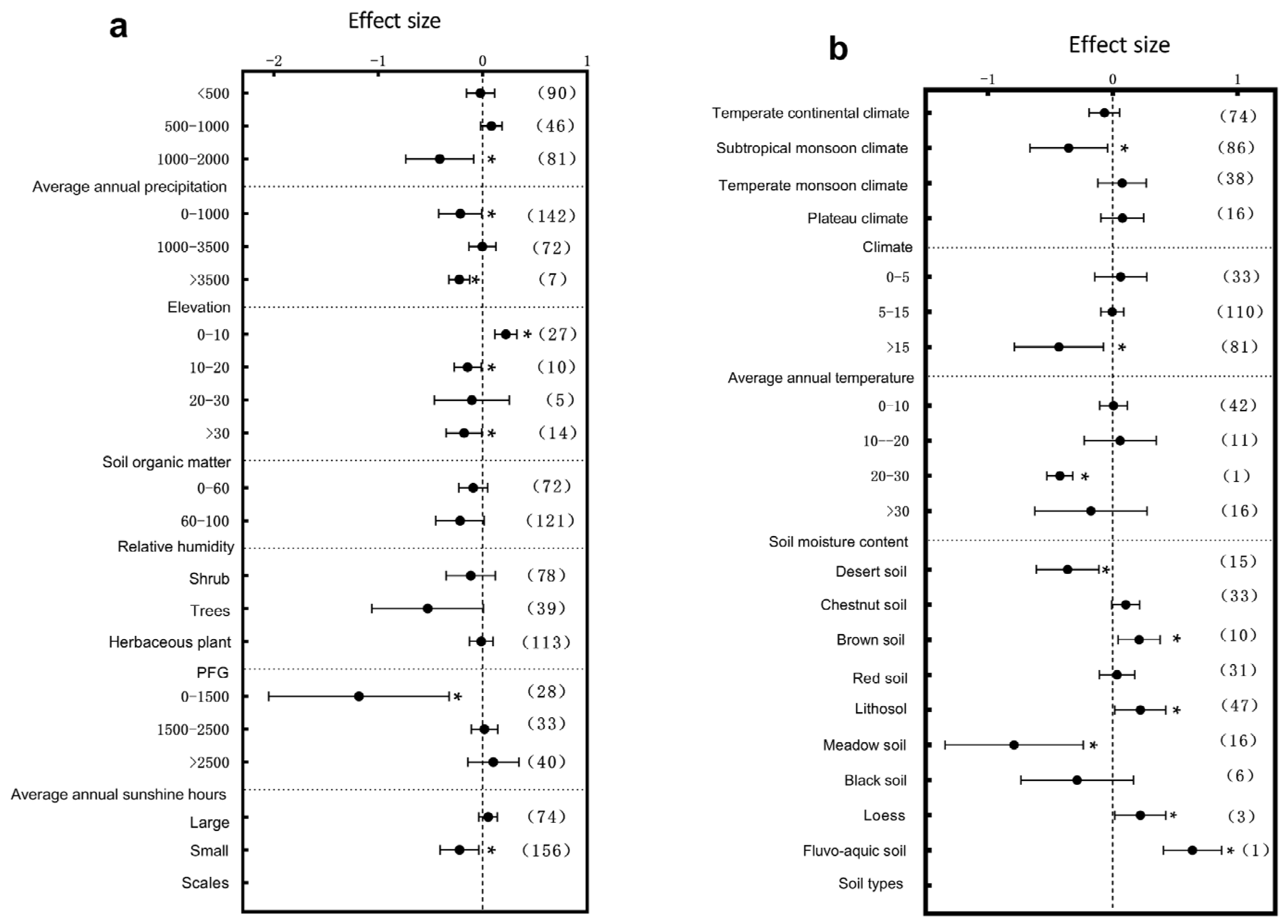
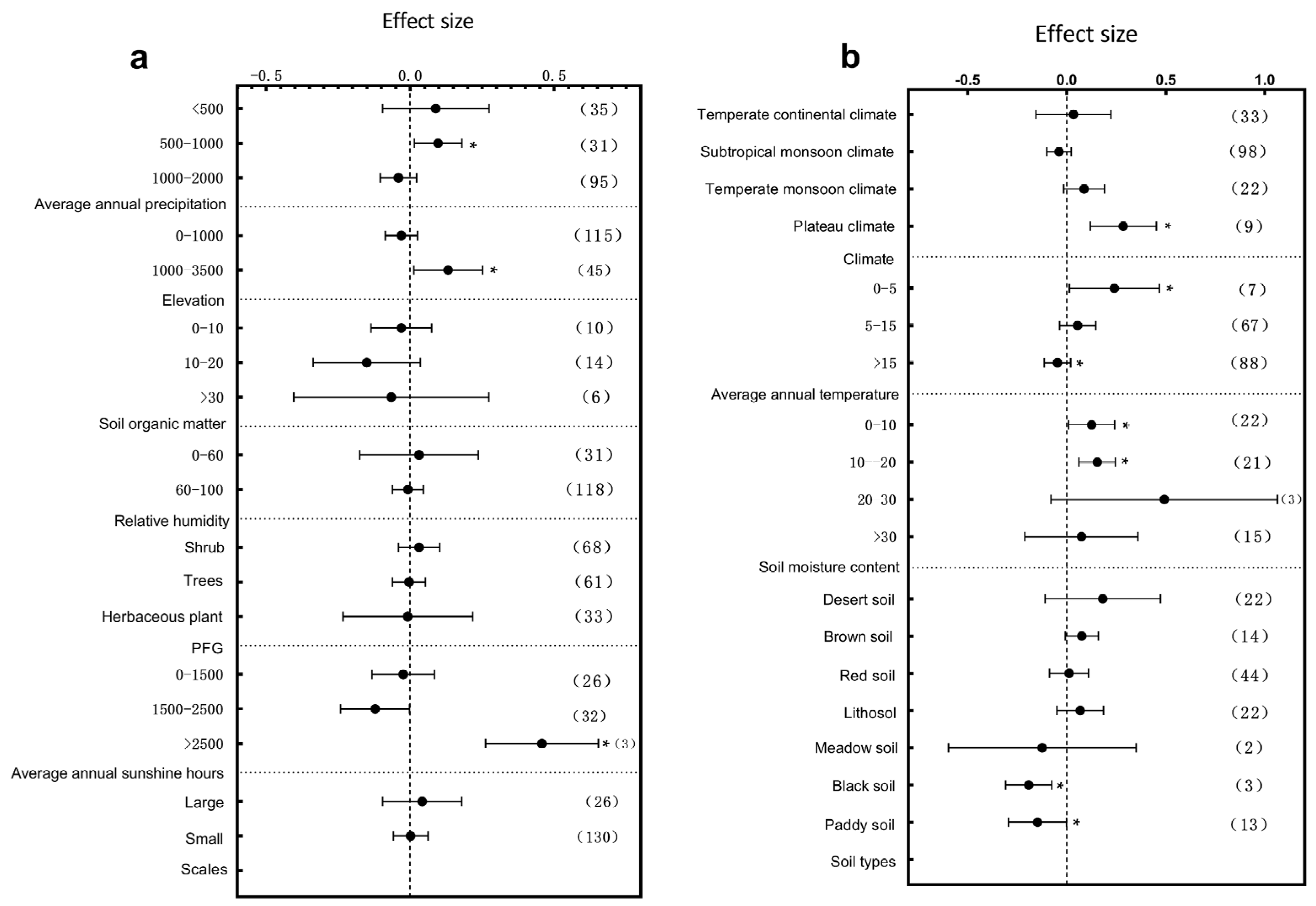
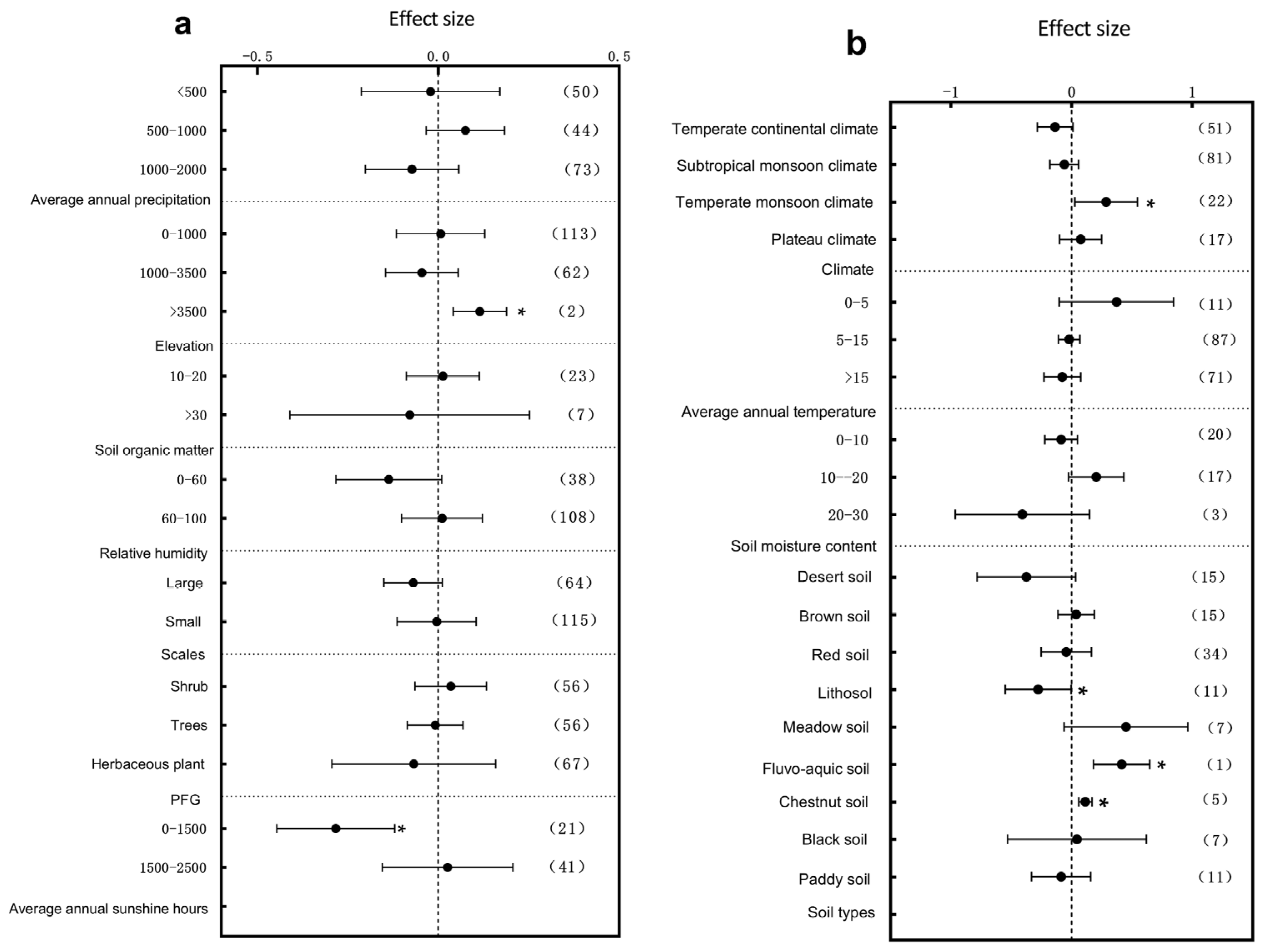
3.1. Subgroup Analysis of Plant Architectural Traits Responding to Environmental Change
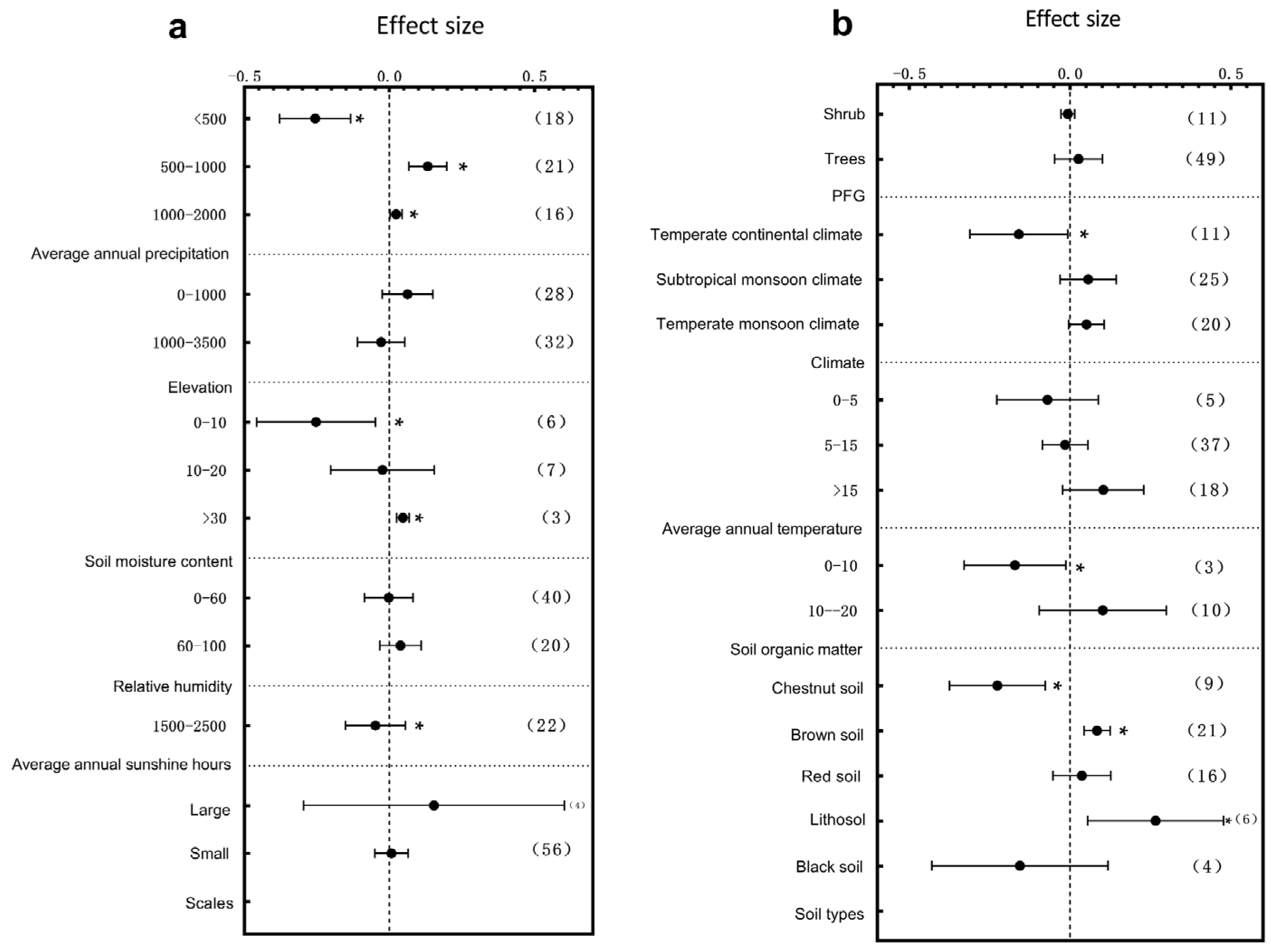
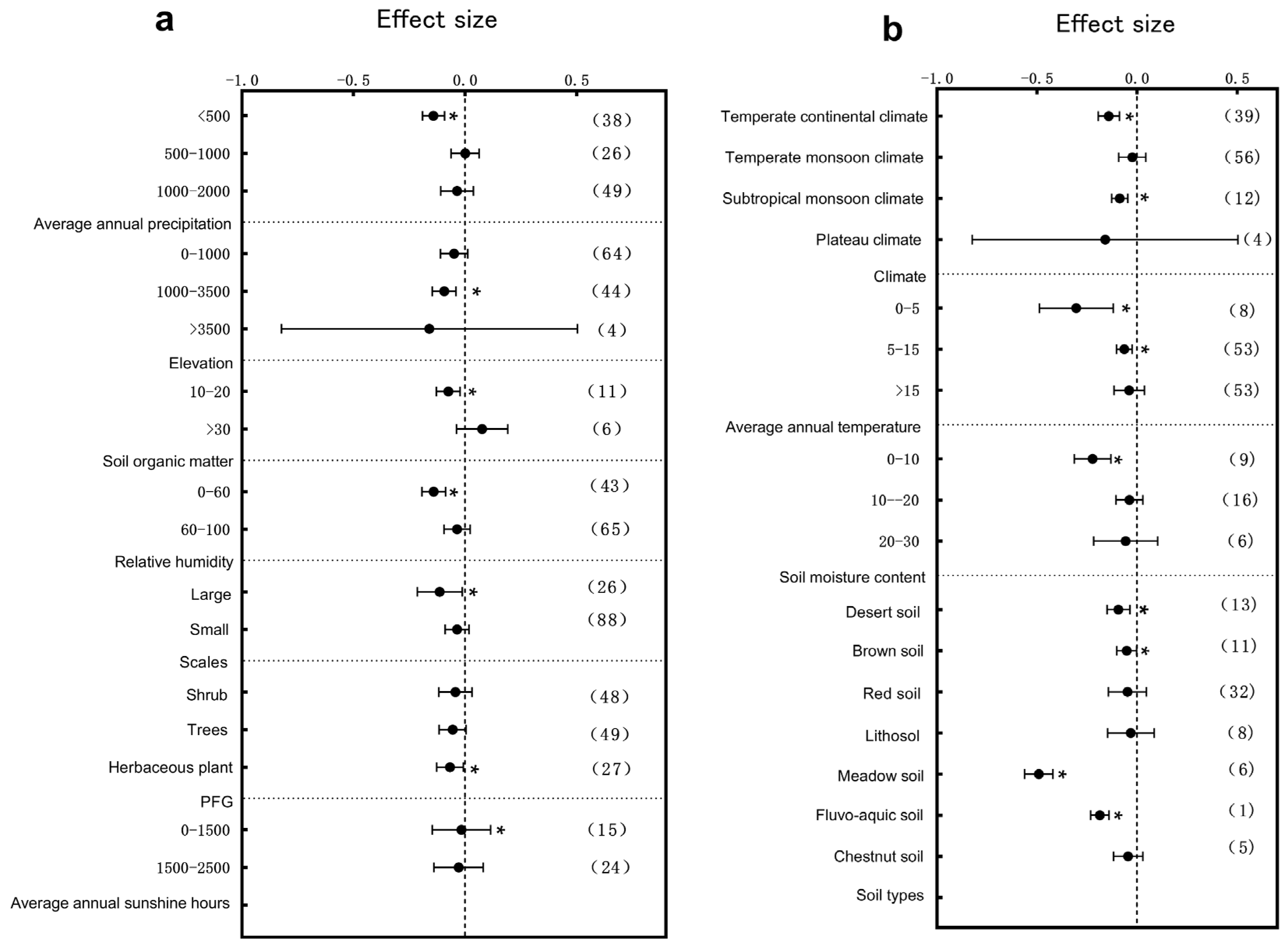
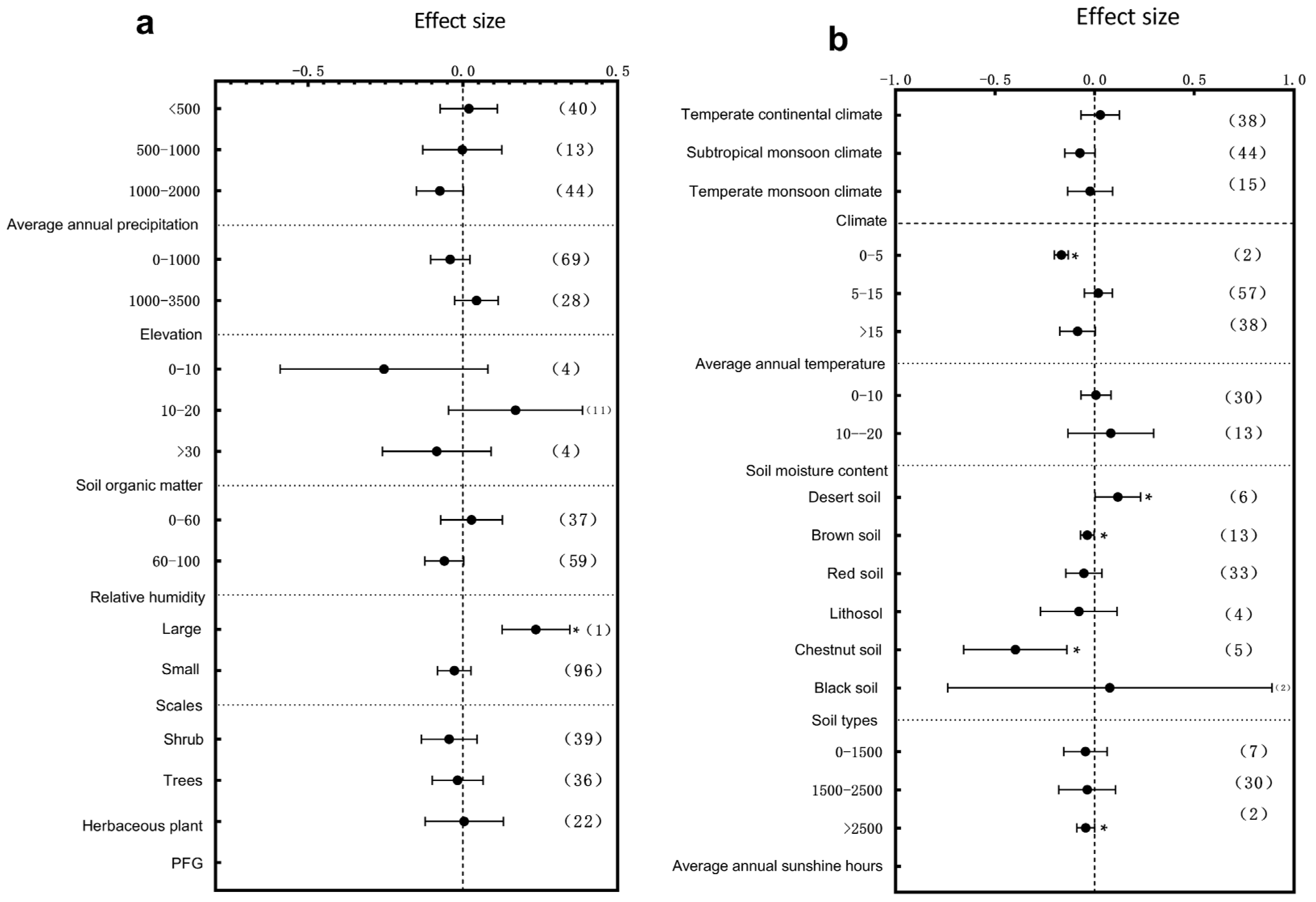
3.2. Subgroup Analysis of Plant Leaf Traits Responding to Environmental Change
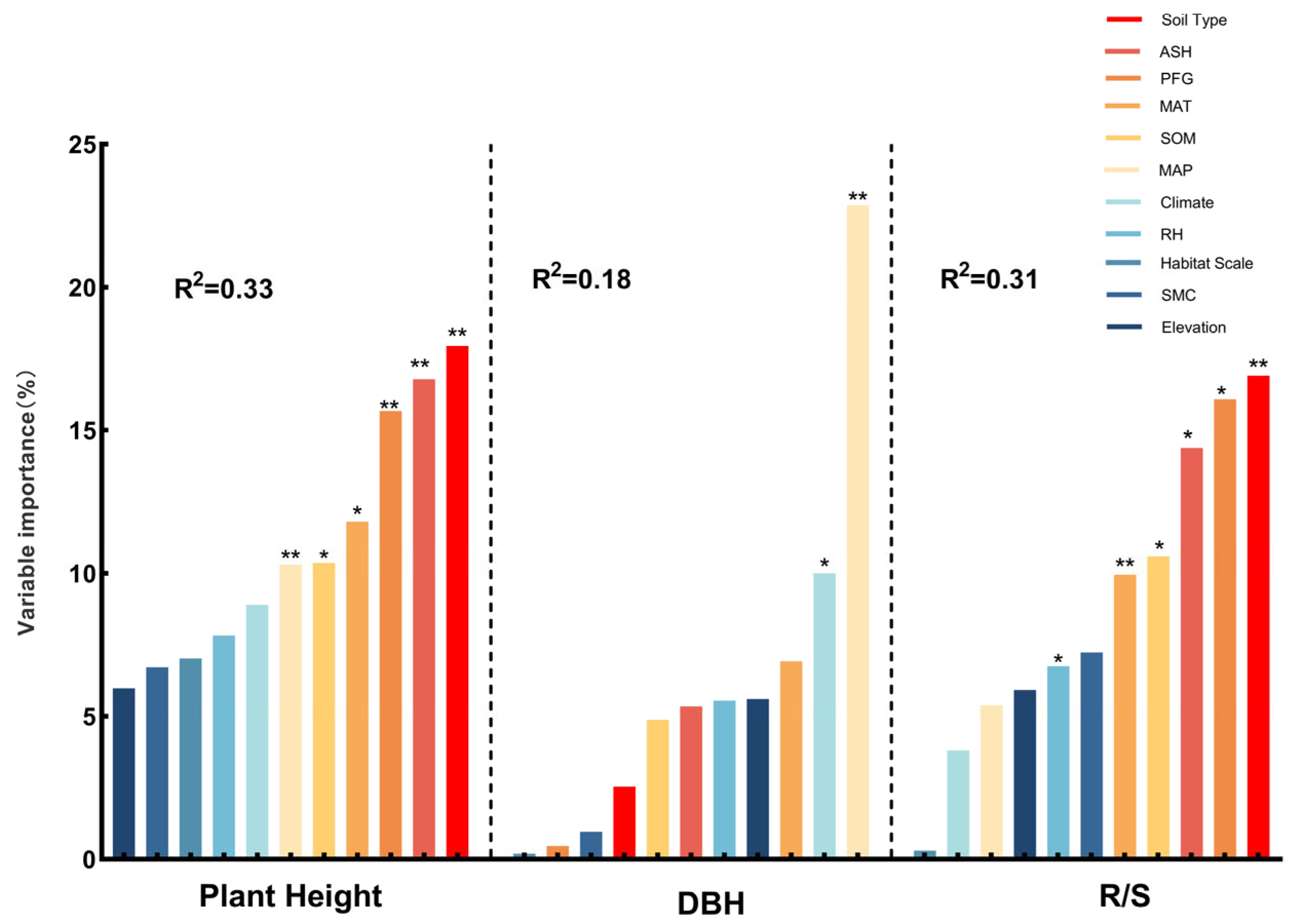
3.3. Primary Factors Influencing the Plant Structure and Leaf Traits
4. Discussion
4.1. The Response of Plant Architectural Structure to Environmental Changes
4.2. The Response of Leaf Traits to Environmental Changes
4.3. Plant Architecture, Leaf Traits and Environmental Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Prentice, I.C. Global Patterns of Plant Functional Traits and Their Relationships to Climate. Commun. Biol. 2024, 7, 1136. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.B.; Brady, S.M. Plant Developmental Responses to Climate Change. Dev. Biol. 2016, 419, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.J.; Thompson, K.E.N.; Hodgson, J.G. Specific Leaf Area and Leaf Dry Matter Content as Alternative Predictors of Plant Strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The Plant Traits That Drive Ecosystems: Evidence from Three Continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Siefert, A.; Fridley, J.D.; Ritchie, M.E. Community Functional Responses to Soil and Climate at Multiple Spatial Scales: When Does Intraspecific Variation Matter? PLoS ONE 2014, 9, e111189. [Google Scholar] [CrossRef]
- Maynard, D.S.; Bialic-Murphy, L.; Zohner, C.M.; Averill, C.; van den Hoogen, J.; Ma, H.; Mo, L.; Smith, G.R.; Acosta, A.T.; Aubin, I. Global Relationships in Tree Functional Traits. Nat. Commun. 2022, 13, 3185. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Bassett, T.; Grman, E.; Brudvig, L.A. Plant Functional Traits and Environmental Conditions Shape Community Assembly and Ecosystem Functioning during Restoration. J. Appl. Ecol. 2017, 54, 1070–1079. [Google Scholar] [CrossRef]
- Albert, C.H.; Grassein, F.; Schurr, F.M.; Vieilledent, G.; Violle, C. When and How Should Intraspecific Variability Be Considered in Trait-Based Plant Ecology? Perspect. Plant Ecol. Evol. Syst. 2011, 13, 217–225. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Z.; Tu, C.; Lv, Z.; Liu, S.; Fan, Y. Response of an Obligate CAM Plant to Competition and Increased Watering Intervals. Physiol. Plant. 2025, 177, e70093. [Google Scholar] [CrossRef]
- Chen, R. Diameter Explains Transient Allocation of Non-Photosynthetic Organs in Trees. Perspect. Plant Ecol. Evol. Syst. 2024, 62, 125763. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, J.; Ji, Y.; Liu, W.; Gao, J. Leaf Nutrient Traits Exhibit Greater Environmental Plasticity Compared to Resource Utilization Traits along an Elevational Gradient. Front. Plant Sci. 2024, 15, 1484744. [Google Scholar] [CrossRef] [PubMed]
- Utkin, A.; Ermolova, L.; Utkina, I.; Dulepova, N.; Rosbakh, S. Utkin et al.’s Dataset on Specific Leaf Area. Ecology 2022, 103, e3714. [Google Scholar] [CrossRef]
- Aneja, P.; Sanyal, R.; Ranjan, A. Leaf Growth in Third Dimension: A Perspective of Leaf Thickness from Genetic Regulation to Ecophysiology. New Phytol. 2025, 245, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant Root-Shoot Biomass Allocation over Diverse Biomes: A Global Synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Smart, S.M.; Glanville, H.C.; Blanes, M.D.C.; Mercado, L.M.; Emmett, B.A.; Jones, D.L.; Cosby, B.J.; Marrs, R.H.; Butler, A.; Marshall, M.R.; et al. Leaf Dry Matter Content Is Better at Predicting Above-ground Net Primary Production than Specific Leaf Area. Funct. Ecol. 2017, 31, 1336–1344. [Google Scholar] [CrossRef]
- Liu, X.J.; Ma, K.P. Advances in research on plant functional traits. Sci. China Life Sci. 2015, 45, 325–339. [Google Scholar]
- Fonseca, C.R.; Overton, J.M.; Collins, B.; Westoby, M. Shifts in Trait-combinations along Rainfall and Phosphorus Gradients. J. Ecol. 2000, 88, 964–977. [Google Scholar] [CrossRef]
- Craine, J.M.; Lee, W.G. Covariation in Leaf and Root Traits for Native and Non-Native Grasses along an Altitudinal Gradient in New Zealand. Oecologia 2003, 134, 471–478. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Midgley, G.; Lavergne, S.; Rebelo, T. Relating plant traits and species distributions along bioclimatic gradients for 88 leucadendron taxa. Ecology 2004, 85, 1688–1699. [Google Scholar] [CrossRef]
- Han, M.; Ji, C.J.; Zuo, W.Y.; He, J.S. CO2 concentration and temperature increase effects on the anatomical traits of leaves in 11 plant species. Acta Ecol. Sin. 2006, 26, 326–333. [Google Scholar]
- Holdrege, M.C.; Beard, K.H.; Kulmatiski, A. Woody Plant Growth Increases with Precipitation Intensity in a Cold Semiarid System. Ecology 2021, 102, e03212. [Google Scholar] [CrossRef] [PubMed]
- Becknell, J.M.; Powers, J.S. Stand Age and Soils as Drivers of Plant Functional Traits and Aboveground Biomass in Secondary Tropical Dry Forest. Can. J. For. Res. 2014, 44, 604–613. [Google Scholar] [CrossRef]
- Meng, T.T.; Ni, J.; Wang, G.H. Plant functional traits, environment, and ecosystem functions. Plant Ecol. J. 2007, 31, 150–165. [Google Scholar]
- Zhao, Y.; Cheng, J.; Wang, Y.; Wan, H.; Hu, X. Effects of Environmental Factors on the Structure of Caragana Shrubland in Semi-Arid Areas. Bull. Soil Water Conserv. 2005, 25, 10–14. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, J.; Sun, W.; Chen, F. Leaf functional traits of Picea schrenkiana at different altitudes and their relationships with soil factors. Acta Ecol. Sin. 2010, 30, 5747–5758. [Google Scholar]
- Du, H.; Peng, W.X.; Song, T.Q.; Zeng, F.P.; Wang, K.L.; Song, M.; Zhang, H. Spatial Pattern of Woody Plants and Their Environmental Interpretation in the Karst Forest of Southwest China. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2015, 149, 121–130. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database-enhanced coverage and open access. Glob. Change Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- Tian, Q.; Yang, F.; Wang, Z.H.; Zhang, Q.Y. Response characteristics and influencing factors of soil CO2 emissions in terrestrial ecosystems to simulated warming. Acta Ecol. Sin. 2024, 44, 1928–1939. [Google Scholar]
- Zhang, L.; Huang, J.; Liu, J.; Pan, H.; Qi, Y.; Li, W.; Liu, X. Response of soil respiration to warming: A meta-analysis. J. Yangtze River Sci. Res. Inst. 2023. Available online: https://link.cnki.net/urlid/42.1171.TV.20231229.1541.004 (accessed on 26 March 2024).
- Yang, F.; Xu, Y.; Cui, Y.; Meng, Y.D.; Dong, Y.; Li, R.; Ma, Y.B. Changes in soil organic matter content in the plow layer of China’s farmland over the past 30 years. Acta Pedol. Sin. 2017, 54, 1047–1056. [Google Scholar]
- Burda, B.U.; O’Connor, E.A.; Webber, E.M.; Redmond, N.; Perdue, L.A. Estimating Data from Figures with a Web-based Program: Considerations for a Systematic Review. Res. Synth. Methods 2017, 8, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, W.I.J.; Luyssaert, S.; Rey, A.; De Angelis, P.; Barton, C.V.M.; Broadmeadow, M.S.J.; Broadmeadow, S.B.; Chigwerewe, K.S.; Crookshanks, M.; Dufrêne, E.; et al. Soil [N] Modulates Soil C Cycling in CO2 -fumigated Tree Stands: A Meta-analysis. Plant Cell Environ. 2010, 33, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Venn, S.E.; Green, K.; Pickering, C.M.; Morgan, J.W. Using Plant Functional Traits to Explain Community Composition across a Strong Environmental Filter in Australian Alpine Snowpatches. Plant Ecol. 2011, 212, 1491–1499. [Google Scholar] [CrossRef]
- Chapin, F.S. The Mineral Nutrition of Wild Plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Yu, L.; Lei, X.D.; Wang, Y.Z.; Yang, Y.J.; Wang, Q.J. Study on the effect of climate on the growth of tree diameter at breast height based on generalized additive models. J. Beijing For. Univ. 2014, 36, 22–32. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass Allocation to Leaves, Stems and Roots: Meta-analyses of Interspecific Variation and Environmental Control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Wang, X.; Ji, M.; Zhang, Y.; Zhang, L.; Akram, M.A.; Dong, L.; Hu, W.; Xiong, J.; Sun, Y.; Li, H.; et al. Plant Trait Networks Reveal Adaptation Strategies in the Drylands of China. BMC Plant Biol. 2023, 23, 266. [Google Scholar] [CrossRef]
- Wang, Y.X. Exploring High Sensitivity Areas in China Based on Multi-Source Soil Moisture Data. Master’s Thesis, Nanjing University, Nanjing, China, 2019. [Google Scholar]
- Liu, W.; Nie, Y.; Luo, Z.; Wang, Z.; Huang, L.; He, F.; Chen, H. Transpiration Rates Decline under Limited Moisture Supply along Hillslopes in a Humid Karst Terrain. Sci. Total Environ. 2023, 894, 164977. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.I.; Newingham, B.; Aschehoug, E.T. Positive Interactions among Alpine Plants Increase with Stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, Z.; Zhou, H.; Ma, L.; Liu, X. Plant Allometric Growth Enhanced by the Change in Soil Stoichiometric Characteristics with Depth in an Alpine Meadow under Climate Warming. Front. Plant Sci. 2022, 13, 860980. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yao, B.; Zhou, H.; Jin, Y.; Yang, Y.; Wang, W.; Dong, S.; Zhang, X. Differential Responses of Belowground Biomass and Carbon Allocation of Two Alpine Meadows to Long-Term Warming on the Qinghai–Tibet Plateau. Chin. Sci. Bull. 2015, 60, 379–388. [Google Scholar]
- Woodward, F.I. Predicting Plant Responses to Global Environmental Change. New Phytol. 1992, 122, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, J.; Wang, J.; Wang, Y.; Lu, Y.; Zheng, Q. Photosynthetic Characteristics and Biomass Allocation of Phoebe bournei Seedlings in Response to Light Conditions. Acta Ecol. Sin. 2012, 32, 3841–3848. [Google Scholar] [CrossRef]
- Cornelissen, J.H.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A. A Handbook of Protocols for Standardised and Easy Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Moradi, J.; Mudrák, O.; Kukla, J.; Vicentini, F.; Šimáčková, H.; Frouz, J. Variations in Soil Chemical Properties, Microbial Biomass, and Faunal Populations as Related to Plant Functional Traits, Patch Types, and Successional Stages at Sokolov Post-Mining Site—A Case Study. Eur. J. Soil Biol. 2017, 83, 58–64. [Google Scholar] [CrossRef]
- Ávila-Lovera, E.; Goldsmith, G.R.; Kay, K.M.; Funk, J.L. Above-and below-Ground Functional Trait Coordination in the Neotropical Understory Genus Costus. AoB Plants 2022, 14, plab073. [Google Scholar] [CrossRef]
- Xie, G.; Wang, Y.; Chen, Z.; Ji, Y.; Lu, Y.; Liang, Y.; Zhou, R.; Tao, J. Response of Plant Leaf Traits to Environmental Factors in Climax Communities at Varying Latitudes in Karst Regions. Plants 2025, 14, 183. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Li, J. Effects of Nitrogen Addition on Plant–Soil Carbon Dynamics in China’s Terrestrial Ecosystems. Acta Ecol. Sin. 2022, 42, 4823–4833. [Google Scholar]
- Zhou, Z.; Wang, C.; Cha, X.; Zhou, T.; Pang, X.; Zhao, F.; Han, X.; Yang, G.; Wei, G.; Ren, C. The Biogeography of Soil Microbiome Potential Growth Rates. Nat. Commun. 2024, 15, 9472. [Google Scholar] [CrossRef]
- Dolezal, J.; Jandova, V.; Macek, M.; Liancourt, P. Contrasting Biomass Allocation Responses across Ontogeny and Stress Gradients Reveal Plant Adaptations to Drought and Cold. Funct. Ecol. 2021, 35, 32–42. [Google Scholar] [CrossRef]
- Ma, W.; Shi, P.; Li, W.; He, Y.; Zhang, X.; Shen, Z. Altitudinal Variation of Plant Traits and Biomass Allocation in Alpine Meadows on the Qinghai–Tibetan Plateau. Sci. China Life Sci. 2010, 40, 533–543. [Google Scholar]
- Wang, L.; Niu, K.; Yang, Y.; Zhou, P. Aboveground and Belowground Biomass Allocation Patterns in China’s Grasslands: An Individual-Level Study. Sci. China Life Sci. 2010, 40, 642–649. [Google Scholar]
- Hoorn, C.; Mosbrugger, V.; Mulch, A.; Antonelli, A. Biodiversity from Mountain Building. Nat. Geosci. 2013, 6, 154. [Google Scholar] [CrossRef]
- Sumner, E.; Venn, S. Plant Responses to Changing Water Supply and Availability in High Elevation Ecosystems: A Quantitative Systematic Review and Meta-Analysis. Land 2021, 10, 1150. [Google Scholar] [CrossRef]
- Ye, J. Global Patterns and Controlling Factors of Forest Root-to-Shoot Ratios. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2022. [Google Scholar] [CrossRef]
- Royer, D.L.; Miller, I.M.; Peppe, D.J.; Hickey, L.J. Leaf Economic Traits from Fossils Support a Weedy Habit for Early Angiosperms. Am. J. Bot. 2010, 97, 438–445. [Google Scholar] [CrossRef]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of Leaf Hydraulic Conductance with Dehydration: Relationship to Leaf Size and Venation Architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Groom, P.K.; Hikosaka, K.; Lee, W.; Lusk, C.H.; Niinemets, Ü.; Oleksyn, J.; et al. Modulation of Leaf Economic Traits and Trait Relationships by Climate. Glob. Ecol. Biogeogr. 2005, 14, 411–421. [Google Scholar] [CrossRef]
- Jager, M.M.; Richardson, S.J.; Bellingham, P.J.; Clearwater, M.J.; Laughlin, D.C. Soil Fertility Induces Coordinated Responses of Multiple Independent Functional Traits. J. Ecol. 2015, 103, 374–385. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Brasil, S.N.R.; de Carvalho, C.E.; de Araujo, F.S.; Loiola, M.I.B. Leaf Traits as Predictors of Climatic Adaptation and Distributional Range in Wide-and Narrow-Range Species. Flora 2025, 326, 152708. [Google Scholar] [CrossRef]
- Li, R.; Shan, L.; Xie, T.; Ma, L.; Yang, J.; Li, Q. Variation of Leaf Functional Traits of Typical Desert Shrubs Along a Precipitation Gradient. Arid Zone Res. 2023, 40, 425–435. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S. Advances in Research on Plant Leaf Trait Responses to Climate Change. Acta Phytoecol. Sin. 2015, 39, 206–216. [Google Scholar]
- Wang, C.; Lv, W.W.; Sun, J.; Zhou, Y.; Jiang, L.; Li, B.; A, W.; Zhang, S.R.; Xia, L.; Wang, Q.; et al. Response of leaf traits of alpine plants to simulated precipitation changes. Acta Ecol. Sin. 2021, 41, 9760–9772. [Google Scholar]
- Stevenson, G. Plant Functional Traits Associated with Frost Susceptibility; Lincoln University: Philadelphia, PA, USA, 2015. [Google Scholar]
- Pietsch, K.A.; Ogle, K.; Cornelissen, J.H.C.; Cornwell, W.K.; Bönisch, G.; Craine, J.M.; Jackson, B.G.; Kattge, J.; Peltzer, D.A.; Penuelas, J.; et al. Global Relationship of Wood and Leaf Litter Decomposability: The Role of Functional Traits within and across Plant Organs. Glob. Ecol. Biogeogr. 2014, 23, 1046–1057. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-540-00347-2. [Google Scholar]
- Tardieu, F.; Granier, C.; Muller, B. Modelling Leaf Expansion in a Fluctuating Environment: Are Changes in Specific Leaf Area a Consequence of Changes in Expansion Rate? New Phytol. 1999, 143, 33–43. [Google Scholar] [CrossRef]
- Adams, S.R.; Langton, F.A. Photoperiod and Plant Growth: A Review. J. Hortic. Sci. Biotechnol. 2005, 80, 2–10. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to Sun and Shade: A Whole-Plant Perspective. Funct. Plant Biol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Y. Responses of Leaf Traits of Korean Pine (Pinus koraiensis) Seedlings of Different Ages to Temperature and Light Changes. Acta Ecol. Sin. 2017, 37, 5662–5672. [Google Scholar]
- Chen, Z.Y. Chloroplast Genomic and Transcriptomic Studies on the Adaptation of Three Cushion Plants to Extreme Environments on the Tibetan Plateau; Wuhan University: Wuhan, China, 2024. [Google Scholar]
- Sun, M.; Su, T.; Zhang, S.-B.; Li, S.-F.; Anberree-Lebreton, J.; Zhou, Z.-K. Variations in Leaf Morphological Traits of Quercus Guyavifolia (Fagaceae) Were Mainly Influenced by Water and Ultraviolet Irradiation at High Elevations on the Qinghai-Tibet Plateau, China. Int. J. Agric. Biol. 2016, 18, 266–273. [Google Scholar] [CrossRef]
- Wang, X.; Li, R.; Li, X.; Ma, F.; Sun, B.; Wu, J.; Wang, Y. Variations in Leaf Characteristics of Three Species of Angiosperms with Changing of Altitude in Qilian Mountains and Their Inland High-Altitude Pattern. Sci. China Earth Sci. 2014, 57, 662–670. [Google Scholar] [CrossRef]
- Fenollosa, E.; Fernandes, P.; Hector, A.; King, H.; Lawson, C.S.; Jackson, J.; Salguero-Gómez, R. Differential Responses of Community-level Functional Traits to Mid- and Late-season Experimental Drought in a Temperate Grassland. J. Ecol. 2024, 112, 2292–2306. [Google Scholar] [CrossRef]
- Kawecki, T.J. Adaptation to Marginal Habitats. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 321–342. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to Stand Still: Adaptation and the Response of Plants to Rapid Climate Change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic Plasticity for Plant Development, Function and Life History. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Ploughe, L.W.; Jacobs, E.M.; Frank, G.S.; Greenler, S.M.; Smith, M.D.; Dukes, J.S. Community Response to Extreme Drought (CRED): A Framework for Drought-induced Shifts in Plant–Plant Interactions. New Phytol. 2019, 222, 52–69. [Google Scholar] [CrossRef]
- Yue, X.; Zuo, X.; Yu, Q.; Xu, C.; Lv, P.; Zhang, J.; Knapp, A.K.; Smith, M.D. Response of Plant Functional Traits of Leymus Chinensis to Extreme Drought in Inner Mongolia Grasslands. Plant Ecol. 2019, 220, 141–149. [Google Scholar] [CrossRef]
- Kramp, R.E.; Liancourt, P.; Herberich, M.M.; Saul, L.; Weides, S.; Tielbörger, K.; Májeková, M. Functional Traits and Their Plasticity Shift from Tolerant to Avoidant under Extreme Drought. Ecology 2022, 103, e3826. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, L.; Liu, B.; Liu, R.; Xiao, Z.; Liu, W. Quantitative Analysis of Vegetation Sensitivity to Climate Change in China. Acta Ecol. Sin. 2025, 45, 2412–2423. [Google Scholar] [CrossRef]
- Zhao, B.; Bao, G.; Yuan, Z.; Wendu, R.; Tong, S.; Guo, E.; Huang, X. Study on the Sensitivity of Vegetation Growth Peak Periods and Peaks to Climate in the Northern Hemisphere Mid-and High Latitudes (2001–2020). Prog. Geogr. Sci. 2023, 42, 1809–1824. [Google Scholar]
- Zhang, K.-L.; Leng, Y.-N.; Hao, R.-R.; Zhang, W.-Y.; Li, H.-F.; Chen, M.-X.; Zhu, F.-Y. Adaptation of High-Altitude Plants to Harsh Environments: Application of Phenotypic-Variation-Related Methods and Multi-Omics Techniques. Int. J. Mol. Sci. 2024, 25, 12666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, Y.; Landis, J.B.; Shen, J.; Zhang, H.; Kuang, T.; Sun, W.; Sun, J.; Tiamiyu, B.B.; Deng, T. Transcriptomes of Saussurea (Asteraceae) Provide Insights into High-Altitude Adaptation. Plants 2021, 10, 1715. [Google Scholar] [CrossRef]
- Trošt Sedej, T.; Turk, T. Alchemilla monticola Opiz. Functional Traits Respond to Diverse Alpine Environmental Conditions in Karavanke, Slovenia. Plants 2022, 11, 2527. [Google Scholar] [CrossRef]
- Li, F.; Bao, W.; Wu, N. Ecological Anatomy of Cotinus szechuanensis Leaves Along an Altitudinal Gradient in the Arid Valleys of the Upper Minjiang River. Chin. J. Appl. Environ. Biol. 2007, 13, 486–491. [Google Scholar]
- Yang, Y.; Chen, J.; Song, B.; Niu, Y.; Peng, D.; Zhang, J.; Deng, T.; Luo, D.; Ma, X.; Zhou, Z.; et al. Research Progress on Periglacial Plant Diversity and Adaptation Mechanisms on the Qinghai–Tibet Plateau. Chin. Sci. Bull. 2019, 64, 2856–2864. [Google Scholar]
- Bardgett, R.D. Plant Trait-Based Approaches for Interrogating Belowground Function. In Biology and Environment: Proceedings of the Royal Irish Academy; Royal Irish Academy: Dublin, Ireland, 2018; Volume 118. [Google Scholar]
- Sierra, C.A.; Trumbore, S.E.; Davidson, E.A.; Vicca, S.; Janssens, I. Sensitivity of Decomposition Rates of Soil Organic Matter with Respect to Simultaneous Changes in Temperature and Moisture. J. Adv. Model. Earth Syst. 2015, 7, 335–356. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Tian, L.; Lou, B.; Zhang, X.; Liu, T.; Liu, X.; Sun, H. Advances in the Relationship Between Soil Aggregates, Microorganisms, and Organic Matter in Salinized Soils. Chin. J. Eco-Agric. Biling. 2023, 31, 364–372. [Google Scholar]
- Murphy, B.W. Impact of Soil Organic Matter on Soil Properties—A Review with Emphasis on Australian Soils. Soil Res. 2015, 53, 605–635.1. [Google Scholar] [CrossRef]


| Categorical Variables | Subgroups | |||
|---|---|---|---|---|
| Elevation [m] | 0–1000 | 1000–3500 | >3500 | |
| Plant Functional Group (PFG) | Tree | Shrub | Herbaceous Plant | |
| Mean Annual Temperature [°C] | 0–5 | 5–15 | >15 | |
| Mean Annual Precipitation [mm] | 0–500 | 500–1000 | 1000–2000 | |
| Scale | Big | Small | ||
| Soil Organic Matter [g/kg−1] | 0–10 | 10–20 | 20–30 | >30 |
| Soil Moisture Content [%] | 0–10 | 10–20 | 20–30 | >30 |
| Mean Annual Sunshine Hours [h] | 0–1500 | 1500–2500 | >2500 | |
| Relative Humidity [%] | 0–60 | 60–100 | ||
| Climate | Temperate Continental Climate | Temperate Monsoon Climate | Subtropical Monsoon Climate | Plateau Climate |
| Soil type | Desert Soil Chestnut Soil Paddy Soil | Red Soil Fluvo-aquic Soil Lithosol | Brown Soil Meadow Soil Alpine Soil | Black Soil Loess |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Cheng, X.; Dai, P.; Zhang, M.; Li, M.; Chen, J.; Hassan, W.u.; Wang, Y. Plant Architectural Structure and Leaf Trait Responses to Environmental Change: A Meta-Analysis. Plants 2025, 14, 1717. https://doi.org/10.3390/plants14111717
Li R, Cheng X, Dai P, Zhang M, Li M, Chen J, Hassan Wu, Wang Y. Plant Architectural Structure and Leaf Trait Responses to Environmental Change: A Meta-Analysis. Plants. 2025; 14(11):1717. https://doi.org/10.3390/plants14111717
Chicago/Turabian StyleLi, Runze, Xiping Cheng, Pengyue Dai, Mengting Zhang, Minxuan Li, Jing Chen, Wajee ul Hassan, and Yanfang Wang. 2025. "Plant Architectural Structure and Leaf Trait Responses to Environmental Change: A Meta-Analysis" Plants 14, no. 11: 1717. https://doi.org/10.3390/plants14111717
APA StyleLi, R., Cheng, X., Dai, P., Zhang, M., Li, M., Chen, J., Hassan, W. u., & Wang, Y. (2025). Plant Architectural Structure and Leaf Trait Responses to Environmental Change: A Meta-Analysis. Plants, 14(11), 1717. https://doi.org/10.3390/plants14111717






