Fatty Acid-Rich Fraction of Hibiscus syriacus L. Alleviates Atopic Dermatitis-like Skin Lesions Mouse Model via Inflammatory Pathway Modulation: Integrative Docking and Experimental Validation
Abstract
1. Introduction
2. Results
2.1. Identification of Major Compounds and Molecular Docking Analysis of Hibiscus syriacus Fatty Acid-Rich Fraction
2.2. Effects of Hibiscus Syriacus Fatty Acid-Rich Fraction on NO and ROS Production
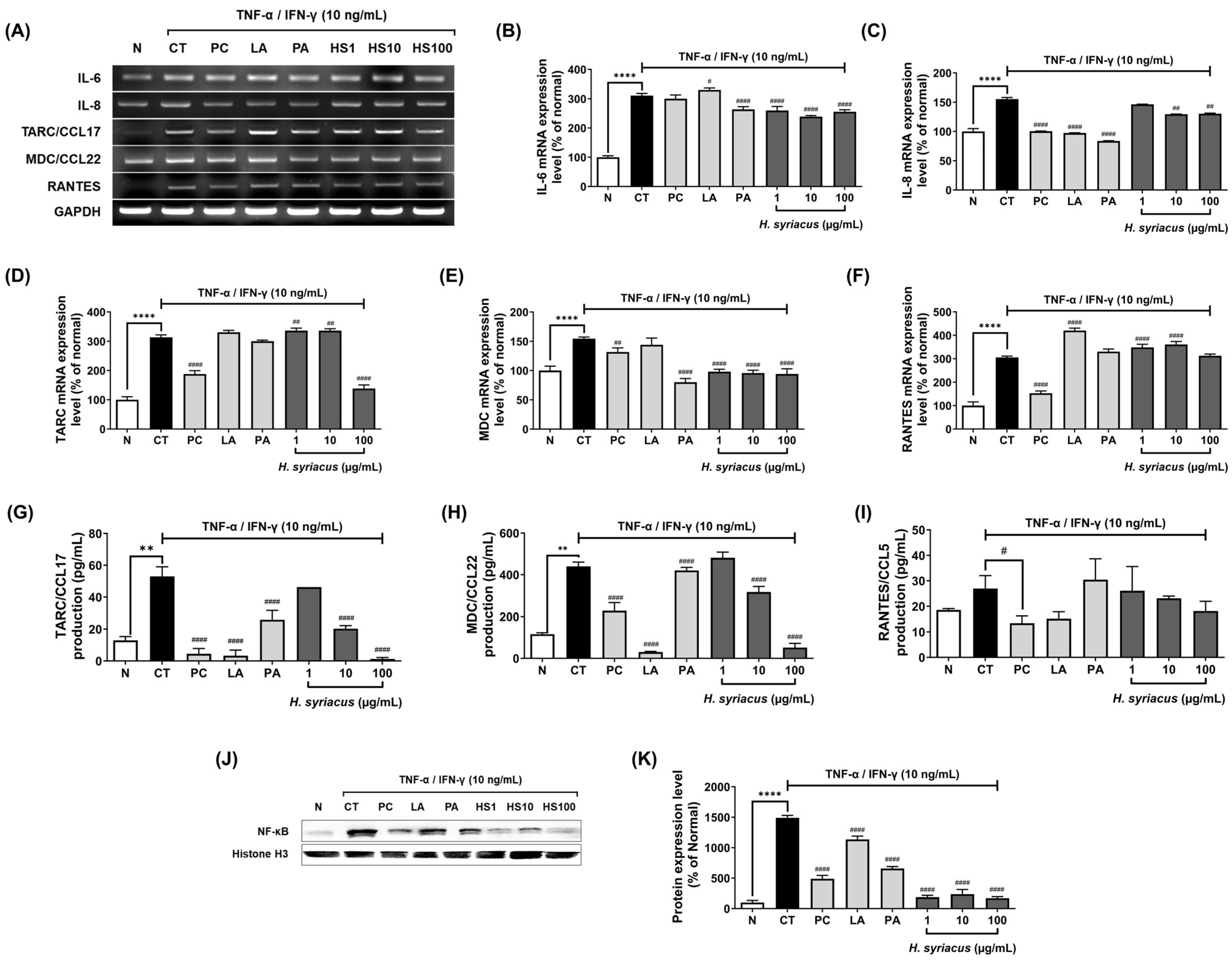
2.3. Hibiscus syriacus Fatty Acid-Rich Fraction Suppresses Pro-Inflammatory Cytokine and Chemokine Expression
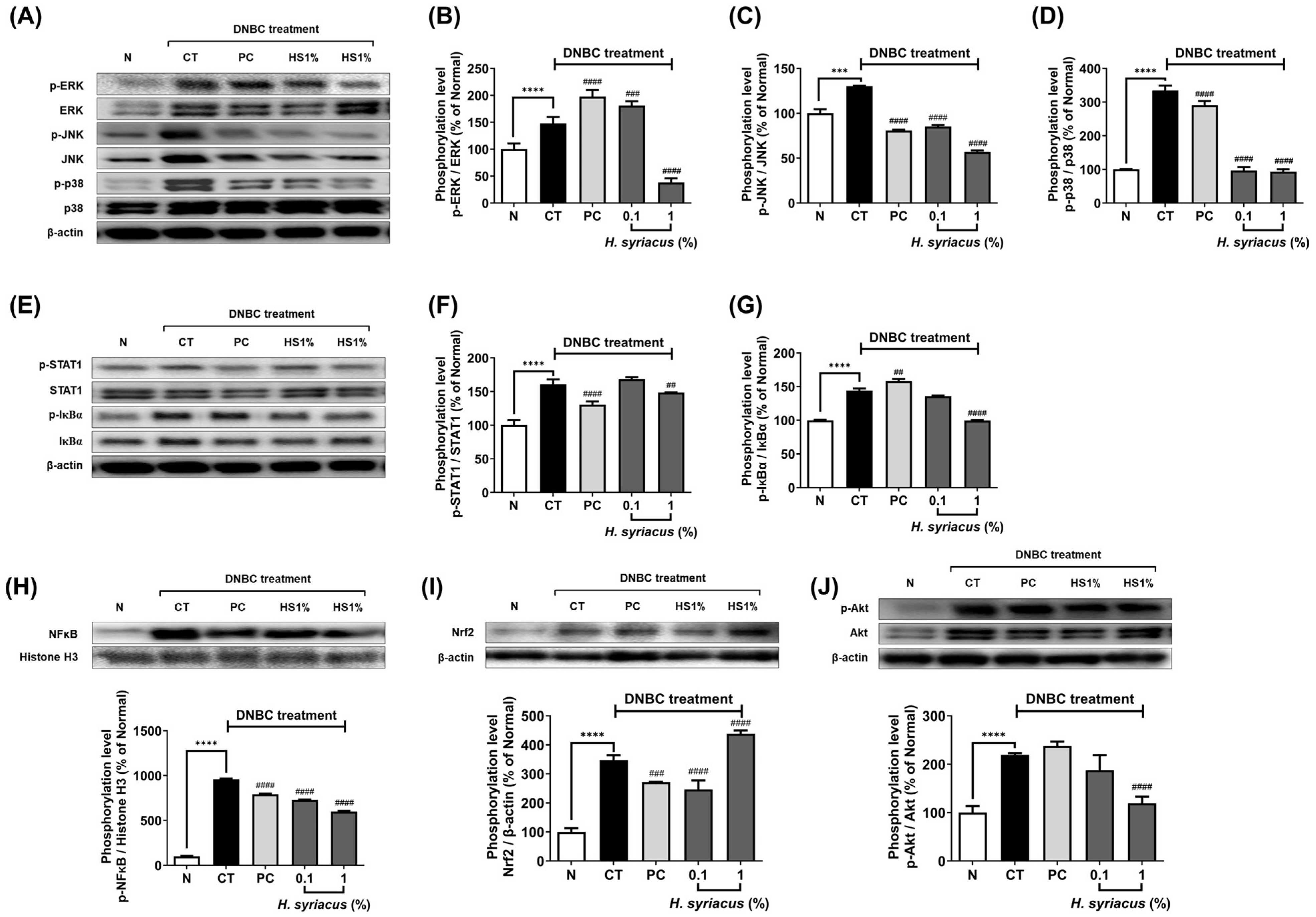
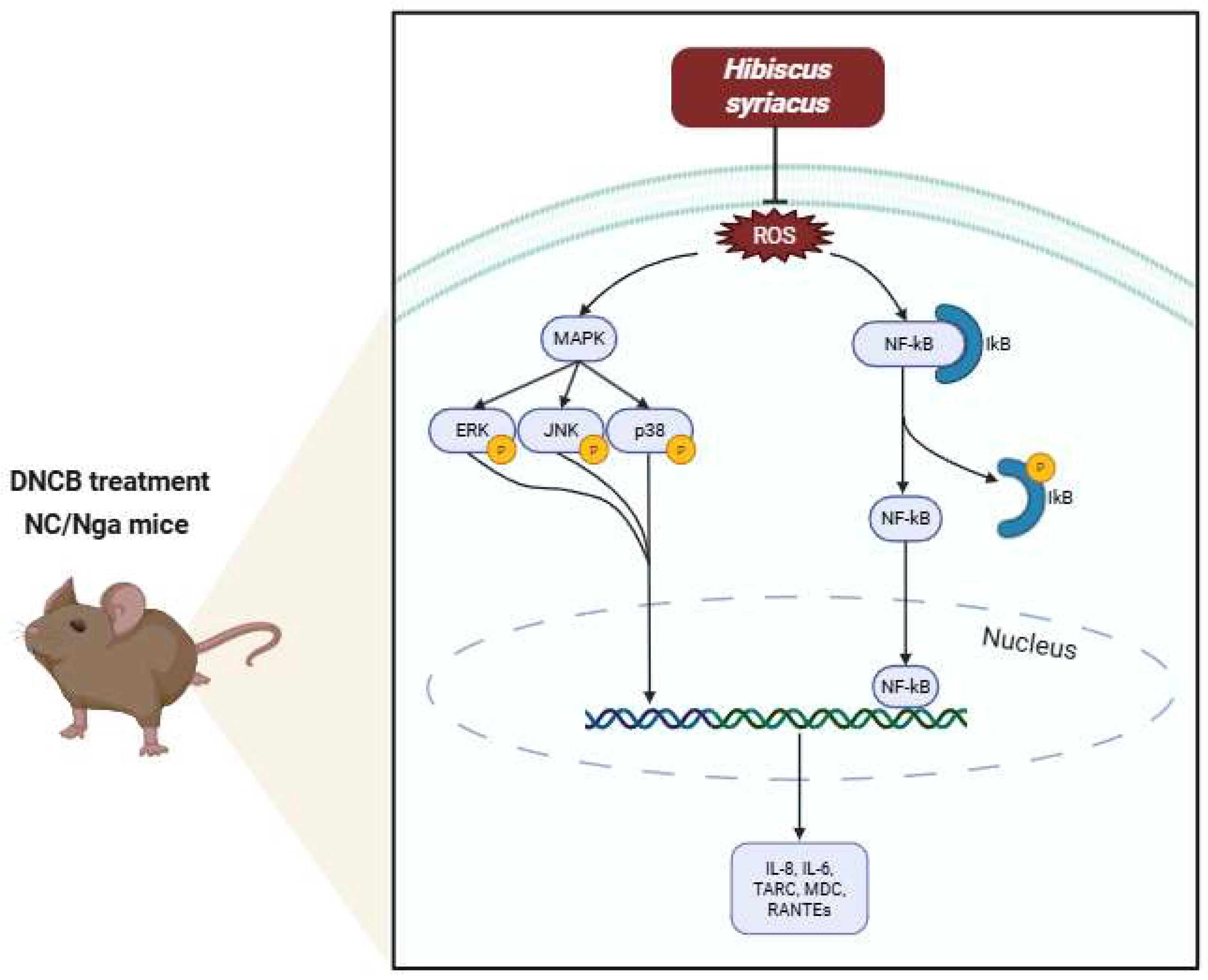
2.4. Impact of Hibiscus syriacus Fatty Acid-Rich Fraction on Pro-Inflammatory Signaling
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Hibiscus syriacus Fatty Acid-Rich Fraction
4.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
4.4. Computational Docking
4.4.1. Identification of Active Sites of the Receptors
4.4.2. Receptor Grid Generation
4.4.3. Molecular Docking Simulation
4.4.4. ADME and QikProp Analyses
4.5. Cell Culture and Treatments
4.6. Measurement of Nitric Oxide (NO) Production
4.7. Measurement of Reactive Oxygen Species (ROS)
4.8. Cell Viability Assay
4.9. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Western Blot
4.12. Animals and Experimental Design
4.13. Evaluation of Atopic Dermatitis-like Symptoms
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic Dermatitis |
| NO | Nitric Oxide |
| ROS | Reactive Oxygen Species |
| TNF-α | Tumor Necrosis Factor-Alpha |
| IFN-γ | Interferon-Gamma |
| TARC | Thymus and Activation-Regulated Chemokine (CCL17) |
| MDC | Macrophage-Derived Chemokine (CCL22) |
| RANTES | Regulated on Activation, Normal T Cell Expressed and Secreted (CCL5) |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| MAPK | Mitogen-Activated Protein Kinase |
| ERK | Extracellular Signal-Regulated Kinase |
| JNK | c-Jun N-Terminal Kinase |
| p38 | p38 Mitogen-Activated Protein Kinase |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| IKKβ | Inhibitor of Nuclear Factor Kappa-B Kinase Subunit Beta |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| AKT | Protein Kinase B |
| DNCB | 2,4-Dinitrochlorobenzene |
| TEWL | Transepidermal Water Loss |
References
- Fadadu, R.P.; Chee, E.; Jung, A.; Chen, J.Y.; Abuabara, K.; Wei, M.L. Air Pollution and Global Healthcare Use for Atopic Dermatitis: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. JEADV 2023, 37, 1958–1970. [Google Scholar] [CrossRef]
- Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; Kim, M.S.; GBD 2019 Allergic Disorders Collaborators; Shin, J.I.; Yon, D.K. Global, Regional, and National Burden of Allergic Disorders and Their Risk Factors in 204 Countries and Territories, from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Allergy 2023, 78, 2232–2254. [Google Scholar] [CrossRef]
- Peters, N.; Peters, A.T. Atopic Dermatitis. Allergy Asthma Proc. 2019, 40, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Arkwright, P.D.; Brüggen, M.-C.; Busse, W.; Gadina, M.; Guttman-Yassky, E.; Kabashima, K.; Mitamura, Y.; Vian, L.; Wu, J.; et al. Type 2 Immunity in the Skin and Lungs. Allergy 2020, 75, 1582–1605. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Schneider, L.; Asiniwasis, R.N.; Boguniewicz, M.; De Benedetto, A.; Ellison, K.; Frazier, W.T.; Greenhawt, M.; Huynh, J.; Kim, E.; et al. Atopic Dermatitis (Eczema) Guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–Based Recommendations. Ann. Allergy. Asthma. Immunol. 2024, 132, 274–312. [Google Scholar] [CrossRef]
- Zablotsky, B.; Black, L.; Akinbami, L. Diagnosed Allergic Conditions in Children Aged 0–17 Years: United States, 2021; National Center for Health Statistics (U.S.): Hyattsville, MD, USA, 2023. [Google Scholar]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Nascimento Júnior, J.A.C.; Oliveira, A.M.S.; Porras, K.D.L.; Menezes, P.D.P.; Araujo, A.A.d.S.; Nunes, P.S.; Aragón, D.M.; Serafini, M.R. Exploring Trends in Natural Product-Based Treatments to Skin Burn: A Comprehensive Review. Phytomedicine Int. J. Phytother. Phytopharm. 2025, 139, 156481. [Google Scholar] [CrossRef]
- Dao, T.T.P.; Song, K.; Kim, J.Y.; Kim, Y.S. Igalan from Inula Helenium (L.) Suppresses the Atopic Dermatitis-like Response in Stimulated HaCaT Keratinocytes via JAK/STAT3 Signaling. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2020, 69, 309–319. [Google Scholar] [CrossRef]
- Balkrishna, A.; Mishra, S.; Singh, A.; Srivastava, D.; Singh, S.; Arya, V. Hibiscus Syriacus L.: A Critical Review of Medicinal Utility & Phytopharmacology with Mechanistic Approach. J. Phytopharm. 2022, 11, 204–210. [Google Scholar] [CrossRef]
- Park, Y.; Kwon, S.; Jang, Y.L.; Lee, D.; Yang, S.; Eo, H.J.; Park, G.H.; Kwon, H. Nutritional Composition and Phytochemical Screening in Different Parts of Hibiscus Syriacus L. Food Sci. Nutr. 2022, 10, 3034–3042. [Google Scholar] [CrossRef]
- Simard, M.; Tremblay, A.; Morin, S.; Martin, C.; Julien, P.; Fradette, J.; Flamand, N.; Pouliot, R. α-Linolenic Acid and Linoleic Acid Modulate the Lipidome and the Skin Barrier of a Tissue-Engineered Skin Model. Acta Biomater. 2022, 140, 261–274. [Google Scholar] [CrossRef]
- Mieremet, A.; Helder, R.; Nadaban, A.; Gooris, G.; Boiten, W.; El Ghalbzouri, A.; Bouwstra, J.A. Contribution of Palmitic Acid to Epidermal Morphogenesis and Lipid Barrier Formation in Human Skin Equivalents. Int. J. Mol. Sci. 2019, 20, 6069. [Google Scholar] [CrossRef]
- Kim, H.N.; Park, S.B.; Park, G.H.; Eo, H.J.; Song, J.H.; Kwon, H.Y.; Jeong, J.B. Anti-Inflammatory Effect of the Root Extracts from Hibiscus Syriacus in LPS-Stimulated RAW264.7 Cells. Korean J. Plant Resour. 2018, 31, 211–217. [Google Scholar] [CrossRef]
- Bakr, R.O.; Amer, R.I.; Attia, D.; Abdelhafez, M.M.; Al-Mokaddem, A.K.; El-Gendy, A.E.-N.G.; El-Fishawy, A.M.; Fayed, M.A.A.; Gad, S.S. In-Vivo Wound Healing Activity of a Novel Composite Sponge Loaded with Mucilage and Lipoidal Matter of Hibiscus Species. Biomed. Pharmacother. 2021, 135, 111225. [Google Scholar] [CrossRef]
- Yang, J.-E.; Park, S.; Ngo, H.T.; Seo, S.; Go, E.; Hwang, J.-S.; Hwang, E.; Yi, T.-H. Skin-Protective and Anti-Inflammatory Effects of Hibiscus Syriacus L. (Mugunghwa): A Comparative Study of Five Parts of the Plant. Pharmacogn. Mag. 2020, 16, 183. [Google Scholar] [CrossRef]
- Yang, J.-E.; Ngo, H.T.T.; Hwang, E.; Seo, S.A.; Park, S.W.; Yi, T.-H. Dietary Enzyme-Treated Hibiscus Syriacus L. Protects Skin against Chronic UVB-Induced Photoaging via Enhancement of Skin Hydration and Collagen Synthesis. Arch. Biochem. Biophys. 2019, 662, 190–200. [Google Scholar] [CrossRef]
- di Martino, O.; Tito, A.; De Lucia, A.; Cimmino, A.; Cicotti, F.; Apone, F.; Colucci, G.; Calabrò, V. Hibiscus Syriacus Extract from an Established Cell Culture Stimulates Skin Wound Healing. BioMed Res. Int. 2017, 2017, 7932019. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R. Tachyphylaxis to Topical Corticosteroids: The More You Use Them, the Less They Work? Clin. Dermatol. 2006, 24, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H. Potential Adverse Effects of the Inhaled Corticosteroids. J. Allergy Clin. Immunol. 2003, 112, 469–478. [Google Scholar] [CrossRef]
- Lax, S.J.; Harvey, J.; Axon, E.; Howells, L.; Santer, M.; Ridd, M.J.; Lawton, S.; Langan, S.; Roberts, A.; Ahmed, A.; et al. Strategies for Using Topical Corticosteroids in Children and Adults with Eczema. Cochrane Database Syst. Rev. 2022, 3, CD013356. [Google Scholar] [CrossRef]
- Baldo, A.; Cafiero, M.; Di Caterino, P.; Di Costanzo, L. Tacrolimus Ointment in the Management of Atopic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2009, 2, 1–7. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and Safety of Dupilumab in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from Two Multicentre, Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Phase 3 Trials. Lancet Lond. Engl. 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Kychygina, A.; Cassagne, M.; Tauber, M.; Galiacy, S.; Paul, C.; Fournié, P.; Simon, M. Dupilumab-Associated Adverse Events During Treatment of Allergic Diseases. Clin. Rev. Allergy Immunol. 2022, 62, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Schuyler, M.R. Prednisone and T-Cell Subpopulations. Arch. Intern. Med. 1984, 144, 973. [Google Scholar] [CrossRef]
- Suga, H.; Sugaya, M.; Miyagaki, T.; Ohmatsu, H.; Kawaguchi, M.; Takahashi, N.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; et al. Skin Barrier Dysfunction and Low Antimicrobial Peptide Expression in Cutaneous T-Cell Lymphoma. Clin. Cancer Res. 2014, 20, 4339–4348. [Google Scholar] [CrossRef]
- Miajlovic, H.; Fallon, P.G.; Irvine, A.D.; Foster, T.J. Effect of Filaggrin Breakdown Products on Growth of and Protein Expression by Staphylococcus Aureus. J. Allergy Clin. Immunol. 2010, 126, 1184–1190.e3. [Google Scholar] [CrossRef]
- Morgner, B.; Werz, O.; Wiegand, C.; Tittelbach, J. Bilayered Skin Equivalent Mimicking Psoriasis as Predictive Tool for Preclinical Treatment Studies. Commun. Biol. 2024, 7, 1529. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of Care for the Management of Atopic Dermatitis. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef]
- Auwardt, R.B.; Mudge, S.J.; Chen, C.G.; Power, D.A. Regulation of Nuclear Factor kappaB by Corticosteroids in Rat Mesangial Cells. J. Am. Soc. Nephrol. 1998, 9, 1620–1628. [Google Scholar] [CrossRef]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by Glucocorticoids: Inhibition of NF-Kappa B Activity through Induction of I Kappa B Synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef]
- Annevelink, C.E.; Sapp, P.A.; Petersen, K.S.; Shearer, G.C.; Kris-Etherton, P.M. Diet-Derived and Diet-Related Endogenously Produced Palmitic Acid: Effects on Metabolic Regulation and Cardiovascular Disease Risk. J. Clin. Lipidol. 2023, 17, 577–586. [Google Scholar] [CrossRef]
- Yang, J.; Xue, X.; Yang, Z.; Hao, F.; Chen, B. Neural-Inflammation Mechanism of Spinal Palmitic Acid Promoting Atopic Dermatitis in Mice. J. Inflamm. Res. 2025, 18, 7907–7919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Wang, X.; Zhang, L.; Hu, M.; Le, Y.; Chen, L.; Zheng, J. Topical Emollient Prevents the Development of Atopic Dermatitis and Atopic March in Mice. Exp. Dermatol. 2023, 32, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, Y.M.; Yoo, H.J.; Suh, D.I.; Shin, Y.H.; Kim, K.W.; Ahn, K.; Hong, S. Gut Linoleic Acid Is Associated with the Severity of Atopic Dermatitis and Sensitization to Egg White/Milk in Infants. Pediatr. Allergy Immunol. 2021, 32, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Tawar, A.; Dipak, T.; Ankita, B.; Vikrant, B.; Walke, R. Mayuri Chandrawanshi From Tradition to Innovation: Hibiscus in Modern Anti-Aging Skincare. Int. J. Adv. Res. Sci. Commun. Technol. 2025, 660–664. [Google Scholar] [CrossRef]
- Liang, G.; Qian, H.; Sun, C.; Zhang, H.; Li, Z.; Li, S.; Jing, K.; Zhao, C.; Wang, Y.; Xiang, R.; et al. Dupilumab, Corticosteroids and Their Combination for the Treatment of Bullous Pemphigoid. An. Bras. Dermatol. 2025, 100, 243–252. [Google Scholar] [CrossRef]
- Farrell, A.M.; Antrobus, P.; Simpson, D.; Powell, S.; Chapel, H.M.; Ferry, B.L. A Rapid Flow Cytometric Assay to Detect CD4+ and CD8+ T-Helper (Th) 0, Th1 and Th2 Cells in Whole Blood and Its Application to Study Cytokine Levels in Atopic Dermatitis before and after Cyclosporin Therapy. Br. J. Dermatol. 2001, 144, 24–33. [Google Scholar] [CrossRef]
- Rho, N.-K.; Kim, W.-S.; Lee, D.-Y.; Lee, J.-H.; Lee, E.-S.; Yang, J.-M. Immunophenotyping of Inflammatory Cells in Lesional Skin of the Extrinsic and Intrinsic Types of Atopic Dermatitis. Br. J. Dermatol. 2004, 151, 119–125. [Google Scholar] [CrossRef]
- Humphreys, N.E. Assessment of Cumulative Allergen-Activated Lymph Node Cell Proliferation Using Flow Cytometry. Toxicol. Sci. 2003, 73, 80–89. [Google Scholar] [CrossRef][Green Version]
- Wang, P.; Sun, M.; Ren, J.; Djuric, Z.; Fisher, G.J.; Wang, X.; Li, Y. Gas Chromatography-Mass Spectrometry Analysis of Effects of Dietary Fish Oil on Total Fatty Acid Composition in Mouse Skin. Sci. Rep. 2017, 7, 42641. [Google Scholar] [CrossRef]
- Hwang, E.; Sun, Z.-W.; Lee, T.H.; Shin, H.-S.; Park, S.-Y.; Lee, D.-G.; Cho, B.-G.; Sohn, H.; Kwon, O.W.; Kim, S.Y.; et al. Enzyme-Processed Korean Red Ginseng Extracts Protects against Skin Damage Induced by UVB Irradiation in Hairless Mice. J. Ginseng Res. 2013, 37, 425–434. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.; Lee, H.J.; Lee, T.Y.; Sun, Z.; Yi, T.H. Gallic Acid Regulates Skin Photoaging in UVB-exposed Fibroblast and Hairless Mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
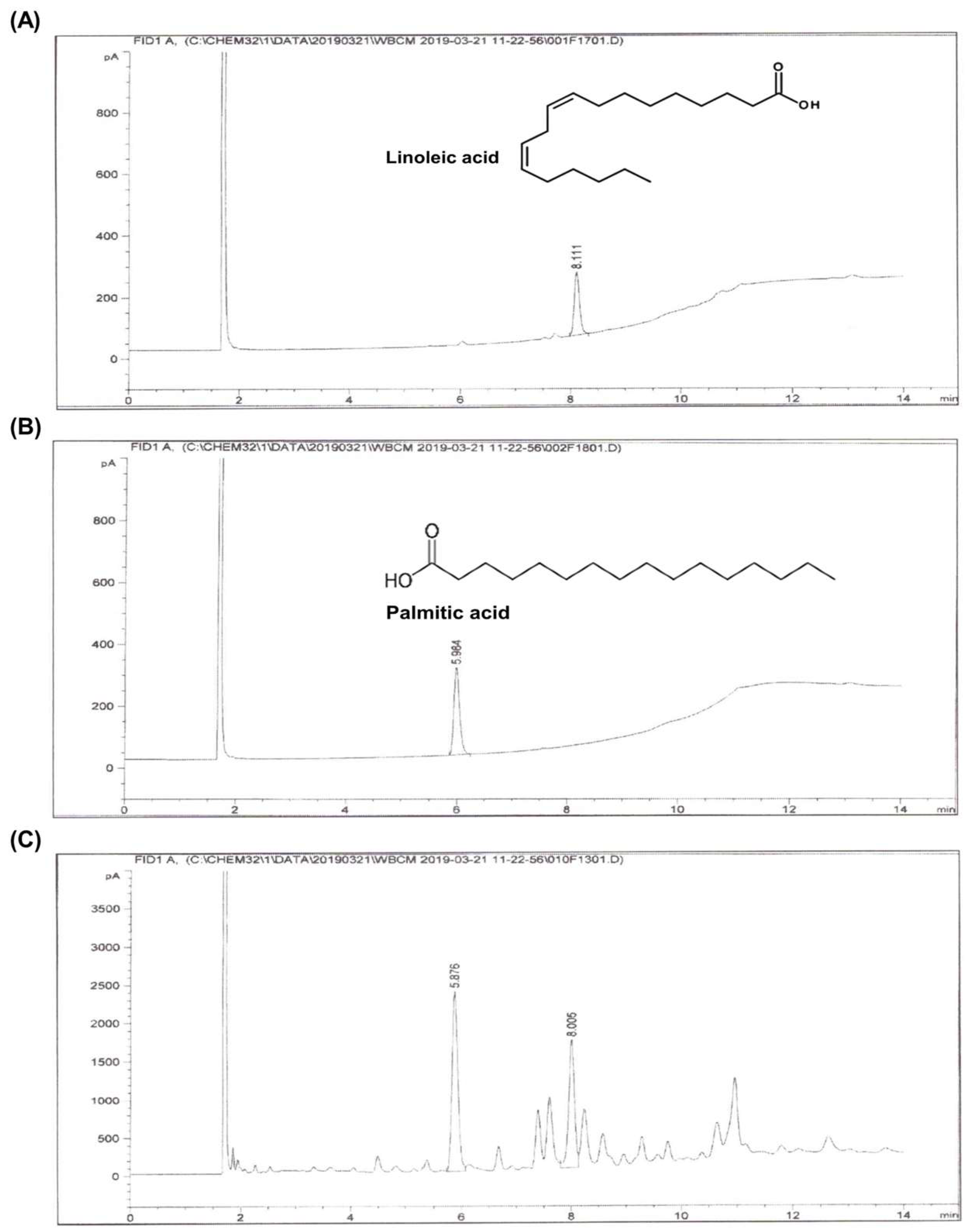
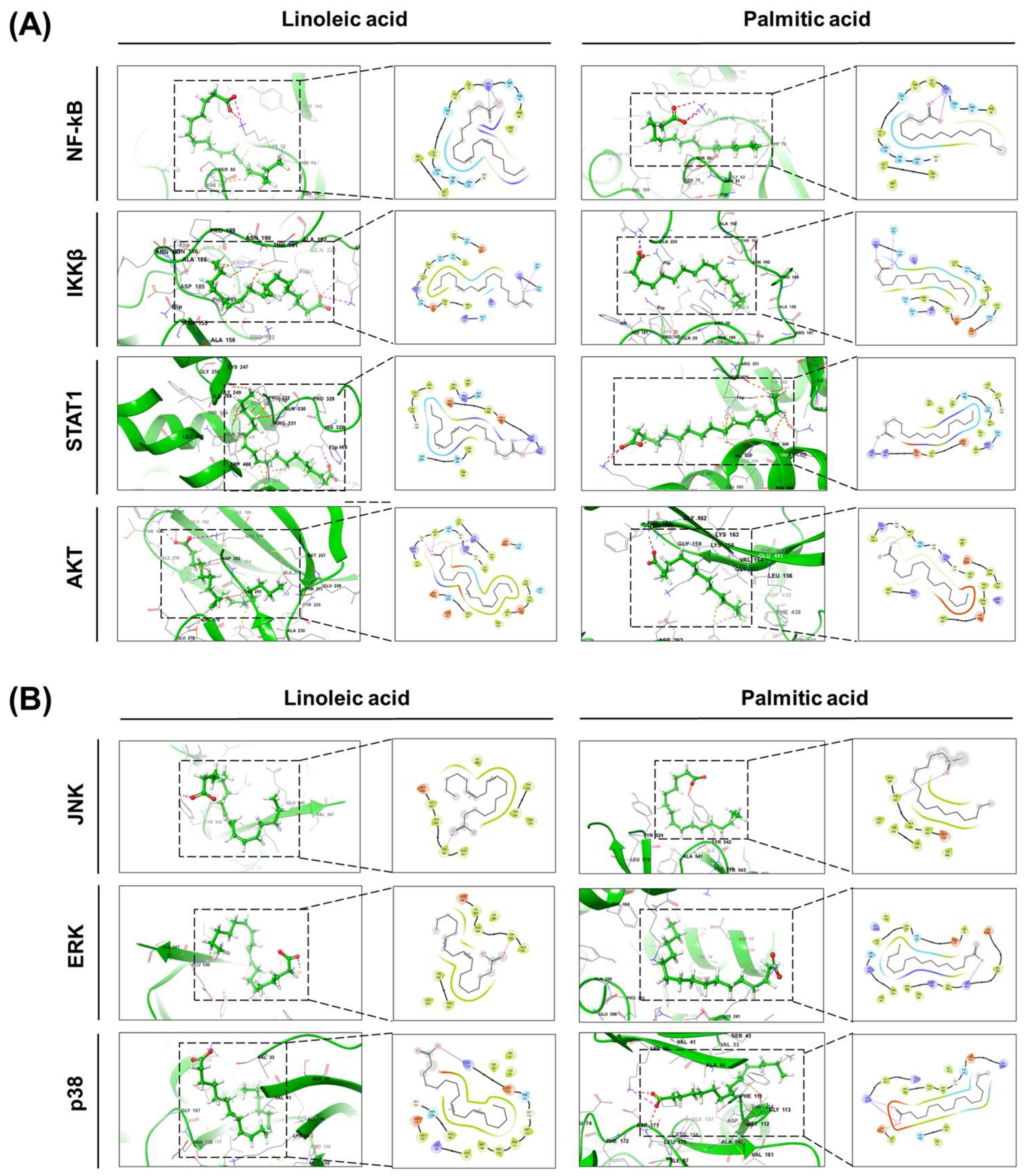


| Category | Linoleic Acid | Palmitic Acid |
|---|---|---|
| Molecular weight | 280.45 | 256.43 |
| LogP (QPlogPo/w) | 5.291 → highly lipophilic | 5.251 → highly lipophilic |
| LogS (QPlogS) | −4.635 → poorly soluble | −5.497 → very poorly soluble |
| Human oral absorption | 3 (high)/87.5% | 3 (high)/87.2% |
| # of H-bond donors | 1 | 1 |
| # of H-bond acceptors | 2 | 2 |
| Dipole moment | 6.52 | 6.83 |
| Polar surface area | 37.5 Å2 | 0.0 Å2 (due to lack of polar groups) |
| CNS activity | −2 (inactive) | −2 (inactive) |
| QPPCaco (permeability) | 239.37 nm/s → good | 235.46 nm/s → good |
| # Rule of 5 violations | 1 | 1 |
| # of rotatable bonds | 14 | 14 |
| Solvent accessible SA | 624.03 Å2 | 670.70 Å2 |
| Protein | Linoleic Acid | Palmitic Acid |
|---|---|---|
| NF-κB | −26.656 | −17.991 |
| IKKβ | −22.503 | −22.781 |
| STAT1 | −23.324 | −19.525 |
| AKT | −35.18 | −22.423 |
| JNK | −15.747 | −12.312 |
| ERK | −26.669 | −18.114 |
| p38 | −30.551 | −24.713 |
| Gene | Primer Type | Sequence (5′-3′) |
|---|---|---|
| GAPDH | Forward | 5′-ACCACAGTCCATGCCATCAC-3′ |
| Reverse | 5′-CCACCACCCTGTTGCTGTAC-3′ | |
| IL-6 | Forward | 5′-CTCCTTCTCCACAAGCGCC-3′ |
| Reverse | 5′-GCCGAAGAGCCCTCAGGC-3′ | |
| IL-8/CXCL8 | Forward | 5′-TCAGTGCATAAAGACATACTCC-3′ |
| Reverse | 5′-TGGCATCTTCACTGATTCTTG-3′ | |
| TARC/CCL17 | Forward | 5′-ATGGCCCCACTGAAGATGCT-3′ |
| Reverse | 5′-TGAACACCAACGGTGGAGGT-3′ | |
| RANTES/CCL5 | Forward | 5′-CCCCGTGCCGAGCACATCAAGGAGTATTT-3′ |
| Reverse | 5′-CGTCCAGCCTGGGGAAGGTTTTTGTA-3′ | |
| MDC/CCL22 | Forward | 5′-AGGACAGAGCATGGCTCGCCTACAGA-3′ |
| Reverse | 5′-AATGGCAGGGAGGTAGGGCTCCTGA-3′ |
| Compositions | Normal (n = 5) | Control (n = 5) | Prednisolone 0.01% (n = 5) | H. syriacus 0.1% (n = 5) | H. syriacus 1% (n = 5) |
| Casein | 230 | 230 | 230 | 230 | 230 |
| L-cystine | 3 | 3 | 3 | 3 | 3 |
| Corn oil | 100 | 100 | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 | 50 | 50 |
| Vitamin mix | 10 | 10 | 10 | 10 | 10 |
| Mineral mix | 35 | 35 | 35 | 35 | 35 |
| Sucrose | 200 | 200 | 200 | 200 | 200 |
| Corn starch | 372 | 372 | 372 | 371 | 362 |
| H. syriacus | − | − | − | 1 | 10 |
| DNCB | − | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.M.; Park, B.; Jin, X.; Zheng, Q.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. Fatty Acid-Rich Fraction of Hibiscus syriacus L. Alleviates Atopic Dermatitis-like Skin Lesions Mouse Model via Inflammatory Pathway Modulation: Integrative Docking and Experimental Validation. Plants 2025, 14, 2447. https://doi.org/10.3390/plants14152447
Nguyen TTM, Park B, Jin X, Zheng Q, Yi G-S, Yang S-J, Yi T-H. Fatty Acid-Rich Fraction of Hibiscus syriacus L. Alleviates Atopic Dermatitis-like Skin Lesions Mouse Model via Inflammatory Pathway Modulation: Integrative Docking and Experimental Validation. Plants. 2025; 14(15):2447. https://doi.org/10.3390/plants14152447
Chicago/Turabian StyleNguyen, Trang Thi Minh, Bom Park, Xiangji Jin, Qiwen Zheng, Gyeong-Seon Yi, Su-Jin Yang, and Tae-Hoo Yi. 2025. "Fatty Acid-Rich Fraction of Hibiscus syriacus L. Alleviates Atopic Dermatitis-like Skin Lesions Mouse Model via Inflammatory Pathway Modulation: Integrative Docking and Experimental Validation" Plants 14, no. 15: 2447. https://doi.org/10.3390/plants14152447
APA StyleNguyen, T. T. M., Park, B., Jin, X., Zheng, Q., Yi, G.-S., Yang, S.-J., & Yi, T.-H. (2025). Fatty Acid-Rich Fraction of Hibiscus syriacus L. Alleviates Atopic Dermatitis-like Skin Lesions Mouse Model via Inflammatory Pathway Modulation: Integrative Docking and Experimental Validation. Plants, 14(15), 2447. https://doi.org/10.3390/plants14152447










