Abstract
Sambucus williamsii Hance (Viburnaceae), the Korean elderberry, is widely used in herbal medicine and in the food industry. It is known to have various pharmacological effects, including antitumor, antioxidant, anti-inflammatory, and antimicrobial activities. During our search for anti-Helicobacter pylori compounds from natural resources, the methanol extract of the S. williamsii branch significantly inhibited the growth of H. pylori. Three phenolic and four lignan compounds were isolated from the methylene chloride fraction that had shown the most potent anti-H. pylori activity among the hexane, methylene chloride, ethyl acetate, butanol, and water fractions. The chemical structures were identified to be three phenolics of sylvopinol (1), dihydroconiferyl alcohol (2), and (7S,8R)-guaiacylglycerol (3) and four lignans of boehmenan (4), (7S,8S)-guaiacylglycerol β-coniferyl ether (6) and lawsonicin (7) with a new lignan, (7R,8R)-sambucanol (5), the structure of which was established by 1H- and 13C-NMR, and HRESI-MS, as well as quantum chemical electronic circular dichroism (ECD) calculations. Among the isolates, compounds 3 and 4 exhibited significant anti-H. pylori activity against strains 51 and 26695. Compound 3 displayed more potent antibacterial activity with MIC values of 3.13 and 6.25 μM, and MIC50 values of 28.5 and 56.8 μM against the two strains, respectively. Their inhibitory activities were higher than those of a positive control, quercetin. Furthermore, these two compounds showed moderate urease inhibitory activity. A molecular docking simulation revealed the high binding ability of 3 and 4 to the active site of H. pylori urease. These results will provide further insights into the design of more potent natural products for eradicating H. pylori.
1. Introduction
Gastric cancer ranks as the fifth most prevalent disease globally, characterized by a correspondingly high mortality rate. Notably, as of 2020, East Asian countries exhibit the highest incidence of gastric cancer worldwide [1,2]. The primary causes of gastric cancer include dietary and lifestyle factors, stress, and various other influences. Meanwhile, infection with Helicobacter pylori is considered to be the most significant and direct causative factor [3]. H. pylori is a spiral-shaped, Gram-negative bacterium that colonizes the stomachs of humans and animals. It was discovered in 1983 by J. Robin Warren and Barry J. Marshall [4]. Although the stomach is expected to be inhospitable to microorganisms due to its firm acidity, H. pylori possesses urease, which allows it to neutralize gastric acid and inhabit the mucosal layer and the mucus [5]. More than half of the global population is infected with H. pylori, with over 50% of the adult population in the Republic of Korea reported to be infected [6]. This bacterium is responsible for various gastrointestinal disorders, including gastric cancer, gastric ulcers, gastritis, and gastric MALT lymphoma. The International Agency for Research on Cancer has classified H. pylori as a Group 1 carcinogen [7,8]. Upon invading the gastric environment, H. pylori produces several inflammatory substances, including urease, CagA, exotoxin (VacA), and lipopolysaccharide (LPS) [9]. Urease, a ureolytic enzyme, hydrolyzes urea into carbon dioxide and ammonia. By generating urease while residing between the gastric mucosal and mucus layers, H. pylori alters the pH of the gastric mucosa, undermining its defensive mechanisms [10,11].
Current treatment options for H. pylori infection include triple therapy, which combines proton pump inhibitors (PPIs) with amoxicillin, clarithromycin, or metronidazole, and quadruple therapy, which consists of PPIs, bismuth, metronidazole, and tetracycline. However, there is a growing global concern regarding antibiotic resistance and associated side effects, leading to a decrease in treatment efficacy [12,13,14]. Consequently, the need for new drug development is increasing, and there is a rising interest in natural products-based pharmaceuticals, which are associated with relatively fewer side effects than synthetic drugs.
Sambucus williamsii Hance, commonly known as Korean elderberry, belongs to the family Viburnaceae. This deciduous shrub is widely distributed in East Asia, including Korea, China, and Japan, typically reaching heights of 5 to 6 m. The leaves are pinnately compound, featuring a palmate arrangement. The flowers are arranged in panicles and bloom in ivory-colored clusters between April and May. The fruits, which are either red or black, mature in July [15]. Traditionally, the stems and branches of this plant and its related species have been utilized in folk medicine under the name “Jeop-Gol-Mok”, primarily for the treatment of fractures [16]. In a previous study, the CHCl3 fraction of S. ebulus leaves and the fruit extract of American elderberry showed moderate anti-H. pylori activity [17,18]. Moreover, S. williamsii and S. pendula demonstrated more potent antibacterial activity against Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Pseudomonas fluorescens, and Salmonella typhimurium) than Gram-positive bacteria (Bacillus cereus, Bacillus subtilis, and Listeria monocytogenes) [19]. Despite their antibacterial activity, there is no research on the anti-H. pylori compounds of Sambucus species or anti-H. pylori activity of Sambucus species branches.
To date, various classes of compounds have been isolated from the stems and branches of S. williamsii, including lignans, terpenoids, iridoids, and phenolics [20]. Studies have demonstrated significant biological activities associated with these compounds, encompassing anti-inflammatory, antibacterial, anticancer, anti-aging, antioxidant, and gastroprotective effects [20,21,22,23,24]. The present study aims to evaluate anti-H. pylori activity of S. williamsii and identify the active compounds as potential agents for treatment of H. pylori.
2. Results and Discussion
The branches of S. williamsii were extracted with 100% methanol, and the extract was fractionated according to the solvent polarity (hexane, CH2Cl2, EtOAc, n-BuOH, and water). Among the fractions, the CH2Cl2 fraction showed the most potent anti-H. pylori activity against the two strains 51 and 26695 (Table 1). To identify the anti-H. pylori substituents of S. williamsii, seven compounds (1−7) were isolated from the CH2Cl2 fraction by open column chromatography (CC) using Sephadex LH-20 resin, medium-pressure liquid chromatography (MPLC) and preparative high-performance liquid chromatography (HPLC) with a reversed-phase C18 column (Figure 1 and Figure S1). The chemical structures of these compounds were determined based on ESI-QTOF-MS, 1H- and 13C-NMR, and ECD spectroscopy data (Table 2, Figure 2 and Figures S2–S22).

Table 1.
Anti-Helicobacter pylori activity of S. williamsii fractions.
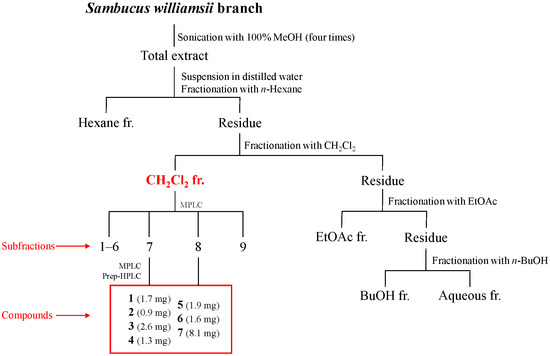
Figure 1.
Isolation of compounds 1–7 from Sambucus williamsii branch.

Table 2.
1H (300 MHz) and 13C-NMR (100 MHz) data of compounds 5 and 6 in CD3OD (δ ppm).
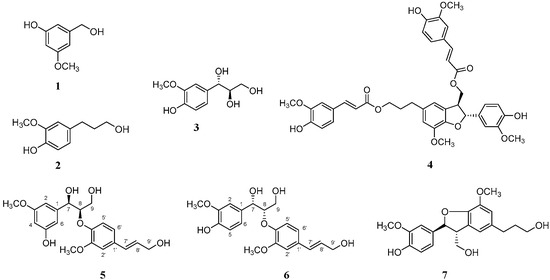
Figure 2.
Chemical structures of compounds 1–7.
2.1. Structural Elucidations of the Isolated Compounds 1–7
Compound 5 was obtained as a white amorphous powder. In the ESI-QTOF-MS spectrum, a quasimolecular ion peak at m/z 399.1379 corresponding to [M + Na]+ was observed, suggesting a molecular formula of C20H24O7 (calcd. C20H24O7Na, 399.1412). The 1H-NMR spectrum revealed proton signals for a 1,3,5- and a 1,2,4-trisubstituted aromatic rings at δ 7.01 (2H, d, J = 1.4 Hz, H-4, 6) and 6.88 (2H, d, J = 1.4 Hz, H-2, 2′), 6.83 (1H, dd, J = 8.1, 1.4 Hz, H-6′) and 6.73 (1H, d, J = 8.1 Hz, H-5′) ppm, indicating meta- and ortho-coupling, respectively. The signals at δ 6.53 (1H, dt, J = 15.9, 1.4 Hz, H-7′) and 6.25 (1H, td, J = 15.9, 5.7 Hz, H-8′) ppm were attributed to two protons coupled to a trans double-bond. Additionally, the 1H-NMR spectrum showed peaks at δ 4.84 (1H, d, J = 5.7 Hz, H-7) and 4.37 (1H, td, J = 5.7, 3.8 Hz, H-8) ppm, corresponding to oxymethine protons. The peaks at δ 3.82 (3H, s, 3-OCH3) and 3.81 (3H, s, 3′-OCH3) ppm were assigned to the two methoxy groups. The 13C-NMR spectrum revealed a total of 20 carbon peaks, including 12 corresponding to the two aromatic rings. The peaks at δ 72.8, 62.4, and 60.8 ppm indicated the presence of three hydroxyl-substituted carbons, while those at δ 55.1 and 55.9 ppm were attributed to the two methoxy groups. These NMR data of compound 5 exhibited a similar pattern to those of an 8-O-4′-neolignan glucoside compound 4a (4b, a peracetylated derivative) isolated from Arum italicum [25]. Structurally, the reference compound 4a possesses an O-β-D-glucose moiety at the C-5 position and a 7S,8S-configuration based on NMR and CD analysis (the positive Cotton effect). In contrast, compound 5 corresponds to the aglycone form, lacking the glucose moiety. In this study, the absolute configuration of compound 5 was determined using ECD spectroscopy (Figure 3). The negative CD curve [λmax 251 nm (Δε −0.06), λmax 279 nm (Δε −0.07)] observed in the ECD spectrum of compound 5 indicated that compound 5 has a 7R,8R-configuration, which is distinct from the 7S,8S-configuration of compound 4a [25]. To verify the absolute configuration of compound 5, two possible isomers, (7R,8R)-compound 5 and (7S,8S)-compound 5, were used for ECD calculations, and the experimental ECD spectrum of compound 5 was in good agreement with the calculated ECD curve of (7R,8R)-compound 5 (Figure 3), indicating the absolute configuration of C-7 and C-8 could be verified as R and R, respectively. Based on these spectroscopic results and comparison with reported data, compound 5 was designated as (7R,8R)-sambucanol, a new neolignan compound that was identified for the first time in this study. Although compound 6 had been previously reported as guaiacylglycerol β-coniferyl ether, its stereochemical configuration had not been determined prior to this study [26].
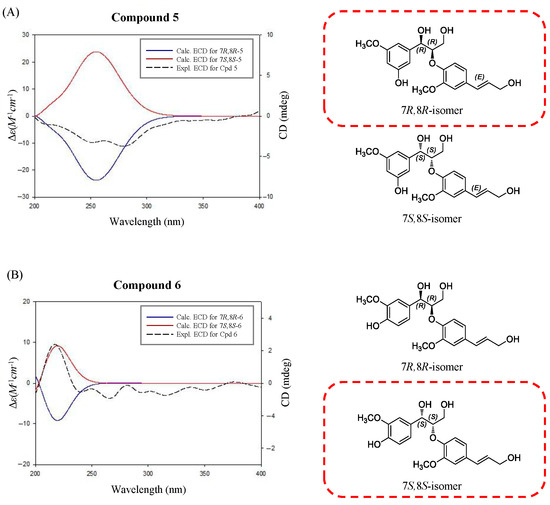
Figure 3.
Comparison of the experimental ECD spectra of compounds 5 (A) and 6 (B) with the calculated ECD spectra of their two possible stereoisomers, (7R,8R)- and (7S,8S)-isomers.
The ECD spectrum of compound 6 showed a positive Cotton effect at 217 nm [λmax 217 nm (Δε +0.06)], supporting the assignment of its absolute stereochemistry as a 7S,8S-configuration [27]. The absolute configuration of compound 6 was confirmed using ECD calculations. The experimental ECD spectrum of compound 6 showed almost the same tendency as that of (7S,8S)-guaiacylglycerol β-coniferyl ether (Figure 3), which suggested that the absolute configuration of compound 6 was 7S,8S. The other five compounds were also isolated from this fraction and identified as the three phenolic compounds of sylvopinol (1) [28], dihydroconiferyl alcohol (2) [29], and (7S,8R)-guaiacylglycerol (3) [30], and the other two lignans of boehmenan (4) [31] and lawsonicin (7) [32] by comparison of their spectroscopic data, such as MS and NMR, with the published literature.
2.2. Anti-H. pylori Activity of Compounds 1−7
The seven isolated compounds were evaluated for their anti-Helicobacter pylori activity (Table 3). Among them, compounds 1, 3, and 4 showed noticeable antibacterial effects, with compounds 3 and 4 exhibiting particularly strong activity. To further investigate their potential, the minimal inhibitory concentration (MIC) values of compounds 3 and 4 were determined against two strains, 51 and 26,695, using the broth dilution method based on a previously reported protocol [33]. As shown in Table 4, both compounds showed significant growth inhibition in a concentration-dependent manner. For compound 3, the MIC50 values were 28.5 μM and 56.8 μM against strains 51 and 26,695, respectively, while the MIC90 values were 97.1 μM and 86.5 μM. Compound 4 also showed inhibitory effects with the MIC50 values of 66.0 μM and 62.0 μM against the two strains.

Table 3.
Anti-Helicobacter pylori activity of compounds 1–7.

Table 4.
Anti-H. pylori activity of compounds 3 and 4.
Although the two phenyl propane compounds 2 and 3 have the same skeleton, they exhibited quite significant differences in their bacterial growth inhibitory activity. Compound 3, which contains each hydroxyl group at C-7 and C-8, exhibited much higher inhibitory activity than compound 2, which has no substitution at C-7 and C-8. This result suggests that the two hydroxyl groups at the C-7 and C-8 positions of 3 increase the inhibitory activity of H. pylori. It is known that the presence of polar functional groups such as hydroxyl and carboxyl moieties increased antibacterial activity [34].
Among the four neolignan isolates (4−7), only boehmeman (4) exhibited significantly potent anti-H. pylori activity. This compound contains two ferulic acid moieties and one oxolane moiety, which may contribute to its enhanced activity. Previous studies have reported the moderate anti-H. pylori activity of ferulic acid [35,36], and it is noteworthy that this moiety is present only in compound 4 among the four lignans. In another study, researchers modified the structure of lignans by introducing acetyl, oxolane, and hydroxymethyl groups, and evaluated their antibacterial activity against Bacillus aryabhattai and Klebsiella species. Among these modifications, lignans containing the oxolane moiety exhibited the most potent antibacterial growth inhibitory activity [37]. Generally, the antimicrobial properties of lignans are attributed to their ability to penetrate bacterial cell walls, resulting in cell wall lysis. This disruption induces oxidative stress, ATP depletion, and a decrease in intracellular pH within bacterial cells, ultimately compromising cellular integrity and leading to cell death [38].
Although compound 6 shares the guaiacylglycerol moiety with compound 3, which showed potent anti-H. pylori activity, its inhibitory activity was very low. This discrepancy can be ascribed to the differing stereochemistry—compound 6 has a stereochemistry of (7S,8S), while compound 3 has (7S,8R). Additionally, compound 6 contains a coniferyl alcohol moiety at the C-7 position. Unlike ferulic acid, coniferyl alcohol, one of the major precursors of lignans, exhibited very weak antibacterial activity, and there are no reports on its anti-H. pylori activity [39]. Similarly, compound 5, which also possesses a coniferyl alcohol moiety, failed to inhibit the bacterial growth.
Our findings provide a valuable basis for further in vitro and in vivo validation studies, which may ultimately advance these isolated compounds closer to preclinical evaluation and clinical translation as natural product-derived therapeutics, increasing the challenges created by H. pylori developing resistance to conventional antibiotics.
2.3. Urease Inhibitory Activity
Urease is recognized as a key virulence factor of H. pylori, catalyzing the hydrolysis of urea into ammonia and carbon dioxide, thereby neutralizing gastric acid and facilitating bacterial survival in the acidic environment of the stomach [10,11]. Therefore, the urease inhibitory activity of compounds 3 and 4, which showed high growth-inhibitory efficacy against H. pylori strains 51 and 26,695, was evaluated. The results revealed that both compounds exhibited moderate inhibition activity (Table 5). The urease inhibitory activity (38.9 ± 1.2%) of compound 4 at the final concentration of 1 mM was higher than that (31.8 ± 2.8%) of compound 3. At this condition, the inhibitory activity of acetohydroxamic acid, a positive control, was 73.5 ± 3%. The anti-urease activity of these two compounds is reported for the first time. Anti-H. pylori urease activity of ferulic acid has been reported, and compound 4 possesses two ferulic acids [40]. So, the moderate anti-urease activity of compound 4 could be derived from the two ferulic acid moieties.

Table 5.
Binding energies, interacting residues, and inhibitory activity of ligand compounds 3 and 4 against H. pylori urease protein.
2.4. Molecular Docking Simulation
Molecular docking is a valuable technique that can accurately predict the optimal orientation of a molecule in relation to its potential target, and it is also a crucial component of the rational drug design process [41]. In this study, among the seven isolated compounds, compounds 3 and 4 were chosen and docked into the binding site of the urease protein due to their significant anti-H. pylori and anti-urease properties. The molecular docking study aimed to identify the binding amino acid residues of these compounds to urease derived from H. pylori and to determine their respective binding energies. The results showed that the ligand molecules effectively bind to the target site. Specifically, compound 3 interacted with the Lys β445 and Gln β471 residues through hydrogen interaction, and with the Val α36, Val α33, Pro β472, and Leu α13 residues through hydrophobic interaction. Compound 4 interacted with the Gln β471, Gln β459, Tyr β32, Phe β441, Glu α80, Gly α82, Thr β469, and His α79 residues through hydrogen interaction, and with the Val β473 residue through hydrophobic interaction (Figure 4). Compound 4 showed lower binding energy compared to compound 3. These findings from the molecular docking analysis are in good agreement with the in vitro urease inhibitory activity of compounds 3 and 4. This suggests that both compounds have the potential to inhibit urease, which may contribute to their anti-H. pylori efficacy. Additionally, there can be other inhibitory mechanisms, considering that compound 3 exhibited more potent anti-H. pylori activity than compound 4.
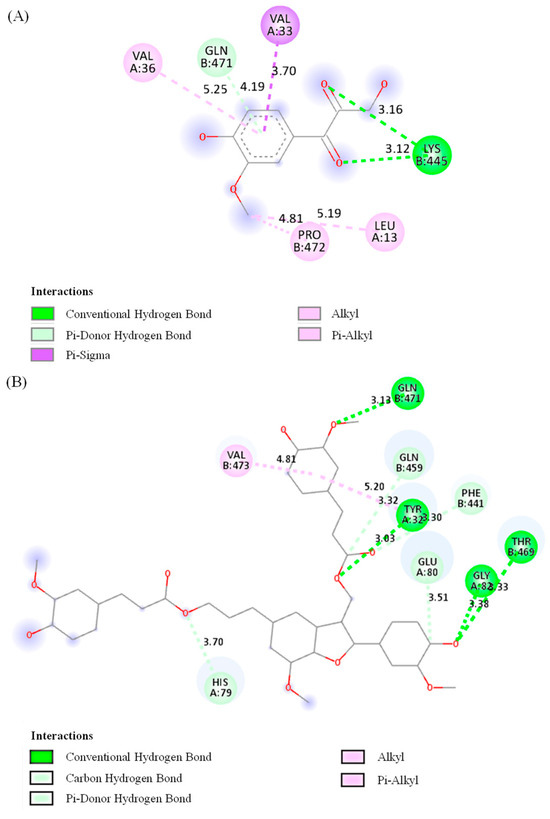
Figure 4.
Two-dimensional interaction diagrams of two compounds 3 (A) and 4 (B) with H. pylori urease. Pink color depicts alkyl or pi–alkyl interaction. In alkyl interaction, Van der Waals forces stabilize the complex through a hydrophobic contact between the ligand and alkyl side chains (such as -CH3, -CH2– groups) of amino acid residues. The pi–alkyl interaction is a hydrophobic contact that improves ligand–receptor packing between alkyl groups and the π-electron system of an aromatic ring. Through close-range interactions, the π-system of an aromatic ring and a sigma (σ) bond interact to produce the pi–sigma interaction, shown in purple. A conventional hydrogen bond is expressed in green. It is an essential component of ligand binding specificity and affinity, and a traditional hydrogen bond involves a shared hydrogen atom between a donor (such as -OH, -NH2) and an acceptor (such as O and N). The carbon hydrogen bond depicted in light green describes the interaction between hydrogen atoms and carbon atoms (carbanions from the neighboring electronegative atoms) present in the ligand. The pi–donor hydrogen bond in light green represents a hydrogen bond in which an electronegative acceptor atom in the protein interacts with an aromatic π-system, which functions as a hydrogen bond donor.
To assess the accuracy of docking program the co-crystallized ligand was removed from the active site and re-docked within the inhibitor binding cavity of H. pylori urease. In this study, the RMSD value was found to be 0.1 Å, showing that our docking method is valid for the studied inhibitors. The grid box parameters were centered on the native ligand’s coordinates.
These results provide a molecular rationale for the urease inhibitory activities observed in compounds 3 and 4 and highlight their potential as lead scaffolds for the development of anti-H. pylori agents.
3. Materials and Methods
3.1. General Experimental Procedures
The 1H and 13C-NMR spectra were recorded on a DRX-300 spectrometer, Avance Neo 400 nanobay, and Avance Neo 600 (Bruker, Billerica, MA, USA), and chemical shifts were measured as δ (ppm) values. All NMR solvents were purchased from Eurisotop (Tewksbury, MA, USA). QTOF mass spectrometry was performed using a Xevo G2-XS TOF (Waters, Framingham, MA, USA) and an X500R (AB SCIEX, Framingham, MA, USA) mass spectrometer. Experimental ECD spectra in EtOH were acquired in a quartz cuvette of 1 mm optical path length on a JASCO J-1500 spectropolarimeter (JASCO, Tokyo, Japan). Column chromatography (CC) was performed with Sephadex LH-20 (Cytiva, Uppsala, Sweden). Medium-pressure liquid chromatography (MPLC) was performed using a SNAP Cartridge KP-SIL 340 g (Biotage, Uppsala, Sweden). HPLC analysis was conducted with an Agilent 1260 HPLC system (Hewlett Packard, Waldbronn, Germany) equipped with a Gemini C18 column (110 Å, 4.6 × 250 mm, 5 µm) (Phenomenex, Torrance, CA, USA). A CO2 incubator (Sanyo, Sakata, Japan) was employed for the cultivation of H. pylori. All standard materials were purchased from Sigma-Aldrich (St. Louis, MO, USA), while other solvents were obtained from Fisher Scientific (Hampton, NH, USA).
3.2. Plant Materials
The Sambucus williamsii branch was collected from Hadong, Gyeongsangnam-do, Republic of Korea. The plant material was authenticated by Dr. Mi-Jeong Ahn (College of Pharmacy, Gyeongsang National University, Republic of Korea), and a specimen (No. PGSC-610) was deposited in the herbarium of College of Pharmacy, Gyeongsang National University.
3.3. Extraction and Isolation
The air-dried branch of S. williamsii (1.5 kg) was finely ground and extracted with methanol at room temperature. The extract was filtered and concentrated through a rotary evaporator. The resulting crude extract was then suspended in water and partitioned with n-hexane, methylene chloride (CH2Cl2), ethyl acetate (EtOAc) and n-butanol (BuOH) successively to yield a hexane fr. (9.2 g), CH2Cl2 fr. (6.0 g), EtOAc fr. (1.8 g), BuOH fr. (6.8 g) and aqueous fr. (11.8 g).
The CH2Cl2 fraction was subjected to MPLC using different solvents of increasing polarity (hexane, ethyl acetate, and methanol, 100:0:0 → 0:100:0 → 0:0:100), resulting in the collection of nine fractions (Fr. 1–Fr. 9). Fr. 7 was subjected to MPLC using a mixture of water and acetonitrile as an eluting solvent to provide seven subfractions (Fr. 7.1–Fr. 7.7). Fr. 7.1 was subjected to MPLC with a gradient of hexane, ethyl acetate, and methanol (100:0:0 → 0:100:0 → 0:0:100) as the eluting solvents, yielding 11 subfractions (Fr. 7.1.1–Fr. 7.1.11). Compounds 1 (1.7 mg, tR 6.2 min) and 2 (0.9 mg, tR 11.9 min) were isolated from Fr. 7.1.2 by prep-HPLC. The prep-HPLC separation was performed with an Agilent 1260 HPLC system with a Gemini C18 column. The chromatogram was performed by a gradient elution of water (A) and acetonitrile (B) mixture under the following conditions: 0 min, 15%; 0 to 5 min, 15% B; 5 to 40 min, 50% B; and 40 to 50 min, 95% B. The flow rate was 1 mL/min, and the temperature was maintained at 30 °C. Compound 3 (2.6 mg) was isolated from fr. 7.1.4 by performing Sephadex LH-20 column chromatography (CC) using methanol as the eluting solvent. Fr. 7.5 was subjected to Sephadex LH-20 CC using methanol as the eluting solvent, resulting in the isolation of compound 4 (1.3 mg). Fr. 8 was subjected to MPLC using a mixture of hexane, ethyl acetate, and methanol (100:0:0 → 0:100:0 → 0:0:100) as the eluting solvents. This process resulted in the collection of 10 subfractions (Fr. 8.1–Fr. 8.10). Fr. 8.5 was then subjected to Sephadex LH-20 CC with methanol as the eluting solvent, yielding six subfractions (Fr. 8.5.1–Fr. 8.5.6). A subfraction, Fr. 8.5.3, was further separated by prep-HPLC using the same HPLC system equipped with a Gemini C18 column. The gradient elution was accomplished as follows: 0 min, 10% B; 0 to 5 min, 10% B; 5 to 40 min, 20% B; 40 to 80 min, 50% B; 80 to 90 min, 95% B. This procedure resulted in the isolation of compounds 5 (1.9 mg, tR 31.1 min), 6 (1.6 mg, tR 33.2 min), and 7 (8.1 mg, tR 50.3 min) (Figure S23).
(7R,8R)-Sambucanol (5) White amorphous powder; −51 (c 0.16, EtOH); UV (MeOH) λmax 205 (4.55), 225 (4.21), 250 (3.84), 270 (3.95), 290 (3.80), 335 (3.55, sh) nm; ECD (EtOH) λmax (Δε) 251 (−0.06), 279 (−0.07) nm; 1H (CD3OD, 300 MHz) and 13C (CD3OD, 100 MHz) NMR data, see Table 2; ESI-QTOF-MS (m/z): 399.1379 [M + Na]+ (calcd. C20H24O7Na, 399.1420).
(7S,8S)-Guaiacylglycerol β-coniferyl ether (6) White amorphous powder; −12 (c 0.13, MeOH); UV (MeOH) λmax 205 (4.60), 225 (4.28), 250 (3.91), 270 (4.09), 290 (3.84), 335 (2.98, sh) nm; ECD (MeOH) λmax (Δε) 217 (+0.06); 1H (CD3OD, 300 MHz) and 13C (CD3OD, 100 MHz) NMR data, see Table 2; ESI-QTOF-MS (m/z): 399.1379 [M + Na]+ (calcd. C20H24O7Na, 399.1420).
MS and NMR spectra for the isolated compounds 1–7, see Figures S2–S22.
3.4. ECD Calculation
Initial conformational searches were performed at the MMFF94 force field using the MacroModel (version 2021-4, Schrödinger LLC, New York, NY, USA) program with a mixed torsional/low-mode sampling method, in which a gas phase with a 50 kJ mol−1 energy window and 10,000 maximum iterations were employed. The Polak–Ribiere conjugate gradient protocol was established with 10,000 maximum iterations and a 0.001 kJ (mol Å)−1 convergence threshold on the root-mean-square gradient to minimize conformers. The conformers proposed in this study (found within 20 kJ mol−1 in the MMFF force field) were selected for geometry optimization using TmoleX 4.3.2 with the density functional theory settings of B3-LYP/6-31+G(d,p).
ECD calculations for the (7R,8R)-5 and (7S,8S)-5 conformers (13 conformers each) were performed at an identical theory level and basis sets. The (7R,8R)-6 and (7S,8S)-6 conformers were calculated, with 14 of each. The calculated ECD spectra were simulated by superimposing each transition, where σ is the bandwidth at a height of 1/e. ΔEi and Ri are the excitation energy and rotatory strength for transition i, respectively. In this study, the value of σ was 0.2 eV. The excitation energies and rotational strengths of the ECD spectra were calculated based on the Boltzmann populations of conformers, and ECD visualization was performed using SigmaPlot 14.0.
3.5. Helicobacter pylori Culture
The H. pylori strains 51 and 26,695 utilized in this study were acquired from the Helicobacter pylori Korean Type Culture Collection (HKTCC), Department of Microbiology, College of Medicine, Gyeongsang National University, Republic of Korea. Strain 51 was initially isolated from the stomach of a Korean patient in 1987 and 2000. Strain 26,695, known as KE26695, was identical to the strain isolated in the United Kingdom from the stomach of a patient with gastritis. The strains were cultured in Brucella Broth liquid medium (BD, Franklin Lakes, NJ, USA) supplemented with 10% horse serum (Gibco, Grand Island, NY, USA) and subcultured every 24 h at 37 °C under 10% CO2.
3.6. Anti-Helicobacter pylori Assay
The broth dilution method was used for determination of the minimal inhibitory concentration (MIC) values [42]. A bacterial colony suspension equivalent to 2–3 × 108 cfu mL−1 and serial two-fold dilutions of each isolate and reference compound were prepared. The culture medium containing twenty microliters of bacterial inoculum and twenty microliters of sample solution was incubated at 37 °C for 24 h. After incubation, MIC, MIC50, and MIC90 were defined as the lowest concentration of compounds at which bacterial growth was inhibited, and inhibited by 50% and 90%, respectively. Analysis of growth was achieved by reading the optical density at 600 nm. All of the values were obtained from three independent experiments. The MIC50 and MIC90 values were calculated using GraphPad Version 5.01 (GraphPad Software, Inc., San Diego, CA, USA). DMSO was used as the negative control, while quercetin and metronidazole (Sigma-Aldrich, St. Louis, MO, USA) were used as the positive control. The following formula was used to calculate the inhibitory activity.
Inhibition (%) = [(Absorbance of negative control − Absorbance of sample solution)/Absorbance of
negative control] × 100
negative control] × 100
3.7. Measurement of Urease Inhibitory Activity
The anti-urease assay was conducted using phenol red reagent, referring to the previously reported paper [33]. The culture fluid of H. pylori strain 51 was centrifuged at 500× g for two minutes, and the pellet was resuspended in lysis buffer (Invitrogen, Middlesex County, MA, USA). After one minute of ultrasonic disruption, the suspension was incubated on ice for ten minutes. This process was carried out three times. After centrifuging the mixture for one minute at 100× g, 20 μL of the supernatant was combined with 50 μL of 10 μM urea and 20 μL of sample solution and incubated for ten minutes at 37 °C with 10% CO2. The final concentration of sample was 1 mM. The incubation was carried out for 30 min after the concentration was changed to 0.1 M urea and 1 mM phenol red. Absorbance was measured at 570 nm. Acetohydroxamic acid (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control [43]. The same formula for MIC values was used to calculate the urease inhibitory activity.
3.8. Molecular Docking
In the study, the AutoDock Vina tool (version 1.5.7) was used to investigate the molecular interaction between target protein and the selected ligand. The Crystallographic structure of H. pylori urease (PDB ID: 1E9Y) was taken from Protein Data Bank. Before docking analysis, BIOVIA Discovery Studio 2021 program used for the optimization of structure of the enzyme by removing excess ligands and water molecules. The selected compounds were drawn in ChemDraw 12.0 (Cambridgesoft, Cambridge, MA, USA) and then all compounds were optimized for energy using the Spartan 14 (Version 1.1.4) program. Polar hydrogens were added to the protein using the AutoDock vina 1.5.7 tool, and Kollman charges were determined using Compute Gasteiger. BIOVIA Discovery was used to determine the active sites of proteins. Finally, the molecular interactions and types of bond between the selected compound and target protein were investigated using the Discovery Studio visualizer programs.
3.9. Statistical Analysis
All experiments were performed in triplicate, and the results are presented as the mean ± standard deviation (SD). One-way analysis of variance (one-way ANOVA) was conducted using the SPSS Statistics 24.0 software (IBM, Armonk, NY, USA). The significance level was evaluated at * p < 0.05.
4. Conclusions
In this study, three phenolic compounds and four lignans were isolated from the CH2Cl2 fraction of S. williamsii branch extract. Among the isolated compounds, (7R,8R)-sambucanol (5) was reported here for the first time in nature. The compounds (7S,8S)-Guaiacylglycerol β-coniferyl ether (6) and lawsonicin (7) were also newly identified within the Sambucus species and Viburnaceae family, respectively. We also documented the anti-H. pylori and anti-urease activities of (7S,8R)-guaiacylglycerol (3) and boehmenan (4) for the first time. These findings provide new insight into the phytochemical diversity of S. williamsii and support its potential as a natural therapeutic agent against H. pylori. Furthermore, the stereochemical configurations of these compounds have been thoroughly determined for the first time, which contributes to our knowledge of their structural diversity and advances the field of natural product chemistry. The identification of (7S,8R)-guaiacylglycerol and boehmenan as key active components of S. williamsii branch opens up new avenues for future research into natural product-based treatments for H. pylori infections, particularly in light of growing concerns about antibiotic resistance and the side effects associated with conventional therapies. Further investigations will aim to isolate additional anti-H. pylori constituents from S. williamsii and elucidate their mechanisms of action through in vitro and in vivo assays and molecular docking studies for the design of more potent natural products for eradicating H. pylori.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14162558/s1, Figure S1: Isolation of compounds 1–7 from Sambucus williamsii branch; Figure S2: The ESI-QTOF mass spectrum of compound 1; Figure S3: The 1H-NMR spectrum of compound 1 (CD3OD, 400 MHz); Figure S4: The 13C-NMR spectrum of compound 1 (CD3OD, 100 MHz); Figure S5: The ESI-QTOF mass spectrum of compound 2; Figure S6: The 1H-NMR spectrum of compound 2 (CD3OD, 400 MHz); Figure S7: The 13C-NMR spectrum of compound 2 (CD3OD, 100 MHz); Figure S8: The ESI-QTOF mass spectrum of compound 3; Figure S9: The 1H-NMR spectrum of compound 3 (CD3OD, 400 MHz); Figure S10: The 13C-NMR spectrum of compound 3 (CD3OD, 100 MHz); Figure S11: The ESI-QTOF mass spectrum of compound 4; Figure S12: The 1H-NMR spectrum of compound 4 (CD3OD, 600 MHz); Figure S13: The 13C-NMR spectrum of compound 4 (CD3OD, 150 MHz); Figure S14: The ESI-QTOF mass spectrum of compound 5; Figure S15: The 1H-NMR spectrum of compound 5 (CD3OD, 300 MHz); Figure S16: The 13C-NMR spectrum of compound 5 (CD3OD, 100 MHz); Figure S17: The ESI-QTOF mass spectrum of compound 6; Figure S18: The 1H-NMR spectrum of compound 6 (CD3OD, 300 MHz); Figure S19: The 13C-NMR spectrum of compound 6 (CD3OD, 100 MHz); Figure S20: The ESI-QTOF mass spectrum of compound 7; Figure S21: The 1H-NMR spectrum of compound 7 (CD3OD, 300 MHz); Figure S22: The 13C-NMR spectrum of compound 7 (CD3OD, 100 MHz); Figure S23: Preparative HPLC chromatograms for the isolation of compounds.
Author Contributions
Conceptualization, M.-J.A.; methodology, W.-J.J., D.-M.K. and M.-J.A.; software, N.-I.Y.; validation, W.-J.J., D.-M.K., A.A.K.K., B.D.N. and K.-H.K.; formal analysis, W.-J.J., D.-M.K. and N.-I.Y.; investigation, W.-J.J., D.-M.K. and B.D.N.; resources, M.-J.A.; data curation, B.D.N. and S.-J.C.; writing—original draft preparation, W.-J.J. and D.-M.K.; writing—review and editing, A.A.K.K., S.-J.C. and K.-H.K.; visualization, N.-I.Y.; supervision, K.-H.K. and M.-J.A.; project administration, M.-J.A.; funding acquisition, M.-J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the project Establishment of a specialized production cluster for Platycodon grandiflorus and development of high-functionality product technology (RS-2025-14383142) of the Rural Development Administration.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Brenner, H.; Rothenbacher, D.; Arndt, V. Epidemiology of stomach cancer. Cancer Epidemiol. 2009, 472, 467–477. [Google Scholar]
- Warren, J.R.; Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983, 321, 1273–1275. [Google Scholar] [CrossRef]
- Johnson, K.S.; Ottemann, K.M. Colonization, localization, and inflammation: The roles of H. pylori chemotaxis in vivo. Curr. Opin. Microbiol. 2018, 41, 51–57. [Google Scholar] [CrossRef]
- Jung, H.-K.; Kang, S.J.; Lee, Y.C.; Yang, H.-J.; Park, S.-Y.; Shin, C.M.; Kim, S.E.; Lim, H.C.; Kim, J.-H.; Nam, S.Y.; et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver 2021, 15, 168–195. [Google Scholar] [CrossRef] [PubMed]
- Sathianarayanan, S.; Ammanath, A.V.; Biswas, R.; Anita, B.; Sukumaran, S.; Venkidasamy, B. A new approach against Helicobacter pylori using plants and its constituents: A review study. Microb. Pathog. 2022, 168, 105594. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group. Schistosomes, Liver Flukes, and Helicobacter pylori; IARC Monographs on The Evaluation of The Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1994; Volume 61, pp. 177–240. [Google Scholar]
- Charitos, A.I.; D’Agostino, D.; Topi, S.; Bottalico, L. 40 Years of Helicobacter pylori: A revolution in biomedical thought. Gastroenterol. Insights 2021, 12, 111–135. [Google Scholar] [CrossRef]
- Eaton, K.A.; Brooks, L.C.; Morgan, D.R.; Krakowka, S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 1991, 59, 2470–2475. [Google Scholar] [CrossRef]
- Athmann, C.; Zeng, N.; Kang, T.; Marcus, E.A.; Scott, D.R.; Rektorschek, M.; Sachs, G. Local pH elevation mediated by the intrabacterial urease of Helicobacter pylori cocultured with gastric cells. J. Clin. Investig. 2000, 106, 339–347. [Google Scholar] [CrossRef]
- Gene, E.; Calvet, X.; Azagra, R.; Gisbert, J.P. Triple vs. quadruple therapy for treating Helicobacter pylori infection: A meta-analysis. Aliment. Pharmacol. Ther. 2003, 17, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Jung, H.-K.; Lee, H.L.; Jang, J.Y.; Lee, H.; Kim, C.G.; Shin, W.G.; Shin, E.S.; Lee, Y.C. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J. Gastroenterol. Hepatol. 2014, 29, 1371–1386. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, J.Y.; Choi, K.D.; Jung, H.-Y.; Kim, J.M.; Baik, G.H.; Kim, B.-W.; Park, J.C.; Jung, H.-K.; Cho, S.J.; et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter 2019, 24, e12592. [Google Scholar] [CrossRef]
- Lee, C.B. Color Illustrated Flora of Korea; Hyangmunsa: Seoul, Republic of Korea, 1985; Volume 2, p. 228. [Google Scholar]
- Kang, D.-M.; Kwon, J.-M.; Jeong, W.-J.; Neupane, D.B.; Ahn, M.-J. Antioxidant compounds of Sambucus pendula stem. Nat. Prod. Sci. 2024, 30, 275–281. [Google Scholar] [CrossRef]
- Yesilada, E.; Gurbuz, I.; Shibata, H. Screening of turkish anti-ulcerogenic folk remedies for anti-Helicobacter pylori activity. J. Ethnopharmacol. 1999, 66, 289–293. [Google Scholar] [CrossRef]
- Chatterjee, A.; Yasmin, T.; Bagchi, D.; Stohs, S. Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. J. Mol. Cell Biochem. 2004, 265, 19–26. [Google Scholar] [CrossRef]
- Seo, K.-S.; Yun, K.W. In vitro antimicrobial and antioxidant activities of Sambucus williamsii and Sambucus pendula. Int. J. Second. Metab. 2024, 11, 191–199. [Google Scholar] [CrossRef]
- Xiao, H.H.; Zhang, Y.; Cooper, R.; Yao, S.X.; Wong, S.M. Phytochemicals and potential health effects of Sambucus williamsii Hance (Jiegumu). Chin. Med. 2016, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Choi, H.; Hwang, I.S.; Kim, A.R.; Woo, E.R.; Lee, D.G. Synergistic antibacterial and antibiofilm effect between (+)-medioresinol and antibiotics In Vitro. Appl. Biochem. Biotechnol. 2013, 170, 1934–1941. [Google Scholar] [CrossRef]
- Kim, H.H.; Lee, S.; Kim, S.H.; Yim, S.H. Triterpenoid constituents and Their Anti-cancer activity from stems and branches of Sambucus williamsii var. coreana Nakai (Caprifoliaceae). Food Sci. Technol. 2021, 42, e76021. [Google Scholar] [CrossRef]
- Kang, X.; Lee, J.A. Development of anti-aging cream containing green tea extract fermented with lactic acid bacteria and evaluation of its skin improvement effects. J. Soc. Cosmet. Sci. Korea 2023, 13, 123–133. [Google Scholar]
- Waswa, E.; Li, J.; Mkala, E.; Wanga, V.; Mutinda, E.; Nanjala, C.; Odago, W.; Katumo, D.; Gichua, M.; Gituru, R.; et al. Ethnobotanical study of medicinal plants used by traditional health practitioners in the management of diabetes in kakamega county, Kenya. J. Ethnopharmacol. 2022, 292, 115102. [Google Scholar] [CrossRef]
- Greca, M.D.; Molinaro, A.; Monaco, P.; Previtera, L. Lignans from Arum italicum. Phytochemistry 1994, 35, 777–779. [Google Scholar] [CrossRef]
- Yao, G.-D.; Wang, J.; Song, X.-Y.; Zhou, L.; Lou, L.-L.; Zhao, W.-Y.; Lin, B.; Huang, X.-X.; Song, S.-J. Stereoisomeric guaiacylglycerol-β-coniferyl aldehyde ether induces distinctive apoptosis by downregulation of MEK/ERK pathway in hepatocellular carcinoma cells. Biroong. Chem. 2018, 81, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lundquist, K.; Wallis, A.F.A. Revised structure for a neolignan from Brucea javanica. Phytochemistry 1998, 49, 2125–2128. [Google Scholar] [CrossRef]
- Tyukavkina, A.N.; Lutskii, I.V.; Borodina, M.N.; Voronov, K.V. A new phenol from Pinus sylvestris. Chem. Nat. Compd. 1970, 6, 199–201. [Google Scholar] [CrossRef]
- Harmatha, J.; Lubke, H.; Rybarik, I.; Mahdalík, M. cis-Coniferyl alcohol and its glucoside from the bark of beech (Fagus silvatica L.). Chem. Commun. 1978, 43, 774–780. [Google Scholar] [CrossRef]
- Comte, G.; Vercauteren, J.; Chulia, J.A.; Allais, P.D.; Delage, C. Phenylpropanoids from leaves of Juniperus phoenicea. Phytochemistry 1997, 45, 1679–1682. [Google Scholar] [CrossRef]
- Rudiyansyah, R.; Masriani, M.; Mudianta, I.; Garson, M. Isolation and absolute configuration of boehmenan from Durio affinis Becc. Rec. Nat. Prod. 2014, 8, 195–198. [Google Scholar]
- Li, L.; Seeram, P.N. Maple syrup phytochemicals include lignans, coumarins, a stilbene, and other previously unreported antioxidant phenolic compounds. J. Agric. Food Chem. 2010, 58, 11673–11679. [Google Scholar] [CrossRef]
- Kang, D.-M.; Khalil, K.A.A.; Park, W.S.; Kim, H.-J.; Akter, K.-M.; Bae, J.-Y.; Büyüker, M.S.; Kim, J.-H.; Kang, K.K.; Ahn, M.-J. Anti-Helicobacter pylori activity of six major compounds isolated from Rumex acetosa. ACS Omega 2023, 8, 42548–42554. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H.; Lv, W.; Xu, P.; Zhu, J.; Xie, J.; Liu, B.; Lou, Z. Antibacterial properties of anthraquinones extracted from rhubarb against Aeromonas hydrophila. Fish. Sci. 2011, 77, 375–384. [Google Scholar] [CrossRef]
- Siddaraju, M.N.; Dharmesh, S.M. Inhibition of Gastric H+, K+- ATPase and Helicobacter pylori growth by phenolic antioxidants of Curcuma amada. J. Agric. Food Chem. 2007, 55, 7377–7386. [Google Scholar] [CrossRef]
- Garro, M.F.; Ibáñez, A.G.S.; Vega, A.E.; Sosa, A.C.A.; Pelzer, L.; Saad, J.R.; Maria, A.O. Gastroprotective effects and antimicrobial activity of Lithraea molleoides and isolated compounds against Helicobacter pylori. J. Ethnopharmacol. 2015, 176, 469–474. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K.; Sharma, P. Antioxidant and antibacterial activities of sugarcane bagasse lignin and chemically modified lignins. Sugar Tech 2017, 19, 675–680. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.M.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.M.; Puglia, D. Valorization of acid isolated high yield lignin nanoparticles as innovative antioxidant/antimicrobial organic materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Barder, M.S.; McConnell, V.S.; DeCaux, B.S. Antimicrobial intermediates of the general phenylpropanoid and lignin specific pathways. Phytochemistry 2000, 54, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Abu-Qatouseh, L.F.; Ahmad, M.I.A.; Amorim, C.G.; Al-Adham, I.S.I.; Collier, P.J.; Montenegro, M.C.B.S.M. Insights into the molecular antimicrobial properties of ferulic acid against Helicobacter pylori. J. Appl. Microbiol. 2025, 2, 136. [Google Scholar] [CrossRef] [PubMed]
- Drews, J. Drug discovery: A historical perspective. Science 2000, 287, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Kaderabkova, N.; Mahmood, A.J.S.; Mavridou, D.A.I. Antibiotic susceptibility testing using minimum inhibitory concentration (MIC) assays. npj Antimicrob. Resist. 2024, 2, 37. [Google Scholar] [CrossRef]
- Svane, S.; Sigurdarson, J.J.; Finkenwirth, F.; Eitinger, T.; Karring, H. Inhibition of urease activity by different compounds provides insight into the modulation and association of bacterial nickel import and ureolysis. Sci. Rep. 2020, 10, 8503. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).