Hydraulic Traits Constrain Salinity-Dependent Niche Segregation in Mangroves
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site and Study Species
2.2. Leaf Water Potential and Stomatal Conductance
2.3. Hydraulic Vulnerability Curves
2.4. Specific Leaf Area and Wood Density
2.5. Statistical Analyses
3. Results
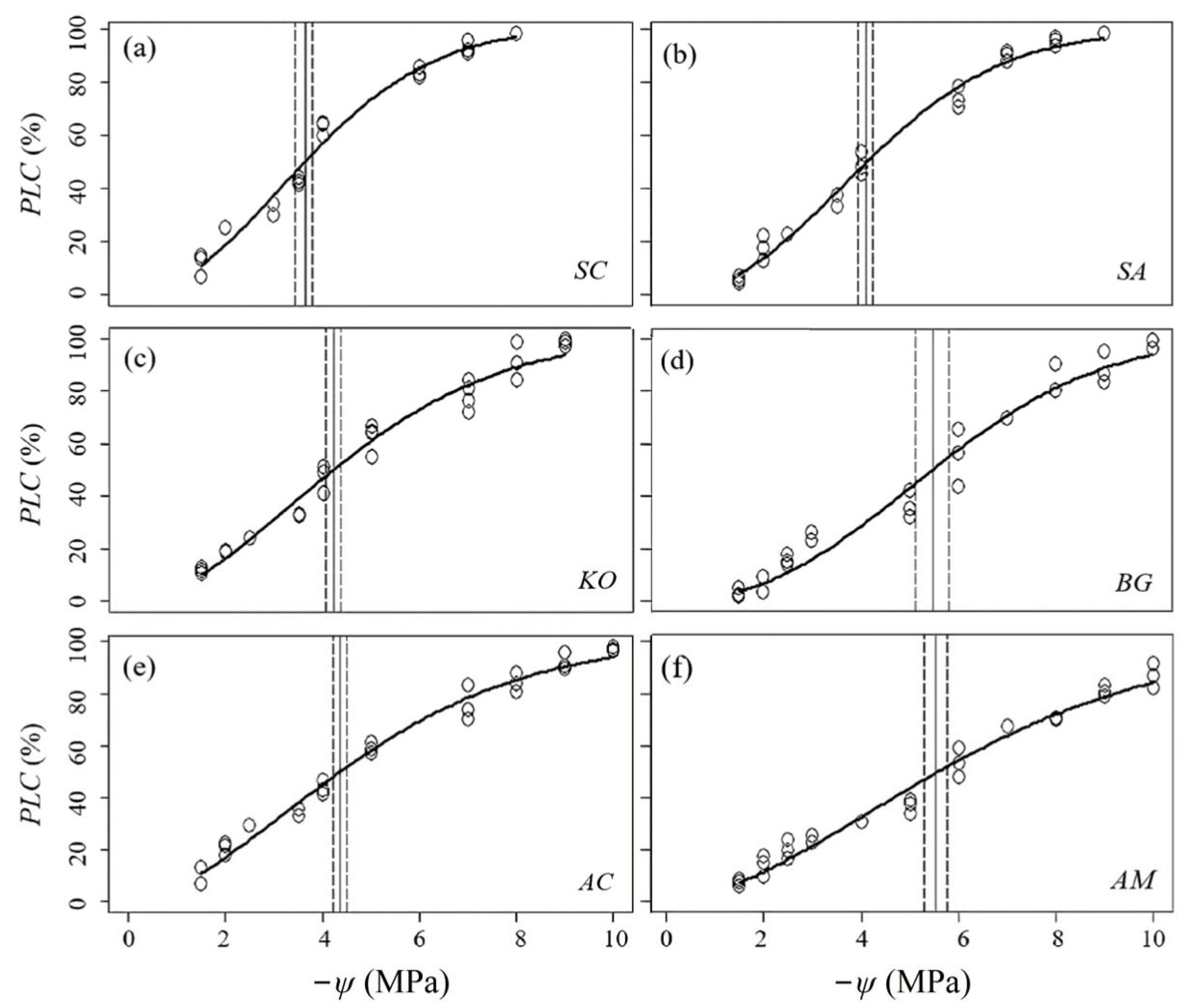
3.1. Hydraulic Vulnerability and Sensitivity
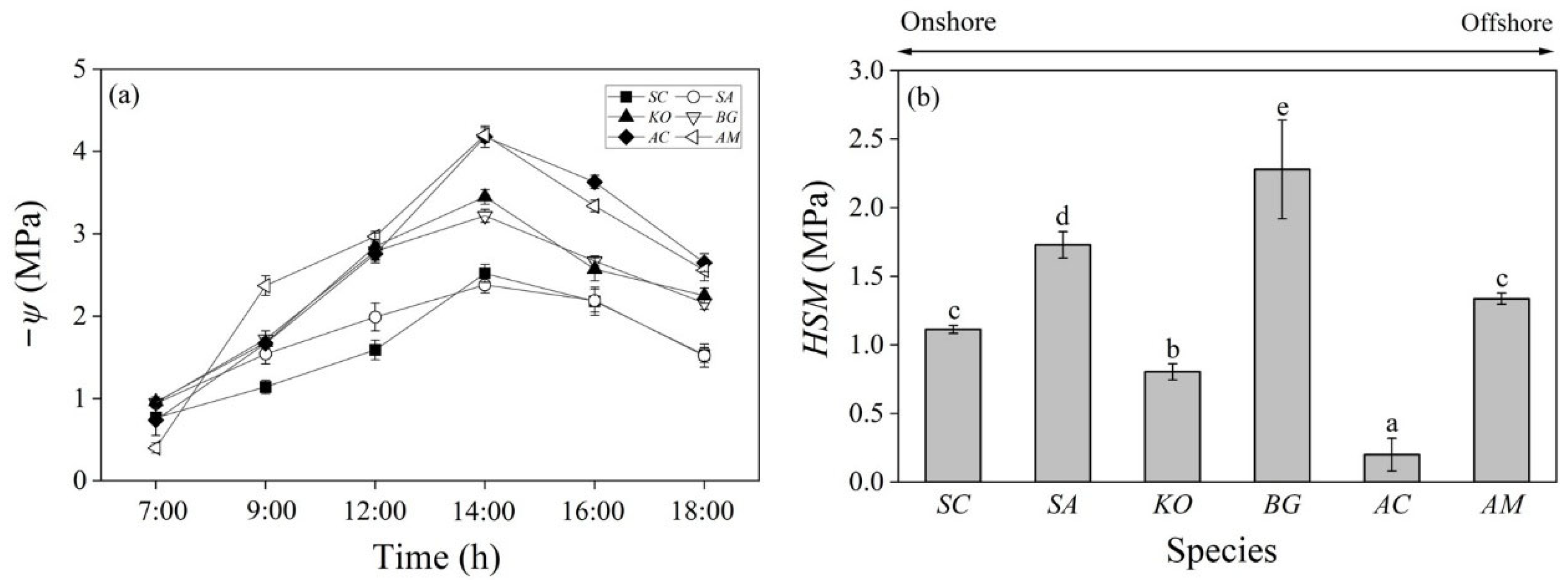
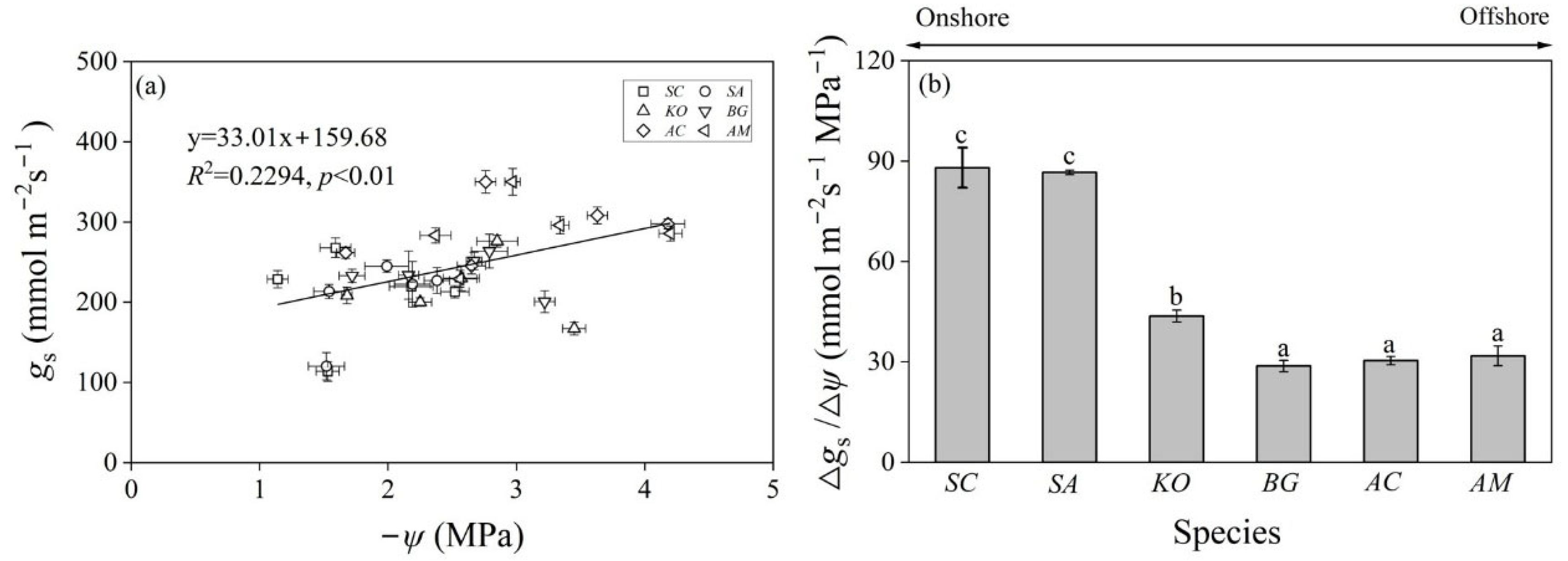
3.2. Stomatal Conductance and Water Potential
3.3. Hydraulic Safety Margin
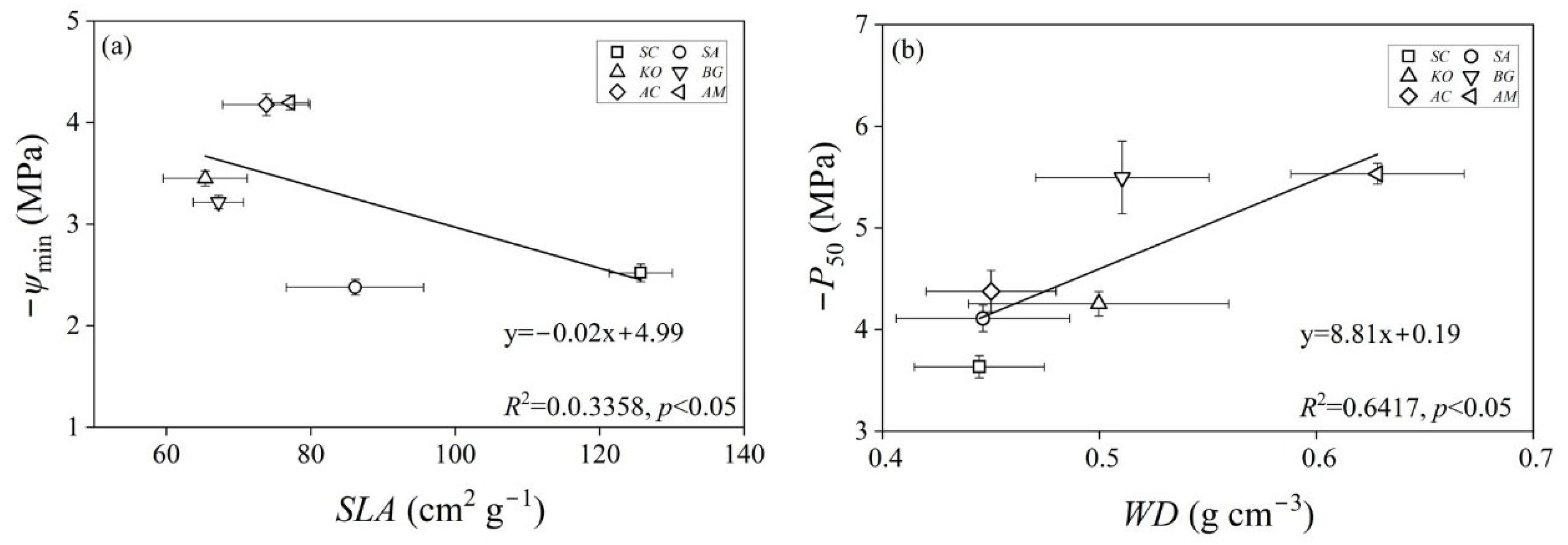
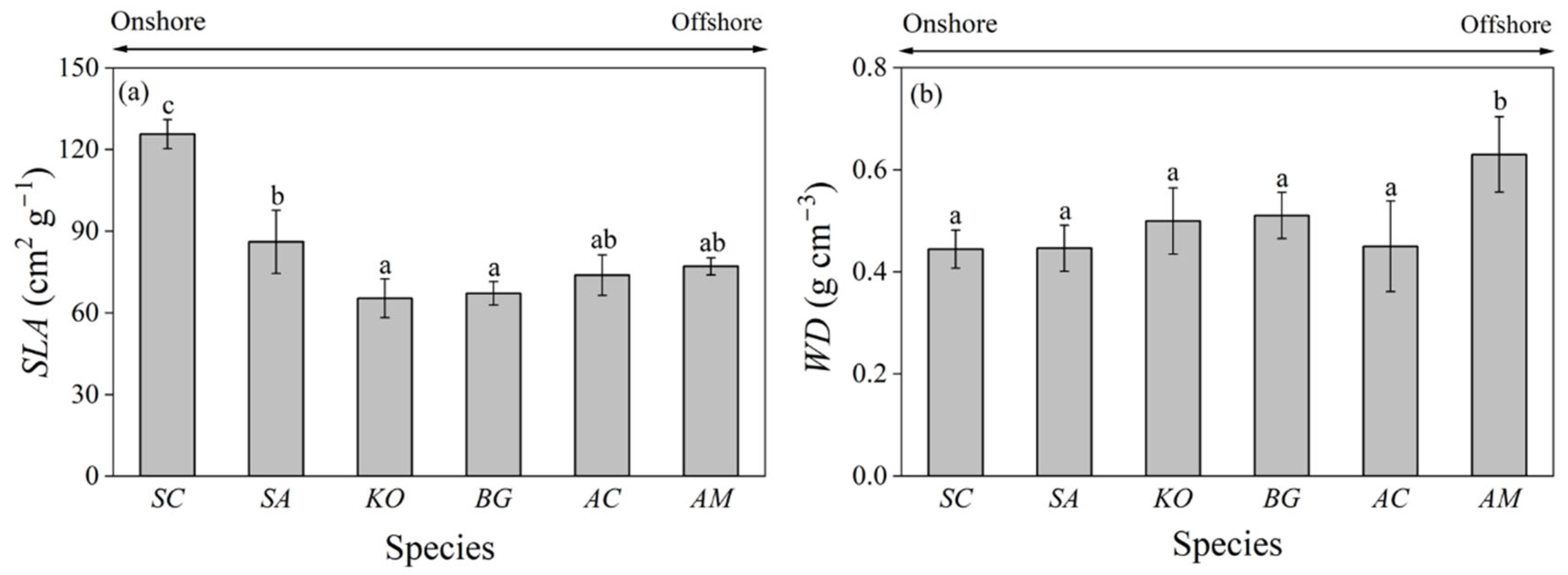
3.4. Specific Leaf Area and Wood Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Species/Time | 06:00–08:00 | 8:00–10:00 | 10:00–13:00 | 13:00–15:00 | 15:00–17:00 | 17:00–19:00 |
|---|---|---|---|---|---|---|
| SC | −0.77 ± 0.02 b | −1.14 ± 0.08 d | −1.59 ± 0.12 c | −2.52 ± 0.11 d | −2.18 ± 0.17 d | −1.53 ± 0.09 c |
| SA | −0.94 ± 0.05 a | −1.54 ± 0.12 c | −1.99 ± 0.17 b | −2.38 ± 0.10 d | −2.19 ± 0.14 d | −1.52 ± 0.14 c |
| KO | −0.96 ± 0.06 a | −1.68 ± 0.04 bc | −2.85 ± 0.16 a | −3.45 ± 0.09 b | −2.57 ± 0.14 c | −2.25 ± 0.09 b |
| BG | −0.95 ± 0.03 a | −1.72 ± 0.1 b | −2.79 ± 0.14 a | −3.22 ± 0.08 c | −2.67 ± 0.06 c | −2.16 ± 0.08 b |
| AC | −0.74 ± 0.04 b | −1.67 ± 0.07 bc | −2.76 ± 0.08 a | −4.18 ± 0.13 a | −3.63 ± 0.08 a | −2.65 ± 0.11 a |
| AM | −0.40 ± 0.06 c | −2.37 ± 0.12 a | −2.97 ± 0.06 a | −4.20 ± 0.09 a | −3.34 ± 0.07 b | −2.56 ± 0.13 a |
| Traits | SC | SA | KO | BG | AC | AM |
|---|---|---|---|---|---|---|
| SLA | 125.68 ± 5.37 c | 86.11 ± 11.65 b | 65.36 ± 7.11 a | 67.19 ± 4.27 a | 73.84 ± 7.42 ab | 77.12 ± 3.13 ab |
| WD | 0.44 ± 0.04 a | 0.45 ± 0.05 a | 0.50 ± 0.06 a | 0.51 ± 0.05 a | 0.45 ± 0.09 a | 0.63 ± 0.07 b |
| HSM | 1.11 ± 0.03 c | 1.73 ± 0.10 d | 0.8 ± 0.06 b | 2.28 ± 0.36 e | 0.20 ± 0.10 a | 1.34 ± 0.04 c |
References
- Wang, W.Q.; Yan, Z.Z.; You, S.Y.; Zhang, Y.H.; Chen, L.Z.; Lin, G.H. Mangroves: Obligate or facultative halophytes? A review. Trees-Struct. Funct. 2011, 25, 953–963. [Google Scholar] [CrossRef]
- Basha, S.K.C. An overview on global mangroves distribution. Indian J. Mar. Sci. 2018, 47, 766–772. [Google Scholar]
- Robert, E.M.R.; Koedam, N.; Beeckman, H.; Schmitz, N. A safe hydraulic architecture as wood anatomical explanation for the difference in distribution of the mangroves Avicennia and Rhizophora. Funct. Ecol. 2009, 23, 649–657. [Google Scholar] [CrossRef]
- Barik, J.; Mukhopadhyay, A.; Ghosh, T.; Mukhopadhyay, S.K.; Chowdhury, S.M.; Hazra, S. Mangrove species distribution and water salinity: An indicator species approach to Sundarban. J. Coastal Conserv. 2018, 22, 361–368. [Google Scholar] [CrossRef]
- Reef, R.; Lovelock, C.E. Regulation of water balance in mangroves. Ann. Bot. 2015, 115, 385–395. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Roddy, A.B.; Wason, J.W.; McElrone, A.J. Functional status of xylem through time. Annu. Rev. Plant Biol. 2019, 70, 407–433. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Jha, B. Salt tolerance mechanisms in mangroves: A review. Trees-Struct. Funct. 2010, 24, 199–217. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Cano-Delgado, A.I. The physiology of plant responses to drought. Sci. 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature. 2012, 491, 752–756. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Konings, A.G.; Trugman, A.T.; Yu, K.; Bowling, D.R.; Gabbitas, R.; Karp, D.S.; Pacala, S.; Sperry, J.S.; Sulman, B.N.; et al. Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature 2018, 561, 538–541. [Google Scholar] [CrossRef]
- Tan, F.-S.; Song, H.-Q.; Fu, P.-L.; Chen, Y.-J.; Siddiq, Z.; Cao, K.-F.; Zhu, S.-D. Hydraulic safety margins of co-occurring woody plants in a tropical karst forest experiencing frequent extreme droughts. Agric. For. Meteorol. 2020, 292, 108107. [Google Scholar] [CrossRef]
- Yan, C.-L.; Ni, M.-Y.; Cao, K.-F.; Zhu, S.-D. Leaf hydraulic safety margin and safety-efficiency trade-off across angiosperm woody species. Biol. Lett. 2020, 16, 20200456. [Google Scholar] [CrossRef] [PubMed]
- Delzon, S.; Cochard, H. Recent advances in tree hydraulics highlight the ecological significance of the hydraulic safety margin. New Phytol. 2014, 203, 355–358. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, X.; Jiang, J.; DeAngelis, D.L.; Fu, Z.; Zhang, J. Similarity of plant functional traits and aggregation pattern in a subtropical forest. Ecol. Evol. 2017, 7, 4086–4098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, M.; Zhang, H.; Ren, T.; Liu, C.; He, N. Divergent response and adaptation of specific leaf area to environmental change at different spatio-temporal scales jointly improve plant survival. Global Change Biol. 2022, 29, 1144–1159. [Google Scholar] [CrossRef]
- Uchiya, P.; Escaray, F.J.; Bilenca, D.; Pieckenstain, F.; Ruiz, O.A.; Menendez, A.B. Salt effects on functional traits in model and in economically important Lotus species. Plant Biol. 2016, 18, 703–709. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Greenwood, S.; Ruiz-Benito, P.; Martinez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bönisch, G.; et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef]
- Del Campo, J.T.F.; Olvera-Vargas, M.; Figueroa-Rangel, B.L.; Cuevas-Guzman, R.; Casanoves, F. Intraspecific functional variability and functional changes along environmental gradients associated to mangrove forest zonation in West-Central Mexico. Wetlands. 2022, 42, 115. [Google Scholar] [CrossRef]
- Virgulino-Junior, P.C.C.; Gardunho, D.C.L.; Silva, D.N.C.; Fernandes, M.E.B. Wood density in mangrove forests on the Brazilian Amazon coast. Trees-Struct. Funct. 2020, 34, 51–60. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Fu, X.; Meinzer, F.C. Metrics and proxies for stringency of regulation of plant water status (iso/anisohydry): A global data set reveals coordination and trade-offs among water transport traits. Tree Physiol. 2019, 39, 122–134. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Murray, M.; Soh, W.K.; Yiotis, C.; Batke, S.; Parnell, A.C.; Spicer, R.A.; Lawson, T.; Caballero, R.; Wright, I.J.; Purcell, C.; et al. Convergence in maximum stomatal conductance of C3 woody angiosperms in natural ecosystems across bioclimatic zones. Front. Plant Sci. 2019, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Dominguez, C.M.; Forner, A.; Martorell, S.; Choat, B.; Lopez, R.; Peters, J.M.R.; Pfautsch, S.; Mayr, S.; Carins-Murphy, M.R.; McAdam, S.A.M.; et al. Leaf water potential measurements using the pressure chamber: Synthetic testing of assumptions towards best practices for precision and accuracy. Plant Cell Environ. 2022, 45, 2037–2061. [Google Scholar] [CrossRef]
- Jiang, Z.-K.; Tuo, L.; Huang, D.-L.; Osterman, I.A.; Tyurin, A.P.; Liu, S.-W.; Lukyanov, D.A.; Sergiev, P.V.; Dontsova, O.A.; Korshun, V.A.; et al. Diversity novelty antimicrobial activity of endophytic actinobacteria from mangrove plants in Beilun Estuary National Nature Reserve of Guangxi China. Front. Microbiol. 2018, 9, 868. [Google Scholar] [CrossRef]
- Secchi, F.; Zwieniecki, M.A. Analysis of xylem sap from functional (nonembolized) and nonfunctional (embolized) vessels of Populus nigra: Chemistry of refilling. Plant Physiol. 2012, 160, 955–964. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Roark, L.C.; Pittermann, J. The physiological implications of primary xylem organization in two ferns. Plant Cell Environ. 2012, 35, 1898–1911. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Song, J.; Wang, M.; Li, N.; Niu, C.Y.; Hao, G.Y. Coordination of xylem hydraulics and stomatal regulation in keeping the integrity of xylem water transport in shoots of two compound-leaved tree species. Tree Physiol. 2015, 35, 1333–1342. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef] [PubMed]
- Duursma, R.; Choat, B. Fitplc—An R package to fit hydraulic vulnerability curves. Hydrol. 2017, 4, e-002. [Google Scholar] [CrossRef]
- Pammenter, N.W.; Vander Willigen, C. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol. 1998, 18, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Fuenzalida, T.I.; Brothers, N.; Mencuccini, M.; Sack, L.; Binks, O.; Ball, M.C. Shifting access to pools of shoot water sustains gas exchange and increases stem hydraulic safety during seasonal atmospheric drought. Plant Cell Environ. 2021, 44, 2898–2911. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Zheng, Y.; Takeuchi, W. Estimating mangrove forest gross primary production by quantifying environmental stressors in the coastal area. Sci. Rep. 2022, 12, 2238. [Google Scholar] [CrossRef]
- Wang, Y.S.; Gu, J.D. Ecological responses, adaptation and mechanisms of mangrove wetland ecosystem to global climate change and anthropogenic activities. Int. Biodeterior. Biodegrad. 2021, 162, 105248. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Peñuelas, J. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.M.; Chen, J.; Feng, L.; Li, F.B.; Yu, L.F. Study on the relationship between functional characteristics and environmental factors in karst plant communities. Ecol. Evol. 2022, 12, e9335. [Google Scholar] [CrossRef]
- Ma, J.G.; Wang, X.B.; Hou, F.J. A general pattern of plant traits and their relationships with environmental factors and microbial life-history strategies. Sci. Total Environ. 2024, 931, 172670. [Google Scholar] [CrossRef] [PubMed]
- Costa-Saura, J.M.; Martinez-Vilalta, J.; Trabucco, A.; Spano, D.; Mereu, S. Specific leaf area and hydraulic traits explain niche segregation along an aridity gradient in Mediterranean woody species. Perspect Plant Ecol. 2016, 21, 23–30. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Gazol, A.; Anderegg, W.R.L.; Martinez-Vilalta, J.; Mencuccini, M.; Julio Camarero, J. Wood density and hydraulic traits influence species’ growth response to drought across biomes. Global. Change Biol. 2022, 28, 3871–3882. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sarker, S.K.; Friess, D.A.; Kamruzzam, M.; Jacobs, M.; Islam, A.; Alam, A.; Suvo, M.J.; Sani, N.H.; Dey, T.; et al. Salinity reduces site quality and mangrove forest functions. From monitoring to understanding. Sci. Total Environ. 2022, 853, 158662. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Granda, E.; Camarero, J.J.; Vila-Cabrera, A.; Jump, A.S.; Sanchez-Salguero, R.; Sangüesa-Barreda, G.; Imbert, J.B.; Gazol, A.; Jucker, T. Impacts of recurrent dry and wet years alter long-term tree growth trajectories. J. Ecol. 2021, 109, 1561–1574. [Google Scholar] [CrossRef]
- Powell, T.L.; Wheeler, J.K.; de Oliveira, A.A.R.; Lola da Costa, A.C.; Saleska, S.R.; Meir, P.; Moorcroft, P.R. Differences in xylem and leaf hydraulic traits explain differences in drought tolerance among mature Amazon rainforest trees. Global Change Biol. 2017, 23, 4280–4293. [Google Scholar] [CrossRef]
- Kotowska, M.M.; Link, R.M.; Roell, A.; Hertel, D.; Hoelscher, D.; Waite, P.-A.; Moser, G.; Tjoa, A.; Leuschner, C.; Schuldt, B. Effects of wood hydraulic properties on water use and productivity of tropical rainforest trees. Front. For. Global. Change 2021, 3, 598759. [Google Scholar] [CrossRef]
- Jiang, C.P.; Pauly, D.; Wang, W.Q.; Du, J.G.; Cheng, J.H.; Wang, M. A preliminary model of the mangrove ecosystem of Dongzhaigang Bay, Hainan, (China) based on Ecopath and Ecospace. Front. Mar. Sci. 2023, 10, 1277226. [Google Scholar] [CrossRef]
- Jiang, D.A.; Nie, T.; He, Q.Y.; Yan, J.; Feng, E.H.; Ye, Q. A trade-off between leaf carbon economics and plant size among mangrove species in Dongzhaigang, China. Ecol. Evol. 2024, 14, e70559. [Google Scholar] [CrossRef]
- Wu, S.; Gu, X.; Zheng, Y.; Chen, L. Nocturnal sap flow as compensation for water deficits: An implicit water-saving strategy used by mangroves in stressful environments. Front. Plant Sci. 2023, 14, 1118970. [Google Scholar] [CrossRef]
- Santiago, L.S.; De Guzman, M.E.; Baraloto, C.; Vogenberg, J.E.; Brodie, M.; Herault, B.; Fortunel, C.; Bonal, D. Coordination and trade-offs among hydraulic safety, efficiency and drought avoidance traits in Amazonian rainforest canopy tree species. New Phytol. 2018, 218, 1015–1024. [Google Scholar] [CrossRef]
- Fuchs, S.; Leuschner, C.; Mathias Link, R.; Schuldt, B. Hydraulic variability of three temperate broadleaf tree species along a water availability gradient in central Europe. New Phytol. 2021, 231, 1387–1400. [Google Scholar] [CrossRef]
- Jiang, G.-F.; Brodribb, T.J.; Roddy, A.B.; Lei, J.-Y.; Si, H.-T.; Pahadi, P.; Zhang, Y.-J.; Cao, K.-F. Contrasting water use, stomatal regulation, embolism resistance, and drought responses of two co-occurring mangroves. Water. 2021, 13, 1945. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Bi, M.-H.; Nie, Z.-F.; Jiang, H.; Liu, X.-D.; Fang, X.-W.; Brodribb, T.J. Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Duursma, R.A.; Farrior, C.E.; Medlyn, B.E.; Feng, X. Optimal stomatal drought response shaped by competition for water and hydraulic risk can explain plant trait covariation. New Phytol. 2020, 225, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cushman, J.C.; Borland, A.M.; Liu, Q. Editorial: Systems biology and synthetic biology in relation to drought tolerance or avoidance in Plants. Front. Plant Sci. 2020, 11, 394. [Google Scholar] [CrossRef]
- Martinez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef]
- Zhu, S.D.; Chen, Y.J.; Ye, Q.; He, P.C.; Liu, H.; Li, R.H.; Fu, P.-L.; Jiang, G.-F.; Cao, K.-F. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol. 2018, 38, 658–663. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Marchin, R.M.; Abit, P.; Lau, O.L. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Global Change Biol. 2011, 17, 2731–2742. [Google Scholar] [CrossRef]
- Garcia-Forner, N.; Adams, H.D.; Sevanto, S.; Collins, A.D.; Dickman, L.T.; Hudson, P.J.; Zeppel, M.J.; Jenkins, M.W.; Powers, H.; Martínez-Vilalta, J.; et al. Responses of two semiarid conifer tree species to reduced precipitation and warming reveal new perspectives for stomatal regulation. Plant Cell Environ. 2016, 39, 38–49. [Google Scholar] [CrossRef]
- Skelton, R.P.; West, A.G.; Dawson, T.E. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc. Natl. Acad. Sci. USA 2015, 112, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
| Symbols | Descriptions | Units |
|---|---|---|
| K | xylem hydraulic conductivity | kg m−1 s−1 Mpa−1 |
| Kini | initial xylem hydraulic conductivity | kg m−1 s−1 Mpa−1 |
| Kmax | maximum xylem hydraulic conductivity | kg m−1 s−1 Mpa−1 |
| Px | xylem water potential corresponding to x% loss in hydraulic conductivity | Mpa |
| P12 | xylem water potential corresponding to 12% loss in hydraulic conductivity | Mpa |
| P50 | xylem water potential corresponding to 50% loss in hydraulic conductivity | Mpa |
| P88 | xylem water potential corresponding to 88% loss in hydraulic conductivity | Mpa |
| Sx | slope of the hydraulic vulnerability curve at Px | % Mpa−1 |
| S12 | slope of the hydraulic vulnerability curve at P12 | % Mpa−1 |
| S50 | slope of the hydraulic vulnerability curve at P50 | % Mpa−1 |
| S88 | slope of the hydraulic vulnerability curve at P88 | % Mpa−1 |
| ψ | leaf water potential | Mpa |
| ψmin | minimum leaf water potential | Mpa |
| △ψ | range from the minimum to the maximum leaf water potential | Mpa |
| gs | leaf stomatal conductance | mmol m−2 s−1 |
| △gs | range from the minimum to the maximum leaf stomatal conductance | mmol m−2 s−1 |
| HSM | hydraulic safety margin | Mpa |
| SLA | specific leaf area | cm2 g−1 |
| WD | wood density | g cm−3 |
| Species | P12 (Mpa) | P50 (Mpa) | P88 (Mpa) | S12 (% Mpa−1) | S50 (% Mpa−1) | S88 (% Mpa−1) |
|---|---|---|---|---|---|---|
| SC | −1.59 [−1.40, −1.77] a | −3.64 [−3.46, −3.80] a | −6.31 [−5.93, −6.59] a | 14.42 [13.90, 15.11] a | 19.34 [17.97, 21.74] a | 8.19 [7.31, 9.67] a |
| SA | −1.87 [−1.69, −1.9] ab | −4.10 [−3.94, −4.24] b | −6.90 [−6.58, −7.20] a | 12.95 [12.56, 13.40] b | 18.17 [16.89, 19.67] ab | 7.93 [7.04, 8.93] a |
| KO | −1.66 [−1.51, −1.83] a | −4.22 [−4.06, −4.39] b | −7.81 [−7.35, −8.29] b | 12.29 [11.85, 12.76] bc | 14.91 [13.67, 16.33] c | 5.91 [5.16, 6.85] b |
| BG | −2.65 [−2.27, −3.16] c | −5.47 [−5.09, −5.79] c | −8.83 [−8.33, −9.34] c | 9.90 [9.47, 10.51] d | 14.79 [13.09, 17.05] bc | 6.73 [5.66, 8.38] ab |
| AC | −1.62 [−1.47, −1.76] a | −4.37 [−4.20, −4.51] b | −8.49 [−8.07, −8.90] bc | 11.83 [11.45, 12.31] c | 13.36 [12.41, 14.40] c | 5.05 [4.50, 5.71] bc |
| AM | −2.07 [−1.86, −2.34] bc | −5.53 [−5.29, −5.79] c | −10.59 [−10.11, −11.19] d | 9.34 [8.99, 9.77] d | 10.79 [9.91, 11.61] d | 4.14 [3.64, 4.77] c |
| Species/Time | 06:00–08:00 | 8:00–10:00 | 10:00–13:00 | 13:00–15:00 | 15:00–17:00 |
|---|---|---|---|---|---|
| SC | 228.93 ± 10.75 bc | 268.10 ± 12.21 ab | 213.33 ± 7.63 bc | 219.67 ± 5.85 a | 113.93 ± 12.21 a |
| SA | 213.57 ± 8.90 ab | 245.13 ± 7.60 a | 227.23 ± 16.07 c | 222.70 ± 28.38 a | 120.47 ± 17.00 a |
| KO | 208.67 ± 10.35 a | 276.27 ± 7.35 b | 167.43 ± 8.14 a | 230.27 ± 15.43 ab | 200.40 ± 5.36 b |
| BG | 233.13 ± 8.20 c | 263.90 ± 21.11 ab | 200.87 ± 13.66 b | 251.73 ± 10.92 b | 233.93 ± 30.05 c |
| AC | 261.87 ± 6.89 d | 350.23 ± 13.98 c | 297.80 ± 6.15 d | 308.30 ± 10.42 c | 245.63 ± 10.23 c |
| AM | 283.47 ± 9.30 e | 350.37 ± 16.61 c | 285.87 ± 9.49 d | 296.13 ± 10.73 c | 229.33 ± 10.45 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Chen, Y.; Peng, Y.; Wei, M.; Niu, J. Hydraulic Traits Constrain Salinity-Dependent Niche Segregation in Mangroves. Plants 2025, 14, 1850. https://doi.org/10.3390/plants14121850
Cheng H, Chen Y, Peng Y, Wei M, Niu J. Hydraulic Traits Constrain Salinity-Dependent Niche Segregation in Mangroves. Plants. 2025; 14(12):1850. https://doi.org/10.3390/plants14121850
Chicago/Turabian StyleCheng, Haijing, Yinjie Chen, Yunhui Peng, Mi Wei, and Junfeng Niu. 2025. "Hydraulic Traits Constrain Salinity-Dependent Niche Segregation in Mangroves" Plants 14, no. 12: 1850. https://doi.org/10.3390/plants14121850
APA StyleCheng, H., Chen, Y., Peng, Y., Wei, M., & Niu, J. (2025). Hydraulic Traits Constrain Salinity-Dependent Niche Segregation in Mangroves. Plants, 14(12), 1850. https://doi.org/10.3390/plants14121850






