Abstract
[Background] Brasenia schreberi is a perennial floating leaf aquatic plant with high ecological protection value and potential for economic development, and thus, its endangered mechanisms are of great concern. The rapid endangerment of this species in modern times may be primarily attributed to the deterioration of water and soil environmental conditions, as its growth relies on high-quality water and soil. [Objective] Exploring the responses of B. schreberi to water and soil conditions from the perspective of functional traits is of great significance for understanding its endangered mechanisms and implementing effective conservation strategies. [Methods] This study was conducted in the Tengchong Beihai Wetland, which has the largest natural habitat of B. schreberi in China. By measuring the key functional traits of B. schreberi and detecting the water and soil parameters at the collecting sites, the responses of these functional traits to the water and soil conditions have been investigated. [Results] (1) The growth status of B. schreberi affects the expression of its functional traits. Compared with sporadic distribution, B. schreberi in continuous patches have significantly higher stomatal conductance, intercellular CO2 concentration, transpiration rate, and vein density, while these plants have significantly smaller leaf area and perimeter. (2) Good water quality directly promotes photosynthetic, morphological, and structural traits. However, high soil carbon, nitrogen, and phosphorus contents can inhibit the photosynthetic rate. The net photosynthetic rate is significantly positively correlated with dissolved oxygen content, pH value, ammonia nitrogen, and nitrate nitrogen contents in the water, as well as the magnesium, zinc, and silicon contents in the soil. In contrast, the net photosynthetic rate is significantly negatively correlated with the total phosphorus content in water and the total carbon, total nitrogen, and total phosphorus content in the soil. (3) Leaf area and perimeter show positive correlations with various water parameters, including the depth, temperature, pH value, dissolved oxygen content, ammonium nitrogen, and nitrate nitrogen content, yet they are negatively correlated with total phosphorus content, chemical oxygen demand, biological oxygen demand, and permanganate index of water. [Conclusions] This study supports the idea that B. schreberi thrives in oligotrophic water environments, while the notion that fertile soil is required for its growth still needs to be investigated more thoroughly.
1. Introduction
Brasenia schreberi, a perennial floating leaf plant in the family Nymphaeaceae, is a relic and rare species from the Tertiary flora, characterized by its submerged structures being covered with mucilage produced by epidermal glandular hairs [1]. The mucilage primarily consists of polysaccharides and is particularly abundant in the tender tissues of B. schreberi, such as buds and young stems [2,3]. B. schreberi exhibits a distribution primarily confined to subtropical regions below 40° N latitude and at elevations below 2500 m, classifying it as belonging to the group of thermophilic aquatic species [4,5]. As a pioneer species in freshwater ecosystems, B. schreberi often becomes the dominant species in water deeper than the growth limit of emergent aquatic plants, due to its strong asexual reproduction ability and the inhibitory effect of the mucilage on other plants [6,7]. This plant is also a traditional edible and medicinal aquatic plant in Asia, especially in China, with its edible parts mainly being the tender buds and stems that have a thick and transparent gelatinous substance, giving it a unique texture [3,8,9]. During the early 20th century, B. schreberi exhibited a broad distribution across unpolluted freshwater habitats, including ponds, marshes, lakes, and even agricultural wetlands, spanning Asia, North America, and Australia [1,10]. In recent decades, the suitable aquatic habitats for wild B. schreberi have significantly decreased, leading to a dramatic shrinkage in its distribution area and a marked decline in population size and numbers, affected by environmental pollution, overharvesting, and climate change, and as a result, it has been listed as a key protected wild plant species by many countries [1,11]. Understanding the mechanisms of the endangered status of B. schreberi is crucial for its scientific conservation and utilization, and conducting research on its adaptability to the growth environment is an important aspect in exploring these mechanisms.
Several studies have explained the reasons for the disappearance of natural B. schreberi populations by exploring the adaptability of its phenological, reproductive, morphological, and structural characteristics to the environment. For example, the extinction of B. schreberi in Europe during geological periods may have been primarily due to its meristematic tissues, such as winter buds and rhizome apices, being unable to withstand freezing temperatures and thus failing to produce winter buds for overwintering during glacial and interglacial periods [1,12]. The flowers of B. schreberi exhibit unisexual sequential blooming (dioecious), a mechanism to avoid self-pollination, but cross-pollination may be hindered due to the reduced number of plants and weakened individuals, which is another potential reason for its endangered status [13]. Anatomical analysis of B. schreberi reveals that its tender parts are covered by a fragile barrier of hydrophilic mucilage and a discontinuous cuticle, and it is hypothesized that this makes its epidermal glandular trichomes and the underlying parenchyma cells susceptible to high ionic strength and organic pollutants in contaminated water, damaging the plant’s barrier tissues and preventing normal growth and development, thus leading to its endangerment [10,14,15]. In summary, the findings indicate that over large temporal and spatial scales, climate changes (e.g., temperature) and reproductive strategies may significantly impact B. schreberi, while the rapid contemporary endangerment of B. schreberi is closely linked to water and soil conditions.
B. Schreberi is adapted to oligotrophic aquatic environments, requiring clean, flowing water for growth, habitat, and water environment protection are crucial for maintaining its populations [1,16,17]. B. schreberi thrives best in waters with near-neutral pH, low conductivity, and low turbidity [18,19]. Small amounts of fertilizer and pesticides that are discharged into the water in the field can cause the leaves of B. schreberi to rot within a few days [20]. Low levels of dissolved oxygen in water will have specific negative impacts on the growth of B. schreberi [21]. As residue and COD in water increase, B. schreberi shows more decayed leaves and black roots, changes from dark green to grayish green, and declines from vigorous to slender growth or even die off [22]. The permanganate index, total N content, and conductivity of water are all negatively correlated with the mucilage components, thickness, and single bud weight of B. schreberi [17]. Studies on the artificial propagation of B. schreberi have also indicated that, in addition to good water quality, B. schreberi requires soil rich in organic matter content for its growth. Brasenia schreberi grows best in slightly acidic paddy soils with high organic matter and total nitrogen, adequate nitrogen supply, deep soil, good surface soil structure, soft soil body, and a thick mud layer [22]. Applying chemical and organic fertilizers in soil can boost the growth of B. schreberi and enhance its yield and quality [23]. The mucilage content and individual bud weight of B. schreberi are significantly positively correlated with the organic matter and total nitrogen content of the sediment [17]. These studies provide some explanations for the ecological adaptation mechanisms of B. schreberi. However, they mainly focus on growth performance and mucilage characteristics to explore the environmental impacts on it, with limited research on other morphological, structural, and photosynthetic physiological parameters closely related to resource capture, growth rate, ecological strategies, and environmental responses. Intensifying research on these parameters under water and soil microenvironment conditions can more directly explain the growth status and endangerment mechanisms of B. schreberi.
China has the largest scale and longest history of cultivating and using B. schreberi. In China, it mainly occurs in provinces south of the Yangtze River, growing in nature reserves with good natural environments. Tengchong Beihai Wetland is one of the most important areas for wild B. schreberi in China. In recent years, local departments have implemented projects such as afforestation around wetlands, integrated protection and management, and biodiversity conservation and restoration. These initiatives have led to an increase in the wild B. schreberi population in Tengchong Beihai Wetland, expanding its distribution area from 0.2 ha to over 100 ha, which now exceeds the combined area of other provinces. This study examines wild B. schreberi in the Tengchong Beihai Wetland Provincial Nature Reserve, Yunnan, China, measuring its photosynthetic and morphological parameters to explore its physiological and ecological responses to water and soil conditions from a physiological ecology perspective. This study hypothesizes that Brasenia schreberi growth correlates positively with good water quality parameters (e.g., dissolved oxygen, pH) and negatively with water pollution indicators (e.g., total nitrogen, total phosphorus, BOD, COD). Additionally, based on the experience of artificial propagation of Brasenia schreberi, its growth also requires moderately fertile soil. However, this conclusion still needs to be verified by specific experiments and may lead to different conclusions due to the differences in soil nutrient levels of different wetlands. This study will investigate the responses of B. schreberi to water and soil from a physiological and ecological perspective, and explore its mechanisms of endangerment.
2. Results
2.1. Differences in Functional Traits of Brasenia schreberi Between Two Clustering Groups
The clustering results of B. schreberi’s functional traits are in line with its natural growth conditions. Cluster I sampling sites have sparsely distributed B. schreberi, while Cluster II sites have densely distributed plants (Figure 1), showing that the functional traits of B. schreberi are affected by its growth status. Compared to sparsely distributed B. schreberi, clustered-distribution B. schreberi has significantly higher stomatal conductance, intercellular CO2 concentration, transpiration rate, and vein density (p < 0.05); yet, its leaf area, perimeter, length to width ratio, air cavity area, and aerenchyma area are significantly smaller (p < 0.05; Figure 2). No significant differences were observed in other functional traits between groups. These results indicate that the different growth statuses of B. schreberi may influence its physiological and morphological traits as adaptive responses to environmental changes.
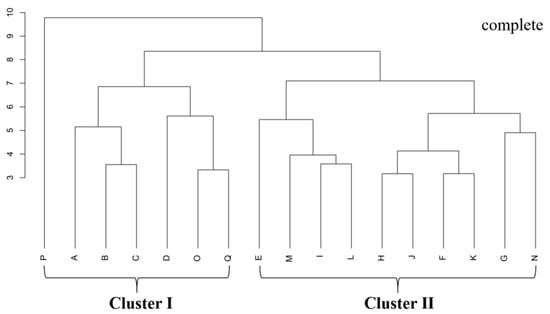
Figure 1.
Cluster diagram of Brasenia schreberi based on the functional traits. The vertical axis values represent the Euclidean distance. Letters A~Q are sampling points, Cluster I is a B. schreberi community with sporadic distribution, and Cluster II is a B. schreberi community with continuous distribution.
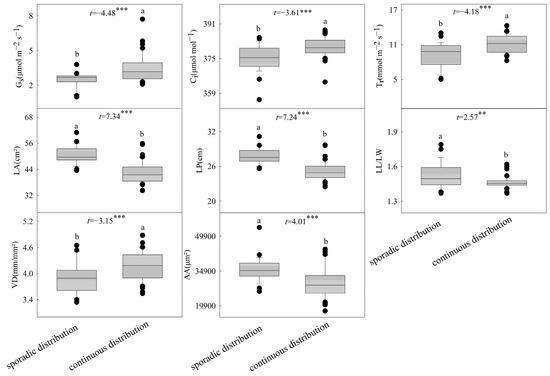
Figure 2.
Differences in functional traits between the two cluster groups in Brasenia schreberi. Pn, net photosynthetic rate; Gs, stomatal conductance; Ci, intercellular carbon dioxide concentration; Tr, transpiration rate; LA, leaf area; LP, leaf perimeter; L/W, ratio of leaf length to width; LP2/LA, ratio of square of leaf perimeter to area; SD, stomatal density; SL, stomatal length; SW, stomatal width; SA, stomatal area; VD, leaf vein density; BA, vascular bundle area; AS, air space area; AA, spiracle area; CT, cuticle thickness; ET, epidermal thickness. **, p < 0.01; ***, p < 0.001.
2.2. Ecological Response of Functional Traits of Brasenia schreberi to Water and Soil Environmental Factors
The water environment and soil nutrient parameters significantly influence the functional trait expression of B. schreberi. Random forest analysis revealed that most aquatic parameters (WH, WT, NH4+, NO3−, CODMn, COD, and Nwater) affected photosynthetic traits, while the primary soil factors influencing photosynthesis were Psoil, K, and Zn (Figure S2). Among them, the main parameters affecting Pn were three water environmental parameters (COD, WH, and CODMn), the main parameters affecting Gs were Psoil, WH, and Nwater, the main parameters affecting Ci were NO3−, K, and Zn, and the main parameters affecting Tr were WT, Psoil, and NH4+ (Figure S2).
The parameters affecting the size and shape of B. schreberi leaves were mainly DO, WT, WH, NH4+, Psoil, Nsoil, and Ca (Figure S2). Among them, LA was mainly affected by DO, WT, and WH; LP was mainly affected by DO, WT, and Ca; L/W was mainly affected by WH, DO, and Psoil; and LP2/LA was mainly affected by pH, Nsoil, and NH4+ (Figure S2).
The main water environment parameters affecting stomata were DO, NH4+, and pH, and the main soil nutrient parameters affecting stomata were Nsoil, Psoil, and Ca (Figure S2). Among them, the main parameters affecting SD were pH and Nsoil and Psoil; those affecting SL were DO, Psoil, and Ca; those affecting SW were NH4+, DO, and Psoil; and those affecting SA were NH4+, Nsoil, and Psoil (Figure S2).
The water environment factors affecting the anatomical structural traits of B. schreberi were mainly WT, DO, WH, pH, and Pwater, while the soil factors affecting these functional traits were mainly Psoil and trace elements, including Si, Zn, Mg, and K (Figure S2), suggesting that the structural trait parameters were more susceptible to the influence of soil trace elements. Among them, WT, DO, and Si were the main parameters affecting VD; WT, pH, and DO were the main parameters affecting BA; WH, K, and Zn were the main parameters affecting AS; WT, DO, and Si were the main parameters affecting AA; WT, Mg, and Psoil were the main parameters affecting CT; and Pwater, NH4+, and K were the main parameters affecting ET (Figure S2).
Overall, the water environmental parameters of water depth, water temperature, dissolved oxygen content, ammoniacal nitrogen, pH, Nsoil, and Psoil in the substrate parameters had a greater effect on the functional characteristics of B. schreberi, while the water pollution indicators of Nwater, COD, and CODMn mainly affected the photosynthetic parameters, and the soil trace elements mainly affected the anatomical and structural characteristics of B. schreberi (Figure S2).
Further bivariate correlation analysis between functional traits and environmental factors showed that B. schreberi Pn was significantly positively correlated with pH, DO, NH4+, and NO3− in water and Mg, Zn, and Si in soil, while it was significantly negatively correlated with Pwater in water and Csoil, Nsoil, and Psoil in soil (p < 0.05); Gs was significantly negatively correlated with Nwater (p < 0.05); Tr was significantly positively correlated with BOD, COD, and CODMn of water and K, Zn, and Si of soil, while it was significantly negatively correlated with WH, WT, Csoil, Nsoil, and Psoil of soil (p < 0.05); Ci was significantly negatively correlated with pH, DO, NH4+, and NO3− of water, while there were significant positive correlations (p < 0.05) between Ci and Pwater and COD of water (Table 1). In the relationship between photosynthetic parameters and environmental factors, the overall trend was consistent among Pn, Gs, and Tr, while their overall relationship with Ci was opposite (Table 1).

Table 1.
Correlations between leaf functional traits of Brasenia schreberi and environmental parameters.
Leaf size parameters, including leaf area and leaf perimeter, were significantly and positively correlated with WH, WT, pH, DO, NH4+, and NO3−, and significantly and negatively correlated with Pwater, BOD, COD, and CODMn (p < 0.05; Table 1). There was also a significant positive correlation between L/W and DO and a significant positive correlation between LP2/LA and NH4+ (p < 0.05; Table 1).
Among 190 potential relationships between 10 structural traits and 19 hydroedaphic parameters, only 18 showed statistically significant correlations (Table 1). Among the relationships of these functional traits with water and environmental parameters, significant trait environment correlations were identified: SL showed positive relationships with pH, NH4+, and NO3−; SA positively correlated with NO3−; VD negatively associated with WT, DO, and NO3−; AA positively linked to DO but negatively to Pwater; CT decreased with WT; ET negatively correlated with Pwater but positively with NH4+ (Table 1). Among these functional traits, only stomatal parameters and VD exhibited statistically significant correlations with soil parameters (Table 1); SD showed a significant positive correlation with Psoil, whereas stomatal size parameters, including SL, SW and SA, showed significant negative correlations with Psoil; and VD showed a significant positive correlation with soil Ca and a significant negative correlation with Mg and Si (Table 1).
Integrated analysis revealed that hydroedaphic conditions predominantly influenced photosynthetic traits in B. schreberi, while morphological parameters were mainly affected by aquatic factors. Anatomical traits showed limited significant correlations with either water or soil parameters (Table 1). Among the abiotic parameters, there was generally a negative correlation between Nwater, Pwater, BOD, COD, and CODMn, which indicate the degree of water pollution, and Pn, Gs, Tr, and leaf size, which indicate the photosynthetic productivity of B. schreberi, while there was a positive correlation between these traits and abiotic parameters such as DO, pH, NH4+, and NO3−, which indicate good water quality conditions (Table 1). Meanwhile, there were negative correlations between photosynthetic parameters in general and soil Csoil, Nsoil, Psoil, and Ca, while positive correlations were found with soil trace elements such as Mg, Zn, and Si (Table 1).
Principal component analysis of B. schreberi’s functional traits and water and soil factors showed that WH, WT, pH, DO, NH4+, NO3−, BOD, COD, and CODMn contributed more to the total variance, while Nwater contributed less to the total variance; soil parameters Csoil, Nsoil, Psoil, K, Ca, Mg, Zn, and Si all contributed more to the total variance; all functional traits were far from the origin and contributed more to the total variance (Figure 3).
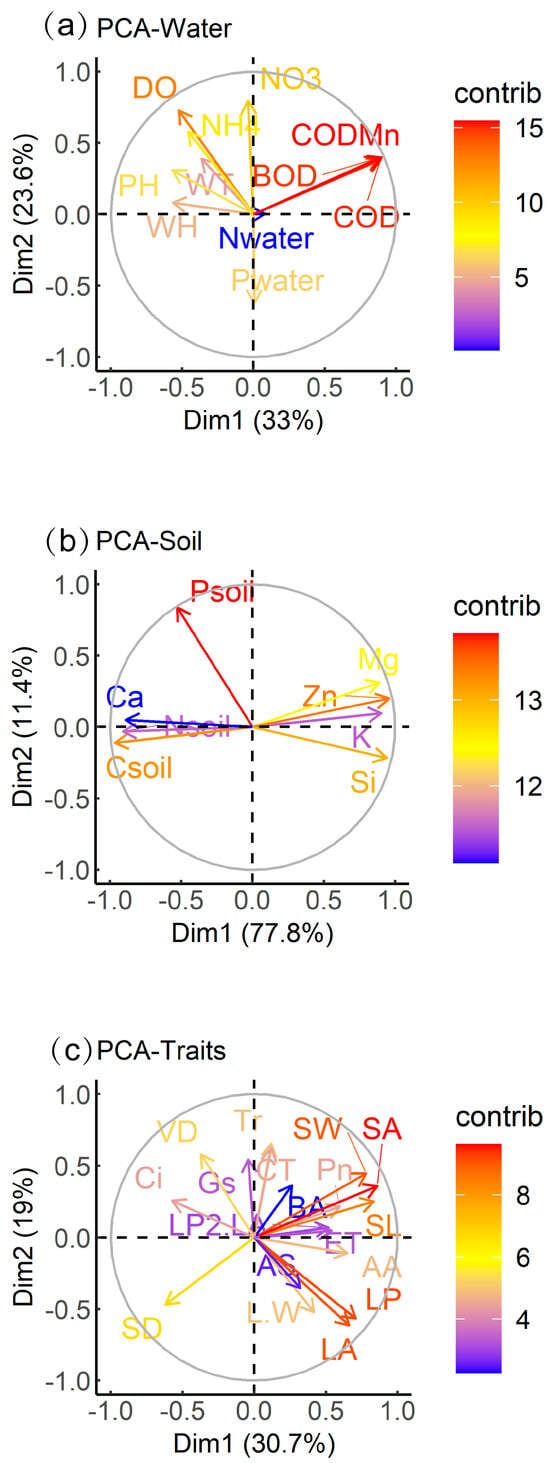
Figure 3.
Principal component analysis of plant traits, water factors, and sediment factors. Abbreviations of functional traits and environmental parameters in the figure are consistent with those in Table 1.
Based on the functional traits and environmental parameters selected by PCA, we further constructed a structural equation model to explore the causal relationship between the functional traits of B. schreberi and water and soil factors. Applied partial least squares path modeling revealed the different pathways influencing the functional traits of B. schreberi (Figure 4). Water and soil factors directly influence the photosynthetic, morphological, and structural traits of B. schreberi. Water quality parameters significantly promote its morphological, structural, and other traits (p < 0.05), while soil nutrient conditions somewhat inhibit its structural and photosynthetic traits (Figure 4). By integrating random forest models and “trait environment” bivariate correlations, it is suggested that parameters representing good water quality conditions (e.g., DO, WH, WT, pH, NH4+, NO3−) play a more prominent role in indicating water environment conditions. These water environment parameters, by influencing the morphology and structure of B. schreberi, promote its photosynthetic productivity and leaf size. Among soil parameters, soil nutrient content (Csoil, Nsoil, and Psoil) plays a more prominent role in the index of soil nutrient conditions. When these soil nutrient levels are too high, they can limit the structural traits of B. schreberi, which in turn can be detrimental to its photosynthetic productivity and leaf expansion.
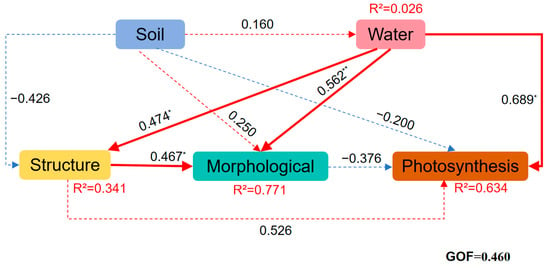
Figure 4.
Partial least squares path model (PLS-PM) of plant functional traits and environmental factors. Solid red and blue arrows indicate positive and negative relationships; the numbers on the arrows are normalized path coefficients. The model performance was assessed using a goodness-of-fit (GOF) index. *, p < 0.05; **, p < 0.01.
2.3. Correlations Between Functional Traits in Brasenia schreberi Leaves
During the adaptation of B. schreberi to water and soil conditions, there are functional linkages among different traits, manifested as significant correlations between traits (Figure 5).
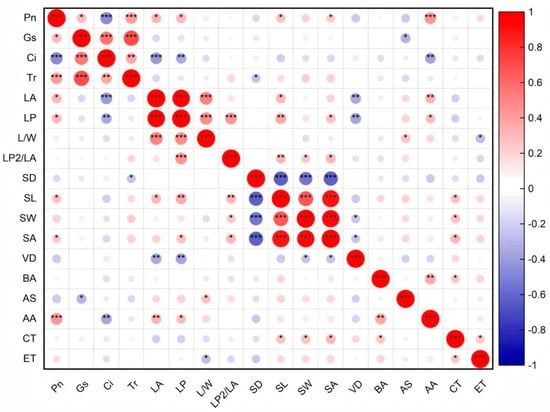
Figure 5.
Correlations among leaf functional traits of Brasenia schreberi. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Pn, net photosynthetic rate; Gs, stomatal conductance; Ci, intercellular carbon dioxide concentration; Tr, transpiration rate; LA, leaf area; LP, leaf perimeter; L/W, ratio of leaf length to width; LP2/LA, ratio of square of leaf perimeter to area; SD, stomatal density; SL, stomatal length; SW, stomatal width; SA, stomatal area; VD, leaf vein density; BA, vascular bundle area; AS, air space area; AA, spiracle area; CT, cuticle thickness; ET, epidermal thickness.
Overall, the structural traits of B. schreberi significantly influence its morphological traits, and together they affect its photosynthetic performance. (p < 0.05; Figure 4). Specifically, there were significant positive correlations (p < 0.05) between Pn and Gs, Tr, LA, LP, and stomatal size (Figure 6), indicating that there was a positive correlation between B. schreberi’s photosynthetic carbon assimilation rate and stomatal water and CO2 exchange capacity and leaf light capture capacity, and a negative correlation between stomatal sensitivity and stomatal and leaf vein transport efficiency.
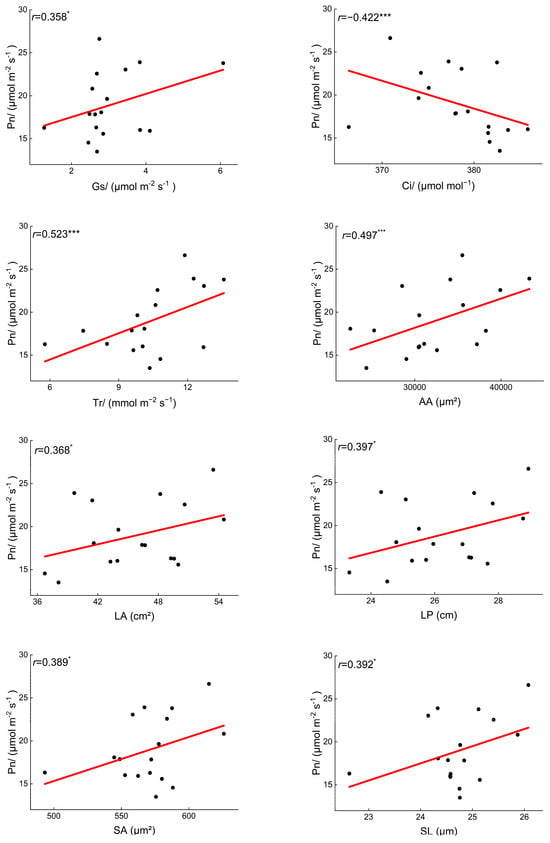
Figure 6.
Significant correlations between net photosynthetic rate and other leaf functional traits. Pn, net photosynthetic rate; Gs, stomatal conductance; Ci, intercellular carbon dioxide concentration; Tr, transpiration rate; LA, leaf area; LP, leaf perimeter; SL, stomatal length; SA, stomatal area. *, p < 0.05; ***, p < 0.001.
Leaf size parameters, including leaf area and perimeter, show a significant positive correlation with stomatal length and stomatal area, and a significant negative correlation with vein density. (p < 0.05; Figure 5). The vascular bundle area, stomatal area, and cuticle thickness are positively correlated (Figure 5), indicating a coordinated role in the transport capacity of vascular bundles, air transmission via stomata, and the barrier function of the cuticle.
3. Research Area and Methods
3.1. Study Area Overview
The research was conducted in Beihai Wetland Provincial Nature Reserve (25°06′42″–25°08′49″ N, 98°30′55″–98°35′02″ E), a protected volcanic wetland ecosystem in Tengchong, Yunnan Province, China (Figure 7). Beihai Wetland, a volcanic dam lake formed by andesite lava flows blocking the Daying River Valley during the Quaternary Period, is located at the southwest edge of the northern subtropics. Its rainfall is influenced by both the Pacific winter monsoon and the Indian Ocean summer monsoon. According to Tengchong Weather Station, the area has an average annual temperature of 14.7 °C, average annual precipitation of 1527.1 mm, evaporation of 1591.3 mm, relative humidity of 79%, drought index of 0.2–0.75, prevailing southwest winds, and wind speeds of 1.6–3.5 m/s.
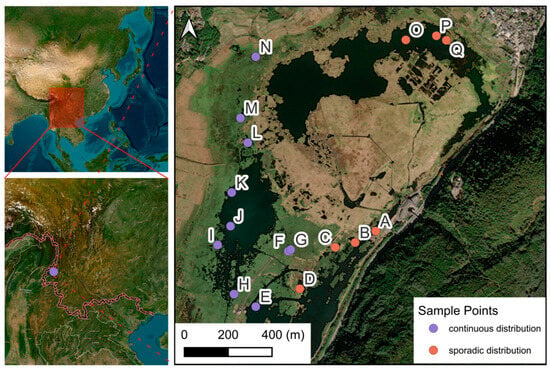
Figure 7.
Research sites of Brasenia schreberi in Tengchong Beihai Wetland. A–Q, sampling point number; red dots, sporadic distribution; purple dots, continuous distribution.
The long-term water level elevation of Beihai Wetland is 1730 m, and its water surface area is about 200 hm2. According to the background value research report of Beihai Wetland water quality from 2022 to 2024, Beihai Wetland has clear water and good water environment quality, generally belonging to Class II water, with some sites being Class III water. Its eutrophication level ranges from mild to moderate. The lake supports abundant submerged vegetation (Utricularia vulgaris, Hydrilla verticillata, Myriophyllum spicatum, Ottelia acuminata) and floating leaved plants (Brasenia schreberi, Trapa maximowiczii Korsdh), indicating superior water quality.
Currently, Beihai Wetland is mainly divided into two parts: the north and the south. The northern part, the original Beihai Wetland, has an average water depth of 3 m and a maximum depth of 7 m. Within this area, Brasenia schreberi is sparsely distributed in regions where the water depth is less than 3 m, while in the northeast, there are extensive areas of marshy floating-mat-type meadows. The southern part was originally paddy fields reclaimed by farmers along the lakeside and has fertile soil. It was restored to wetland by the local government through a “farmland to wetland” project in 2013. At present, the water depth in the southern part is 0.5–2 m, and there is a large-scale, continuous distribution of Brasenia schreberi, with a cover of 100%. The southern reclaimed wetland area is also the main distribution area of Brasenia schreberi in Beihai Wetland. Based on the comparison between the two parts, the water depth in the northern part is deeper, while the soil in the southern part is more fertile. The water environment does not differ significantly between the two parts.
3.2. Research Materials
In this study, the natural B. schreberi in Beihai Wetland in Tengchong, Western Yunnan, was used as the research object. According to the field survey, B. schreberi in Beihai Wetland mainly showed two growth states, one was B. schreberi growing in the northern part of Beihai Wetland, which was sporadically distributed (Figure 8A,C), and the other was B. schreberi growing in the southwestern part of Beihai Wetland and the southern part of the hiking trail, which was in a large continuous and overlapping distribution (Figure 8B). In this study, based on the growth condition of B. schreberi, 17 study sites were evenly selected in the distribution area of B. schreberi in Beihai Wetland, of which 7 study sites were sporadically distributed and 10 study sites had a large continuous and overlapping distribution (Figure 7). Since the propagation strategy of its underground stems, the selection of samples should try to keep each repeat at more than 5 m, after thorough investigation and consultation with the managers and manufacturers of artificially cultivated Brasenia schreberi. Therefore, this study selected five healthy and similarly growing plants at each study site, with a minimum of 5 m between each plant to prevent the interference of asexual reproduction in B. schreberi.
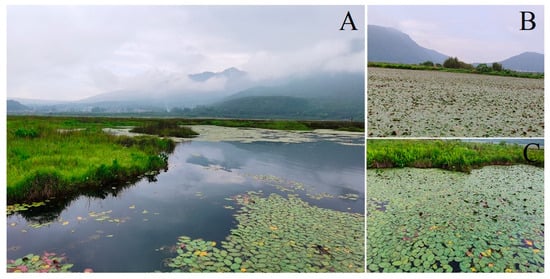
Figure 8.
The landscape and its natural communities of Brasenia schreberi in Tengchong Beihai wetland. (A) The landscape of Tengchong Beihai Wetland; (B) B. schreberi is densely distributed in the southern and southwestern parts of the Tengchong Beihai Wetland; (C) B. schreberi is sporadically distributed in the northern and eastern parts of the Tengchong Beihai Wetland.
3.3. Measurement of Plant Traits
Photosynthetic parameters of B. schreberi were measured during the peak growth period of B. schreberi from June to July 2022–2023, with daily measurements from 9:00 a.m. to 12:00 a.m., and the determination of photosynthetic parameters at all sampling sites was completed within one week. Net photosynthetic rate (Pn, μmol m−2 s−1), stomatal conductance (Gs, mol m−2 s−1), intercellular CO2 concentration (Ci, μmol mol−1), and transpiration rate (Tr, mmol m−2 s−1) were measured on healthy mature leaves using an LI-6800 photosynthetically active fluorescence meter (LI-6800, LICOR, Lincoln, NE, USA) under field conditions. Prior to measurements, a small CO2 bottle was installed, and the instrument was warmed up for 30 min. The CO2 concentration in the leaf chamber was adjusted to 400 μmol mol−1. The temperature of the leaf chamber (25–27 °C) and the relative humidity of the air (75–80%) were kept at a natural level. For measurement, B. schreberi were first induced with 1800 μmol m−2 s−1 light for 2 min to maintain maximum stomatal conductance, and then the light intensity was adjusted to 1500 μmol m−2 s−1, and the CO2 concentration in the leaf chamber was allowed to equilibrate. The photosynthetic parameters of each plant were measured three times, and the average value was taken as the photosynthetic value for one replicate. The average value of all replicates at each sampling site was used as the photosynthetic value for that site.
In the laboratory, after the determination of photosynthetic parameters, B. schreberi leaves were scanned in plan view with an 11000XL (Epson Expression) flatbed scanner in the presence of a ruler (Figure 9A) and then manually measured with ImageJ (https://imagej.net/contribute/citing) (accessed on 21 March 2024) [24] for leaf length (LL, μm), width (LW, μm), perimeter (LP, μm), and area (LA, μm2), and other morphological parameters were measured manually using ImageJ. Leaf shape index is an important index to measure the morphology of plant leaves and their degree of variability. In this study, the ratio of leaf length to width (L/W) and the ratio of leaf perimeter squared to leaf area (LP2/LA) were calculated as leaf shape index.
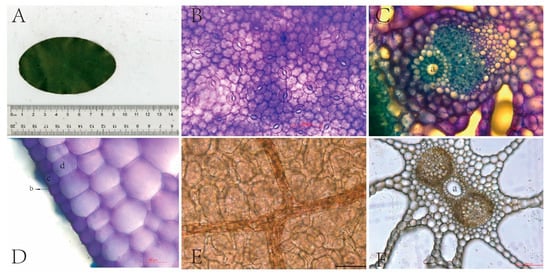
Figure 9.
Anatomical structure of Brasenia schreberi. (A) leaf morphology; (B) stomata; (C) vascular bundle structure; (D) epidermal structure; (E) vein; (F) air hole. a, air cavity; b, cuticle; c, epidermic cell; d, mesophyll cell.
After scanning the leaves, the centre of one side (approximately 8 mm × 6 mm) was cut for freehand transverse sectioning, and the remainder of the leaves were placed in centrifuge tubes filled with 50% alcohol. Sections were dehydrated, stained, and diluted to produce temporary water slides, which were placed under a Leica microscope (Leica Corp DM6B, Germany) to observe and photograph leaf vascular structures (400×), leaf cuticle and epidermal cells (400×), and leaf apertures (100×) (Figure 9 and Figure S1). Leaf vascular bundle area (BA, μm2), air aperture area (AA, μm2), air space area (AS, μm2), cuticle thickness (CT, μm), and epidermal thickness (ET, μm) were measured and counted using Image J software (https://imagej.net/contribute/citing).
The other half of the blades were removed from the centrifuge tube, and the appropriate size of the two blades was placed in a weighing bottle (40 × 25), with a mass fraction of 25% sodium hydroxide immersion. The solution was changed every 2 days, and the samples were subjected to continuous immersion for one week. After the colour of the sodium hydroxide solution had stopped changing, the sample was rinsed 5 times with distilled water and then soaked in distilled water for 30 min. The distilled water was then poured off and replaced with graded concentrations of alcohol (25%, 50%, 75%, 98%) for 30 min each time.
For the determination, the leaves were removed and placed on two microscope slides, one with the upper epidermis facing up to observe the stomata and the other with the lower epidermis facing up to observe the veins. The leaves were then stained with 1% toluidine blue solution, and after one minute the leaves and slides were lightly rinsed with 50% alcohol to remove excess toluidine blue solution, and then the excess solution around the slide was absorbed with absorbent paper and a temporary water slide was made and photographed under a light microscope. Stomata were observed and clear pictures of stomata were taken at 400× magnification (Figure 9 and Figure S1); leaf veins were observed and clear pictures of leaf veins were taken at 200× magnification (Figure 9 and Figure S1). Stomatal length (SL, μm), stomatal width (SW, μm), and stomatal area (SA, μm2) were later measured and recorded using Image J software, and the number of stomata was counted. Stomatal density (SD, no./mm2) was counted as the number of stomata per unit leaf area, and vein density (VD, mm/mm2) was counted as the total length of veins per unit area. Five plants were selected from each sampling site, and six values for each trait were counted for each plant to ensure that 30 values were counted for each trait at each study site.
3.4. Determination of Soil Elemental Content
After measuring the photosynthetic parameters of Brasenia schreberi, soil samples from each sampling site were collected immediately to ensure the consistency of sampling time. Soil samples of 1 kg were collected at each sampling point using a fixed-depth peat auger (Eikel Kampala 0423SA, The Netherlands). The samples were then returned to the laboratory for natural drying. After air drying, samples were ground using a soil grinder and sieved through a 100 mesh sieve, then sealed for storage. These soil samples were sent to a third-party testing organisation at the Xishuangbanna Tropical Botanical Garden of the Chinese Academy of Sciences to determine the mass fraction (ω, g kg−1) of eight elements, namely carbon (Csoil), nitrogen (Nsoil), phosphorus (Psoil), potassium (K), calcium (Ca), magnesium (Mg), zinc (Zn), and silicon (Si), which were counted as the corresponding elemental content.
3.5. Determination of Water Parameters
A multi-parameter water quality analyzer (YSI 650 MDS) was used to measure dissolved oxygen (DO, mg L−1), pH (pH, mol L−1), and water temperature (WT, °C) on site at each sampling point. Water depth (WH, m) was measured by vertically inserting a bamboo pole from the water surface to the substrate and using a tape measure to measure the length of the submerged part. The measurement of the above-mentioned water environment parameters was carried out simultaneously with the measurement of the photosynthetic parameters of Brasenia schreberi. Then, 500 mL water samples were collected from each sampling point and transported back to the laboratory. In the laboratory, 40 mL of water from each sampling site was filtered and then analysed using a continuous flow analyser (Germany SEAL Analytical AA3) to determine and calculate the volume fraction of total nitrogen (Nwater, mg L−1), total phosphorus (Pwater, mg L−1), ammoniacal nitrogen (NH4+, mg L−1), ammonium nitrogen (NO3−, mg L−1), and nitrate nitrogen (NO3−, mg L −1). The remainder of the water samples were sent to a third-party professional testing organisation for analysis of Biochemical Oxygen Demand (BOD, mg L−1), 5-day Chemical Oxygen Demand (COD, mg L−1), and Potassium Permanganate Index (CODMn, mg L−1).
3.6. Data Analysis
Data from this study were analysed using SPSS (v.27, https://spss.en.softonic.com), Canoco (5.0, https://www.canoco5.com) [25] and R (4.3.1, https://cran.r-project.org/src/) (accessed on 16 July 2024) [26] with built-in ‘vegan’ [27] and ‘plspm’ [28] packages. Based on the functional characteristics of Brasenia schreberi at 17 sampling points, cluster analysis was performed using the ”complete” method. The results of the cluster analysis were consistent with the distribution of B. schreberi, which was significantly clustered into two groups: Group I was the sporadic distribution of points, including 7 sampling points; and Group II was the large area of continuous distribution of points, including 10 research points. According to the clustering results, the differences in functional traits of the two B. schreberi clusters were compared using an independent samples t-test. Pearson correlation analysis was used to evaluate the relationships between B. schreberi functional traits and environmental parameters, as well as the relationships among different functional traits. A random forest model was utilized to analyze the key water and soil factors influencing functional traits. The randomForest [29] package was employed to rank the importance of each trait and environmental factor, with the increase in MSE serving as the criterion to identify the level of contribution. Based on the screening of principal component analysis (PCA), variables with small contributions to the total variance of principal components were removed. The remaining variables were used to construct a structural equation model (SEM) to evaluate the causal relationships between the functional traits of B. schreberi and water and soil factors. During the model construction, the functional traits of B. schreberi were classified into three categories: morphological, structural, and physiological functions. According to the functional connections of these three types of traits, the influence direction was set as structural parameters affecting morphological expression, and both of them jointly influencing physiological functions. Meanwhile, environmental parameters were divided into two major categories: water and soil parameters. All images in this study were drawn using Adobe Illustrator 2024 and Origin 2024.
4. Discussion
4.1. Differences Between Growth Stages in the Functional Characteristics of Brasenia schreberi
Stomatal conductance and transpiration rate are generally positively correlated with the photosynthetic rate, because they both reflect the water exchange capacity during photosynthetic carbon assimilation [30]. Compared to sporadic distribution, continuous B. schreberi has much higher stomatal conductance, intercellular CO2 concentration, transpiration rate, and leaf vein density (Figure 2), indicating stronger photosynthetic water and CO2 exchange abilities and thus a potentially higher photosynthetic rate. However, there was no significant difference in the net photosynthetic rate of B. schreberi between the two different coverage sites. The water and CO2 use efficiencies of plant leaves can be calculated, respectively, through the ratios of net photosynthetic rate to transpiration rate and stomatal conductance. Therefore, the above research results indicate that, compared with sporadically distributed B. schreberi (2.11 and 7.81, respectively), continuous distribution reduces the water and CO2 use efficiencies of B. schreberi (1.66 and 5.37, respectively). That is, achieving the same photosynthetic capacity consumes more water and CO2 at a higher coverage [31]. In addition, in sporadic B. schreberi, larger aerenchyma and air cavity areas (Figure 2) may enhance air delivery, reducing oxygen stress and channeling more photosynthetic products into growth, thus producing larger leaves [32,33]. A larger leaf area maximizes light harvesting, boosting photosynthesis, water use efficiency, and plant competitiveness [34]. A high coverage of floating leaf plants reduces light penetration and water re-oxygenation, lowers water body oxygen and CO2 fixation rates, and thus inhibits photosynthesis [31,35]. An increase in aerenchyma pore area not only enhances short-term air delivery to the lower parts of B. schreberi but also boosts CO2 uptake, ensuring normal photosynthesis.
4.2. Response of Functional Traits of Brasenia schreberi to Aqueous Environmental Factors
Water and soil, key sources of matter and energy for aquatic plant growth, are major environmental factors affecting plant functional traits [36]. The response of B. schreberi functional traits to water environment factors confirms our first hypothesis, which is that the growth of B. schreberi needs good water quality. Parameters like water pH, dissolved oxygen, and ammonia and nitrate nitrogen, which indicate good water quality, significantly promote the photosynthetic rate and leaf size of B. schreberi (Figure S2, Figure 3 and Figure 4, Table 1). For aquatic plants, within a certain pH range (typically between 6.5–7.5), increasing pH can enhance the activity of photosynthetic enzymes like Rubisco, enabling more efficient substrate (e.g., CO2) binding and promoting photosynthesis [37]. In addition, within the optimal pH range, aquatic plants have high ion channel permeability in cell membranes, properly functioning carrier proteins, smooth CO2 entry into chloroplasts, and efficient O2 release, all of which boost photosynthesis and promote larger leaf formation [38,39]. Dissolved oxygen in water significantly boosts the photosynthetic rate and leaf size of B. schreberi (Figure S2, Figure 3 and Figure 4, Table 1). When water dissolved oxygen is high, plant roots can respire aerobically, producing adequate ATP that is transported to photosynthetic organs like leaves, ensuring efficient photosynthesis [40]. For floating leaf plants in a high dissolved oxygen environment, the stomata on the plant leaves are more likely to stay open, which not only favors CO2 uptake but also lessens the feedback inhibition of photosynthesis caused by oxygen accumulation. Additionally, aquatic plants possess a well-developed system of channels, such as aerenchyma and vascular bundles. In high dissolved oxygen water, plant cells proliferate and enlarge, forming bigger structures like aerenchyma (e.g., the aerenchyma size of B. schreberi is positively correlated with water dissolved oxygen) to ensure internal oxygen supply, and larger vascular bundles (with lower vein density) to boost water and nutrient transport [41]. The expansion of these channel structures allows the smooth transport of photosynthetic products to all parts of the aquatic plants, promoting their growth and reproduction.
Nitrogen is a key component of many important biological macromolecules and compounds in plants, such as proteins, nucleic acids, and chlorophyll. Ammonium nitrogen (NH4+) and nitrate nitrogen (NO3−) are the two major inorganic nitrogen sources absorbable and utilizable by plants, providing the nitrogen atoms needed for the synthesis of the above-mentioned biomacromolecules [42]. When levels of these two nitrogen forms in water are sufficient, aquatic plants show a marked rise in leaf chlorophyll. This helps plants capture more light energy for the photosynthetic light reaction, supplying ample energy and reducing power. Meanwhile, leaves synthesize more photosynthetic enzymes like Rubisco, accelerating CO2 fixation and reducing the Calvin cycle, thus promoting carbon assimilation and boosting the photosynthetic rate [43]. However, excessive NH4+ concentrations can also be phytotoxic [44], so it is hypothesized that the NH4+ concentrations in Tengchong Beihai Wetland have not yet reached levels that would harm B. schreberi. Although nitrate and ammonium nitrogen promote photosynthetic rate and leaf size in B. schreberi, excessively high water total nitrogen and phosphorus levels may lead to opposing results [43,45]. This study also revealed that high total phosphorus levels in water significantly restrict the net photosynthetic rate and leaf size of B. schreberi, and there is a significant negative correlation between total nitrogen levels and stomatal conductance (Figure S2, Table 1). Excess nitrogen and phosphorus in water can inhibit a plant’s absorption of other nutrients, causing nutritional imbalance, which in turn weakens the photosynthetic ability and growth potential of the plant. For example, excess phosphorus can lead to excessive accumulation of phosphates in plants, which interferes with zinc uptake [46,47]. B. schreberi requires large amounts of zinc to form zinc-rich tissues during growth. Zinc is the main nutrient in its young parts, so restricted zinc absorption may hinder their development. Excessive total nitrogen and phosphorus can also cause eutrophication, leading to algae blooms that consume dissolved oxygen and create hypoxic conditions. This triggers oxidative stress in aquatic plants, generating reactive oxygen species that damage cellular structures and functions, thus inhibiting photosynthesis [48].
High water BOD, COD, and permanganate index also significantly hinder the photosynthetic productivity and leaf growth of B. schreberi (Figure S2, Table 1). High water BOD, COD, and permanganate index usually indicate a greater amount of organic pollutants in water. The decomposition of these pollutants consumes a lot of dissolved oxygen, causing a shortage, and releases toxic substances like nitrite, which inhibits photosynthesis and reduces leaf growth and development [45]. High levels of these water parameters can also lead to changes in the physiological metabolism and resource allocation of aquatic plants [49]. Plants may consume significant energy and resources to adjust their antioxidant and defense systems against pollutant toxicity, or reallocate more resources to roots and underground stems, reducing leaf resource investment and affecting their growth and development [49]. The significant positive correlation between the transpiration rate of B. schreberi and these parameters (Table 1) may be a response to the restriction of plant photosynthesis in more polluted situations. Plants increase transpiration to improve the supply of water for photosynthesis, thereby maintaining a stable net photosynthetic rate and alleviating the pressure on photosynthetic product requirements for growth and reproduction.
In addition to the impacts of the aforementioned water quality parameters on the functional traits of B. schreberi, this study also found that water temperature and depth are significantly negatively correlated with transpiration rate and significantly positively correlated with leaf size (Figure S2, Table 1). Within the suitable temperature range, increased water temperature enhances aquatic plant enzyme activity, benefits photosynthesis, and enables more efficient light energy utilization for organic matter synthesis, thus offering more materials for leaf growth and promoting leaf enlargement [50]. Meanwhile, the increased enzyme activity helps with plant cell division, elongation, and differentiation, and also promotes the growth and enlargement of leaves [50]. However, while rising water temperatures enhance photosynthesis, they can also cause physiological water stress in plants due to excessive water loss from transpiration [51]. In response to physiological water deficit, plants close their stomata to reduce transpiration water loss, which is consistent with previous findings on seagrasses that water temperature affects the balance between photosynthesis and respiration in plants by influencing the photosynthesis irradiance relationship [52]. The impact of water depth on B. schreberi may mainly relate to its growth status and interspecific as well as intraspecific competition. In deep waters (>1.5 m) of Tengchong Beihai Wetland, B. schreberi is sporadically distributed, with no emergent plants and few submerged plants. Its leaves have enough space to expand, ensuring sufficient water vapour exchange and a photosynthetic rate that meets growth needs. In shallow areas (<1.5 m) of Tengchong Beihai Wetland, B. schreberi mainly grows in dense patches competing with submerged plants like bladderwort, horned pondweed, and black algae, leading to intense interspecific and intraspecific competition. In these shallow water areas, B. schreberi leaves overlap, so the lower layer leaves cannot obtain enough sunlight, and the plant’s transpiration is also stronger. (Figure 8). To maintain a stable photosynthetic rate, plants increase stomatal conductance and transpiration, but this simultaneous rise reduces water- and CO2-use efficiency.
4.3. Response of Functional Traits of Brasenia schreberi to Soil Nutrient Conditions
Floating leaf plants primarily acquire nutrients from sediments through their root systems [53], making sediment nutrient conditions crucial in determining plant growth strategies [54,55]. Artificial cultivation of B. schreberi shows that its growth requires nutrient-rich soil, especially with abundant organic matter, nitrogen, and phosphorus [17]. However, this study shows that sediment organic carbon, nitrogen, and phosphorus levels are negatively correlated with the net photosynthetic and transpiration rates of B. schreberi (Figure 5, Table 1), indicating that the photosynthesis of B. schreberi is sensitive to soil nutrient conditions, which inhibit photosynthesis. This finding is a key discovery of this study, although it is in conflict with the second research hypothesis.
Soil is the primary nutrient source for most rooted plants, and its nutrient conditions are a key factor limiting normal plant growth. In most cases, especially in terrestrial habitats, soil nutrients tend to be deficient, and increasing soil nutrients can significantly enhance plant photosynthesis and growth [56,57]. However, wetlands, as nutrient sinks, are frequently subject to eutrophication due to the influx of nutrients like nitrogen and phosphorus. Moderately adding nitrogen and phosphorus to soil can promote chlorophyll synthesis in aquatic plant leaves and increase stomatal opening, thereby raising photosynthetic and transpiration rates [58]. However, excessive soil nitrogen and phosphorus not only cause nitrogen waste and pollution but also negatively affect plant nutrition, stomatal conductance, and photosynthetic efficiency, ultimately reducing the net photosynthetic rate [59]. The south, north, and west of Tengchong Beihai Wetland are farmland and villages, and the east is a major tourist spot. With constant nutrient input from these sources, the soil nutrient levels in the wetland are already high. The main distribution location of B. schreberi in Tengchong Beihai Wetland is in the wetland restoration area in the south of the wetland. Long-term farming has made the soil in these areas fertile, with high carbon (135.05 g kg−1), nitrogen (9.17 g kg−1), and phosphorus (1.29 g kg−1) content. These levels are significantly higher than in other B. schreberi production areas, such as the soil in Hunan Chaling (total nitrogen: 5.05 g kg−1, total phosphorus: 0.82 g kg−1, organic matter: 205.00 g kg−1) [60]. Consequently, it can be inferred that the existing soil nutrient levels in Tengchong Beihai Wetland are sufficiently high to sustain the robust growth of B. schreberi. Any additional increase in soil nutrients would surpass the optimal tolerance threshold of this species, thereby resulting in suboptimal growth.
Although most morphological parameters are not significantly related to sediment element content, soil total phosphorus significantly impacts stomatal density and size. Stomatal density shows a significant positive correlation with soil total phosphorus, while stomatal size (length, width, area) exhibits a significant negative correlation (Figure S2, Figure 3 and Figure 4, Table 1). This indicates that stomatal morphological parameters are sensitive to soil phosphorus content, implying that soil phosphorus affects stomatal function. Stomata are the main pathways for water and CO2 exchange in plants and the primary route for water loss [61,62]. Under conditions of high soil fertility, the increase in stomatal density may enhance water vapor exchange, thereby compensating for the decrease in photosynthetic and transpiration rates and maintaining the stability of leaf photosynthesis. A high phosphorus environment may promote epidermal cell proliferation via the cytokinin signaling pathway (e.g., ARR12—mediated cell proliferation), raising stomatal density per unit leaf area [47]. There exists a certain trade-off relationship between stomatal size and density. As stomatal density escalates, stomatal size tends to diminish correspondingly in order to preserve normal stomatal function and maintain leaf structural integrity [63]. Overly large stomata may compromise the stability of the leaf internal architecture, thereby disrupting the normal physiological processes [63]. Smaller stomata are more efficient at reducing water loss when closed, thus enhancing plant water-use efficiency [64]. In the context of elevated soil phosphorus levels, plants may preferentially augment stomatal density to boost photosynthetic efficiency while concurrently reducing stomatal size to optimize water use efficiency, thereby attaining a balance between growth and water management [64].
Other soil nutrients, such as meso- and micro-elements, are also crucial for plant growth, as they can influence photosynthesis by modulating one or more steps in plant physiological processes [65]. For instance, potassium can boost root growth, helping roots absorb more water and nutrients from deeper soil layers and promoting the plant’s vertical growth [66]. Calcium, a vital cell wall component, binds with pectic substances to strengthen cell wall mechanical strength and promote cell splitting and expansion [66]. Magnesium, a component of chlorophyll and a cofactor for many enzymes, directly engages in respiration and photosynthesis. It also regulates the absorption and utilization of potassium, calcium, nitrogen, etc., thus promoting plant growth [67]. Zinc, as a cofactor of carbonic anhydrase, catalyzes the hydration of CO2 to form HCO3−, indirectly boosting CO2 availability around Rubisco and enhancing photosynthesis. Silicon deposited in plant cell walls as silicified walls enhances leaf mechanical strength, adjusts stomatal opening and closing sensitivity, and thus boosts transpiration rate. Furthermore, silicon can form silica polymers within leaf cells that alleviate damage to photosynthetic structures caused by environmental stress, thereby maintaining normal photosynthetic function [68]. In this study, the net photosynthetic and transpiration rates of B. schreberi generally showed a positive correlation with soil levels of potassium, magnesium, zinc, and silicon, and a negative correlation with soil calcium levels (Figure S2, Table 1). This indicates that potassium, magnesium, zinc, and silicon enhance photosynthetic productivity either by directly participating in the photosynthetic processes of B. schreberi or by promoting chlorophyll synthesis. Furthermore, more calcium helps B. schreberi enhance its mechanical strength. This is a plant adaptation to adversity, implying that under unfavorable conditions, photosynthesis weakens, and more photosynthetic products are consumed.
Unlike photosynthetic parameters, vein density shows a significantly positive correlation with soil calcium and negative correlations with magnesium and silicon content (Figure S2, Table 1). Veins are key transport channels for water, nutrients, and photosynthetic products in plants and play a critical role in ensuring the stability of photosynthesis [69]. In addition to providing structural support for leaves and keeping them spread out, veins require carbohydrates and proteins for their development. This may create competition with chlorophyll synthesis, so when magnesium levels rise, vein density can decrease [69]. Silicon from sediments precipitates in plant mechanical tissues as silicified cell walls, which may restrict vein expansion and reduce vein density [70]. Conversely, calcium may enhance cell wall mechanical strength, promoting cell division and expansion, and thus increase vein construction and stability. The response of veins to soil nutrients (Figure 10) also indicates the degree of adversity that B. schreberi faces.
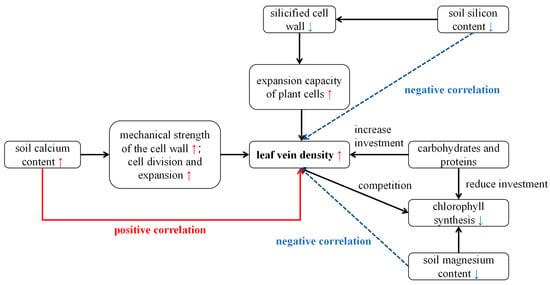
Figure 10.
The relationship between vein density and soil nutrients. Red arrows indicate positive correlations, while blue arrows indicate negative correlations.
4.4. Functional Linkages Among Brasenia schreberi Traits
During responses to water and soil environmental changes, B. schreberi functional traits exhibit certain functional linkages. Many studies have indicated a positive correlation between net photosynthetic rate, stomatal conductance, and transpiration rate. This study also found the same relationships in B. schreberi (Figure 5 and Figure 6), reflecting the dependence of photosynthesis on stomatal water and CO2 conductance. Stomata reflect the trade-off between plant H2O protection and CO2 capture [71]. High stomatal conductance means strong water conduction, higher transpiration rates, and ample CO2 supply for photosynthesis, thus supporting higher photosynthetic rates. As photosynthesis utilizes more CO2, the intercellular CO2 concentration decreases. Additionally, net photosynthetic rate correlates positively with leaf size, stomatal size, and pore size (Figure 5 and Figure 6), aligning with previous studies [35]. Larger leaves often mean a bigger photosynthetic area, allowing for more light absorption and higher photosynthetic efficiency. Stomata, which facilitate gas exchange by letting CO2 in and releasing oxygen and water vapor, directly impact intercellular CO2 concentration and, consequently, photosynthesis [72]. Larger stomata can supply more CO2, enhancing photosynthesis. However, they often have lower opening/closing sensitivity [72,73]. This means that under stress, plants may suffer greater photosynthetic damage, partly explaining B. schreberi’s endangered status. Aquatic plants can fill tissues with air via aerenchyma pores to increase buoyancy. This helps them float and reduces hypoxia stress. Larger aerenchyma pores enhance breathing and photosynthetic activity [32,74]. The three traits—leaf size, stomatal size, and aerenchyma pore size—in B. schreberi, due to their similarly positive effects on photosynthetic function, are significantly functionally coordinated.
5. Conclusions
The functional traits of B. schreberi are closely related to its growth patterns. Compared to sporadically distributed individuals, plants in continuous patches exhibit higher stomatal conductance, transpiration rate, and vein density, but significantly smaller leaf size, reduced aerenchyma area, and diminished aerial pore area. This indicates that when B. schreberi grows too densely, its water and CO2 utilization efficiency, leaf size, and gas transport ability decrease, while transport efficiency increases. The photosynthetic productivity and leaf size of B. schreberi are positively correlated with water temperature, dissolved oxygen, nitrate, and ammonium nitrogen in water, and negatively correlated with total nitrogen, total phosphorus, BOD, COD, and permanganate index. This indicates that B. schreberi requires good water quality for growth. The photosynthetic productivity of B. schreberi shows a significantly negative correlation with soil organic carbon, nitrogen, and phosphorus, but a significantly positive correlation with soil magnesium, zinc, and silicon. This indicates that, while B. schreberi requires certain micronutrients for optimal growth, its demand for major soil nutrients like carbon, nitrogen, and phosphorus is somewhat limited. The soil in Tengchong Beihai Wetland is fertile and rich in nutrients. Adding more soil nutrients can be harmful to B. schreberi. When adapting to water and soil changes, there are functional links among its functional traits. Stomatal conduction of water and CO2, leaf light harvesting, and aerenchyma gas transmission are functionally linked. Enhanced performance in these processes can significantly boost B. schreberi’s photosynthetic carbon assimilation. These findings demonstrate that alterations in aquatic environments and soil conditions significantly impact the growth and survival of B. schreberi. Specifically, water quality degradation coupled with eutrophication of water and soil may impair the photosynthetic productivity of B. schreberi and reduce its efficiency in water and CO2 utilization, ultimately leading to suboptimal growth or even extinction of this species. These findings offer physiological explanations for the endangered mechanisms of B. schreberi and are highly significant for its scientific conservation and utilization. Based on the results of this study, it is necessary to pay special attention to the content of nutrients in water and soil, especially the input of nitrogen and phosphorus, in the conservation and management of B. schreberi. In addition, appropriately increasing the content of trace elements such as magnesium, zinc, and silicon in the soil can play a certain role in improving the photosynthetic productivity of B. schreberi. When carrying out habitat restoration and species reintroduction, improving water quality and reducing the content of the three main soil nutrients, carbon, nitrogen, and phosphorus, are the keys to the success of reintroduction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14132072/s1, Figure S1. Workflow diagram of anatomical and morphological characteristics. Figure S2. Randomforest modeling of functional traits of B. schreberi leaves in relation to water and substrate. Photosythetics parameters in relation to water and substrate(a); form parameters in relation to water and substrate(b); structure parameters in relation to water and substrate(c). Pn, net photosynthetic rate; Gs, stomatal conductance; Ci, intercellular carbon dioxide concentration; Tr, transpiration rate; LA, leaf area; LP, leaf perimeter; L/W, ratio of leaf length to width; LP2/LA, ratio of square of leaf perimeter to area; SD, stomatal density; SL, stomatal length; SW, stomatal width; SA, stomatal area; VD, leaf vein density; BA, vascular bundle area; AS, air space area; AA, spiracle area; CT, cuticle thickness; ET, epidermal thickness; WH, water height; WT, water temperature; PH, acidity and alkalinity; DO, dissolved oxygen; Nwater, total nitrogen; Pwater, total phosphorus; NH4+, ammonia nitrogen; NO3−, nitrate nitrogen; BOD, biological oxygen demand; COD, chemical oxygen demand; CODMn, potassium permanganate; Csoil, total soil carbon; Nsoil, total soil nitrogen; Psoil, total soil phosphorus; K, soil potassium mass fraction; Ca, soil calcium mass fraction; Mg, soil magnesium mass fraction; Zn, soil zinc mass fraction; Si, soil silicon mass fraction.
Author Contributions
M.S. designed the research plan and organized the research work; J.Y. (Jingyu Yao) and M.S. wrote the manuscript; J.Y. (Junbao Yu) and Z.L. measured the functional traits; Z.L., R.X. and J.L. detected the water and sediment elements; Y.Z., M.S., J.Y. (Jingyu Yao), and Y.X. analyzed the data; M.S., J.Y. (Jingyu Yao), and J.Y. (Junbao Yu) revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Key Research and Development Program of Science and Technology Planning Project of the Science and Technology Department of Yunnan Province, China (202303AC100012), the “Xingdian Talent” Youth Talent Project of Yunnan Province, China (XDYC-QNRC-2023-0218), the Yunnan Agricultural Basic Research Joint Special Project (202101BD070001-099), and the Scientific Research Project of the Yunnan Biodiversity Foundation, China.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please contact the author at sm0510215@163.com.
Acknowledgments
The fieldwork of this study was conducted in the Beihai Wetland Provincial Nature Reserve in Tengchong City, Yunnan Province, China. We sincerely thank the following reserve managers: Qiubai Yin, Wei Zhang, Qifan Li, Du Kang, and Tianxiang Fang. We also sincerely thank the boatmen for their service in rowing boats for us.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Drzymulska, D. On the history of Brasenia Schreb. in the European Pleistocene. Veg. Hist. Archaeobotany 2018, 27, 527–534. [Google Scholar] [CrossRef]
- Lu, B.; Shi, T.; Chen, J. Chromosome-level genome assembly of watershield (Brasenia schreberi). Sci. Data 2023, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Cai, X.; Fan, Y.; Luo, A. Antioxidant activity of water-soluble polysaccharides from Brasenia schreberi. Pharmacogn. Mag. 2016, 12, 193. [Google Scholar] [PubMed]
- Zhu, H.L.; Du, J.; Liu, Z.W.; Sun, Y.L.; Li, M.H.; Peng, J.; Zhou, K.; Ke, W.D. Investigation and genetic diversity of wild water shield (Brasenia schreberi) in China. J. Plant Genet. Resour. 2020, 21, 1586–1595. [Google Scholar]
- Stachowicz-Rybka, R.; Pidek, I.A.; Żarski, M. New palaeoclimate reconstructions based on multidisciplinary investigation in the Ferdynandów 2011 stratotype site (eastern Poland). Geol. Q. 2017, 61, 276–290. [Google Scholar] [CrossRef]
- Thompson, K.A.; Sora, D.M.; Cross, K.S.; St. Germain, J.M.; Cottenie, K. Mucilage reduces leaf herbivory in Schreber’s watershield, Brasenia schreberi (Cabombaceae). Botany 2014, 92, 412–416. [Google Scholar] [CrossRef]
- Grasset, C.; Delolme, C.; Arthaud, F.; Bornette, G. Carbon allocation in aquatic plants with contrasting strategies: The role of habitat nutrient content. J. Veg. Sci. 2015, 26, 946–955. [Google Scholar] [CrossRef]
- Ai, T.; Wan, J.; Yu, X.; Liu, J.; Yin, C.; Yang, L.; Yang, L.; Liu, H.; Qin, R. The non-denatured processing of Brasenia schreberi mucilage—Characteristics of hydrodynamic properties and the effect on in vivo functions. Foods 2024, 13, 1824. [Google Scholar] [CrossRef]
- Feng, S.; Ning, K.; Luan, D.; Lu, S.; Sun, P. Chemical composition and antioxidant capacities analysis of different parts of Brasenia schreberi. J. Food Process. Preserv. 2019, 43, e14014. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Seago, J.L., Jr.; Wang, Q. Anatomical and histochemical features of Brasenia schreberi (Cabombaceae) shoots. Flora 2020, 263, 151524. [Google Scholar] [CrossRef]
- Li, J.F. The Impact of Simulated Warming on the Growth and Reproduction of Brasenia schreberi. Doctoral Dissertation, Nanjing University, Nanjing, China, 2021. [Google Scholar]
- Adams, F.S. Winterbud production and function in Brasenia schreberi. Rhodora 1969, 71, 417–433. [Google Scholar]
- Thien, L.B.; Bernhardt, P.; Devall, M.S.; Chen, Z.D.; Luo, Y.B.; Fan, J.H.; Yuan, L.C.; Williams, J.H. Pollination biology of basal angiosperms (ANITA grade). Am. J. Bot. 2009, 96, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Zhang, F.; Wang, X.; Wang, Q. Structure and ion physiology of Brasenia schreberi glandular trichomes in vivo. PeerJ 2019, 7, e7288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, C.; Wang, X.; Zhang, X.; Zhou, C. Physiological study on structure and ion permeability of mucilaginous hairs of Brasenia schreberi in Vivo. J. Yangtze Univ. Nat. Sci. Ed. 2020, 17, 85–90. [Google Scholar]
- Li, Z.Z.; Gichira, A.W.; Wang, Q.F.; Chen, J.M. Genetic diversity and population structure of the endangered basal angiosperm Brasenia schreberi (Cabombaceae) in China. PeerJ 2018, 6, e5296. [Google Scholar] [CrossRef]
- Xie, C.; Li, J.; Pan, F.; Fu, J.; Zhou, W.; Lu, S.; Li, P.; Zhou, C. Environmental factors influencing mucilage accumulation of the endangered Brasenia schreberi in China. Sci. Rep. 2018, 8, 17955. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Yu, C.; Chen, Y.; Tang, H.; Zhang, L. Water lilies as emerging models for Darwin’s abominable mystery. Hortic. Res. 2017, 4, 17051. [Google Scholar] [CrossRef]
- Kim, Y.D. Characterization of water and sediment environment in water shield (Brasenia schreberi) habitats. Korean J. Ecol. 1996, 19, 209–216. [Google Scholar]
- Zhang, Y.; Wang, L.; Hu, Y. Water organic pollution and eutrophication influence soil microbial processes, increasing soil respiration of estuarine wetlands: Site study in Jiuduansha wetland. PLoS ONE 2015, 10, e0126951. [Google Scholar] [CrossRef]
- Zaman, T.; Asaeda, T. Effects of NH4−N concentrations and gradient redox level on growth and allied biochemical parameters of Elodea nuttallii (Planch.). Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 211–219. [Google Scholar] [CrossRef]
- Wang, C.Q.; LI, H.X.; Peng, G.H.; Zhou, Y.H.; Li, T.X. Relationship between the conditions of soil and water quality and the growth of water shield (Brasenia schreberi). J. Sichuan Agric. Univ. 2000, 18, 265–268. [Google Scholar]
- Zheng, Y.X.; Xiang, Q.; Zhang, W.; Zhao, S.; Yan, Z.; Xiao, L.; Wang, D.; Qu, Z. Effects of formula fertilization on growth, yield and quality of water shield. Yangtze River Veg. 2018, 14, 65–67. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5. Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The vegan package. Community Ecol. Package 2007, 10, 631–637. [Google Scholar]
- Sanchez, G. PLS path modeling with R. Berkeley Trowchez Ed. 2013, 383, 551. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Jarvis, A.J.; Davies, W.J. The coupled response of stomatal conductance to photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 399–406. [Google Scholar] [CrossRef]
- Oliveira-Junior, E.S.; Tang, Y.; van den Berg, S.J.; Cardoso, S.J.; Lamers, L.P.; Kosten, S. The impact of water hyacinth (Eichhornia crassipes) on greenhouse gas emission and nutrient mobilization depends on rooting and plant coverage. Aquat. Bot. 2018, 145, 1–9. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef]
- Constable, J.V.; Longstreth, D.J. Aerenchyma carbon dioxide can be assimilated in Typha Iatifolia L. leaves. Plant Physiol. 1994, 106, 1065–1072. [Google Scholar] [CrossRef]
- Cochrane, G.; Karsch-Mizrachi, I.; Takagi, T.; Sequence Database Collaboration, I.N. The international nucleotide sequence database collaboration. Nucleic Acids Res. 2016, 44, D48–D50. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, H.; Chen, H.; Zhang, W.; Liu, Z.; Li, Q.; Sun, M. Growth of Brasenia schreberi requries good water quality and appropriate sediment nitrogen content. Front. Plant Sci. 2025, 16, 1535395. [Google Scholar] [CrossRef]
- Gavrilescu, M. Water, soil, and plants interactions in a threatened environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Galka, M.M.; Rajagopalan, N.; Buhrow, L.M.; Nelson, K.M.; Switala, J.; Cutler, A.J.; Palmer, D.R.; Loewen, P.C.; Abrams, S.R.; Loewen, M.C. Identification of interactions between abscisic acid and ribulose-1, 5-bisphosphate carboxylase/oxygenase. PLoS ONE 2015, 10, e0133033. [Google Scholar] [CrossRef]
- Andreeva, L.; David, L.; Rawson, S.; Shen, C.; Pasricha, T.; Pelegrin, P.; Wu, H. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell 2021, 184, 6299–6312. [Google Scholar] [CrossRef] [PubMed]
- Terashima, I.; Hanba, Y.T.; Tholen, D.; Niinemets, Ü. Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 2011, 155, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Striker, G.G. An overview of oxygen transport in plants: Diffusion and convection. Plant Biol. 2023, 25, 842–847. [Google Scholar] [CrossRef]
- Nakamura, M.; Gao, Y.; Dominguez, A.A.; Qi, L.S. CRISPR technologies for precise epigenome editing. Nat. Cell Biol. 2021, 23, 11–22. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; Månsson, K.F.; Olsson, M.O. Uptake capacity of amino acids by ten grasses and forbs in relation to soil acidity and nitrogen availability. Environ. Exp. Bot. 2000, 44, 207–219. [Google Scholar] [CrossRef]
- Chen, H.Y.; Sun, M.; Liu, Z.Y.; Yang, H.M. Response of leaf economic traits of natural Brasenia schreberi to water environment in high altitude area. Chin. J. Ecol. 2024, 43, 1763–1771. [Google Scholar]
- Jampeetong, A.; Brix, H. Effects of NH4+ concentration on growth, morphology and NH4+ uptake kinetics of Salvinia natans. Ecol. Eng. 2009, 35, 695–702. [Google Scholar] [CrossRef]
- Shi, J.; Li, S.; Xi, L.; Peng, F.; Li, Y. Characteristics of Brasenia schreberi Community and Its Relationship with Water Environmental Factors in Alpine Wetland of Southern Hunan. Wetland Sci. 2024, 22, 972–980. [Google Scholar]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and strategies for enhancing zinc availability in plants for sustainable agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Hasan, B.R.; Islam, M.S.; Kundu, P.; Mallick, U.K. Modeling the effects of algal bloom on dissolved oxygen in eutrophic water bodies. J. Math. 2023, 2023, 2335570. [Google Scholar] [CrossRef]
- Wang, Q.X.; Peng, S.T.; Gan, W.Y.; Peng, Z.D.; Xu, Q.; Wang, J.; Huang, L.J. Response of reed leaf and fine root functional traits to water and salt environment: A case study of Fuzhou section of the Min River. Acta Ecol. Sin. 2024, 44, 8338–8348. [Google Scholar]
- Hudson, J.M.G.; Henry, G.H.R.; Cornwell, W.K. Taller and larger: Shifts in Arctic tundra leaf traits after 16 years of experimental warming. Glob. Chang. Biol. 2011, 17, 1013–1021. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Huang, J. Effects of Increasing Water Temperature on Water Properties and Aquatic Organisms: A Critical Review. J. Hydroecol. 2020, 41, 100–109. [Google Scholar]
- Said, N.E.; McMahon, K.; Lavery, P.S. Accounting for the influence of temperature and location when predicting seagrass (Halophila ovalis) photosynthetic performance. Estuaries Coasts Shelf Sci. 2021, 257, 107414. [Google Scholar] [CrossRef]
- Delgard, M.L.; Deflandre, B.; Kochoni, E.; Avaro, J.; Cesbron, F.; Bichon, S.; Poirier, D.; Anschutz, P. Biogeochemistry of dissolved inorganic carbon and nutrients in seagrass (Zostera noltei) sediments at high and low biomass. Estuar. Coast. Shelf Sci. 2016, 179, 12–22. [Google Scholar] [CrossRef]
- Kiani, M.; Raave, H.; Simojoki, A.; Tammeorg, O.; Tammeorg, P. Recycling lake sediment to agriculture: Effects on plant growth, nutrient availability, and leaching. Sci. Total Environ. 2021, 753, 141984. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.K.; Sharma, S.; Sharma, A.; Singh, K.K.; Pal, P.K. Integration of biochar with nitrogen in acidic soil: A strategy to sequester carbon and improve the yield of stevia via altering soil properties and nutrient recycling. J. Environ. Manag. 2023, 345, 118872. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, P.; Prasad, M.; Singh, T.B.; Yadav, A.; Goyal, D.; Ali, A.; Dantu, P.K. Role of nutrients in plant growth and development. In Contaminants in Agriculture: Sources, Impacts and Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 43–59. [Google Scholar]
- Pandey, S.N.; Abid, M.; Abid Ali Khan, M.M. Diversity, functions, and stress responses of soil microorganisms. In Plant Microbiome: Stress Response; Springer: Singapore, 2018; pp. 1–19. [Google Scholar]
- Chen, X.N.; Zhao, N.Q.; Duan, N.; Gegen, B.T.; Zhang, J.B.; Shi, S.Y. Plant response to water and nitrogen addition: A review. J. Temp. For. Res. 2022, 5, 8–11. [Google Scholar]
- Ma, D.; Teng, W.; Mo, Y.T.; Yi, B.; Chen, W.L.; Pang, Y.P.; Wang, L. Effects of nitrogen, phosphorus, and potassium fertilization on plant growth, element levels in plants and soil, and the relationships among nutrient concentrations, plant yield, and nutrient status in Erythropalum scandens (Blume). J. Plant Nutr. 2024, 47, 82–96. [Google Scholar] [CrossRef]
- Dong, J.W.; Wang, D.H.; Liu, Z.B.; Yang, J.G.; Chen, T.X.; Wu, S.H.; Li, Q.; Liu, Y. Investigation on the original habitats of Brasenia schreberi in Hunan Province. Hunan Agric. Sci. 2025, 1, 45–52. [Google Scholar]
- Nguyen, T.B.A.; Lefoulon, C.; Nguyen, T.H.; Blatt, M.R.; Carroll, W. Engineering stomata for enhanced carbon capture and water-use efficiency. Trends Plant Sci. 2023, 28, 1290–1309. [Google Scholar] [CrossRef]
- Clark, J.W.; Harris, B.J.; Hetherington, A.J.; Hurtado-Castano, N.; Brench, R.A.; Casson, S.; Williams, T.A.; Gray, J.E.; Hetherington, A.M. The origin and evolution of stomata. Curr. Biol. 2022, 32, R539–R553. [Google Scholar] [CrossRef]
- Ni, R.W.; Gan, Y.T.; Yang, G.M.; Huang, L.J.; Liu, X.Z.; Yan, S.J. Trade-off the characteristics of stomata in subtropical urban vegetation and its relationship with leaf functional traits under heat island effect. Acta Ecol. Sin. 2023, 43, 5336–5346. [Google Scholar]
- Zukswert, J.M.; Vadeboncoeur, M.A.; Yanai, R.D. Responses of stomatal density and carbon isotope composition of sugar maple and yellow birch foliage to N, P and CaSiO3 fertilization. Tree Physiol. 2024, 44, tpad142. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Alaoui, I.; Serbouti, S.; Ahmed, H.; Mansouri, I.; El Kamari, F.; Taroq, A.; Ousaaid, D.; Squalli, W.; Farah, A. The mechanisms of absorption and nutrients transport in plants: A review. Trop. J. Nat. Prod. Res. 2022, 6, 8–14. [Google Scholar]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Krause, M. The plant leaf: A biomimetic resource for multifunctional and economic design. Biomimetics 2023, 8, 145. [Google Scholar] [CrossRef]
- Bauer, P.; Elbaum, R.; Weiss, I.M. Calcium and silicon mineralization in land plants: Transport, structure and function. Plant Sci. 2011, 180, 746–756. [Google Scholar] [CrossRef]
- Pantin, F.; Simonneau, T.; Muller, B. Coming of leaf age: Control of growth by hydraulics and metabolics during leaf ontogeny. New Phytol. 2012, 196, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Beerling, D.J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. USA 2009, 106, 10343–10347. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nakazono, M. Modeling-based age-dependent analysis reveals the net patterns of ethylene-dependent and -independent aerenchyma formation in rice and maize roots. Plant Sci. 2022, 321, 111340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).