Effects of Different Modified Biochars on Growth of Kosteletzkya virginica and Corresponding Transcriptome Analysis
Abstract
1. Introduction
2. Results
2.1. Effects of Different Modified Biochars on the Growth Parameters of K. virginica
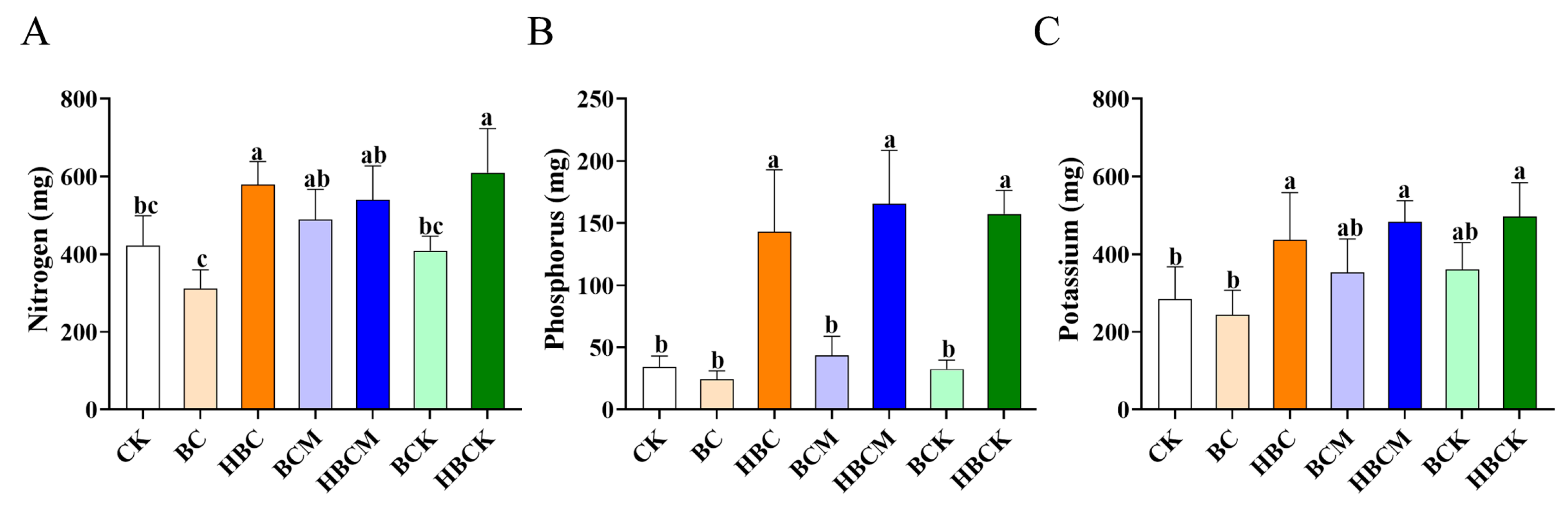
2.2. Analysis of Nutrient Element Indicators in K. virginica with Different Modified Biochars
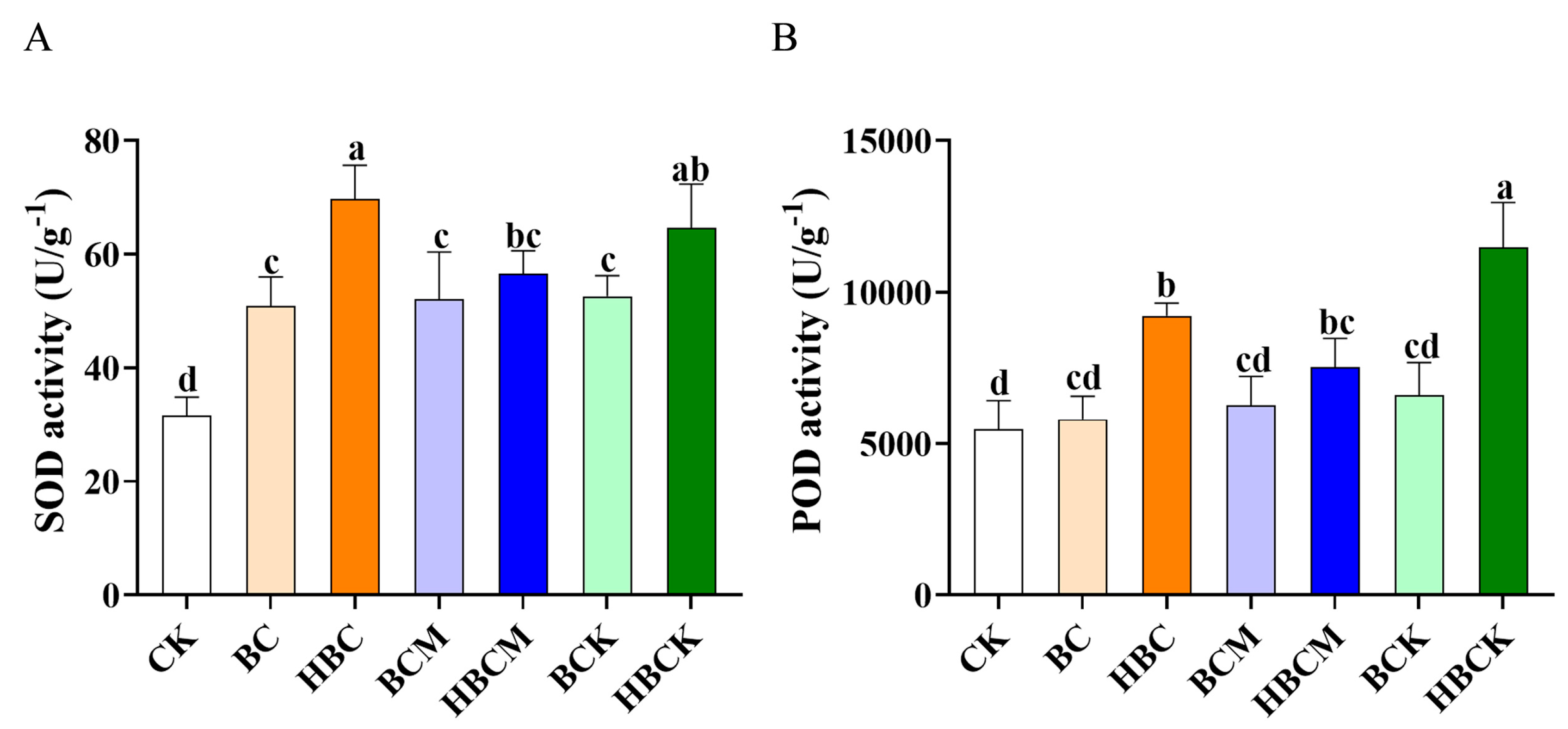
2.3. Impacts of Different Modified Biochars on Antioxidant Enzyme Activity in K. virginica
2.4. Quality Analysis of the Transcriptome Data of K. virginica Roots
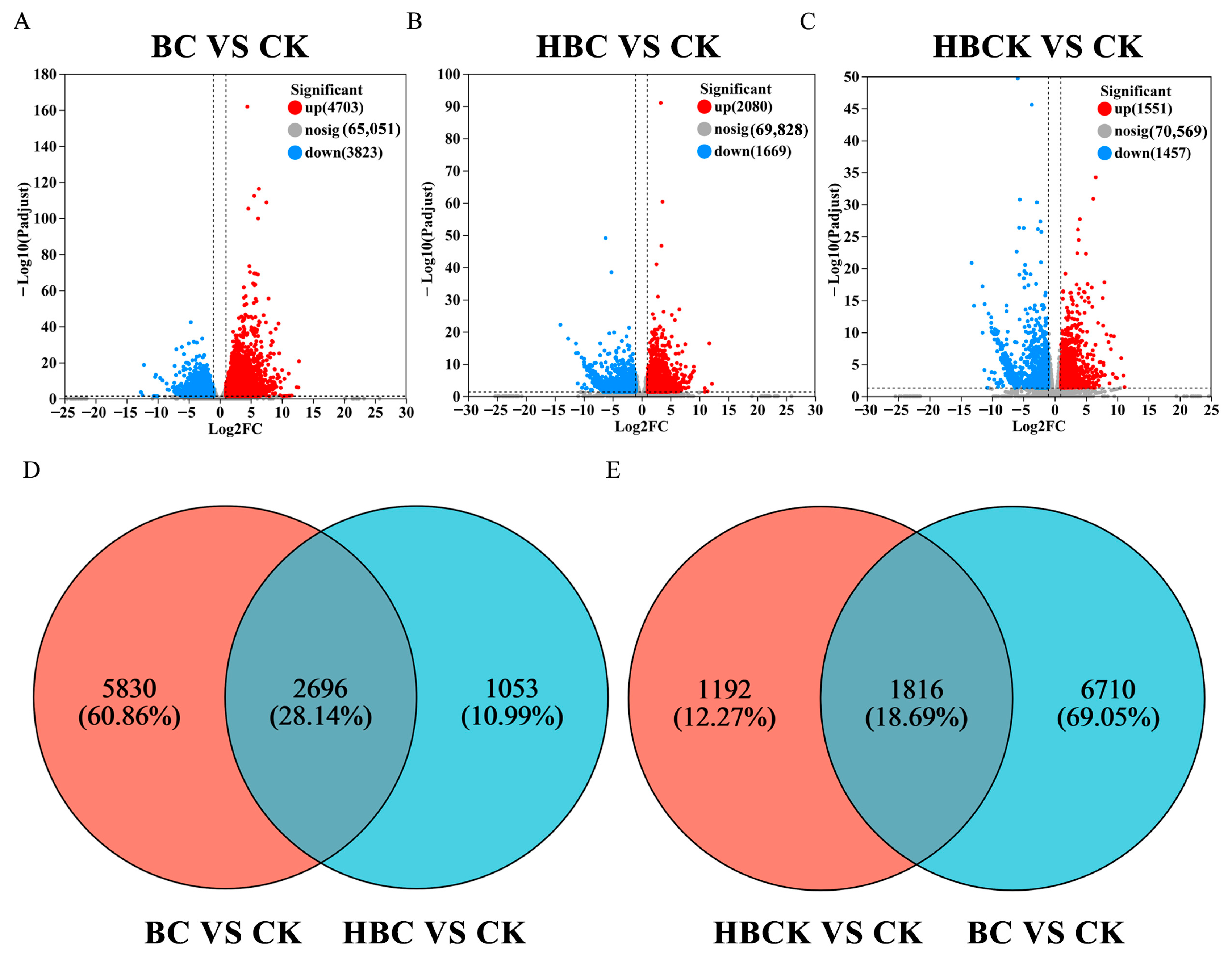
2.5. Gene Function Annotation Analysis and Differential Gene Statistics
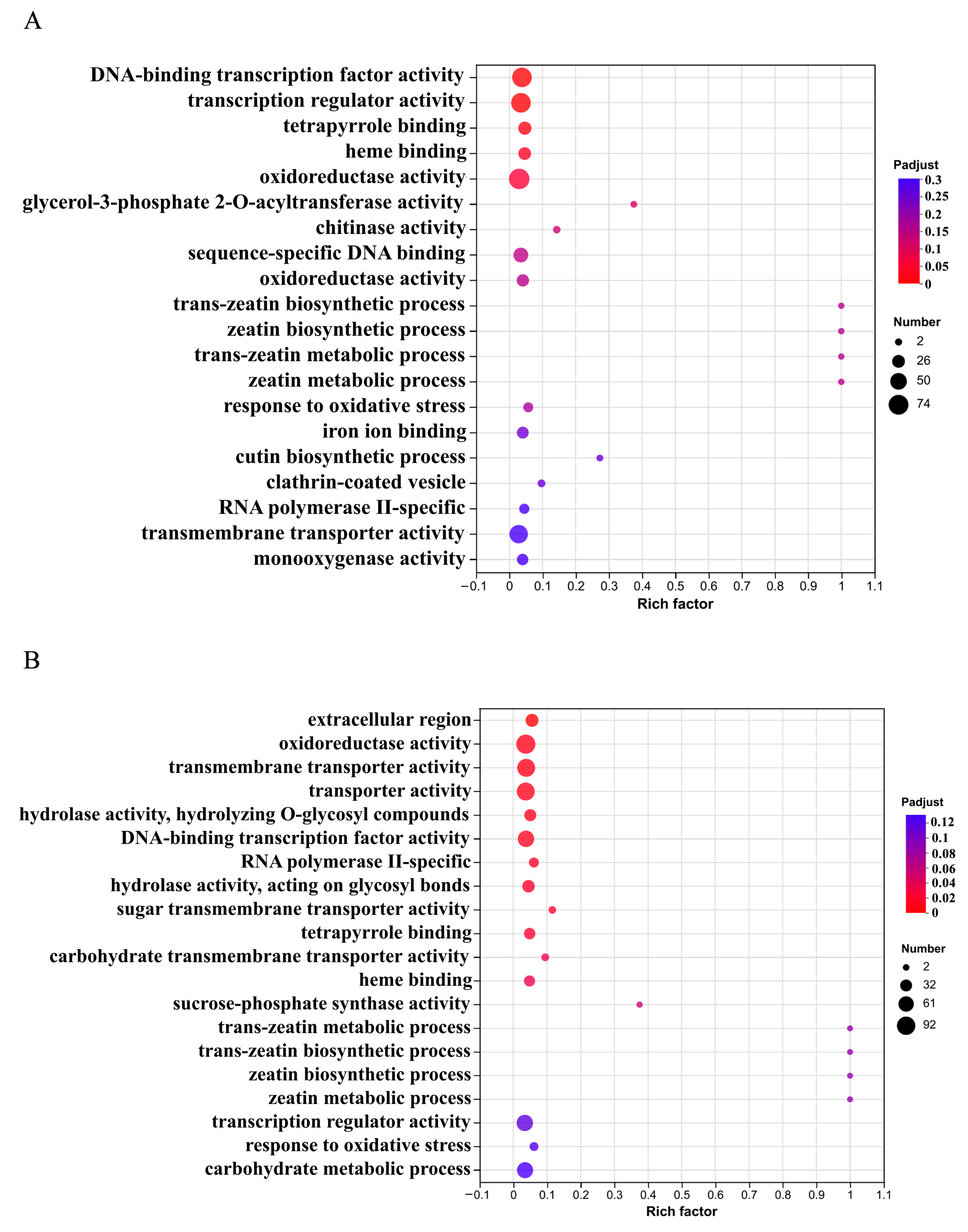
2.6. GO Enrichment Classification of Differentially Expressed Genes
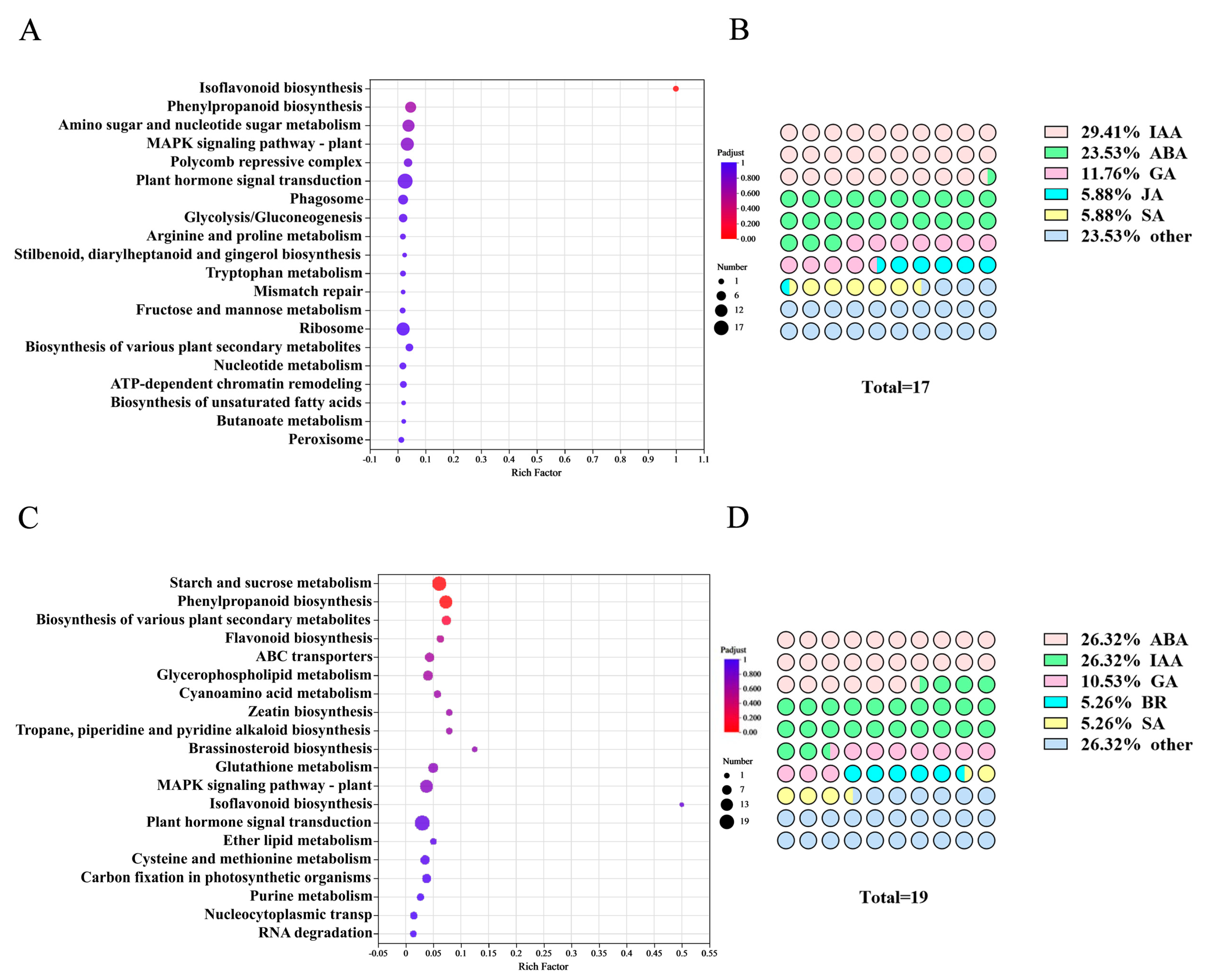
2.7. KEGG Enrichment Analysis of Differentially Expressed Genes
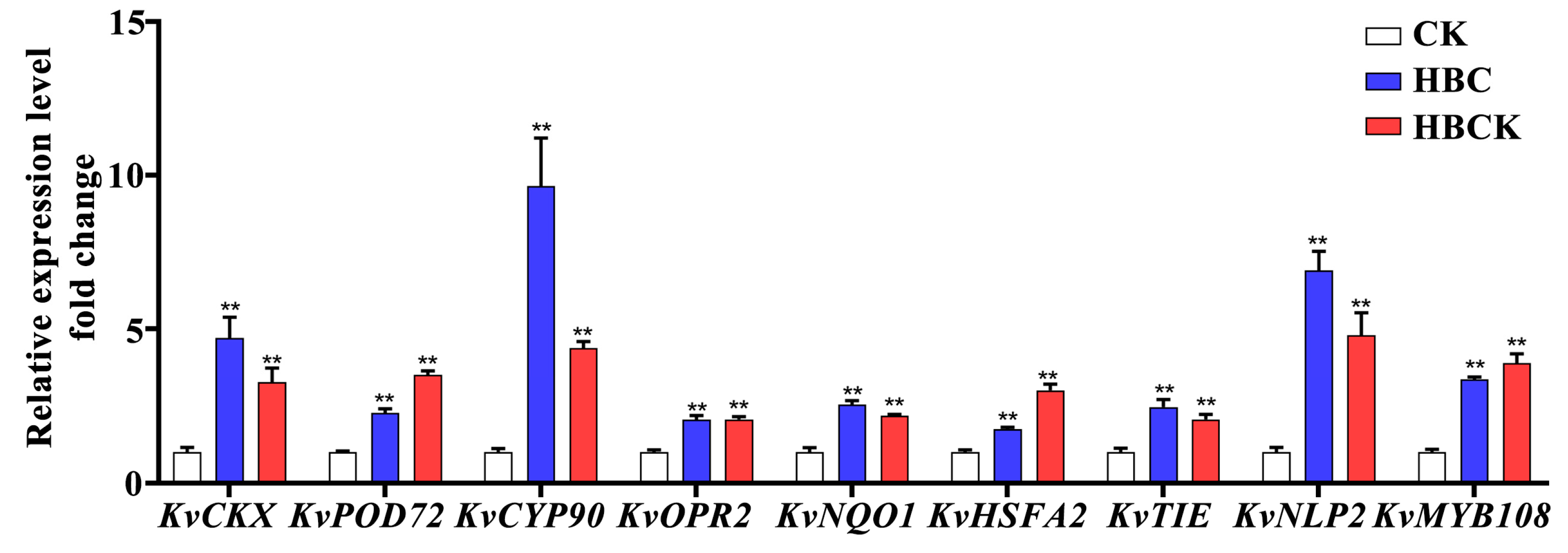
2.8. RT-qPCR Analysis
3. Discussion
3.1. Effects of Different Modified Biochars on K. virginica Growth and Development
3.2. Effects of Modified Biochars on Nutrient Uptake in K. virginica Roots
3.3. HBC and HBCK Enhance Antioxidant Enzyme Activity and Salt–Alkali Stress Tolerance in K. virginica
3.4. Molecular Response Mechanism of K. virginica to HBC and HBCK Application
4. Materials and Methods
4.1. Materials
4.2. Biochar Preparation and Modification
4.3. Pot Experiment
4.4. Determination of Growth Parameters, Nutrient Contents, and Antioxidant Enzyme Activities in K. virginica
4.5. RNA Extraction and Transcriptome Sequencing of K. virginica Roots
4.6. Functional Annotation and Enrichment Analysis of Differentially Expressed Genes (DEGs)
4.7. qRT-PCR Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, P.; Bing, X.; Jiao, L.; Xiao, H.; Li, B.; Sun, H. Amelioration effects of coastal saline-alkali soil by ball-milled red phosphorus-loaded biochar. Chem. Eng. J. 2022, 431, 133904. [Google Scholar] [CrossRef]
- Li, Q.; Xi, M.; Wang, Q.; Kong, F.; Li, Y. Characterization of soil salinization in typical estuarine area of the Jiaozhou Bay, China. Phys. Chem. Earth Parts A/B/C 2018, 103, 51–61. [Google Scholar] [CrossRef]
- Zhang, S.; Hong, Q.; Guo, H. Research on the Impact of Coastal Land Reclamation on Geomorphic Complexity and Tidal Energy Dissipation—A Case Study of Zhoushan Islands, Hangzhou Bay. Eng. Rep. 2025, 7, e13075. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.; Liu, S.; Zhang, X.; Dong, H.; Dai, S.; Chai, L.; Li, H.; Lv, Y.; Li, T.; et al. Trade-offs of organic amendment input on soil quality and crop productivity in saline-alkali land globally: A meta-analysis. Eur. J. Agron. 2025, 164, 127471. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, C.; Chen, L.; Cao, S. Improving food security in China by taking advantage of marginal and degraded lands. J. Clean. Prod. 2018, 171, 1020–1030. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y. Reflections on China’s food security and land use policy under rapid urbanization. Land Use Policy 2021, 109, 105699. [Google Scholar] [CrossRef]
- Shokri-Kuehni, S.; Raaijmakers, B.; Schollenberger, T.; Or, D.; Helmig, R.; Shokri, N. Water Table Depth and Soil Salinization: From Pore-Scale Processes to Field-Scale Responses. Water Resour. Res. 2020, 56, e2019WR026707. [Google Scholar] [CrossRef]
- Zhu, W.; Gu, S.; Jiang, R.; Zhang, X.; Hatano, R. Saline–Alkali Soil Reclamation Contributes to Soil Health Improvement in China. Agriculture 2024, 14, 1210. [Google Scholar] [CrossRef]
- Song, J.; Zhang, S.; Li, G.; Du, Q.; Yang, F. Preparation of montmorillonite modified biochar with various temperatures and their mechanism for Zn ion removal. J. Hazard. Mater. 2020, 391, 121692. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef] [PubMed]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Kumar, M.; Rao, C.S.; Nataraj, K.C.; Singh, G.; Vinu, A.; Bhowmik, A.; Sharma, H.; et al. Biochar modulating soil biological health: A review. Sci. Total Environ. 2024, 914, 169585. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, M.; Li, R.; Rasul, F.; Shahzad, S.; Wu, C.; Shao, J.; Huang, G.; Li, R.; Almari, S.; et al. Soil acidification and salinity: The importance of biochar application to agricultural soils. Front. Plant Sci. 2023, 14, 1206820. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Lehmann, J.; Kuzyakov, Y.; Pan, G.; Ok, Y.S. Biochars and the plant-soil interface. Plant Soil 2015, 395, 1–5. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Cornelis, W.; Zoroufchi Benis, K. Understanding the physicochemical structure of biochar affected by feedstock, pyrolysis conditions, and post-pyrolysis modification methods—A meta-analysis. J. Environ. Chem. Eng. 2024, 12, 114885. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Cui, S.; Kong, F.; Li, Y.; Jiang, Z.; Xi, M. Effect of mineral loaded biochar on the leaching performances of nitrate and phosphate in two contrasting soils from the coastal estuary area. Sci. Total Environ. 2021, 779, 146346. [Google Scholar] [CrossRef]
- He, Z.; Ruan, C.; Qin, P.; Seliskar, D.M.; Gallagher, J.L. Kosteletzkya virginica, a halophytic species with potential for agroecotechnology in Jiangsu Province, China. Ecol. Eng. 2003, 21, 271–276. [Google Scholar] [CrossRef]
- Ruan, C.J.; Li, H.; Guo, Y.Q.; Qin, P.; Gallagher, J.L.; Seliskar, D.M.; Lutts, S.; Mahy, G. Kosteletzkya virginica, an agroecoengineering halophytic species for alternative agricultural production in China’s east coast: Ecological adaptation and benefits, seed yield, oil content, fatty acid and biodiesel properties. Ecol. Eng. 2008, 32, 320–328. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Shao, C.; Shao, H.; Wang, H. Molecular cloning and bioinformatics analysis of a new plasma membrane Na+/H+ antiporter gene from the halophyte Kosteletzkya virginica. Sci. World J. 2014, 2014, 141675. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, L.; Dai, H.; Xie, M.; Wang, Y.; Peng, J.; Sun, H.; Ao, T.; Chen, W. Antioxidant system response, mineral element uptake and safe utilization of Polygonatum sibiricum in cadmium-contaminated soil. Sci. Rep. 2021, 11, 18737. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Nazish, M.; Akbar, M.; Hussain, Q.; Yousra, M.; Haroon, U.; Ausaf, N.; Alkahtani, J.; Munis, M.F.H. Enhancing Sesbania sesban L. (Merr.) growth and metal resilience by synergistic application of Trichoderma harzianum loaded biochar and biochar-zinc oxide nanocomposite. BMC Plant Biol. 2025, 25, 771. [Google Scholar] [CrossRef]
- Li, W.; Gu, X.; Du, Y.; Zheng, X.; Lu, S.; Cheng, Z.; Cai, W.; Chang, T. Optimizing nitrogen, phosphorus, and potassium fertilization regimes to improve maize productivity under double ridge-furrow planting with full film mulching. Agric. Water Manag. 2023, 287, 108439. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, X.; Zong, S.; Zhuo, Y.; Zhang, Y.; Wang, S.; Deng, Y.; Zhang, X.; Li, J. Biochar as a highly efficient adsorption carrier for sewage sludge-derived nutrients and biostimulants: Component fixation and mechanism. Biochar 2024, 6, 42. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, C.; Chen, X.; Hopkins, I.; Zhang, X.; Han, Z.; Jiang, F.; Billy, G. The crucial factors of soil fertility and rapeseed yield—A five year field trial with biochar addition in upland red soil, China. Sci. Total Environ. 2019, 649, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Rasse, D.P.; Weldon, S.; Joner, E.J.; Joseph, S.; Kammann, C.I.; Liu, X.; O’Toole, A.; Pan, G.; Kocatürk-Schumacher, N.P. Enhancing plant N uptake with biochar-based fertilizers: Limitation of sorption and prospects. Plant Soil 2022, 475, 213–236. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Bamagoos, A.; Alharby, H.; Fahad, S. Biochar coupling with phosphorus fertilization modifies antioxidant activity, osmolyte accumulation and reactive oxygen species synthesis in the leaves and xylem sap of rice cultivars under high-temperature stress. Physiol. Mol. Biol. Plants 2021, 27, 2083–2100. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.A.-O.; Gao, G.; Lu, M.; Riaz, M.; Zhang, M.A.-O.; Tong, K.A.-O.; Yu, H.; Yang, Y.; Hao, W.; Niu, Y.A.-O. Insight into the Amelioration Effect of Nitric Acid-Modified Biochar on Saline Soil Physicochemical Properties and Plant Growth. Plants 2024, 13, 3434. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, J.; Song, J.; Jameson, P.A.-O. Cytokinin dehydrogenase: A genetic target for yield improvement in wheat. Plant Biotechnol. J. 2020, 18, 614–630. [Google Scholar] [CrossRef]
- Li, S.; An, Y.; Hailati, S.; Zhang, J.; Cao, Y.; Liu, Y.; Geng, J.; Hu, T.; Yang, P. Overexpression of the Cytokinin Oxidase/dehydrogenase (CKX) from Medicago sativa Enhanced Salt Stress Tolerance of Arabidopsis. J. Plant Biol. 2019, 62, 374–386. [Google Scholar] [CrossRef]
- Li, G.; Manzoor, M.A.; Wang, G.; Huang, S.; Ding, X.; Abdullah, M.; Zhang, M.; Song, C. Comparative analysis of POD genes and their expression under multiple hormones in Pyrus bretschenedri. BMC Genom. Data 2024, 25, 41. [Google Scholar] [CrossRef]
- Xu, W.A.-O.; Zheng, B.; Bai, Q.; Wu, L.; Liu, Y.; Wu, G.A.-O. Functional study of the brassinosteroid biosynthetic genes from Selagnella moellendorfii in Arabidopsis. PLoS ONE 2019, 14, e0220038. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, Y.; Li, G.; Chen, Y.; Li, M.; Yang, R.; Qin, Y.; Chen, Y.; Cheng, J.; Tang, J.; et al. ZmCYP90D1 regulates maize internode development by modulating brassinosteroid-mediated cell division and growth. Crop J. 2024, 12, 58–67. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Xian, W.; Wang, J.; Liao, H. Identification and expression analysis of the Glycine max CYP707A gene family in response to drought and salt stresses. Ann. Bot. 2012, 110, 743–756. [Google Scholar] [CrossRef]
- Wang, M.A.-O.; Yuan, J.; Qin, L.; Shi, W.; Xia, G.; Liu, S. TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnol. J. 2020, 18, 791–804. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, G.; Yuan, S.; Duan, W.; Wang, P.; Bai, J.; Zhang, F.; Gao, S.; Zhang, L.; Zhao, C. TaOPR2 encodes a 12-oxo-phytodienoic acid reductase involved in the biosynthesis of jasmonic acid in wheat (Triticum aestivum L.). Biochem. Biophys. Res. Commun. 2016, 470, 233–238. [Google Scholar] [CrossRef]
- Ross, D.; Kepa, J.K.; Winski, S.L.; Beall, H.D.; Anwar, A.; Siegel, D. NAD(P)H:quinone oxidoreductase 1 (NQO1): Chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem.-Biol. Interact. 2000, 129, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Meng, S.; Wang, B.; Zhao, S.; Liu, Y.; Qi, M.; Wang, Z.; Yin, Z.; Li, T. Exogenous melatonin enhances tomato heat resistance by regulating photosynthetic electron flux and maintaining ROS homeostasis. Plant Physiol. Biochem. 2023, 196, 197–209. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.A.-O.; Hayat, S. Specific Roles of Lipoxygenases in Development and Responses to Stress in Plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, W.; Guan, C.; Guan, M.; He, X. Evolution and functional diversity of lipoxygenase (LOX) genes in allotetraploid rapeseed (Brassica napus L.). Int. J. Biol. Macromol. 2021, 188, 844–854. [Google Scholar] [CrossRef]
- Chang, H.; Wu, T.; Shalmani, A.; Xu, L.; Li, C.; Zhang, W.A.-O.; Pan, R. Heat shock protein HvHSP16.9 from wild barley enhances tolerance to salt stress. Physiol. Mol. Biol. Plants 2024, 30, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liang, W.; Gu, P.; Huang, Z. Salt tolerance function of the novel C2H2-type zinc finger protein TaZNF in wheat. Plant Physiol. Biochem. 2016, 106, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Perotti, M.F.; Ribone, P.A.; Chan, R.A.-O. Plant transcription factors from the homeodomain-leucine zipper family I. Role in development and stress responses. IUBMB Life 2017, 69, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.S.; Um, J.H.; Kim, S.; Kim, K.; Park, H.J.; Lim, J.S.; Kim, W.Y.; Jun, S.E.; Yoon, E.K.; Lim, J.; et al. Arabidopsis thaliana homeobox 12 (ATHB12), a homeodomain-leucine zipper protein, regulates leaf growth by promoting cell expansion and endoreduplication. New Phytol. 2015, 205, 316–328. [Google Scholar] [CrossRef]
- Diao, Y.; Zhan, J.; Zhao, Y.; Liu, L.; Liu, P.; Wei, X.; Ding, Y.; Sajjad, M.; Hu, W.; Wang, P.; et al. GhTIE1 Regulates Branching Through Modulating the Transcriptional Activity of TCPs in Cotton and Arabidopsis. Front Plant Sci. 2019, 10, 1348. [Google Scholar] [CrossRef]
- Yu, J.; Xuan, W.; Tian, Y.; Fan, L.; Sun, J.; Tang, W.; Chen, G.; Wang, B.; Liu, Y.; Wu, W.; et al. Enhanced OsNLP4-OsNiR cascade confers nitrogen use efficiency by promoting tiller number in rice. Plant Biotechnol. J. 2021, 19, 167–176. [Google Scholar] [CrossRef]
- Zhang, F.A.-O.; Wang, J.A.-O.; Ding, T.A.-O.; Lin, X.A.-O.; Hu, H.A.-O.; Ding, Z.A.-O.; Tian, H.A.-O.X. MYB2 and MYB108 regulate lateral root development by interacting with LBD29 in Arabidopsis thaliana. J. Integr. Plant Biol. 2024, 66, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, J.A.-O.; Carrillo-Carrasco, V.P.; Rienstra, J.A.-O.; Tanaka, K.; de Roij, M.A.-O.; Dipp-Álvarez, M.A.-O.; Freire-Ríos, A.; Crespo, I.A.-O.; Boer, R.A.-O.; van den Berg, W.A.M.; et al. Evolutionary origins and functional diversification of Auxin Response Factors. Nat. Commun. 2024, 15, 10909. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Harberd, N.P. The role of GA-mediated signalling in the control of seed germination. Curr. Opin. Plant Biol. 2002, 5, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Liu, Z.; Yu, J.; Teng, X.; Liu, L.; Jia, M.; Xue, J. Assessment of the Characters of a Novel Phosphoric Acid and Mineral-Comodified Biochar Composite and Its Potential Application in Saline–Alkali Soil Improvement. Agriculture 2025, 15, 785. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Sample | Total Reads | Mapped Reads | GC Content | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|
| CK-1 | 25,686,502 | 22,626,954 (88.09%) | 45.3 | 98.8 | 96.34 |
| CK-2 | 25,734,167 | 22,678,505 (88.13%) | 45.08 | 98.8 | 96.35 |
| CK-3 | 24,271,463 | 21,317,319 (87.83%) | 44.99 | 98.76 | 96.25 |
| BC-1 | 22,125,697 | 19,337,469 (87.40%) | 45.13 | 98.74 | 96.13 |
| BC-2 | 24,332,344 | 21,323,199 (87.63%) | 45.36 | 98.75 | 96.18 |
| BC-3 | 23,895,927 | 21,033,680 (88.02%) | 45.09 | 98.77 | 96.24 |
| HBC-1 | 21,542,792 | 18,929,233 (87.87%) | 45.2 | 98.7 | 95.99 |
| HBC-2 | 20,394,966 | 17,864,767 (87.59%) | 45.1 | 98.73 | 96.12 |
| HBC-3 | 22,663,117 | 19,920,356 (87.90%) | 45.14 | 98.71 | 96.07 |
| HBCK-1 | 22,558,390 | 19,912,614 (88.27%) | 45.24 | 98.75 | 96.16 |
| HBCK-2 | 21,589,175 | 19,084,337 (88.40%) | 45.11 | 98.75 | 96.2 |
| HBCK-3 | 26,135,691 | 23,134,737 (88.52%) | 45.26 | 98.79 | 96.31 |
| Annotated Database | Annotated Number | Annotated Percent (%) |
|---|---|---|
| GO | 37,354 | 50.96 |
| KEGG | 18,529 | 25.28 |
| eggNOG | 34,955 | 47.69 |
| NR | 43,828 | 59.79 |
| Swiss-Prot | 32,555 | 44.41 |
| Pfam | 28,317 | 38.63 |
| Total_annotated | 44,137 | 60.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, H.; Jia, M.; Xue, J.; Huang, Y.; Yu, J. Effects of Different Modified Biochars on Growth of Kosteletzkya virginica and Corresponding Transcriptome Analysis. Plants 2025, 14, 1849. https://doi.org/10.3390/plants14121849
Dai H, Jia M, Xue J, Huang Y, Yu J. Effects of Different Modified Biochars on Growth of Kosteletzkya virginica and Corresponding Transcriptome Analysis. Plants. 2025; 14(12):1849. https://doi.org/10.3390/plants14121849
Chicago/Turabian StyleDai, Hao, Mingyun Jia, Jianhui Xue, Yuying Huang, and Jinping Yu. 2025. "Effects of Different Modified Biochars on Growth of Kosteletzkya virginica and Corresponding Transcriptome Analysis" Plants 14, no. 12: 1849. https://doi.org/10.3390/plants14121849
APA StyleDai, H., Jia, M., Xue, J., Huang, Y., & Yu, J. (2025). Effects of Different Modified Biochars on Growth of Kosteletzkya virginica and Corresponding Transcriptome Analysis. Plants, 14(12), 1849. https://doi.org/10.3390/plants14121849






