Alleviation of Plant Abiotic Stress: Mechanistic Insights into Emerging Applications of Phosphate-Solubilizing Microorganisms in Agriculture
Abstract
1. Introduction
2. Roles and Multifunctional Traits of PSMs in Soil
3. Applications of PSMs in Heavy Metal Remediation and Stress Alleviation
3.1. Efficacy of PSMs in Heavy Metal Remediation
3.1.1. Application of PSM Inoculants
3.1.2. Synergistic Application of PSMs with Other Amendments
3.1.3. Synergistic Effects of PSMs on Plant-Assisted Remediation
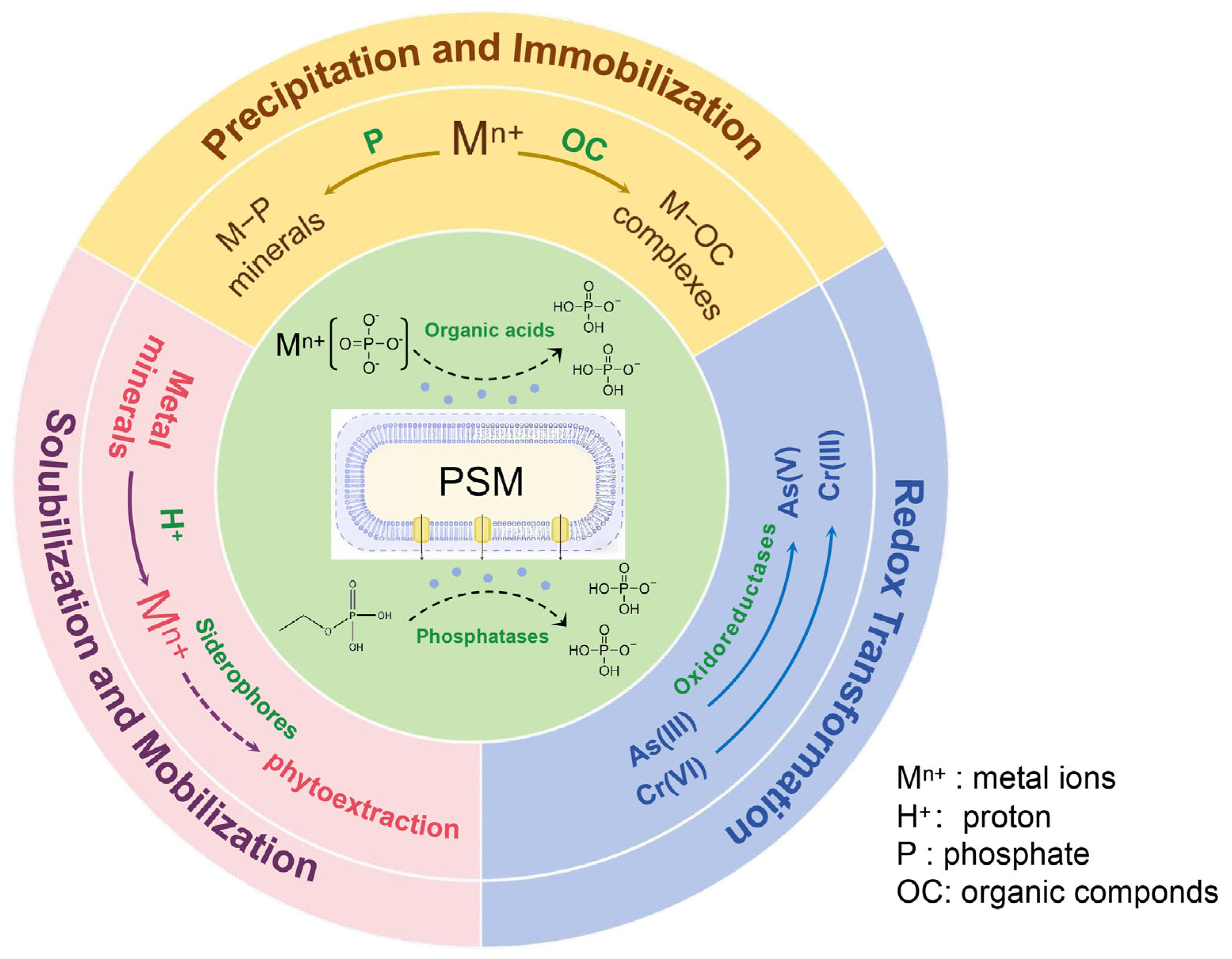
3.2. Mechanisms of Heavy Metal Remediation in Soil by PSMs
3.2.1. Influence of PSMs on the Physicochemical Status of Heavy Metals in Soil
- (1)
- Heavy Metal Precipitation and Immobilization
- (2)
- Heavy Metal Solubilization and Mobilization
- (3)
- Redox Transformation of Heavy Metals
3.2.2. Plant Growth-Promoting Effects of PSMs on Phytoremediation
3.2.3. Indirect Effects of PSMs Through Microbial Community Regulation
4. Applications of PSMs in Drought Stress Mitigation
4.1. Promotion of Root Growth and Nutrient Uptake
4.2. Enhancement of Plant Antioxidant Capacity and Osmotic Regulation
4.3. Formation of Biofilm and Soil Aggregation
5. Applications of PSMs in Saline–Alkaline Stress Mitigation
5.1. Enhancement of Microbial-Mediated Soil Nutrient Cycling
5.2. Plant-Growth Promotion and Antioxidant Effects of PSMs Under Salt Stress
5.3. Regulation of Plant Ion Homeostasis
6. Challenges and Future Perspectives
- (1)
- Elucidation of Underlying Genetic Mechanisms
- (2)
- Targeted Screening and Optimization of PSM Strains
- (3)
- Synergism with Other Biological and Agronomic Managements
- (4)
- Long-Term Ecological Assessment
- (5)
- Large-Scale Production and Commercialization
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSM | Phosphate-solubilizing microorganism |
| PSB | Phosphate-solubilizing bacteria |
| IAA | Indole-3-acetic acid |
| ACC | 1-aminocyclopropane-1-carboxylate |
| EPS | Extracellular polymeric substances |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| ROS | Reactive oxygen species |
| AMF | Arbuscular mycorrhizal fungi |
| APX | Ascorbate peroxidase |
| PPO | Polyphenol oxidase |
References
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Corwin, D.L. Dealing with the impact of climate change-induced drought on the management of soil salinity under irrigated agriculture. Adv. Agron. 2024, 184, 67–124. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Wang, L.; Rinklebe, J.; Tack, F.M.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil. Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Azhar, U.; Ahmad, H.; Shafqat, H.; Babar, M.; Munir, H.M.S.; Sagir, M.; Khoo, K.S. Remediation techniques for elimination of heavy metal pollutants from soil: A review. Environ. Res. 2022, 214, 113918. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-J.; Li, Q.; Peng, H.; Zhang, J.-X.; Chen, W.-J.; Zhou, B.-C.; Chen, M. Remediation of heavy metal-contaminated soils with soil washing: A review. Sustainability 2022, 14, 13058. [Google Scholar] [CrossRef]
- Dey, G.; Banerjee, P.; Sharma, R.K.; Maity, J.P.; Etesami, H.; Shaw, A.K.; Huang, Y.-H.; Huang, H.-B.; Chen, C.-Y. Management of phosphorus in salinity-stressed agriculture for sustainable crop production by salt-tolerant phosphate-solubilizing bacteria-A review. Agronomy 2021, 11, 1552. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Yang, T.; Li, L.; Wang, B.; Tian, J.; Shi, F.; Zhang, S.; Wu, Z. Isolation, mutagenesis, and organic acid secretion of a highly efficient phosphate-solubilizing fungus. Front. Microbiol. 2022, 13, 793122. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil. Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef] [PubMed]
- Saranya, K.; Sundaramanickam, A.; Manupoori, S.; Vinodh Kanth, S. Screening of multi-faceted phosphate-solubilising bacterium from seagrass meadow and their plant growth promotion under saline stress condition. Microbiol. Res. 2022, 261, 127080. [Google Scholar] [CrossRef]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.-X.; Dong, D.-F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef]
- Alemneh, A.A.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Is phosphate solubilizing ability in plant growth-promoting rhizobacteria isolated from chickpea linked to their ability to produce ACC deaminase? J. Appl. Microbiol. 2021, 129, 1133–1156. [Google Scholar] [CrossRef]
- Afzal, J.; Shah, Z.; Depar, N.; Arshad, M.; Rao, S.S.; Rajpar, I.; Shah, A.N. Wheat response to ACC-deaminase-containing rhizobacterial strains with varying phosphate-solubilizing activity under different levels of phosphatic fertilizer. J. Anim. Plant Sci. 2014, 24, 1834–1839. [Google Scholar]
- Zhang, J.; Feng, L.; Ouyang, Y.; Hua, R.; Xu, H.; Wang, J. Phosphate-solubilizing bacteria and fungi in relation to phosphorus availability under different land uses for some latosols from Guangdong, China. Catena 2020, 195, 104686. [Google Scholar] [CrossRef]
- Xu, S.; Jia, K.; Zheng, Y.; Chen, W.; Wang, Z.; Wei, D.; Sun, B.; Cheng, M.; Fan, B.; Li, J.; et al. Phosphorus transformation behavior and phosphorus cycling genes expression in food waste composting with hydroxyapatite enhanced by phosphate-solubilizing bacteria. Bioresour. Technol. 2023, 376, 128882. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiyab, C.S.; Kalra, A. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J. Plant Physiol. 2014, 171, 884–894. [Google Scholar] [CrossRef]
- Misra, S.; Dixit, V.K.; Khan, M.H.; Mishra, S.K.; Dviwedi, G.; Yadav, S.; Lehri, A.; Chauhan, P.S. Exploitation of agro-climatic environment for selection of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing salt tolerant indigenous plant growth promoting rhizobacteria. Microbiol. Res. 2017, 205, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hii, Y.S.; San, C.Y.; Lau, S.W.; Danquah, M.K. Isolation and characterisation of phosphate solubilising microorganisms from peat. Biocatal. Agric. Biotechnol. 2020, 26, 101643. [Google Scholar] [CrossRef]
- Park, J.H.; Bolan, N.; Megharaj, M.; Naidu, R. Isolation of phosphate solubilising bacteria and their potential for lead immobilisation in soil. J. Hazard. Mater. 2011, 185, 829–836. [Google Scholar] [CrossRef]
- Li, H.Z.; Peng, J.; Yang, K.; Zhang, Y.; Chen, Q.L.; Zhu, Y.G.; Cui, L. Single-cell exploration of active phosphate-solubilising bacteria across diverse soil matrices for sustainable phosphorus management. Nat. Food 2024, 5, 673–683. [Google Scholar] [CrossRef]
- Lacava, P.T.; Machado, P.C.; de Andrade, P.H.M. Phosphate solubilisation by endophytes from the tropical plants. In Endophytes: Mineral Nutrient Management; Springer: Cham, Switzerland, 2021; Volume 3, pp. 207–226. [Google Scholar]
- Berza, B.; Sekar, J.; Vaiyapuri, P.; Pagano, M.C.; Assefa, F. Evaluation of inorganic phosphate solubilising efficiency and multiple plant growth promoting properties of endophytic bacteria isolated from root nodules Erythrina brucei. BMC Microbiol. 2022, 22, 276. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Wang, S.; Li, S.; Lei, X.; Li, M. Roles of bacterial biomass, physiology and community in sediment phosphorus solubilising at varying hydrostatic pressures. J. Clean. Prod. 2021, 282, 124531. [Google Scholar] [CrossRef]
- Wang, X.; Gao, L.; Ma, K.; Wei, L.; Pi, Y. Isolation of inorganic-phosphate-solubilising bacteria and analysis of their effect on phosphorus release from lagoon sediments. Geomicrobiol. J. 2023, 40, 46–59. [Google Scholar] [CrossRef]
- Armandeh, M.; Mahmoudi, N.; Fallah Nosratabad, A.R. Screening and evaluation of phosphate-solubilising bacteria isolated from aquaculture ponds in a step-by-step strategy as potential biofertiliser. J. Appl. Microbiol. 2022, 133, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Doilom, M.; Guo, J.W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Xu, J.C. Screening of phosphate-solubilising fungi from air and soil in Yunnan, China: Four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Naresh Kumar, G.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil. 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Della Mónica, I.F.; Stefanoni Rubio, P.J.; Cina, R.P.; Recchi, M.; Godeas, A.M.; Scervino, J.M. Effects of the phosphate-solubilizing fungus Talaromyces flavus on the development and efficiency of the Gigaspora rosea-Triticum aestivum symbiosis. Symbiosis 2014, 64, 25–32. [Google Scholar] [CrossRef]
- Lei, Y.; Kuai, Y.; Guo, M.; Zhang, H.; Yuan, Y.; Hong, H. Phosphate-solubilizing microorganisms for soil health and ecosystem sustainability: A forty-year scientometric analysis (1984–2024). Front. Microbiol. 2025, 16, 1546852. [Google Scholar] [CrossRef]
- Gupta, R.; Singal, R.; Shankar, A.; Kuhad, R.C.; Saxena, R.K. A modified plate assay for screening phosphate solubilising microorganisms. J. Gen. Appl. Microbiol. 1994, 40, 255–260. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilising microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Du, P.; Liu, X.; Zhu, C. An effective method for screening and testing the true phosphate-solubilising fungus that enhances corn growth. J. Agric. Sci. 2014, 6, 60. [Google Scholar]
- Rahim, I.; Kuswinanti, T.; Asrul, L.; Rasyid, B. Screening of fungal rot isolates from cocoa as phosphate-dissolving and their growth ability on three types of media. Procedia Food Sci. 2015, 3, 104–111. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, S.; Wang, D.; Zhang, L.; Chen, H.; Ye, X. Heavy metal remediation using phosphate-solubilising fungi: From bioprocess to application. Agronomy 2024, 14, 2638. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. From isolation of phosphate solubilising microbes to their formulation and use as biofertilisers: Status and needs. Front. Bioeng. Biotechnol. 2020, 7, 425. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilising fungi–current perspective. Arch. Agron. Soil. Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Jain, R.; Saxena, J.; Sharma, V. Effect of phosphate-solubilising fungi Aspergillus awamori S29 on mungbean (Vigna radiata cv. RMG 492) growth. Folia Microbiol. 2012, 57, 533–541. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, Z.; Zou, M.; Cirenlamu; Chen, X.; Sun, X.; Wang, Y.; Jia, Z. Culture Media and Generations Influence Diversity Assessment of Soil Culturable Phosphate-Solubilising Bacteria. Acta Pedol. Sin. 2024, 61, 1680–1693. [Google Scholar]
- Wei, Y.; Wei, Z.; Cao, Z.; Zhao, Y.; Zhao, X.; Lu, Q.; Wang, X.; Zhang, X. A regulating method for the distribution of phosphorus fractions based on environmental parameters related to the key phosphate-solubilising bacteria during composting. Bioresour. Technol. 2016, 211, 610–617. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilising bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Santos-Torres, M.; Romero-Perdomo, F.; Mendoza-Labrador, J.; Gutiérrez, A.Y.; Vargas, C.; Castro-Rincon, E.; Estrada-Bonilla, G.A. Genomic and phenotypic analysis of rock phosphate-solubilising rhizobacteria. Rhizosphere 2021, 17, 100290. [Google Scholar] [CrossRef]
- Kim, C.H.; Han, S.H.; Kim, K.Y.; Cho, B.H.; Kim, Y.H.; Koo, B.S.; Kim, Y.C. Cloning and expression of pyrroloquinoline quinone (PQQ) genes from a phosphate-solubilising bacterium Enterobacter intermedium. Curr. Microbiol. 2003, 47, 457–461. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for using phosphate-solubilising microorganisms as natural fertilisers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Dafale, N.A.; Purohit, H.J. Regulatory rewiring through global gene regulations by PhoB and alarmone (p) ppGpp under various stress conditions. Microbiol. Res. 2019, 227, 126309. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Bent, E.; Entz, M.H.; Dunfield, K.E. Soil bacterial phoD gene abundance and expression in response to applied phosphorus and long-term management. Soil Biol. Biochem. 2015, 88, 137–147. [Google Scholar] [CrossRef]
- Cui, H.; Wang, S.; Wei, T.; Yang, X.; Li, X.; Fan, M.; Sun, W. Soil phoD-harboring bacteria mediate the responses of phosphorus availability to N addition and mowing among soil aggregates. Geoderma 2025, 454, 117170. [Google Scholar] [CrossRef]
- Ghosh, R.; Barman, S.; Mandal, N.C. Phosphate deficiency induced biofilm formation of Burkholderia on insoluble phosphate granules plays a pivotal role for maximum release of soluble phosphate. Sci. Rep. 2019, 9, 5477. [Google Scholar] [CrossRef] [PubMed]
- Lucero, C.T.; Lorda, G.S.; Halliday, N.; Ambrosino, M.L.; Cámara, M.; Taurian, T. Impact of quorum sensing from native peanut phosphate solubilising Serratia sp. S119 strain on interactions with agronomically important crops. Symbiosis 2023, 89, 107–121. [Google Scholar] [CrossRef]
- Pan, L.; Cai, B. Phosphate-solubilising bacteria: Advances in their physiology, molecular mechanisms and microbial community effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilisation and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Wang, Y.; Lang, J.; Ye, M.; Liu, Q.; Zhou, N. Phosphorus-solubilising fungi promote the growth of Fritillaria taipaiensis PY Li by regulating physiological and biochemical reactions and protecting enzyme system–related gene expression. Front. Genet. 2025, 15, 1459191. [Google Scholar] [CrossRef]
- Park, M.S.; Singvilay, O.; Seok, Y.S.; Chung, J.B.; Ahn, K.S.; Sa, T.M. Effect of Phosphate Solubilizing Fungion P Uptake and Growth of Tobacco in Rock Phosphate Applied Soil. Korean J. Soil Sci. Fert. 2003, 36, 233–238. [Google Scholar]
- Grant, C.A.; Bailey, L.D.; Harapiak, J.T.; Flore, N.A. Effect of phosphate source, rate and cadmium content and use of on phosphorus, zinc and cadmium concentration in durum wheat grain. J. Sci. Food Agric. 2002, 82, 1097–1104. [Google Scholar] [CrossRef]
- Barea, J.M.; Navarro, E.; Montoya, E. Production of plant growth regulators by rhizosphere phosphate-solubilising bacteria. J. Appl. Bacteriol. 1976, 40, 129–134. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, L.; Li, S.; Xie, J.; Xue, X.; Jiang, Y. Screening of phosphate-solubilising bacteria and their abilities of phosphorus solubilisation and wheat growth promotion. BMC Microbiol. 2022, 22, 296. [Google Scholar] [CrossRef]
- Khiangte, L.; Lalfakzuala, R. Effects of heavy metals on phosphatase enzyme activity and Indole-3-Acetic Acid (IAA) production of phosphate solubilising bacteria. Geomicrobiol. J. 2021, 38, 494–503. [Google Scholar] [CrossRef]
- Kaur, M.; Vyas, P.; Rahi, P.; Sharma, S. Chlorpyrifos- and Carbofuran-Tolerant Phosphate-Solubilising Arthrobacter oxydans and Bacillus flexus Improved Growth and Phosphorus Content in Potato in Pesticide-Amended Soils. Potato Res. 2022, 65, 213–231. [Google Scholar] [CrossRef]
- Cao, H.; Peng, T.; Zhao, W.; Huang, H.; Yu, S.; Zhu, Y. Whole-genome sequencing uncovers the plant growth-promoting potential of Bacillus licheniformis G41, isolated from the rhizosphere soil of Gannan navel orange. Ann. Microbiol. 2025, 75, 8. [Google Scholar] [CrossRef]
- Abu-Zaitoon, Y.M.; Al-Ramamneh, E.A.D.M.; Al Tawaha, A.R.; Alnaimat, S.M.; Almomani, F.A. Comparative coexpression analysis of indole synthase and tryptophan synthase a reveals the independent production of auxin via the cytosolic free indole. Plants 2023, 12, 1687. [Google Scholar] [CrossRef]
- Nonhebel, H.M. Tryptophan-independent indole-3-acetic acid synthesis: Critical evaluation of the evidence. Plant Physiol. 2015, 169, 1001–1005. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus spp.: Potent microfactories of bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tian, B.; Xiong, J.; Lin, G.; Cheng, L.; Zhang, T.; Li, X. Exploring IAA biosynthesis and plant growth promotion mechanism for tomato root endophytes with incomplete IAA synthesis pathways. Chem. Biol. Technol. Agric. 2024, 11, 187. [Google Scholar] [CrossRef]
- Valetti, L.; Iriarte, L.; Fabra, A. Growth promotion of rapeseed (Brassica napus) associated with the inoculation of phosphate solubilising bacteria. Appl. Soil. Ecol. 2018, 132, 1–10. [Google Scholar] [CrossRef]
- Katznelson, H.; Peterson, E.A.; Rouatt, J.W. Phosphate-dissolving microorganisms on seed and in the root zone of plants. Can. J. Bot. 1962, 40, 1181–1186. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Bargaz, A. Phosphate solubilising rhizobacteria could have a stronger influence on wheat root traits and aboveground physiology than rhizosphere P solubilisation. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, C.; Feng, B.; Chi, R. Phosphate rock solubilization and the potential for lead immobilization by a phosphate-solubilizing bacterium (Pseudomonas sp.). J. Environ. Sci. Health Part A 2020, 55, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kumar, V.; Usmani, Z.; Rani, R.; Chandra, A.; Gupta, V.K. Implications of plant growth promoting Klebsiella sp. CPSB4 and Enterobacter sp. CPSB49 in luxuriant growth of tomato plants under chromium stress. Chemosphere 2020, 240, 124944. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, H. Phosphate solubilizing microorganism: A green measure to effectively control and regulate heavy metal pollution in agricultural soils. Front. Microbiol. 2023, 14, 1193670. [Google Scholar] [CrossRef]
- Park, J.H.; Bolan, N.; Megharaj, M.; Naidu, R. Concomitant rock phosphate dissolution and lead immobilization by phosphate solubilizing bacteria (Enterobacter sp.). J. Environ. Manag. 2011, 92, 1115–1120. [Google Scholar] [CrossRef]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; Coba de la Peña, T.; Pueyo, J.J.; Talebi, M. Isolation and Characterization of Pb-Solubilizing Bacteria and Their Effects on Pb Uptake by Brassica juncea: Implications for Microbe-Assisted Phytoremediation. J. Microbiol. Biotechnol. 2018, 28, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Zou, C.; Shi, Z.; Yang, Y.; Zhang, J.; Hou, Y.; Zhang, N. The characteristics, enrichment, and migration mechanism of cadmium in phosphate rock and phosphogypsum of the Qingping phosphate deposit, Southwest China. Minerals 2023, 13, 107. [Google Scholar] [CrossRef]
- Huang, S.W.; Jin, J.Y. Status of heavy metals in agricultural soils as affected by different patterns of land use. Environ. Monit. Assess. 2008, 139, 317–327. [Google Scholar] [CrossRef]
- Cheraghi, M.; Lorestani, B.; Merrikhpour, H. Investigation of the effects of phosphate fertilizer application on the heavy metal content in agricultural soils with different cultivation patterns. Biol. Trace Elem. Res. 2012, 145, 87–92. [Google Scholar] [CrossRef]
- Yuquan, W.; Yue, Z.; Mingzi, S.; Zhenyu, C.; Qian, L.; Tianxue, Y.; Yuying, F.; Zimin, W. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar]
- Areesha, A.; Syed, F.M.; Iram, L.; Sadia, S.; Faiz, M.; Talat, M.; Urooj, Z. Isolation, Solubilization of Inorganic Phosphate, and Production of Organic Acids by Individual and Co-inoculated Microorganisms. Geomicrobiol. J. 2023, 40, 111–121. [Google Scholar]
- Yuan, Z.; Yi, H.; Wang, T.; Zhang, Y.; Zhu, X.; Yao, J. Application of phosphate solubilizing bacteria in immobilization of Pb and Cd in soil. Environ. Sci. Pollut. Res. 2017, 24, 21877–21884. [Google Scholar] [CrossRef]
- Miretzky, P.; Fernandez-Cirelli, A. Phosphates for Pb immobilization in soils: A review. Environ. Chem. Lett. 2008, 6, 121–133. [Google Scholar] [CrossRef]
- Han, H.; Kan, D.; Tian, M.; Ruan, Y. Phosphate-solubilizing bacteria reshaped the rhizosphere microbiome and metabolic profile of wheat to inhibit Cd absorption. Environ. Exp. Bot. 2024, 226, 105929. [Google Scholar] [CrossRef]
- Tao, Y.; Han, S.; Zhang, Q.; Yang, Y.; Shi, H.; Akindolie, M.S.; Jiao, Y.; Qu, J.; Jiang, Z.; Han, W.; et al. Application of biochar with functional microorganisms for enhanced atrazine removal and phosphorus utilization. J. Clean. Prod. 2020, 257, 120535. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Jiang, Y.; Ding, C.; Liu, J.; Zhu, C. Synergistic remediation of lead pollution by biochar combined with phosphate solubilizing bacteria. Sci. Total Environ. 2023, 861, 160649. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Wu, D.; Hu, Z. Enhanced phosphorus availability and cadmium remediation using phosphate-solubilizing bacteria-loaded biochar in contaminated soils. Environ. Technol. Innov. 2024, 36, 103878. [Google Scholar] [CrossRef]
- Teng, Z.; Zhao, X.; Yuan, J.; Li, M.; Li, T. Phosphate functionalized iron-based nanomaterials coupled with phosphate solubilizing bacteria as an efficient remediation system to enhance lead passivation in soil. J. Hazard. Mater. 2021, 419, 126433. [Google Scholar] [CrossRef]
- Qu, J.; Wei, S.; Liu, Y.; Zhang, X.; Jiang, Z.; Tao, Y.; Zhang, G.; Zhang, B.; Wang, L.; Zhang, Y. Effective lead passivation in soil by bone char/CMC-stabilized FeS composite loading with phosphate-solubilizing bacteria. J. Hazard. Mater. 2022, 423, 127043. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Zhu, X.; Zhu, F.; Ke, W.; Huang, Y.; Wu, C.; Xu, X.; Guo, J.; Xue, S. Organic acid release and microbial community assembly driven by phosphate-solubilizing bacteria enhance Pb, Cd, and As immobilization in soils remediated with iron-doped hydroxyapatite. J. Hazard. Mater. 2025, 488, 137340. [Google Scholar] [CrossRef]
- Montreemuk, J.; Stewart, T.N.; Prapagdee, B. Bacterial-assisted phytoremediation of heavy metals: Concepts, current knowledge, and future directions. Environ. Technol. Innov. 2024, 33, 103488. [Google Scholar] [CrossRef]
- Pouresmaieli, M.; Ataei, M.; Forouzandeh, P.; Azizollahi, P.; Mahmoudifard, M. Recent progress on sustainable phytoremediation of heavy metals from soil. J. Environ. Chem. Eng. 2022, 10, 108482. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Q.; Li, Y.; Chen, Y.; Jia, B.; Zhang, J.; Li, F.Y. Bio-organic fertilizer facilitated phytoremediation of heavy metal(loid)s-contaminated saline soil by mediating the plant-soil-rhizomicrobiota interactions. Sci. Total Environ. 2024, 922, 171278. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, Z.; Glick, B.R.; He, S.; Huang, C.; Wu, L. Co-occurrence patterns of microbial communities affected by inoculants of plant growth-promoting bacteria during phytoremediation of heavy metal-contaminated soils. Ecotoxicol. Environ. Saf. 2019, 183, 109504. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Gholamhosseini, M.; Yaghoubian, Y.; Pirdashti, H. Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul. 2020, 90, 123–136. [Google Scholar] [CrossRef]
- He, H.; Ye, Z.; Yang, D.; Yan, J.; Xiao, L.; Zhong, T.; Yuan, M.; Cai, X.; Fang, Z.; Jing, Y. Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere 2013, 90, 1960–1965. [Google Scholar] [CrossRef]
- He, T.; Xu, Z.-J.; Wang, J.-F.; Wang, F.-P.; Zhou, X.-F.; Wang, L.-L.; Li, Q.-S. Improving cadmium accumulation by Solanum nigrum L. via regulating rhizobacterial community and metabolic function with phosphate-solubilizing bacteria colonization. Chemosphere 2022, 287, 132209. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, H.; He, Y.; Chen, Z.; Yao, L.; Han, H. Improving radish phosphorus utilization efficiency and inhibiting Cd and Pb uptake by using heavy metal-immobilizing and phosphate-solubilizing bacteria. Sci. Total Environ. 2023, 868, 161685. [Google Scholar] [CrossRef]
- Li, K.; Ramakrishna, W. Effect of multiple metal resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J. Hazard. Mater. 2011, 189, 531–539. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, J.; Wang, G.; Hu, Z.; Luo, W.; Zhao, X.; Guo, Y.; Ji, X.; Hu, W.; Li, M. Phosphate-solubilizing bacteria improve the antioxidant enzyme activity of Potamogeton crispus L. and enhance the remediation effect on Cd-contaminated sediment. J. Hazard. Mater. 2024, 470, 134305. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sau, G.B.; Mukherjee, S.K. Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J. Microbiol. Biotechnol. 2009, 25, 1829–1836. [Google Scholar] [CrossRef]
- Vaxevanidou, K.; Christou, C.; Kremmydas, G.F.; Georgakopoulos, D.G.; Papassiopi, N. Role of indigenous arsenate and iron(III) respiring microorganisms in controlling the mobilization of arsenic in a contaminated soil sample. Bull. Environ. Contam. Toxicol. 2015, 94, 282–288. [Google Scholar] [CrossRef]
- Bruno, L.B.; Anbuganesan, V.; Karthik, C.; Kumar, A.; Banu, J.R.; Freitas, H.; Rajkumar, M. Enhanced phytoextraction of multi-metal contaminated soils under increased atmospheric temperature by bioaugmentation with plant growth promoting Bacillus cereus. J. Environ. Manag. 2021, 289, 112553. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S. Bacillus species enhance growth parameters of chickpea (Cicer arietinum L.) in chromium stressed soils. Food Chem. Toxicol. 2010, 48, 3262–3267. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, Z.; Zhang, N.; Deng, X.; Thomashow, L.S.; Lidbury, I.; Kowalchuk, G.A. Phosphorus availability influences disease-suppressive soil microbiome through plant-microbe interactions. Microbiome 2024, 12, 185. [Google Scholar] [CrossRef]
- Kumar, K.V.; Singh, N.; Behl, H.M.; Srivastava, S. Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere 2008, 72, 678–683. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Song, J.; Min, L.; Wu, J.; He, Q.; Chen, F.; Wang, Y. Response of the microbial community to phosphate-solubilizing bacterial inoculants on Ulmus chenmoui Cheng in Eastern China. PLoS ONE 2021, 16, e0247309. [Google Scholar] [CrossRef]
- He, T.; Xu, Z.-M.; Wang, J.-F.; Zhang, K.; Wang, F.-P.; Li, W.-L.; Tian, P.; Li, Q.-S. Inoculation of Escherichia coli enriched the key functional bacteria that intensified cadmium accumulation by halophyte Suaeda salsa in saline soils. J. Hazard. Mater. 2023, 458, 131922. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, L.; Lu, H.; Shao, Y.; Liu, S.; Fu, S. Drought promotes soil phosphorus transformation and reduces phosphorus bioavailability in a temperate forest. Sci. Total Environ. 2020, 732, 139295. [Google Scholar] [CrossRef]
- Christian, J.I.; Martin, E.R.; Basara, J.B.; Furtado, J.C.; Otkin, J.A.; Lowman, L.E.L.; Hunt, E.D.; Mishra, V.; Xiao, X. Global projections of flash drought show increased risk in a warming climate. Commun. Earth Environ. 2023, 4, 165. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Fujita, M.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Inter-tissue and inter-organ signaling in drought stress response and phenotyping of drought tolerance. Plant J. 2022, 109, 342–358. [Google Scholar] [CrossRef]
- Breitkreuz, C.; Buscot, F.; Tarkka, M.; Reitz, T. Shifts between and among populations of wheat rhizosphere Pseudomonas, Streptomyces and Phyllobacterium suggest consistent phosphate mobilization at different wheat growth stages under abiotic stress. Front.Microbiol. 2020, 10, 3109. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M.S.; Rizwan, M.R. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Chacon, N.; Flores, S.; Gonzalez, A. Implications of iron solubilization on soil phosphorus release in seasonally flooded forests of the lower Orinoco River, Venezuela. Soil Biol. Biochem. 2006, 38, 1494–1499. [Google Scholar] [CrossRef]
- Ibrahim, M.; Iqbal, M.; Tang, Y.T.; Khan, S.; Guan, D.X.; Li, G. Phosphorus mobilization in plant-soil environments and inspired strategies for managing phosphorus: A review. Agronomy 2022, 12, 2539. [Google Scholar] [CrossRef]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A. Benefits of phosphate solubilizing bacteria on belowground crop performance for improved crop acquisition of phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef]
- Khourchi, S.; Elhaissoufi, W.; Loum, M.; Ibnyasser, A.; Haddine, M.; Ghani, R.; Barakat, A.; Zeroual, Y.; Rchiad, Z. Phosphate solubilizing bacteria can significantly contribute to enhance P availability from polyphosphates and their use efficiency in wheat. Microbiol. Res. 2022, 262, 127094. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Raklami, A.; Tahiri, A.I.; Benidire, L.; Gottfert, M.; Oufdou, K. Phosphate-solubilizing rhizobacteria and their effects on the growth and phosphorus uptake by wheat plants. J. Plant Nutr. 2024, 47, 2811–2823. [Google Scholar] [CrossRef]
- Alemneh, A.A.; Cawthray, G.R.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Ability to produce indole acetic acid is associated with improved phosphate solubilising activity of rhizobacteria. Arch. Microbiol. 2021, 203, 3825–3837. [Google Scholar] [CrossRef]
- Yahya, M.; Islam, E.U.; Rasul, M.; Farooq, I.; Mahreen, N.; Tawab, A.; Irfan, M.; Rajput, L.; Amin, I.; Yasmin, S. Differential root exudation and architecture for improved growth of wheat mediated by phosphate solubilizing bacteria. Front. Microbiol. 2021, 12, 744094. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Rezaei, K.; Fayyaz, P.; Naghiha, R.; Namvar, Z. The effect of indigenous phosphate-solubilizing bacteria on Quercus brantii seedlings under water stress. J. Sustain. For. 2021, 40, 733747. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Batool, F.; Muhammad, M.; Zaman, W.; Mikhlef, R.M.; Qaddoori, S.Q.; Ullah, S.; Abdi, G.; Saqib, S. Unveiling the complex molecular dynamics of arbuscular mycorrhizae: A comprehensive exploration and future perspectives in harnessing phosphate-solubilizing microorganisms for sustainable progress. Environ. Exp. Bot. 2024, 219, 105633. [Google Scholar] [CrossRef]
- Ghorchiani, M.; Etesami, H.; Alikhani, H.A. Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric. Ecosyst. Environ. 2018, 258, 59–70. [Google Scholar] [CrossRef]
- Nacoon, S.; Seemakram, W.; Ekprasert, J.; Jogloy, S.; Kuyper, T.W.; Mongkolthanaruk, W.; Riddech, N.; Somdee, T.; Boonlue, S. Promoting growth and production of sunchoke(Helianthus tuberosus) by co-inoculation with phosphate solubilizing bacteria and arbuscular mycorrhizal fungi under drought. Front. Plant Sci. 2022, 13, 1022319. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Ding, X.; He, X.; Zhang, F.; Feng, G. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol. Biochem. 2014, 74, 177e–183. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Mahdavikia, H.; Subramanian, S.; Alipour, H.; Siddique, K.H.M.; Smith, D.L. Co-inoculation of Phosphate-Solubilizing Bacteria and Mycorrhizal Fungi: Effect on Seed Yield, Physiological Variables, and Fixed Oil and Essential Oil Productivity of Ajowan (Carum copticum L.) Under Water Deficit. J. Soil. Sci. Plant Nutr. 2021, 21, 3159–3179. [Google Scholar] [CrossRef]
- Petrillo, C.; Vitale, E.; Ambrosino, P.; Arena, C.; Isticato, R. Plant growth-promoting bacterial consortia as a strategy to alleviate drought stress in Spinacia oleracea. Microorganisms 2022, 10, 1798. [Google Scholar] [CrossRef]
- Culotta, V.C.; Yang, M.; O'Halloran, T.V. Activation of superoxide dismutatses: Putting the metal to the pedal. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 747–758. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, B.; Bisht, N.; Tewari, L. Role of beneficial microbial gene pool in mitigating salt/nutrient stress of plants in saline soils through underground phytostimulating signalling molecules. Pedosphere 2023, 33, 153–171. [Google Scholar] [CrossRef]
- Piotrwska-Niczyporuk, A.; Bajguz, A.; Kotowska, U.; Bralska, M.; Talarek-Karwel, M. Growth, metabolite profile, oxidative status, and phytohormone levels in the green alga Acutodesmus obliquus exposed to exogenous auxins and cytokinins. J. Plant Growth Regul. 2018, 37, 1159–1174. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Bacterial indole-3-acetic acid: A key regulator for plant growth, plant-microbe interactions, and agricultural sustainability. Soil. Biol. Biochem. 2022, 168, 127602. [Google Scholar]
- Kerchev, P.I.; Van Breusegem, F. Improving oxidative stress resilience in plants. Plant J. 2022, 109, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Sahandi, M.S.; Mehrafarin, A.; Badi, H.N.; Khalighi-Sigaroodi, F.; Sharifi, M. Improving growth, phytochemical, and antioxidant characteristics of peppermint by phosphate-solubilizing bacteria along with reducing phosphorus fertilizer use. Ind. Crops Prod. 2019, 141, 111777. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, M.-A.; Hamayun, M.; Kim, L.-R.; Kwon, E.-H.; Kang, Y.-S.; Kim, K.-Y.; Park, J.-J.; Lee, I.-J. Phosphate-solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 rescues alfalfa’s growth under post-drought stress. Agriculture 2021, 11, 485. [Google Scholar] [CrossRef]
- Azizi, A.; Bagnazari, M.; Mohammadi, M. Seaweed and phosphate-solubilizing bacteria biofertilizers ameliorate physiochemical traits and essential oil content of Calendula officinalis L. under drought stress. Sci. Hortic. 2024, 328, 112653. [Google Scholar] [CrossRef]
- Osman, H.S.; Rady, A.M.S.; Awadalla, A.; Omara, A.E.D.; Hafez, E.M. Improving the antioxidants system, growth, and sugar beet quality subjected to long-term osmotic stress by phosphate solubilizing bacteria and compost tea. Int. J. Plant Prod. 2022, 16, 119135. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana(L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere 2018, 28, 227–240. [Google Scholar] [CrossRef]
- Hu, Z.; Wei, S.; Li, W.; Wu, T.; Ullah, S.; Yang, M. Effect of inoculation with rhizosphere phosphate-solubilizing bacteria on the growth and physiological characteristics of Parashorea chinensis. Forests 2024, 15, 1932. [Google Scholar] [CrossRef]
- Haque, M.M.; Khatuna, M.; Mosharaf, M.K.; Rahman, A.; Haque, M.A.; Nahar, K. Biofilm producing probiotic bacteria enhance productivity and bioactive compounds in tomato. Biocatal. Agric. Biotechnol. 2023, 50, 102673. [Google Scholar] [CrossRef]
- Ameen, F.; AlYahya, S.A.; AlNadhari, S.; Alasmari, H.; Alhoshani, F.; Wainwright, M. Phosphate solubilising bacteria and fungi in desert soils: Species, limitations and mechanisms. Arch. Agron. Soil. Sci. 2019, 65, 1446–1459. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, V. Value added phytoremediation of metal stressed soils using phosphate solubilizing microbial consortium. World J. Microbiol. Biotechnol. 2017, 33, 9. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, D.; Talukdar, N.C.; Goswami, C.; Hazarika, S.; Kalita, M.C.; Bending, G.D. Evaluation of rice- legume- rice cropping system on grain yield, nutrient uptake, nitrogen fixation, and chemical, physical, and biological properties of soil. Biol. Fertil. Soils 2008, 45, 237–251. [Google Scholar] [CrossRef]
- Beheshti, M.; Alikhani, H.A.; Pourbabaee, A.A.; Etesami, H.; Asadi Rahmani, H.; Norouzi, M. Periphytic biofilm and rice rhizosphere phosphate-solubilizing bacteria and fungi: A possible use for activating occluded P in periphytic biofilms in paddy fields. Rhizosphere 2021, 19, 100395. [Google Scholar] [CrossRef]
- Lucero, C.T.; Lorda, G.S.; Luduena, L.M.; Anzuay, M.S.; Taurian, T. Motility and biofilm production involved in the interaction of phosphate solubilizing endophytic strains with peanut, maize and soybean plants. Rhizosphere 2020, 15, 100228. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Liu, H.; Todd, J.L.; Luo, H. Turfgrass salinity stress and tolerance—A review. Plants 2023, 12, 925. [Google Scholar] [CrossRef]
- Li, Z.; Kekeli, M.A.; Jiang, Y.; Rui, Y. Progress and Prospect of Saline-Alkaline Soil Management Technology: A Review. Appl. Sci. 2025, 15, 4567. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.C.; Ridoutt, B.G.; Wang, X.C.; Ren, P.A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Munns, R. Evolution of approaches to increase the salt tolerance of crops. Crit. Rev. Plant Sci. 2022, 41, 128–160. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Q.; Huang, L.; Xu, S.; Fu, Y.; Hou, D.; Feng, Y.; Yang, X. Cadmium phytoextraction through Brassica juncea L. under different consortia of plant growth-promoting bacteria from different ecological niches. Ecotoxicol. Environ. Saf. 2022, 237, 113541. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant growth enhancement using rhizospheric halotolerant phosphate solubilizing bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, X.; Zheng, F.; Zhang, Z.; Wang, Z.; Qu, L.; Hong, X. Salt- alkali-resistant phosphate-solubilizing bacterium: Kushneria sp. YCWA18 improves soil available phosphorus and promotes the growth of Suaeda salsa. J. Plant Growth Regul. 2024, 43, 272282. [Google Scholar] [CrossRef]
- Tchakounté, G.V.T.; Berger, B.; Patz, S.; Becker, M.; Fankem, H.; Taffouo, V.D.; Ruppel, S. Selected rhizosphere bacteria help tomato plants cope with combined phosphorus and salt stresses. Microorganisms 2020, 8, 1844. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Gonzalez-Mendoza, D. Isolation and identification of phosphate solubilizing Bacillus spp. from Tamarix ramosissima rhizosphere and their effect on growth of Phaseolus vulgaris under salinity stress. Geomicrobiol. J. 2020, 37, 901–908. [Google Scholar] [CrossRef]
- Chegeni, A.R.; Fatehi, F.; Ebrahimi, A.; Maleki, M. Phosphate-solubilizing bacteria modulated salinity stress in the presence of phosphorous through improving growth, biochemical properties, and gene expression of chickpea (Cicer arietinum L.). J. Soil Sci. Plant Nutr. 2023, 23, 4450–4462. [Google Scholar] [CrossRef]
- Belkebla, N.; Bessai, S.A.; Melo, J.; Caeiro, M.F.; Cruz, C.; Nabti, E.H. Restoration of Triticum aestivum growth under salt stress by phosphate-solubilizing bacterium isolated from southern Algeria. Agronomy 2022, 12, 2050. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Datta, R. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Ding, J.; Zhu, D.; Hu, H.-W.; Delgado-Baquerizo, M.; Ma, Y.-B.; He, J.-Z.; Zhu, Y.-G. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol. Biochem. 2020, 141, 107686. [Google Scholar] [CrossRef]
- Li, M.; Zhou, W.; Sun, M.; Shi, W.; Lun, J.; Zhou, B.; Gao, Z. Decoupling soil community structure, functional composition, and nitrogen metabolic activity driven by salinity in coastal wetlands. Soil Biol. Biochem. 2024, 198, 109547. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P.; et al. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Tunesi, S.; Poggi, V.; Gessa, C. Phosphate adsorption and precipitation in calcareous soils: The role of calcium ions in solution and carbonate minerals. Nutr. Cycl. Agroecosyst. 1999, 53, 219–227. [Google Scholar] [CrossRef]
- Bi, W.; Weng, B.; Yan, D.; Wang, H.; Wang, M.; Yan, S.; Jing, L.; Liu, T.; Chang, W. Responses of Phosphate-Solubilizing Microorganisms Mediated Phosphorus Cycling to Drought-Flood Abrupt Alternation in Summer Maize Field Soil. Front. Microbiol. 2022, 12, 768921. [Google Scholar] [CrossRef]
- Raymond, N.S.; Gómez-Muñoz, B.; van der Bom, F.J.T.; Nybroe, O.; Jensen, L.S.; Müller-Stöver, D.S.; Oberson, A.; Richardson, A.E. Phosphate-solubilising microorganisms for improved crop productivity: A critical assessment. New Phytol. 2021, 229, 1268–1277. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Saleem, M.H.; Ali, B.; Mussart, M.; Ullah, R.; Arif, M.; Ahmad, M.; Shah, W.A.; Romman, M.; et al. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12, 11997. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Wang, Y.; Wang, X.; Liu, P.; Han, M.; Zhou, W. Improving soil phosphorus availability in saline areas by marine bacterium Bacillus paramycoides. Environ. Sci. Pollut. Res. 2023, 30, 112385–112396. [Google Scholar] [CrossRef]
- Yu, D.; Miao, Q.; Shi, H.; Feng, Z.; Feng, W. Effects of combined application of organic and inorganic fertilizers on physical and chemical properties in saline-alkali soil. Agronomy 2024, 14, 2236. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, Z.; Han, F.; Chen, S.; Zhou, W. Integrated application of phosphorus-accumulating bacteria and phosphorus-solubilizing bacteria to achieve sustainable phosphorus management in saline soils. Sci. Total Environ. 2023, 885, 163971. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, P.; Wang, T.; Chi, X.; Wang, M.; Chen, M.; Chen, N.; Pan, L. Role of halotolerant phosphate-solubilising bacteria on growth promotion of peanut (Arachis hypogaea) under saline soil. Ann. Appl. Biol. 2019, 174, 20–30. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D.; Arsey, S.; Yadav, D.K.; Gubre, D.F.; Naroju, S.P.; Saini, P. Biochemical characterization and metabolic activities of salt tolerant phosphate solubilizing bacteria isolated from red soil. Cogent Food Agric. 2025, 11, 2472246. [Google Scholar] [CrossRef]
- Luo, H.; Riu, M.; Ryu, C.-M.; Yu, J.M. Volatile organic compounds emitted by Burkholderia pyrrocinia CNUC9 trigger induced systemic salt tolerance in Arabidopsis thaliana. Front. Microbiol. 2022, 13, 1050901. [Google Scholar] [CrossRef]
- Joe, M.M.; Devaraj, S.; Benson, A.; Sa, T. Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum& Thonn: Evaluation of plant growth promotion and antioxidant activity under salt stress. J. Appl. Res. Med. Aromat. Plants 2016, 3, 71–77. [Google Scholar]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil. Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Lal, R. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Hasanuzzaman, M. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Dai, Y.; Chen, Y.; Tian, Y.; Huo, Z. Isolation and screening of phosphorus solubilizing bacteria from saline alkali soil and their potential for Pb pollution remediation. Front. Bioeng. Biotechnol. 2023, 11, 1134310. [Google Scholar] [CrossRef] [PubMed]
- Tripti; Kumar, A.; Maleva, M.; Borisova, G.; Rajkumar, M. Amaranthus biochar-based microbial cell composites for alleviation of drought and cadmium stress: A novel bioremediation approach. Plants 2023, 12, 1973. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Jaime-Pérez, N.; Pineda, B.; Garcia-Sogo, B.; Atares, A.; Athman, A.; Byrt, C.S.; Belver, A. The sodium transporter encoded by the HKT1;2 gene modulates sodium/potassium homeostasis in tomato shoots under salinity. Plant Cell Environ. 2017, 40, 658–671. [Google Scholar] [CrossRef]
- Ramakrishna, P.; Gámez-Arjona, F.M.; Bellani, E.; Martin-Olmos, C.; Escrig, S.; De Bellis, D.; Meibom, A. Elemental cryo-imaging reveals SOS1-dependent vacuolar sodium accumulation. Nature 2025, 637, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Safdarian, M.; Askari, H.; Nematzadeh, G.; Sofo, A. Halophile plant growth-promoting rhizobacteria induce salt tolerance traits in wheat seedlings (Triticum aestivum L.). Pedosphere 2020, 30, 684–693. [Google Scholar] [CrossRef]
- Asins, M.J.; Villalta, I.; Aly, M.M.; Olías, R.; Álvarez de Morales, P.; Huertas, R.; Li, J.; Jaime-Pérez, N.; Haro, R.; Raga, V.; et al. Two closely linked tomato HKT coding genes are positional candidates for the major tomato QTL involved in Na+/K+ homeostasis. Plant Cell Environ. 2013, 36, 1171–1191. [Google Scholar] [CrossRef]
- Jia, Q.; Zheng, C.; Sun, S.; Amjad, H.; Liang, K.; Lin, W. The role of plant cation/proton antiporter gene family in salt tolerance. Biol. Plantarum 2018, 62, 617–629. [Google Scholar] [CrossRef]
- Arroyo-Olarte, R.D.; Bravo Rodriguez, R.; Morales-Rios, E. Genome editing in bacteria: CRISPR-Cas and beyond. Microorganisms 2021, 9, 844. [Google Scholar] [CrossRef]
- Silva, U.C.; Cuadros-Orellana, S.; Silva, D.R.; Freitas-Júnior, L.F.; Fernandes, A.C.; Leite, L.R.; Santos, V.L. Genomic and phenotypic insights into the potential of rock phosphate solubilizing bacteria to promote millet growth in vivo. Front. Microbiol. 2021, 11, 574550. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodriguez, S.; Garcia-Aznar, J.M.; Gonzalo-Asensio, J. Microfluidic devices for studying bacterial taxis, drug testing and biofilm formation. Microb. Biotechnol. 2022, 15, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.C.; An, B.; Huang, Y.; Vasikaran, S.; Wang, Y.; Jiang, X.; Zhong, C. Materials design by synthetic biology. Nat. Rev. Mater. 2021, 6, 332–350. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Rehman, A.U.; Umar, U.U.D. Synergistic interplay of microbial probiotics in rice rhizosphere: A sustainable strategy for bacterial blight management through microbiome engineering. Physiol. Mol. Plant Pathol. 2025, 136, 102568. [Google Scholar] [CrossRef]
- Saxena, J.; Saini, A.; Ravi, I.; Chandra, S.; Garg, V. Consortium of phosphate-solubilising bacteria and fungi for promotion of growth and yield of chickpea (Cicer arietinum). J. Crop Improv. 2015, 29, 353–369. [Google Scholar] [CrossRef]
- Li, J.T.; Lu, J.L.; Wang, H.Y.; Fang, Z.; Wang, X.J.; Feng, S.W.; Wang, Z.W.; Yuan, T.; Zhang, S.C.; Ou, S.N.; et al. A comprehensive synthesis unveils the mysteries of phosphate-solubilizing microbes. Biol. Rev. 2021, 96, 2771–2793. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, X.; Wang, L.; Han, M.; Zhang, C.; Ye, X. Lead remediation is promoted by phosphate-solubilizing fungi and apatite via the enhanced production of organic acid. Front. Bioeng. Biotechnol. 2023, 11, 1180431. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, Z.; Ma, T.; Zhang, X.; Wang, R.; Liu, Y.; Li, J. Phosphorus excess changes rock phosphate solubilization level and bacterial community mediating phosphorus fractions mobilization during composting. Bioresour. Technol. 2021, 337, 125433. [Google Scholar] [CrossRef]
- Hu, M.; Peñuelas, J.; Sardans, J.; Tong, C.; Chang, C.T.; Cao, W. Dynamics of phosphorus speciation and the phoD phosphatase gene community in the rhizosphere and bulk soil along an estuarine freshwater-oligohaline gradient. Geoderma 2020, 365, 114236. [Google Scholar] [CrossRef]
- Wang, G.; George, T.S.; Pan, Q.; Feng, G.; Zhang, L. Two isolates of Rhizophagus irregularis select different strategies for improving plants phosphorus uptake at moderate soil P availability. Geoderma 2022, 421, 115910. [Google Scholar] [CrossRef]
- Zhang, K.; Teng, Z.; Shao, W.; Wang, Y.; Li, M.; Lam, S.S. Effective passivation of lead by phosphate solubilizing bacteria capsules containing tricalcium phosphate. J. Hazard. Mater. 2020, 397, 122754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, X.; Gou, Y.; Zhang, W.; Cui, B.; Xing, R.; Tang, Z. Living-loaded hydrogel: Strategies for loading living, interactions between loaded living and hydrogel, and applications. Eur. Polym. J. 2024, 213, 113130. [Google Scholar] [CrossRef]


| PSM Strain | Strain Source | Plant Species | Effects | Mechanisms | Reference |
|---|---|---|---|---|---|
| Bacillus pumilus | Quinoa fields in Morocco | Chenopodium quinoa | Seed germination rate increased by 305%; seedling length increased by 211% | Organic acid secretion; IAA and siderophores production; biofilm formation | [157] |
| Kushneria sp. | Saline soil on the Coast of Yellow Sea of China | Suaeda salsa | Available phosphorus increased by more than 10 times; plant height and biomass increased by 1.5–10 times | Organic acid secretion | [158] |
| Arthrobacter sp.; Bacillus sp. | Rhizosphere of maize in Cameroon | Solanum lycopersicum | Plant height increased by 24.1%; dry weight increased by 73.5%; total biomass increased by 115% | Organic acid secretion | [159] |
| Bacillus megaterium | Rhizosphere of Tamarix ramosissima in Mexicali valley | Phaseolus vulgaris | Root length increased by 151%; root dry weight increased by 188%; phosphorus content increased by 114% | Organic acid secretion; enhanced photosynthesis | [160] |
| Bacillus pumilus; Bacillus amyloliquefaciens | Laboratory collection | Cicer arientnum | Shoot dry weight increased by 34%; leaf phosphorus content increased by 600%; total chlorophyll content increased by 32% | Organic acid secretion; phosphatase production; H+-ATPase activation; enhanced antioxidant system | [161] |
| Pseudomonas azotoformans | Agricultural field in southern Algeria | Triticum aestivum | Wheat seed germination rate increased to 68.88%; fresh weight increased by 99.68% | Organic acid secretion; phosphatase production | [162] |
| Bacillus sp.; Burkholderia sp. | Laboratory collection | Zea mays | Corn height increased by 5.6%; shoot biomass increased by 7.8% | Organic acid secretion; IAA, siderophore, and axine production | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, Z.; Li, Q.; Hu, Z. Alleviation of Plant Abiotic Stress: Mechanistic Insights into Emerging Applications of Phosphate-Solubilizing Microorganisms in Agriculture. Plants 2025, 14, 1558. https://doi.org/10.3390/plants14101558
Wang X, Li Z, Li Q, Hu Z. Alleviation of Plant Abiotic Stress: Mechanistic Insights into Emerging Applications of Phosphate-Solubilizing Microorganisms in Agriculture. Plants. 2025; 14(10):1558. https://doi.org/10.3390/plants14101558
Chicago/Turabian StyleWang, Xiujie, Zhe Li, Qi Li, and Zhenqi Hu. 2025. "Alleviation of Plant Abiotic Stress: Mechanistic Insights into Emerging Applications of Phosphate-Solubilizing Microorganisms in Agriculture" Plants 14, no. 10: 1558. https://doi.org/10.3390/plants14101558
APA StyleWang, X., Li, Z., Li, Q., & Hu, Z. (2025). Alleviation of Plant Abiotic Stress: Mechanistic Insights into Emerging Applications of Phosphate-Solubilizing Microorganisms in Agriculture. Plants, 14(10), 1558. https://doi.org/10.3390/plants14101558






