Soil-Gradient-Derived Bacterial Synthetic Communities Enhance Drought Tolerance in Quercus pubescens and Sorbus domestica Seedlings
Abstract
1. Introduction
2. Results
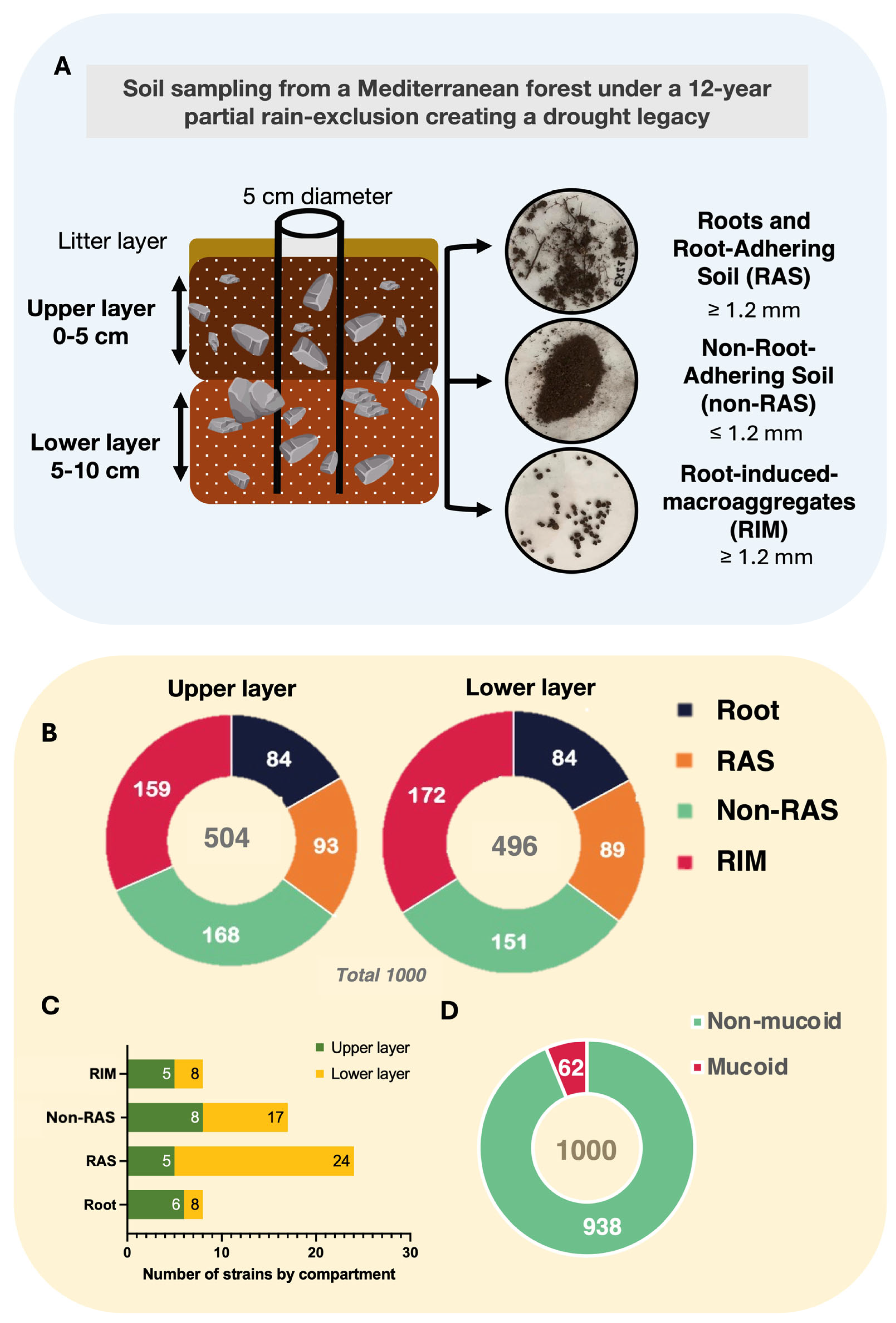
2.1. Bacterial Strain Isolation
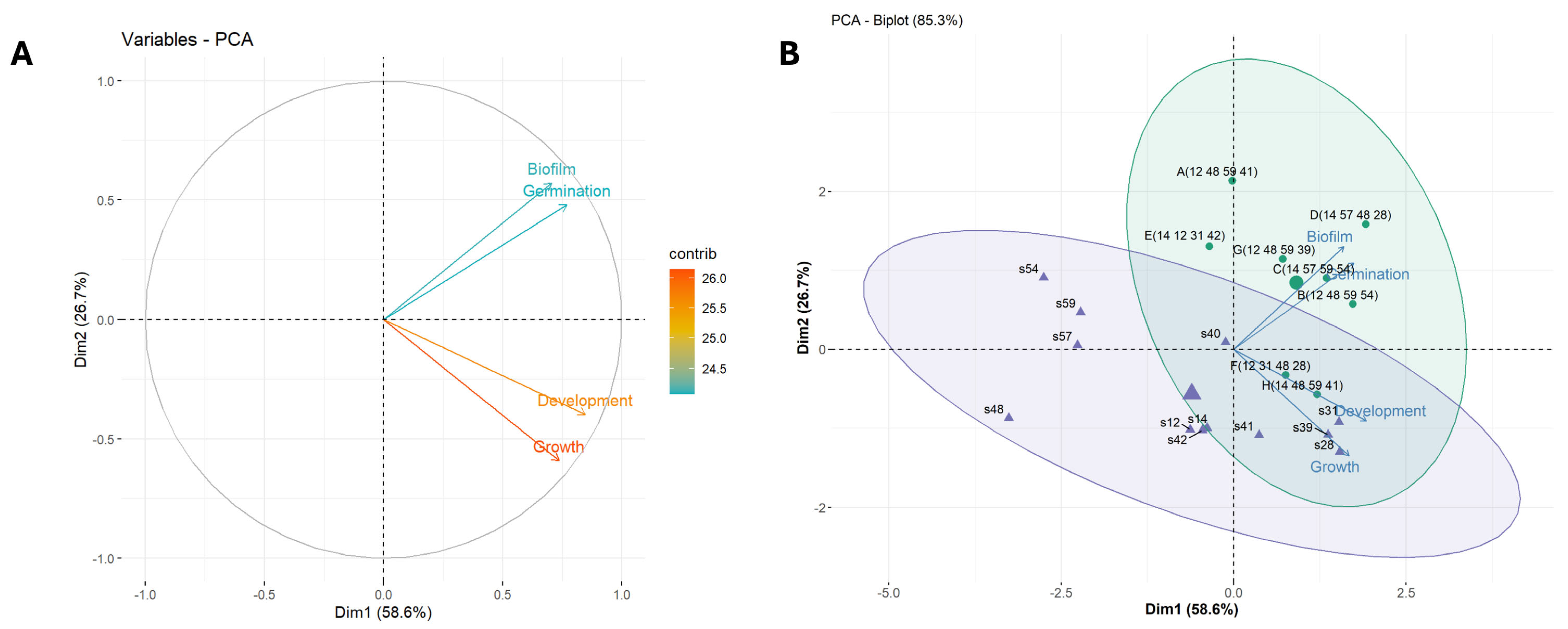
2.2. Multivariate Analysis of Strain Clustering Based on Functional Traits, Spatial and Nutrient Gradients
2.3. Designing Synthetic Communities (SynComs)
2.4. Biofilm Assay
2.5. In Vitro Inoculation of Arabidopsis with Single Bacterial Strains or Consortia
2.6. Data-Driven Selection of Top-Performing SynCom Candidates
2.7. SynComs and Single Inoculants to Mitigate Drought Stress in Tree Seedlings
2.8. Prediction of Inoculant Efficacy for Drought Protection of Quercus and Sorbus Seedlings
2.9. Quercus pubescens
2.10. Sorbus domestica
3. Discussion
3.1. From Soil Niches to Synthetic Communities (SynCom)
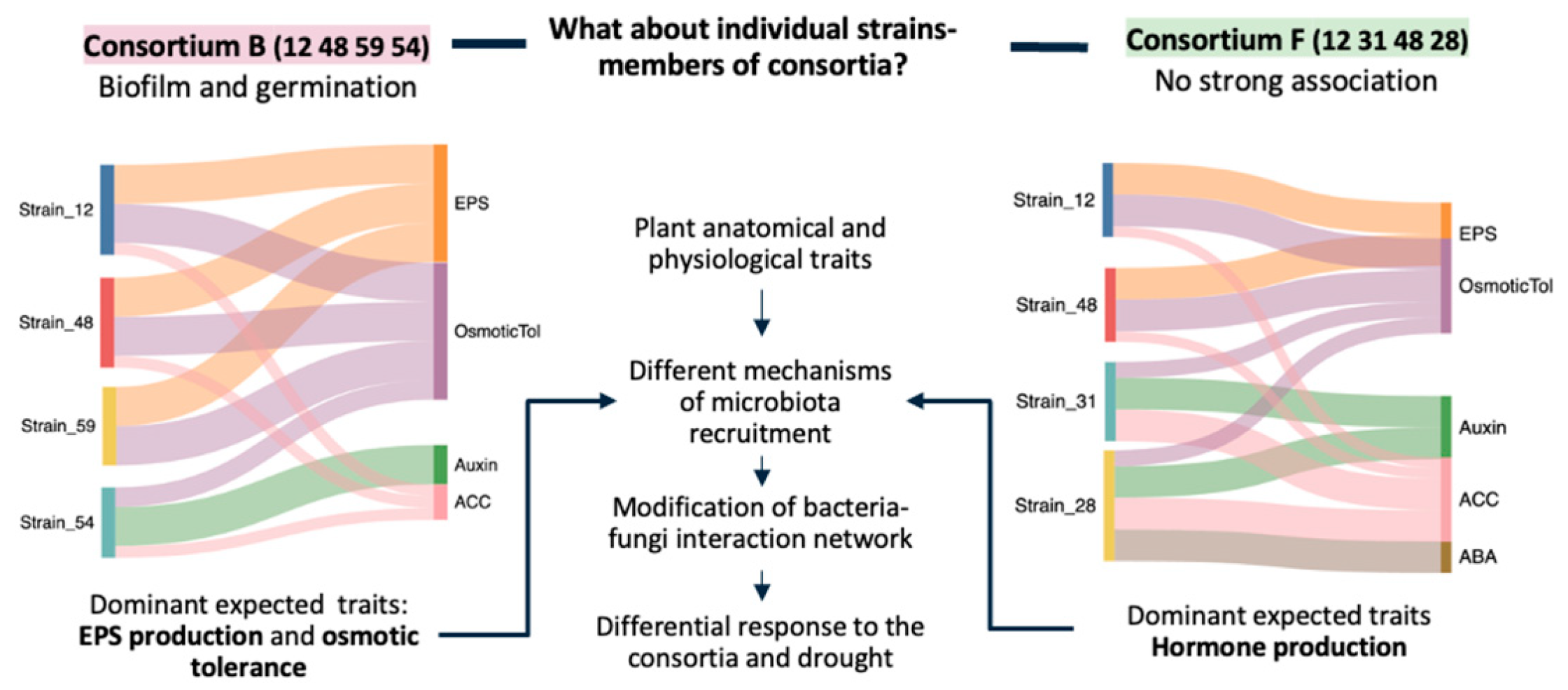
3.2. Stronger Together? How Weak Biofilm Formers Drive Collective Performance
3.3. Teaming up Underground: Optimizing SynCom by Matching Microbial Skills to Root Strategies
3.4. Beyond Survival: Predictive Insights into SynCom Function and Drought Tolerance in Trees
4. Materials and Methods
4.1. Soil Sampling
4.2. Strain Isolation and Growth
4.3. Strain Screening for Specific Traits
4.4. Ability to Form Biofilms
4.5. Strain Identification
4.6. Test of Single Strains and Consortia on Arabidopsis thaliana
4.7. Greenhouse Experiments on Trees
4.8. Statistical Analyses
4.9. Multivariate Analysis-Driven Selection of the Best Candidate Strains for SynComs Selection
4.10. Statistical Analyses of Plant Symptoms and Growth Parameters
4.11. Survival Models
4.12. Multinomial Logistic Regression
4.13. Use of GenAI
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. IPCC Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Cambridge University Press: New York, NY, USA, 2023; ISBN 978-1-009-32584-4. [Google Scholar]
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess Forest Mortality Is Consistently Linked to Drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef] [PubMed]
- Bevacqua, E.; Rakovec, O.; Schumacher, D.; Kumar, R.; Thober, S.; Samaniego, L.; Seneviratne, S.; Zscheischler, J. Direct and Lagged Climate Change Effects Strongly Intensified the Widespread 2022 European Drought. Nat. Geosci. 2024, 17, 1100–1107. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Balachowski, J.A.; Flores, D.; Horning, M.E. Reforestation to Mitigate Changes to Climate: More than Just Planting Seedlings. In XV World Forestry Congress: Building a Green, Healthy, and Resilient Future with Forests, Coex, Seoul, Republic of Korea, 2–6 May 2022; Food and Agriculture Organization of the United Nations: Rome, Italy; Korean Forest Service: Daejeon, Republic of Korea, 2022; p. 7. [Google Scholar]
- Knutzen, F.; Averbeck, P.; Barrasso, C.; Bouwer, L.M.; Gardiner, B.; Grünzweig, J.M.; Hänel, S.; Haustein, K.; Johannessen, M.R.; Kollet, S.; et al. Impacts on and Damage to European Forests from the 2018–2022 Heat and Drought Events. Nat. Hazards Earth Syst. Sci. 2025, 25, 77–117. [Google Scholar] [CrossRef]
- Puértolas, J.; Villar-Salvador, P.; Andivia, E.; Ahuja, I.; Cocozza, C.; Cvjetković, B.; Devetaković, J.; Diez, J.J.; Fløistad, I.S.; Ganatsas, P.; et al. Die-Hard Seedlings. A Global Meta-Analysis on the Factors Determining the Effectiveness of Drought Hardening on Growth and Survival of Forest Plantations. For. Ecol. Manag. 2024, 572, 122300. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Meddich, A.; Baslam, M. Plant-Microbiome Interactions under Drought—Insights from the Molecular Machinist’s Toolbox. Front. Sustain. Food Syst. 2023, 7, 1253735. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, Q.; Hao, Y.; Huang, A.C. Crafting the Plant Root Metabolome for Improved Microbe-assisted Stress Resilience. New Phytol. 2022, 234, 1945–1950. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Iqbal, S.; Wang, X.; Mubeen, I.; Kamran, M.; Kanwal, I.; Diaz, G.A.; Abbas, A.; Parveen, A.; Atiq, M.N.; Alshaya, H.; et al. Phytohormones Trigger Drought Tolerance in Crop Plants: Outlook and Future Perspectives. Front. Plant Sci. 2022, 12, 799318. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Pereira, A.d.E.S.; Aleksieienko, I.; Carmo, G.C.d.; Gohari, G.; Santaella, C.; Fraceto, L.F.; Oliveira, H.C. Encapsulated Plant Growth Regulators and Associative Microorganisms: Nature-Based Solutions to Mitigate the Effects of Climate Change on Plants. Plant Sci. 2023, 331, 111688. [Google Scholar] [CrossRef]
- Tiepo, A.N.; Constantino, L.V.; Madeira, T.B.; Gonçalves, L.S.A.; Pimenta, J.A.; Bianchini, E.; de Oliveira, A.L.M.; Oliveira, H.C.; Stolf-Moreira, R. Plant Growth-Promoting Bacteria Improve Leaf Antioxidant Metabolism of Drought-Stressed Neotropical Trees. Planta 2020, 251, 83. [Google Scholar] [CrossRef] [PubMed]
- Tiepo, A.N.; Hertel, M.F.; Rocha, S.S.; Calzavara, A.K.; De Oliveira, A.L.M.; Pimenta, J.A.; Oliveira, H.C.; Bianchini, E.; Stolf-Moreira, R. Enhanced Drought Tolerance in Seedlings of Neotropical Tree Species Inoculated with Plant Growth-Promoting Bacteria. Plant Physiol. Biochem. 2018, 130, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Heydari, M.; Alikhani, H.A.; Arani, A.M.; Guidi, L.; Bernard, P. Mitigating Negative Impacts of Drought on Oak Seedlings Performances through Plant Growth-Promoting Rhizobacteria. J. Environ. Manag. 2025, 375, 124163. [Google Scholar] [CrossRef]
- Poudel, M.; Mendes, R.; Costa, L.A.; Bueno, C.G.; Meng, Y.; Folimonova, S.Y.; Martins, S.J. The Role of Plant-Associated Bacteria, Fungi, and Viruses in Drought Stress Mitigation. Front. Microbiol. 2021, 12, 743512. [Google Scholar] [CrossRef]
- Carballo-Sánchez, M.P.; Alarcón, A.; Pérez-Moreno, J.; Ferrera-Cerrato, R. Agricultural and Forestry Importance of Microorganism-Plant Symbioses: A Microbial Source for Biotechnological Innovations. Rev. Agric. Sci. 2022, 10, 344–355. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Liu, X.; Mei, S.; Salles, J.F. Inoculated Microbial Consortia Perform Better than Single Strains in Living Soil: A Meta-Analysis. Appl. Soil Ecol. 2023, 190, 105011. [Google Scholar] [CrossRef]
- Mehlferber, E.C.; Arnault, G.; Joshi, B.; Partida-Martinez, L.P.; Patras, K.A.; Simonin, M.; Koskella, B. A Cross-Systems Primer for Synthetic Microbial Communities. Nat. Microbiol. 2024, 9, 2765–2773. [Google Scholar] [CrossRef]
- Xu, X.; Dinesen, C.; Pioppi, A.; Kovács, Á.T.; Lozano-Andrade, C.N. Composing a Microbial Symphony: Synthetic Communities for Promoting Plant Growth. Trends Microbiol. 2025. [Google Scholar] [CrossRef]
- Stock, S.C.; Koester, M.; Boy, J.; Godoy, R.; Nájera, F.; Matus, F.; Merino, C.; Abdallah, K.; Leuschner, C.; Spielvogel, S.; et al. Plant Carbon Investment in Fine Roots and Arbuscular Mycorrhizal Fungi: A Cross-Biome Study on Nutrient Acquisition Strategies. Sci. Total Environ. 2021, 781, 146748. [Google Scholar] [CrossRef]
- Bergmann, J.; Weigelt, A.; van der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M.; et al. The Fungal Collaboration Gradient Dominates the Root Economics Space in Plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zeng, X.; McCormack, M.L.; Fernandez, C.W.; Yang, Y.; Guo, H.; Xi, M.; Liu, Y.; Qi, X.; Liang, S.; et al. Linking root-associated fungal and bacterial functions to root economics. eLife 2024, 13, RP94359. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of Root System Architecture to Water Stress at Multiple Levels: A Meta-Analysis of Trials under Controlled Conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef]
- Bauke, S.L.; Amelung, W.; Bol, R.; Brandt, L.; Brüggemann, N.; Kandeler, E.; Meyer, N.; Or, D.; Schnepf, A.; Schloter, M.; et al. Soil Water Status Shapes Nutrient Cycling in Agroecosystems from Micrometer to Landscape Scales. J. Plant Nutr. Soil Sci. 2022, 185, 773–792. [Google Scholar] [CrossRef]
- Platt, T.G. Community Outcomes Depend on Cooperative Biofilm Structure. Proc. Natl. Acad. Sci. USA 2023, 120, 2221624120. [Google Scholar] [CrossRef]
- Laoué, J.; Havaux, M.; Ksas, B.; Orts, J.P.; Reiter, I.M.; Fernandez, C.; Ormeno, E. A Decade of Rain Exclusion in a Mediterranean Forest Reveals Trade-Offs of Leaf Chemical Defenses and Drought Legacy Effects. Sci. Rep. 2024, 14, 24119. [Google Scholar] [CrossRef]
- Belviso, S.; Reiter, I.M.; Loubet, B.; Gros, V.; Lathière, J.; Montagne, D.; Delmotte, M.; Ramonet, M.; Kalogridis, C.; Lebegue, B.; et al. A Top-down Approach of Surface Carbonyl Sulfide Exchange by a Mediterranean Oak Forest Ecosystem in Southern France. Atmos. Chem. Phys. 2016, 16, 14909–14923. [Google Scholar] [CrossRef]
- Knights, H.E.; Jorrin, B.; Haskett, T.L.; Poole, P.S. Deciphering Bacterial Mechanisms of Root Colonization. Environ. Microbiol. Rep. 2021, 13, 428–444. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, Variation, and Assembly of the Root-Associated Microbiomes of Rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef]
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.A.; Meier, I.C.; Kögel-Knabner, I.; Mueller, C.W. Root Exudates Induce Soil Macroaggregation Facilitated by Fungi in Subsoil. Front. Environ. Sci. 2018, 6, 140. [Google Scholar] [CrossRef]
- Leontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Madesis, P.; Vokou, D.; Karamanoli, K.; Mellidou, I. Plant Growth Promoting Rhizobacteria Isolated from Halophytes and Drought-Tolerant Plants: Genomic Characterisation and Exploration of Phyto-Beneficial Traits. Sci. Rep. 2020, 10, 14857. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, C.; Vitale, E.; Ambrosino, P.; Arena, C.; Isticato, R. Plant Growth-Promoting Bacterial Consortia as a Strategy to Alleviate Drought Stress in Spinacia Oleracea. Microorganisms 2022, 10, 1798. [Google Scholar] [CrossRef] [PubMed]
- Benmrid, B.; Ghoulam, C.; Zeroual, Y.; Kouisni, L.; Bargaz, A. Bioinoculants as a Means of Increasing Crop Tolerance to Drought and Phosphorus Deficiency in Legume-Cereal Intercropping Systems. Commun. Biol. 2023, 6, 1016. [Google Scholar] [CrossRef]
- Ren, D.; Madsen, J.S.; Sørensen, S.J.; Burmølle, M. High Prevalence of Biofilm Synergy among Bacterial Soil Isolates in Cocultures Indicates Bacterial Interspecific Cooperation. ISME J. 2015, 9, 81–89. [Google Scholar] [CrossRef]
- Yang, N.; Nesme, J.; Røder, H.L.; Li, X.; Zuo, Z.; Petersen, M.; Sørensen, S.J. Emergent Bacterial Community Properties Induce Enhanced Drought Tolerance in Arabidopsis. npj Biofilms Microbiomes 2021, 7, 82. [Google Scholar] [CrossRef]
- Lange, M.A. Climate Change in the Mediterranean: Environmental Impacts and Extreme Events. IEMed Mediterr. Yearb. 2020, 2020, 224–229. [Google Scholar]
- Meisner, A.; Jacquiod, S.; Snoek, B.L.; ten Hooven, F.C.; van der Putten, W.H. Drought Legacy Effects on the Composition of Soil Fungal and Prokaryote Communities. Front. Microbiol. 2018, 9, 294. [Google Scholar] [CrossRef]
- Tang, Y.; Winterfeldt, S.; Brangarí, A.C.; Hicks, L.C.; Rousk, J. Higher Resistance and Resilience of Bacterial Growth to Drought in Grasslands with Historically Lower Precipitation. Soil Biol. Biochem. 2023, 177, 108889. [Google Scholar] [CrossRef]
- Alotaibi, F.; St-Arnaud, M.; Hijri, M. In-Depth Characterization of Plant Growth Promotion Potentials of Selected Alkanes-Degrading Plant Growth-Promoting Bacterial Isolates. Front. Microbiol. 2022, 13, 863702. [Google Scholar] [CrossRef]
- Manetsberger, J.; Gómez, N.C.; Soria-Rodríguez, C.; Benomar, N.; Abriouel, H. Simply Versatile: The Use of Peribacillus simplex in Sustainable Agriculture. Microorganisms 2023, 11, 2540. [Google Scholar] [CrossRef]
- Ma, Y.; Yin, Y.; Rong, C.; Chen, S.; Liu, Y.; Wang, S.; Xu, F. Pantoea pleuroti Sp. Nov., Isolated from the Fruiting Bodies of Pleurotus eryngii. Curr. Microbiol. 2016, 72, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, J.; Pukall, R.; Stackebrandt, E. Carbon Source Utilization Patterns of Bacillus simplex Ecotypes Do Not Reflect Their Adaptation to Ecologically Divergent Slopes in ‘Evolution Canyon, Israel. FEMS Microbiol. Ecol. 2008, 66, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Steenkamp, E.T.; Coetzee, M.P.; Avontuur, J.R.; Chan, W.Y.; van Zyl, E.; Venter, S.N. Mixta Gen. Nov., a New Genus in the Erwiniaceae. Int. J. Syst. Evol. Microbiol. 2018, 68, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Dragone, N.B.; Hoffert, M.; Strickland, M.S.; Fierer, N. Taxonomic and Genomic Attributes of Oligotrophic Soil Bacteria. ISME Commun. 2024, 4, 081. [Google Scholar] [CrossRef]
- Verhille, S.; Baida, N.; Dabboussi, F.; Hamze, M.; Izard, D.; Leclerc, H. Pseudomonas gessardii Sp. Nov. and Pseudomonas migulae Sp. Nov., Two New Species Isolated from Natural Mineral Waters. Int. J. Syst. Evol. Microbiol. 1999, 49, 1559–1572. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Evidence of Endophytic Diazotrophic Bacteria in Lodgepole Pine and Hybrid White Spruce Trees Growing in Soils with Different Nutrient Statuses in the West Chilcotin Region of British Columbia, Canada. For. Ecol. Manag. 2018, 430, 558–565. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Dobritsa, A.P.; Linardopoulou, E.V.; Samadpour, M. Transfer of 13 Species of the Genus burkholderia to the Genus Caballeronia and Reclassification of Burkholderia jirisanensis as Paraburkholderia jirisanensis Comb. Nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 3846–3853. [Google Scholar] [CrossRef]
- Zolg, W.; Ottow, J.C. Pseudomonas glathei Sp. Nov., a New Nitrogen-Scavening Rod Isolated from Acid Lateritic Relicts in Germany. J. Comp. Neurol. 1975, 164, 287–299. [Google Scholar]
- Peeters, C.; Meier-Kolthoff, J.P.; Verheyde, B.; Brandt, E.; Cooper, V.S.; Vandamme, P.; Raaijmakers, J.M.; Kuramae, E.E. Phylogenomic Study of Burkholderia glathei-like Organisms, Proposal of 13 Novel Burkholderia Species and Emended Descriptions of Burkholderia sordidicola, Burkholderia zhejiangensis, and Burkholderia grimmiae. Front. Microbiol. 2016, 7, 877. [Google Scholar] [CrossRef]
- Li, R.; Jiang, Y.; Wang, X.; Yang, J.; Gao, Y.; Zi, X.; Zhang, X.; Gao, H.; Hu, N. Psychrotrophic Pseudomonas mandelii CBS-1 Produces High Levels of Poly-β-Hydroxybutyrate. SpringerPlus 2013, 2, 335. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, S.A.; Roberts, R.F.; Ziegler, G.R. Optimization of Exopolysaccharide Production by Lactobacillus delbrueckii Subsp. Bulgaricus RR Grown in a Semidefined medium. Appl. Environ. Microbiol. 1998, 64, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.S.; Furrer, J.M.; Kadilak, A.L.; Hinestroza, H.F.; Gage, D.J.; Cho, Y.K.; Shor, L.M. Bacterial Extracellular Polymeric Substances Amplify Water Content Variability at the Pore Scale. Front. Environ. Sci. 2018, 6, 93. [Google Scholar] [CrossRef]
- Paul, S.; Parvez, S.S.; Goswami, A.; Banik, A. Exopolysaccharides from Agriculturally Important Microorganisms: Conferring Soil Nutrient Status and Plant Health. Int. J. Biol. Macromol. 2024, 262, 129954. [Google Scholar] [CrossRef]
- Vanhaverbeke, C.; Heyraud, A.; Mazeau, K. Conformational Analysis of the Exopolysaccharide from Burkholderia caribensis Strain MWAP71: Impact on the Interaction with Soils. Biopolymers 2003, 69, 480–497. [Google Scholar] [CrossRef]
- Lünsdorf, E.; Abraham, T. ‘Clay Hutches’: A Novel Interaction between Bacteria and Clay Minerals. Environ. Microbiol. 2000, 2, 161–168. [Google Scholar] [CrossRef]
- Santaella, C.; Schue, M.; Berge, O.; Heulin, T.; Achouak, W. The Exopolysaccharide of Rhizobium Sp. YAS34 Is Not Necessary for Biofilm Formation on Arabidopsis thaliana and Brassica napus Roots but Contributes to Root Colonization. Environ. Microbiol. 2008, 10, 2150–2163. [Google Scholar] [CrossRef]
- Driouich, A.; Gaudry, A.; Pawlak, B.; Moore, J.P. Root Cap–Derived Cells and Mucilage: A Protective Network at the Root Tip. Protoplasma 2021, 258, 1179–1185. [Google Scholar] [CrossRef]
- Aznar, A.; Dellagi, A. New Insights into the Role of Siderophores as Triggers of Plant Immunity: What Can We Learn from Animals? J. Exp. Bot. 2015, 66, 3001–3010. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.-M.; Lee, I.-J. Inoculation of Abscisic Acid-Producing Endophytic Bacteria Enhances Salinity Stress Tolerance in Oryza Sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic Bacteria in Sunflower (Helianthus annuus L.): Isolation, Characterization, and Production of Jasmonates and Abscisic Acid in Culture Medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Boiero, L.; Perrig, D.; Masciarelli, O.; Penna, C.; Cassán, F.; Luna, V. Phytohormone Production by Three Strains of Bradyrhizobium japonicum and Possible Physiological and Technological Implications. Appl. Microbiol. Biotechnol. 2007, 74, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Shayanthan, A.; Ordoñez, P.A.C.; Oresnik, I.J. The Role of Synthetic Microbial Communities (SynCom) in Sustainable Agriculture. Front. Agron. 2022, 4, 896307. [Google Scholar] [CrossRef]
- Joni, F.R.; Hamid, H.; Yanti, Y. Effect of Plant Growth Promoting Rhizobacteria (PGPR) on Increasing the Activity of Defense Enzymes in Tomato Plants. Int. J. Environ. Agric. Biotechnol. 2020, 5, 1474–1479. [Google Scholar] [CrossRef]
- Amaya-Gómez, C.V.; Porcel, M.; Mesa-Garriga, L.; Gómez-Álvarez, M.I. A Framework for the Selection of Plant Growth-Promoting Rhizobacteria Based on Bacterial Competence Mechanisms. Appl. Environ. Microbiol. 2020, 86, e00760-20. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Orozco-Mosqueda, M.D.C.; Santos-Villalobos, S.D.L.; Santoyo, G.; Babalola, O.O. Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation. Plants 2022, 11, 3090. [Google Scholar] [CrossRef]
- Haque, M.M.; Mosharaf, M.K.; Khatun, M.; Haque, M.A.; Biswas, M.S.; Islam, M.S.; Siddiquee, M.A. Biofilm Producing Rhizobacteria with Multiple Plant Growth-Promoting Traits Promote Growth of Tomato under Water-Deficit Stress. Front. Microbiol. 2020, 11, 542053. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, H.; Wang, H.; Lu, Z. Interspecies Synergistic Interactions Mediated by Cofactor Exchange Enhance Stress Tolerance by Inducing Biofilm Formation. mSystems 2024, 9, e00884-24. [Google Scholar] [CrossRef]
- Xavier, J.B.; Foster, K.R. Cooperation and Conflict in Microbial Biofilms. Proc. Natl. Acad. Sci. USA 2007, 104, 876–881. [Google Scholar] [CrossRef]
- Sadiq, F.A.; De Reu, K.; Burmølle, M.; Maes, S.; Heyndrickx, M. Synergistic Interactions in Multispecies Biofilm Combinations of Bacterial Isolates Recovered from Diverse Food Processing Industries. Front. Microbiol. 2023, 14, 1159434. [Google Scholar] [CrossRef]
- Deng, Y.; Kong, W.; Zhang, X.; Zhu, Y.; Xie, T.; Chen, M.; Zhu, L.; Sun, J.; Zhang, Z.; Chen, C.; et al. Rhizosphere Microbial Community Enrichment Processes in Healthy and Diseased Plants: Implications of Soil Properties on Biomarkers. Front. Microbiol. 2024, 15, 1333076. [Google Scholar] [CrossRef] [PubMed]
- Matthus, E.; Zwetsloot, M.; Delory, B.M.; Hennecke, J.; Andraczek, K.; Henning, T.; Bergmann, J. Revisiting the Root Economics Space—Its Applications, Extensions and Nuances Advance Our Understanding of Fine-Root Functioning. Plant Soil 2025. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H. Nonlinearity of Root Trait Relationships and the Root Economics Spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Veiga, R.S.L.; Faccio, A.; Genre, A.; Pieterse, C.M.J.; Bonfante, P.; van der Heijden, M.G.A. Arbuscular Mycorrhizal Fungi Reduce Growth and Infect Roots of the Non-Host Plant Arabidopsis thaliana. Plant Cell Environ. 2013, 36, 1926–1937. [Google Scholar] [CrossRef]
- Dl Iorio, A.; Lasserre, B.; Scippa, G.S.; Chiatante, D. Root System Architecture of Quercus Pubescens Trees Growing on Different Sloping Conditions. Ann. Bot. 2005, 95, 351–361. [Google Scholar] [CrossRef]
- Jia, H.; Guan, C.; Zhang, J.; He, C.; Yin, C.; Meng, P. Drought Effects on Tree Growth, Water Use Efficiency, Vulnerability and Canopy Health of Quercus variabilis-Robinia pseudoacacia Mixed Plantation. Front. Plant Sci. 2022, 13, 1018405. [Google Scholar] [CrossRef]
- Pividori, M.; Giannetti, F.; Barbati, A.; Chirici, G. European Forest Types: Tree Species Matrix. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., Ed.; Publications Office of the European Union: Luxembourg, 2016; p. e01f162. [Google Scholar]
- Šajbidorová, V.; Lichtnerová, H.; Paganová, V. The Impact of Different Water Regime on Chlorophyll Fluorescence of Pyrus pyraster L. and Sorbus domestica L. Acta Univ. Agric. Silvic. Mendelianae Brun. 2015, 63, 1575–1579. [Google Scholar] [CrossRef]
- Paganová, V.; Jureková, Z.; Lichtnerová, H. The Nature and Way of Root Adaptation of Juvenile Woody Plants Sorbus and Pyrus to Drought. Environ. Monit. Assess. 2019, 191, 714. [Google Scholar] [CrossRef]
- Schmucker, J.; Skovsgaard, J.P.; Uhl, E.; Pretzsch, H. Crown Structure, Growth, and Drought Tolerance of True Service Tree (Sorbus domestica L.) in Forests and Urban Environments. Urban For. Urban Green. 2024, 91, 128161. [Google Scholar] [CrossRef]
- Rudrappa, T.; Splaine, R.E.; Biedrzycki, M.L.; Bais, H.P. Cyanogenic pseudomonads influence multitrophic interactions in the rhizosphere. PLoS ONE 2008, 3, 2073. [Google Scholar] [CrossRef]
- Jhaveri, R.; Cannanbilla, L.; Bhat, K.S.A.; Sankaran, M.; Krishnadas, M. Anatomical Traits Explain Drought Response of Seedlings from Wet Tropical Forests. Ecol. Evol. 2024, 14, e70155. [Google Scholar] [CrossRef] [PubMed]
- De Swaef, T.; De Schepper, V.; Vandegehuchte, M.W.; Steppe, K. Stem Diameter Variations as a Versatile Research Tool in Ecophysiology. Tree Physiol. 2015, 35, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Kunz, J.; Räder, A.; Bauhus, J. Effects of Drought and Rewetting on Growth and Gas Exchange of Minor European Broadleaved Tree Species. Forests 2016, 7, 239. [Google Scholar] [CrossRef]
- Ellis, C. When to Use Multinomial Regression. Available online: https://crunchingthedata.com/when-to-use-multinomial-regression/ (accessed on 10 April 2025).
- Frost, J. How to Interpret Regression Models That Have Significant Variables but a Low R-Squared—Statistics by Jim. Available online: https://statisticsbyjim.com/regression/low-r-squared-regression/ (accessed on 10 April 2025).
- Emmenegger, B.; Massoni, J.; Pestalozzi, C.M.; Bortfeld-Miller, M.; Maier, B.A.; Vorholt, J.A. Identifying Microbiota Community Patterns Important for Plant Protection Using Synthetic Communities and Machine Learning. Nat. Commun. 2023, 14, 7983. [Google Scholar] [CrossRef]
- Polade, S.D.; Pierce, D.W.; Cayan, D.R.; Gershunov, A.; Dettinger, M.D. The Key Role of Dry Days in Changing Regional Climate and Precipitation Regimes. Sci. Rep. 2014, 4, 4364. [Google Scholar] [CrossRef]
- Allen, R.J.; Waclaw, B. Bacterial Growth: A Statistical Physicist’s Guide. Rep. Prog. Phys. 2019, 82, 016601. [Google Scholar] [CrossRef]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.S.; Fernández, F.J. O-CAS, a Fast and Universal Method for Siderophore Detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef]
- Ustiatik, R.; Nuraini, Y.; Suharjono, S.; Handayanto, E. Siderophore Production of the Hg-Resistant Endophytic Bacteria Isolated from Local Grass in the Hg-Contaminated Soil. J. Ecol. Eng. 2021, 22, 129–138. [Google Scholar] [CrossRef]
- Gomes, A.F.R.; Sousa, E.; Resende, D.I.S.P. A Practical Toolkit for the Detection, Isolation, Quantification, and Characterization of Siderophores and Metallophores in Microorganisms. ACS Omega 2024, 9, 26863–26877. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of Indole-3-Acetic Acid (IAA) Production in Klebsiella by LC-MS/MS and the Salkowski Method. Bio-Protocol 2019, 9, e3230. [Google Scholar] [CrossRef]
- Fricke, W.; Akhiyarova, G.; Veselov, D.; Kudoyarova, G. Rapid and Tissue-specific Changes in ABA and in Growth Rate in Response to Salinity in Barley Leaves. J. Exp. Bot. 2004, 55, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E. Quantification of Water Stress-Induced Osmotic Adjustment and Proline Accumulation for Arabidopsis thaliana Molecular Genetic Studies. Methods Mol. Biol. 2010, 639, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F.; Efe, D.; Gormez, A. Chapter 7—Advantages of Using Halotolerant/Halophilic Bacteria in Agriculture. In Unravelling Plant-Microbe Synergy; Chandra, D., Bhatt, P., Eds.; Developments in Applied Microbiology and Biotechnology; Academic Press: Cambridge, MA, USA, 2023; pp. 133–149. ISBN 978-0-323-99896-3. [Google Scholar]
- Sobue, I.; Tanimoto, K.; Itoh, S. A Scale of Parental Anxiety about Pediatric Emergency Medical Care Services of Japan: Development, Reliability, Validity, Generalizability and Usefulness. Health 2017, 9, 1427–1458. [Google Scholar] [CrossRef][Green Version]
- Isnaldi, E.; Garuti, A.; Cirmena, G.; Scabini, S.; Rimini, E.; Ferrando, L.; Lia, M.; Murialdo, R.; Tixi, L.; Carminati, E.; et al. Clinico-Pathological Associations and Concomitant Mutations of the RAS/RAF Pathway in Metastatic Colorectal Cancer. J. Transl. Med. 2019, 17, 137. [Google Scholar] [CrossRef]
- Sprouffske, K.; Wagner, A. Growthcurver: An R Package for Obtaining Interpretable Metrics from Microbial Growth Curves. BMC Bioinform. 2016, 17, 172. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2019. 0.7.2. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 10 April 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Soft. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves Using “Ggplot2”; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Balci, S. ClinicoPath Jamovi Module. 2020. Available online: https://osf.io/9szud/ (accessed on 10 April 2025).
- The Jamovi Project, Jamovi (Version 2.6). 2025. Available online: https://www.jamovi.org (accessed on 10 April 2025).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksieienko, I.; Fernandes Hertel, M.; Reilhan, J.; de Castro, M.; Légeret, B.; Caixeta Oliveira, H.; Reiter, I.M.; Santaella, C. Soil-Gradient-Derived Bacterial Synthetic Communities Enhance Drought Tolerance in Quercus pubescens and Sorbus domestica Seedlings. Plants 2025, 14, 1659. https://doi.org/10.3390/plants14111659
Aleksieienko I, Fernandes Hertel M, Reilhan J, de Castro M, Légeret B, Caixeta Oliveira H, Reiter IM, Santaella C. Soil-Gradient-Derived Bacterial Synthetic Communities Enhance Drought Tolerance in Quercus pubescens and Sorbus domestica Seedlings. Plants. 2025; 14(11):1659. https://doi.org/10.3390/plants14111659
Chicago/Turabian StyleAleksieienko, Ivan, Mariana Fernandes Hertel, Jérôme Reilhan, Marie de Castro, Bertrand Légeret, Halley Caixeta Oliveira, Ilja M. Reiter, and Catherine Santaella. 2025. "Soil-Gradient-Derived Bacterial Synthetic Communities Enhance Drought Tolerance in Quercus pubescens and Sorbus domestica Seedlings" Plants 14, no. 11: 1659. https://doi.org/10.3390/plants14111659
APA StyleAleksieienko, I., Fernandes Hertel, M., Reilhan, J., de Castro, M., Légeret, B., Caixeta Oliveira, H., Reiter, I. M., & Santaella, C. (2025). Soil-Gradient-Derived Bacterial Synthetic Communities Enhance Drought Tolerance in Quercus pubescens and Sorbus domestica Seedlings. Plants, 14(11), 1659. https://doi.org/10.3390/plants14111659








