Abstract
Plants are an important source of essential bioactive compounds that not only have a beneficial role in human health and nutrition but also act as drivers for shaping gut microbiome. However, the mechanism of their functional attributes is not fully understood despite their significance. One such important plant is Crocus sativus, also known as saffron, which possesses huge medicinal, nutritional, and industrial applications like food and cosmetics. The importance of this plant is grossly attributed to its incredible bioactive constituents such as crocins, crocetin, safranal, picrocrocin, and glycosides. These bioactive compounds possess a wide range of therapeutic activities against multiple human ailments. Since a huge number of studies have revealed negative unwanted side effects of modern-day drugs, the scientific communities at the global level are investigating a large number of medicinal plants to explore natural products as the best alternatives. Taken into consideration, the available research findings indicate that saffron has a huge scope to be further explored to establish alternative natural-product-based drugs for health benefits. In this review, we are providing an update on the role of bioactive compounds of saffron as therapeutic agents (human disorders and antimicrobial activity) and its nutritional values. We also highlighted the role of omics and metabolic engineering tools for increasing the content of key saffron bioactive molecules for its mass production. Finally, pre-clinical and clinical studies seem to be necessary to establish its therapeutic potential against human diseases.
Keywords:
saffron; crocins; crocetin; safranal; picrocrocin; therapeutics; antimicrobial; anti-cancer; nutrients 1. Introduction
Plants have significant metabolic diversity, which are essential for addressing many of the world’s current challenges, such as drug discovery, food security, and ecosystem functioning. Owing to their inimitable biological attributes and health benefits, the identification of novel plant-based bioactive compounds has received great attention among scientists to further explore their biochemical mechanisms. For centuries, traditional remedies have relied on plant-based natural compounds to cure a variety of ailments. Despite the prevalent dominant use of chemically synthesized drugs to treat human diseases, the application of bioactive compounds derived from medicinal plants is also the center of attention due to their fewer side effects [1]. Other reasons for the use of natural compounds include being readily available, being cost-effective, and having very few side effects [2]. Currently, antimicrobial resistance has emerged as a major threat to humans due to the rapid development of antimicrobial resistance (AMR) infection and a lack of new antimicrobial agents. The World Health Organization (WHO) has reported that AMR will lead to high mortality in humans by 2050. Therefore, the identification of novel plant-based bioactive compounds is one of the viable strategies to tackle this problem. Plant-based bioactive molecules (capsaicin, curcumin, resveratrol, catechin, lignans, quercetin, etc.) have received a lot of attention in recent years due to their wide range of pharmacological functions and biological activities, including antioxidant, antimicrobial, anti-inflammatory, anti-stress, and anti-tumor ones, as well as lowering blood glucose and lipids and improving insulin sensitivity [3]. This further supports the notion that plant metabolites or their structural analogues are key for the development of future drugs.
Crocus sativus is a medicinal plant with nutritional and medicinal importance. It is a valuable cash crop grown throughout Europe, the Mediterranean, and central Asia because of its widespread applications as a spice, colorant, and medication [4]. Saffron belongs in the Iridaceae family, which consists of 100 seasonal and perennial species that are cultivated from corm. It is a sterile triploid species (2n = 3x = 24) with a genome size of 3.01 GB. Owing to its wide application in food and cosmetics, it is considered as the world’s most expensive spice, also known as the Golden Condiment. It has been reported that the production of 1kg of dried saffron requires about 80 kg of flowers of C. sativus [5] and 1 pound of saffron requires about 225,000 stigmas. Metabolic profiling of C. sativus has revealed more than 150 volatile compounds that contribute to the aroma, flavor, and color [6]. Nowadays, in the food industry, saffron is mostly used as a coloring agent and for food-grade flavoring. The phytochemical analysis of saffron has revealed various primary and secondary chemical constituents that are present in different concentrations in different parts of saffron. The flower of this plant contains various bioactive compounds with a wide range of medicinal properties [7]. The phytochemical investigation of this wonder plant confirmed the presence of several volatile as well as non-volatile compounds primarily found in stigmas. Apart from the presence of a large number of minerals, sugars, vitamins, and essential proteins, saffron possesses some very special bioactive safranal, picrocrocin, crocin, and crocetin as shown in Table 1 [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Other bioactive compounds found in the stigma include anthocyanins, carotene, lycopene, and glycoside crocins, all possessing a wide range of medicinal properties [27,28,29]. For instance, crocin is reported to possess various medicinal properties such as anti-inflammatory, anti-convulsant, radical scavenger capacity, anti-depressant, and anti-cancer ones, and augments learning and memory [30,31]. Studies reveal that crocin found in stigmas of saffron alone has anti-inflammatory and antioxidant properties [32]. Moreover, crocin and crocetin are also reported to suppress the aggregation of amyloid-β plaques [33,34]. Similarly, essential oils extracted from saffron contain safranal, a monoterpene aldehyde that also possesses several therapeutic properties—antioxidant, anti-hyperglycemic, anti-inflammatory, anxiolytic, and anti-seizure ones [35,36,37]. The current review aims to unravel the composition of bioactive compounds from saffron and their therapeutic values in human diseases. Besides the presence of biologically active compounds, there are various nutrients present in saffron that show a protective role against oxidative stress, harmful microbes, and cancerous ailments in animals [38], thereby signifying its role in the nutraceutical industry [39]. Further, we have summarized the beneficial effects of saffron in Figure 1.

Table 1.
Secondary phytochemical constituents present in different parts of saffron and their medicinal properties.
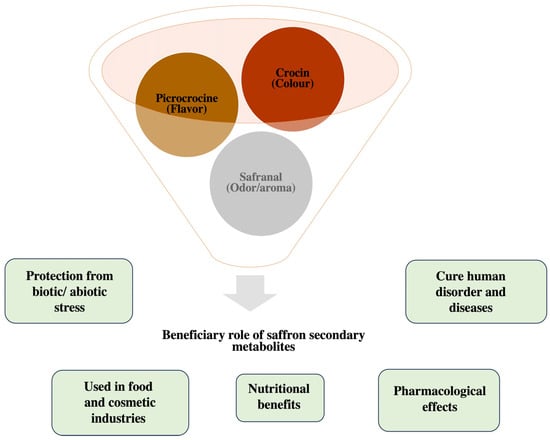
Figure 1.
An illustration of the multifaceted role of saffron bioactive compounds.
2. The Primary and Secondary Metabolites Found in Saffron
The primary chemical constituents and their concentrations present in saffron are mentioned in Table 2. In addition, there are various secondary chemical constituents present in different parts of saffron that have beneficial roles in human life. These secondary chemical constituents include apocarotenoids, monoterpenoids, flavonoids, phenolic acids, and phytosterols.

Table 2.
The primary constituents and their concentrations reported in saffron plants.
2.1. The Apocarotenoids of Saffron
In saffron, apocarotenoid synthesis is a bio-oxidative process and is produced by the CCD enzyme (carotenoid cleavage dioxygenase)-mediated cleavage of carotenoids. Various reports indicate that the stigmas of C. sativus are the rich source of apocarotenoids, i.e., crocetin and crocin [48,49]. These two apocarotenoids play an essential role in enhancing the specific color known as a saffron color and are used by the food industry as a coloring agent [50,51]. It has been reported that crocetin plays a pivotal role in the eradication of cardiovascular diseases, various neuro-disorders, hypersensitive problems, oxidative stress, tumors, and inflammatory problems [38,52,53,54,55]. Besides this, saffron also contains some fat-soluble carotenoids like lycopene, phytoene, phytofluene, a-carotene, b-carotene, and zeaxanthin [56].
The glycosylation of crocetin leads to the formation of a biologically active hydrophilic carotenoid in saffron, which is known as crocin [57]. The α-crocin is a type of crocin that is mostly present in saffron and forms about 10% of its dry weight [58,59]. Reports suggested that the entire contents of crocin form about 6 to 16% dry weight of saffron, which depends on the type of cultivar, origination, and conditions under which its harvesting and processing takes place [60]. Crocin acts as a cellular antioxidative agent and hence reduces lipid peroxidation and maintains membrane integrity [39].
2.2. Saffron-Based Monoterpenoids
The other biologically active secondary phytochemical constituents are monoterpenoids, which include picrocrocin and safranal. These two monoterpenoids are synthesized through the degradation of zeaxanthin. Picrocrocin is very essential for flavor and a bitter taste, while safranal plays a pivotal role in increasing the specific aroma of saffron [61]. Picrocrocin is a monoterpene glycoside that acts as a precursor for safranal and is present as a main constituent in the essential oil of saffron [62,63]. Picrocin is mostly present in the stigma and the petals of C. sativus, which exhibits anti-cancerous and memory-enhancer properties. Besides this, picrocin is used against various diseases like menstrual disorder, cardiovascular disorder, depression, and Alzheimer’s disease [18,28]. However, it has been revealed that safranal is usually present in the stigma part of the flower of C. sativus [61] and has a direct effect on the central nervous system. It acts as an anti-depressant, and anti-convulsant, and also plays an essential role in reducing the withdrawal syndrome [64,65].
2.3. Saffron-Based Flavonoids
The flavonoids and their derivatives are considered the second most biologically active secondary phytochemical constituent present in the stigmas of C. sativus. There are various types of flavonoids and their derivatives present in various parts of C. sativus and these flavonoids show tremendous amounts of medicinal and nutraceutical properties. These flavonoids and their derivatives include vitexin, orientin, kaempferol, isoorientin, naringenin, astragalin, dihydrokaempferol, myricetin, quercetin, rhamnetin, and populin [10,12,27,38,62]. Moreover, saffron-based compounds such as crocin, safranal, picrocrocin, crocusatin D-I, isophorone, lycopene, and crocetin possess a wide range of therapeutic properties (Table 3, [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]).

Table 3.
The biological activities of saffron-based metabolites, which are used as potential therapeutic agents against a wide range of diseases.
2.4. Phenolic Acids in Saffron
The chemical analysis of saffron revealed various types of hydroxycinnamic acids, which include chlorogenic acid, caffeic acid, methylparaben, gallic acid, and pyrogallol [88]. Hydroxybenzoic acids act as the precursor for the biosynthesis of flavonoids and are reported from different parts of C. sativus. Hydroxycinnamic acids like h-coumaric acid, p-hydroxybenzoic acid, sinapic acid, and vanillic acid have been reported from the petals of saffron [89]. However, p-hydroxybenzoic and benzoic acids were also reported from the pollens of C. sativus [88].
2.5. Saffron Phytosterols
There are various types of phytosterols present in the different parts of saffron; however, phytosterols like β-sitosterol and stigmasterol were reported from both stigmas and petals while fucosterol was reported only from the petals of C. sativus [24,47].
3. Role of Saffron Bioactive Compounds against Human Diseases
As per the reports of the World Health Organization (WHO), human beings are paying most of their attention to the use of herbal medicines as they have the least side effects [90,91]. A huge number of medicinal plants are currently being investigated to explore natural products as the best alternative to unsafe drugs [92,93,94]. Regardless of the growth in recent diagnostics and treatment strategies, the prevalence of diseases at the global level is still rising. Although a wide range of therapeutic strategies are satisfactory in treating a large number of diseases, these treatments are failing in many cases such as cancers, cardiovascular diseases, etc., and the worst part of these statements lies in their severe health-complicating side effects. Against this backdrop, scientists are committed to exploring natural therapeutic agents as novel ways to treat a wide range of diseases.
The chemical analysis has reported the presence of about nearly 150 non-volatile and volatile components in saffron. The crocetin, picrocrocin, and crocin are non-volatile and the terpene, terpenoids, and safranal are the volatile components. Among the volatile components, safranal has various therapeutic applications [37]. José Bagur et al. [95] reported that picrocrocin, safranal, and crocin play an essential role in enhancing the bitter taste, aroma, and different colors in saffron, respectively. The different bioactive components associated with different parts of the C. sativus plant have various medicinal properties, which depend on their unique chemical structure. Tong, Y., et al. reported that the highly studied bioactive components like safranal, crocin, and picrocrocin of saffron play an antagonistic role against various diseases like Alzheimer’s, diabetes, cancer, cardiovascular disease, and erectile dysfunction [96]. A large number of investigations reveal that saffron-based bioactive compounds help to act as an anti-metabolic syndrome, antioxidant, memory enhancer, cardioprotective compound, anti-inflammatory compound, anti-depressant, and anti-cancer agent [97,98,99,100]. The following sections of this review are aimed at unraveling the potential of saffron as the source of novel molecules to treat major human diseases.
3.1. Role in the Treatment of Cancers
Saffron-based bioactive compounds are reported to exert anti-cancer effects through the induction of apoptosis, regulation of the immune response, and anti-inflammatory effects. For instance, crocin extracted from saffron was reported to exert anti-cancer effects in colorectal cancer cell lines [101]. Kawabata et al. [102] reported similar results against colitis-associated colon carcinogenesis. The authors further showed that crocin inhibited cancers by downregulating the transcription of cytokines such as tumor necrosis factor-α [TNF-α], nuclear factor-κB [NF-κB], interleukin-6 [IL-6], interferon-γ, COX-2, IL-1β, and inducible NO synthase [iNOS] (Figure 2).
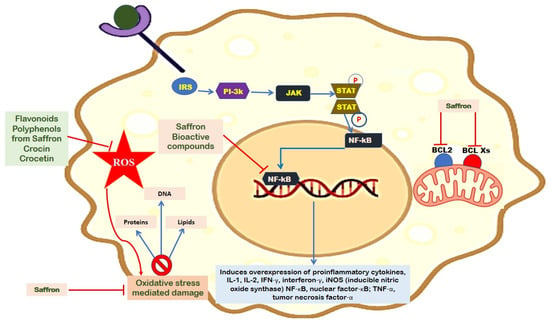
Figure 2.
A schematic illustration shows the inhibitory function of saffron which leads to decrease in the production of reactive oxygen species, IL-17, TNF-α, NF-κB, IL-1β, and NO. The figure also demonstrates that saffron helps in the suppression of apoptosis by inhibiting the function of BCL-2.
Moreover, crocetin treatment inhibited colon cancer by downregulating the levels of vascular endothelial growth factor (VEGF) matrix metalloproteinase-9 and NF-κB [103]. Crocetin was also reported to exert anti-cancer effects on human gastric cancer cells (BGC-823 cells), further validating the therapeutic role of saffron [104].
Further, in vivo studies carried out in rats revealed that saffron functions as a chemoprotective agent by enhancing the caspase-3 cleavage, inhibiting the activity of nuclear factor-κB (NF-κB), and leading to cell cycle arrest [105]. Other studies reported the role of crocin in inhibiting liver cancers by inhibiting the interleukin (IL)-6/STAT3 cascade [106]. For instance, crocetin administration inhibited the growth of proliferating MIA-PaCa-2 cells. Similarly, it was reported that crocin inhibited pancreatic cancer cells [107]. Thus, the current literature strongly suggests that saffron is a source of promising therapeutic agents such as crocin and crocetin employed against several cancers [108]. Consequently, saffron needs special attention by employing pre-clinical investigations to further validate its anti-cancer effects and to troubleshoot the optimum dosage for effective treatments.
3.2. The Defense to Cardiovascular Diseases (CVDs)
CVDs are caused due to dietary and metabolic imbalances affecting heart muscles and the blood circulatory system, resulting in impairment of blood supply to the brain, heart, and other organs. Data reveal that CVDs are the leading cause of human deaths on the global scale [109]. Natural compounds have played a critical role in controlling the progression and development of CVDs and are the best candidates to cure metabolic disorders related to the circulatory system [110]. For instance, crocin, a bioactive constituent in saffron, is reported to regulate normal functions of the cardiovascular system by reducing dyslipidemia and oxidative stress [110]. Similarly, Chahine, et al. [111] reported that saffron bioactive compounds improved the free radical scavenging activity in rabbits due to cardiac dysfunctioning mediated by doxorubicin. The presence of crocin in saffron leads to a decrease in the level of triacylglycerol and cholesterol in plasma, thus aiding in the control of cardiovascular complications [112]. Safranal and crocin can eliminate ROS, thus helping to prevent cancers and cardiovascular diseases [41]. For instance, reports suggest that saffron tea, having lycopene, helps in reducing the risk of CVD development [86]. Similarly, crocin and crocetin were found to activate vasoconstriction during hypertension and were also found to treat endothelial dysfunction and reduce aortic contraction problems [113].
One of the best pharmaceutical effects of saffron is its ability to reduce the levels of serum triglycerides, very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and cholesterol, hence creating low levels of fat and cholesterol deposition, thus reducing the risk of CVDs like atherosclerosis, coronary artery disease, and hypertriglyceridemia [114]. Moreover, crocetin was also reported to have an anti-atherosclerotic effect in model rabbits with atherosclerosis [115]. Similarly, crocetin supplementation reduced the accumulation of oxidatively modified low-density lipoprotein (Ox-LDL) and lessened the formation of atherosclerotic lesions [116]. Previously, it was reported that crocin decreased the expression of Lectin-like oxidized LDL receptor 1 (LOX-1) and nuclear factor kappa B (NF-κB), which in turn reduced the progression of coronary artery disease (CAD). Overall, these reports suggest that saffron-derived compounds have great potential to reduce the negative effects of CADs and regular consumers of saffron-based foods may be protected against the clinical implications of CADs [86,117,118]. Additionally, saffron may serve as a strong functional food or nutraceutical for health as well as commercial purposes.
3.3. Antioxidant and Anti-Inflammatory Properties of Saffron Bioactive Compounds
Antioxidant compounds are very critical to defend against cancers, metabolic disorders, and aging by inhibiting the generation of ROS. Metabolites such as picrocrocin, crocin, and safranal derived from saffron are identified as potential antioxidants to scavenge the ROS [119]. Crocin helps to protect the organs such as the liver, kidneys, and brain from damage induced by oxidative stress [74]. Inflammation in animals may cause severe damage to organ systems, thus leading to functioning impairments [120]. For instance, studies revealed that saffron-based metabolites such as kaempferol, crocetin, crocins, and quercetin are found to inhibit the generation of pro-inflammatory cytokines to prevent damage induced by chronic inflammation [121].
Crocins found in saffron are reported to be a potent anti-inflammatory compound [122]. For instance, the hyperglycemic effect, activation of protein kinase C (PKC), and elevation of ROS trigger the activation and import of NF-κB to induce the expression and coding of pro-inflammatory factors like IL-6, IL-1b, and TNF-a [55]. In this context, in macrophages, the crocin inhibits enzymes such as cyclooxygenase-1 (COX1) and cyclooxygenase-2 (COX2) and inhibits the NF-κB to block the production of prostaglandin-2 (PGE2), hence playing a critical role in the inhibition of inflammatory responses [123].
3.4. Anti-Diabetic Effects of Saffron
Crocin, safranal, and crocetin are the main components found in saffron, which are reported to possess anti-diabetic activity [124]. Studies back up the role of saffron-based metabolites acting as antioxidants to reduce oxidative stress and also negate the effects of hyperglycemia [33]. In an experiment, it was reported that in obese prediabetic individuals, the oral administration of saffron improved the glycemic indices and antioxidant activity [125]. In another study, saffron was found to enhance the uptake of glucose and inhibit the activity of protein tyrosine phosphatase 1B (PTP1B), an inhibitor of the insulin signaling pathway [126]. Similarly, crocin helps in reducing levels of glucose, TG, HbA1c, total cholesterol, IL-1b, IL-2, IL-4, IL-10, and NF-kB and upregulates the synthesis of enzymes such as nuclear respiratory factor 2, manganese superoxide dismutase 1, and catalase (CAT) and heme oxygenase-1 [127]. Moreover, the crocin was found to decrease the ratio of Bax/Bcl-2 to diminish the myocardial apoptosis and additionally increase the AMPK phosphorylation to normalize the autophagy dysfunction in case of a diabetic myocardium. The crocin also inhibits apoptosis by reducing the levels of P53 in cells [128]. In the same way, the administration of saffron to endothelial progenitor cells decreased apoptosis, reduced the caspase 3 levels, and reduced the progression of diabetes by affecting the PI3K/AKT-eNOS and ROS pathways [71].
3.5. Role in Decreasing the Progression of Neurological Disorders
For decades, neurodegenerative diseases have proven to be a significant threat to human health due to the low availability of drugs [129]. Currently, a large number of plants have been explored to screen natural products as the best alternatives to synthetic drugs [130]. Saffron is employed in several studies to prove its potential to treat neurological diseases. Since very limited drugs are available to treat neurological diseases, saffron may be the ray of hope against several neurological diseases, such as AD, anxiety, depression, Parkinson’s disease, etc. The following reports further highlight the role of saffron and derived compounds in decreasing the progression of neurological disorders. For instance, considerable studies demonstrate that saffron-based bioactive compounds have a positive impact on the functioning of cognition and memory in animal models [98,131,132]. In addition, crocin and crocetin were reported to help in neuroprotection by enhancing the viability of cells, suppressing apoptosis as well as the production of ROS, increasing the expression of protein kinase B, activating mTOR, and activating MAPK phosphorylation [133].
The saffron extracts were found to aid in the clearance of Aβs in Alzheimer’s disease models by the upregulation of LRP1 and P-gp [134]. Crocin and crocetin were reported to inhibit LPS-induced NO release, inhibit NF-κB activity, and repress the production of ROS, TNF-α, and IL-1β [67]. The crocin is believed to increase the levels of glutathione peroxidase (GPx) and superoxide dismutase (SOD), which in turn decreases the formation of advanced glycation end products (AGEs) and suppresses ROS production in serum and brain tissues of mice models [133,135]. Saffron-identified compounds such as crocin, flavonoids, saponins, and tannins play a critical role in alleviating the clinical features of AD, due to anti-inflammatory and antioxidative properties [70,136]. The antioxidant effect is primarily displayed by the crocin-induced expression of the γ-glutamyl cysteinyl synthase (γ-GCS) gene, which in turn leads to the synthesis of glutathione (GSH), a strong antioxidant molecule [70]. Both crocetin and crocin are reported to decline in the accumulation of Aβ to enhance neuroprotection in patients with AD [137,138]. Other therapeutic effects of crocin include a significant decline in hippocampal ROS, induction of enhanced activity of superoxide dismutase (SOD), and role in attenuating DNA fragmentation and apoptosis [72]. Pre-clinical reports suggest that saffron extracts impart neuroprotective effects via the regulation of phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) involved in the survival of nervous tissues [80]. Studies also demonstrated the role of saffron extracts in alleviating anxiety and depression in patients with AD [139].
Parkinson’s disease is another neurodegenerative disease still awaiting precise treatment at the global level. This disorder is associated with the formation of Lewy bodies and an ill defense against oxidative stress by dopamine-secreting neurons, leading to the degeneration of neurons. Hence, there is a lack of functioning of dopaminergic neurons in the substantia nigra limbic system [140]. Reports suggest that crocin plays a critical role in stimulating the mTOR signaling, leading in turn to a decreased activation of caspase-9, glycogen synthase kinase-3β (GSK-3β), and forkhead box transcription factor of the O class (FoxO3a) [66]. In addition, crocin leads to an enhanced expression of miRNA-221 and microRNA-7 (miRNA-7), both responsible for the activation of Akt/mTOR and also aiding in the relief of α-synuclein formation and the inhibition of apoptosis [66]. Experiments demonstrated neuroprotective effects of crocin in PD-induced mouse models by decreasing oxidative stress [83]. In PD rat models, improved spatial memory was observed upon treatment with saffron extracts [141]. The saffron extracts were reported to enhance the cellular expression of nuclear factor erythroid 2–related factor 2 (Nrf2), mediating resistance to oxidative stress in rotenone-induced PD models [142]. Inoue E. et al. [143] reported that saffron constituents such as crocin-1, crocin-2, and crocetin play a pivotal role in the inhibition of α-synuclein and Lewy body accumulation.
Huntington disease (HT) is another deadly neurological disorder, characterized by several clinical features such as emotional problems, uncontrolled movements, and loss of thinking ability (cognition), resulting in physical, psychological, and behavioral changes [144]. The genetic basis of HT involves the incorporation of multiple repetitions of cytosine–adenine–guanine (CAG) trinucleotides in the Huntington (HTT) gene, resulting in the synthesis of an abnormally long Huntington protein, which is further cut into smaller fragments, which in turn aggregates together and accumulates in nerve cells [144]. The aggregation and accumulation of these fragments both intracellular and extracellular to neurons results in the impairment of nerve functions; hence, the longer-run outcome is neurodegeneration [145]. The saffron extracts were shown to diminish the modulations caused by 3-Nitropropionic acid (3-NP), resulting in lessening effects of clinical features in Huntington models of mice [81]. Fotoohi et al. [81] also demonstrated that saffron decreases the glutathione (GSH), catalase, and SOD activities and on the other hand prevents an increase in the levels of malondialdehyde (MDA) and nitrite oxide. Zhang et al. [146] also reported that crocin led to enhanced levels of GSHPx, GSH, and SOD and decreased the levels of glutathione disulfide (GSSG) and MDA. Therefore, saffron displays considerable potential to reduce the progression of neurological disorders and this wonder plant needs further attention in terms of research as well as clinical significance. Further well-designed clinical trials are needed to validate the potential benefits of saffron in neurodegenerative diseases and to determine the optimal dosage, treatment duration, and safety profile.
4. Antimicrobial Activities of Saffron
Antimicrobial resistance to traditional antibiotics and its speedy progress have raised serious alarm in the management of infectious diseases across the globe. Numerous studies have been conducted recently with the goal of identifying viable alternatives to combat microbial infections and the antimicrobial resistance threat. One such promising area is harnessing the potential of phytochemical molecules for the development of plant-based drugs. Antimicrobials produced from plants are favored because of their desirable safety and effectiveness profiles. Plant metabolites function by selectively attacking microbial cell membranes; obstructing the production of microbial DNA, RNA, and enzymes; and causing disruptions to the expression of efflux pumps and quorum sensing. Additionally, they boost the antibacterial properties of conventional antibiotics by working in concert with them [147]. Many studies have shown that phytochemicals have great antimicrobial potential with several mechanisms of action against both susceptible and resistant microorganisms [148]. Similarly, it is evident from earlier research that C. sativus petals possess strong antibacterial, antifungal, and antioxidant activity [149,150]. A notable antibacterial activity of saffron petals’ methanolic extract was reported by Asgarpanaha et al. [151] against Escherichia coli, Bacillus cereus, Salmonella typhi, Staphylococcus aureus, and Shigella dysenteriae. Similarly, another study has revealed that saffron extracts showed significant antibacterial activity against P. aeruginosa and S. aureus [152]. Recently, it was reported that saffron petal extracts showed an antibacterial effect against food-borne pathogens such as C. perfringens, C. difficile, and C. botulinum, which further highlights its role as a valuable natural alternative source to conventional preservatives [150]. In addition to antibacterial activity, saffron extracts also show potent antifungal activity against different fungal pathogens such as Aspergillus fumigatus, Candida albicans, and Aspergillus niger [153]. The presences of safranal and crocin have been reported to be the main drivers of antimicrobial and antifungal activity of saffron extracts. However, future studies should further explore the metabolic diversity of saffron with an aim of finding novel drug molecules with antimicrobial potential and that can be viable resources for combating the antimicrobial resistance threat sustainably.
5. Nutritional Benefits of Saffron
Plants are necessary for providing humans with everyday needs like food, shelter, fiber, and medicinal uses. Plants with medicinal and nutritional benefits are key for resources for food and therapeutic usage, especially in developing regions. At present, natural food intake is becoming more and more popular as opposed to processed foods with artificial ingredients. Due to the presence of numerous significant bioactive components, such as crocin (which gives saffron its color), safranal (which gives saffron its smell), and picrocrocin (which gives saffron its taste), the dried stigma of C. sativus is used extensively in cooking as a flavoring and dietary spice. C. sativus possesses huge nutritional benefits in addition to its medicinal properties. It contains proteins and amino acids (12%); minerals (5%); lipids (5%); fibers (5%); various sugars (63%); vitamins [(B1 (riboflavin), B2 (thiamine)]; and different macro- and micronutrients like Ca, K, Mg, P, and Fe [154,155]. Based on available data, we show the nutritional value of C. sativus in Table 2. Owing to its high nutritional importance, it is important to use different omics and metabolic engineering tools to further improve its nutrient components as well as its production.
6. Role of Omics and Metabolic Engineering for Improving Saffron Bioactive Compounds
The application and integration of other omics, such as genomics, proteomics, and metabolomics, can give more insights on the functional attributes of saffron bioactive substances and their biochemical pathways. In the past, a multiomics approach has been an excellent platform to unravel the role of different plant metabolites by integrating the interpretation of vast data from biological processes at the gene, protein, and metabolite levels along with other in silico and analytical analyses. Genome-scale enzyme and pathway annotations and omics technologies have revolutionized research to decrypt plant metabolism and produced a growing list of functionally characterized metabolic genes and pathways in both model and crop plants. Also, advances in metabolic route mapping infrastructure and omics methods make it easier to uncover new pathways in a wide range of plant species [156]. Similarly, transcriptomics can provide a snapshot of the expression profiling of metabolic pathway genes, transcription factors, and their regulation. On the other hand, genome-wide association studies (GWASs) have been frequently used to elucidate the genomic architectures underpinning the phenotypes of interest. Metabolite GWASs (mGWASs) can identify a variety of genes related to metabolic traits, including transcription factors and biosynthetic genes [157]. Therefore, the application of the above-mentioned molecular approaches like omics and metabolite GWASs in saffron can provide novel insights on identifying genes related to metabolic traits. At present, the isolation and identification of key plant bioactive compounds cannot be produced profitably by comprehensive chemical synthesis. As a result, their industrial supply procedures mostly involve extracting end products or precursors from plant resources. In this regard, biotechnological manufacture has led to several benchmark studies on producing key plant-originated bioactive molecules in heterologous hosts. Similarly, a heterologous host system can be used for the production of saffron bioactive compounds for larger-scale production. Crocetin and crocin biosynthesis has received significant attention because of its high scientific and economic interest, and attempts to metabolically engineer their process in different microbial systems have been described with limited success. Therefore, new tools like gene editing are required to further accelerate the metabolic engineering in saffron for bioactive molecule production. In recent years, synthetic biology and metabolic engineering have advanced at a rapid pace, thereby offering new ways for improving targeted metabolite production. One of the key applications of these tools is the building of cell factories to produce high-value-added bioactive compounds [158]. Recently, the CRISPR/Cas tool kit has been commonly used for efficient metabolic engineering in heterogeneous hosts for the production of desired bioactive compounds. Similarly, approaches can be used for producing saffron bioactive compounds like crocin, safranin, etc. However, this will require an in-depth understanding of the metabolic pathways and their encoding gene networks related to different bioactive molecules in saffron. In this regard, the use of multiomics in saffron can help in identifying target genes, transcriptional factors, and proteins that regulate metabolite production (Figure 3). This will also play a key role in metabolic engineering in saffron as well as its heterologous model systems. In comparison to other plants, there have been no investigations on the function of multiomics and metabolic engineering in saffron bioactive chemical synthesis. Because of its economic relevance and demand, it is critical to understand the molecular complexity of saffron metabolic pathways and improve its metabolic traits. Keeping in consideration the critical therapeutic values elucidated by in vitro/in vivo studies using raw extracts in animal cell lines and animal models, the red gold plant (saffron) demands serious attention in terms of medicinal values (Table 4). Also, the impact of climatic changes on saffron cultivation is necessary to develop resilient saffron cultivars to survive under unfavorable conditions.
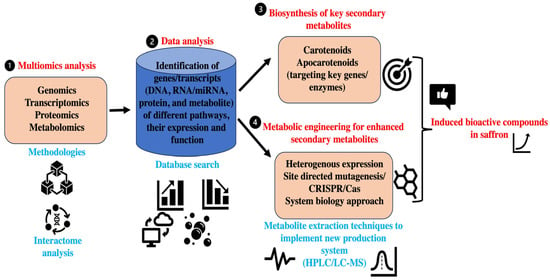
Figure 3.
An illustration of the role of omics and metabolic engineering (CRISPR/Cas) for increasing the production of potential bioactive compounds in saffron.

Table 4.
Therapeutic values, dose, and host applicability of saffron extracts and isolated compounds used under in vivo/in vitro conditions.
7. Regulation of Gut Microbiome by Saffron and its Metabolites: A Concise Account
The human gut harbors many microbial species including fungi, bacteria, archaea, protozoa, and viruses [171]. The existence of microbiomes and their metabolic products plays a critical role in the versatile functions of the gut, which include digestion and absorption and immunity of the gut to pathogenic microbes, and also induces cell proliferation and differentiation. For instance, metabolic products mediate the balance of Treg cells and Th17 cells to regulate inflammation [172]. Gut microbiota can also assist the host by converting dietary nutrients into bioactive metabolites like fermenting nondigestible carbohydrates into short-chain fatty acids (SCFAs), which are the primary metabolites of intestinal microbiota, including butyrate, propionate, and acetate. Recent research indicates that SCFAs, particularly butyrate, have crucial intestine and health-modulating activities. Hence, gut microbiota has a vital function in enhancing host health. Despite a huge number of drugs being designed to treat numerous diseases, the adverse effects such as decreasing the beneficial gut microbiome, risk of infection, toxicity, and malignancies diminish their applicability in humans. Saffron and its derivative compounds are alternatives to eliminating the pro-inflammatory bacteria and regulating the beneficial microbes such as Firmicutes/Bacteroides in mouse models [173]. These studies also demonstrated the role of saffron in regulating microbiome-associated metabolites such as 2 hydroxyglutaric acid, cholesterol, uric acid, 2-hydroxyhexanoic acid, and allantoic acid. In conclusion, saffron plays a pivotal role in maintaining the homeostatic balance in regulating the gut microbiome and healthy state of the gut [173]. Recently, Pontifex et al. [174] reported that the saffron extract reduces anxiety-related behavior in a rat model of low-grade inflammation via modulating the microbiota and gut metabolites. Similarly, in mice, Crocin-I modulates gut microbiota and intestinal inflammation to protect against high-fat-diet-induced obesity. These studies further provide the evidence that saffron plays an important role in modulating gut microbiota and disease treatment in different animal models. These studies necessitate future studies on exploring different saffron bioactive compounds for shaping the gut microbiome, which can have a beneficial impact on human health.
8. Conclusions and Future Directions
The saffron plant is a repository of nutritionally and therapeutically important bioactive compounds. The bioactive constituents improve the antioxidant defense, anti-inflammatory effect, and anti-diabetic effect, and impede the progression of neurodegenerative diseases. However, very limited knowledge is available regarding the activities of saffron in almost all diseases. We strongly believe our current compilation offers a strong up-to-date account of the medicinal applications of saffron and its critical bioactive compounds. Further, the state-of-the-art knowledge regarding this wonderful cash crop will aid in promoting the future research investigation and exploration of saffron-based compounds as therapeutic tools for human ailments. For the elucidation of detailed mechanisms, a further exploration of pathophysiological and molecular experiments is required to validate the therapeutic potential of saffron. However, to elucidate more detailed mechanisms, further studies, especially molecular and pathological experiments warrants future investigation. Still, clinical trials with exclusive results are critical to validate the pharmacological effects of saffron-based compounds. Owing to its multiple benefits, the demand for saffron is rising; therefore, to fulfill market demand, there is a need to use different metabolic engineering tools like CRISPR/Cas for augmenting its metabolite production. Furthermore, as environmental stressors drastically impact C. sativus yield and quality, therefore creating climatically tolerant cultivars is essential to meet future demand.
Author Contributions
Conceptualization, R.A.M., S.A. and M.A.A.; methodology, R.A.M., A.T., S.A. and S.J.H.; software, R.A.M., A.T. and S.A.; validation, R.A.M., A.T., S.A., S.J.H. and R.D.; formal analysis, R.A.M., A.T., S.A., S.J.H., M.T.Z. and R.D.; investigation, R.A.M. and S.A.; resources, R.A.M. and S.A.; data curation, R.A.M., A.T., S.J.H., S.A., M.T.Z. and R.D.; writing—original draft preparation, R.A.M., A.T., S.A., S.J.H. and M.A.A.; writing—review and editing, R.A.M., A.T., S.A., S.J.H., M.A.A., M.T.Z. and R.D.; visualization, R.A.M., A.T. and S.A.; supervision, R.A.M., A.T., S.A. and M.A.A.; project administration, R.A.M., M.A.A. and S.A.; funding acquisition, R.A.M. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khazdair, M.R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective potency of some spice herbs, a literature review. J. Tradit. Complement. Med. 2019, 9, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Dalli, M.; Bekkouch, O.; Azizi, S.-E.; Azghar, A.; Gseyra, N.; Kim, B. Nigella sativa L. phytochemistry and pharmacological activities: A review (2019–2021). Biomolecules 2022, 12, 20. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.I.; Manzoor, M.; Dhar, M. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Gracia, L.; Perez-Vidal, C.; Gracia-López, C. Automated cutting system to obtain the stigmas of the saffron flower. Biosyst. Eng. 2009, 104, 8–17. [Google Scholar] [CrossRef]
- Maggi, M.A.; Bisti, S.; Picco, C. Saffron: Chemical Composition and Neuroprotective Activity. Molecules 2020, 25, 5618. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Boskabady, M.H.; Hosseini, M.; Rezaee, R.; Tsatsakis, A.M. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J. Phytomed 2015, 5, 376–391. [Google Scholar] [PubMed]
- Al-Snafi, A.E. The pharmacology of Crocus sativus—A review. IOSR J. Pharm. 2016, 6, 8–38. [Google Scholar]
- He, M.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Santana-Méridas, O.; Polissiou, M.; Vioque, J.; Astraka, K.; Alaiz, M.; Herraiz-Peñalver, D.; Tarantilis, P.A.; Girón-Calle, J. Polyphenol composition and in vitro antiproliferative effect of corm, tepal, and leaf from Crocus sativus L. on human colon adenocarcinoma cells (Caco-2). J. Funct. Foods 2016, 24, 18–25. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 2016, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Motamedshariaty, V.; Hadizadeh, F. Antidepressant effect of kaempferol, a constituent of saffron (Crocus sativus) petal, mice and rats. Pharmacol. Online 2007, 2, 367–370. [Google Scholar]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wang, J.; Wu, W.; Liu, Q.; Liu, X. Effect of isoorientin on intracellular antioxidant defense mechanisms in hepatoma and liver cell lines. Biomed. Pharmacother. 2016, 81, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018, 2, 9794625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, G.; Sun, C.; Li, H.; Fu, Y.; Xu, W. Apoptosis effects of dihydrokaempferol isolated from Bauhinia championii on Synoviocytes. Evid. Based Complement. Altern. Med. 2018, 2, 9806160. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Maldini, M.; Luciani, L.; Tuberoso, C.I.; Congiu, F.; Pizza, C. Radical scavenging activity and LC-MS metabolic profiling of petals, stamens, and flowers of Crocus sativus L. J. Food Sci. 2012, 77, C893–C900. [Google Scholar] [CrossRef] [PubMed]
- Gismondi, A.; Serio, M.; Canuti, L.; Canini, A. Biochemical, antioxidant and antineoplastic properties of Italian saffron (Crocus sativus L.). Am. J. Plant Sci. 2012, 3, 1573–1580. [Google Scholar] [CrossRef]
- Kim, Y.J. Rhamnetin attenuates melanogenesis by suppressing oxidative stress and pro-inflammatory mediators. Biol. Pharm. Bull. 2013, 36, 1341–1347. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010, 15, 6244–6256. [Google Scholar] [CrossRef]
- Alfaqih, H.; Abu-Bakar, N. The potential of pyrogallol as a possible antimalarial drug candidate. Acad. J. Microbiol. Immun. 2010, 1, 1–4. [Google Scholar]
- Feizy, J.; Reyhani, N. Gas chromatographic determination of phytosterols and fatty acids profile in saffron petals. Can. Chem. Trans. 2016, 4, 389–397. [Google Scholar]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Bouyahya, A. Health Benefits and Pharmacological Properties of Stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Kianbakht, S. A Systematic Review on Pharmacology of Saffron and Its Active Constituents. J. Med. Plants 2008, 4, 1–27. [Google Scholar]
- Aissa, R.; Ibourki, M.; Bouzid, H.A.; Bijla, L.; Oubannin, S.; Jadouali, S.; Hermansyah, A.; Goh, K.W.; Ming, L.C.; Bouyahya, A.; et al. Phytochemistry, quality control and medicinal uses of Saffron (Crocus sativus L.): An updated review. J. Med. Life 2023, 16, 822. [Google Scholar]
- Anaeigoudari, F.; Anaeigoudari, A.; Kheirkhah-Vakilabad, A. A review of therapeutic impacts of saffron (Crocus sativus L.) and its constituents. Physiol. Rep. 2023, 11, e15785. [Google Scholar] [CrossRef]
- Nilakshi, N.; Gadiya, R.V.; Champalal, K.D. Detailed profile of Crocus sativus. Int. J. Pharma Bio Sci. 2011, 2, 189–195. [Google Scholar]
- Azami, S.; Shahriari, Z.; Asgharzade, S.; Farkhondeh, T.; Sadeghi, M.; Ahmadi, F.; Vahedi, M.M.; Forouzanfar, F. Therapeutic potential of saffron (Crocus sativus L.) in ischemia stroke. Evid.-Based Complement. Altern. Med. 2021, 2021, 6643950. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249. [Google Scholar] [CrossRef] [PubMed]
- Tiribuzi, R.; Crispoltoni, L.; Chiurchiù, V.; Casella, A.; Montecchiani, C.; Del Pino, A.M.; Maccarrone, M.; Palmerini, C.A.; Caltagirone, C.; Kawarai, T. Trans-crocetin improves amyloid-β degradation in monocytes from Alzheimer’s disease patients. J. Neurol. Sci. 2017, 372, 408–412. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, X.; Wu, Q.; Chang, F.; Zhou, T.; Zhou, M.; Pei, J. Active constituents of saffron (Crocus sativus L.) and their prospects in treating neurodegenerative diseases. Exp. Ther. Med. 2023, 25, 235. [Google Scholar] [CrossRef] [PubMed]
- Hazman, Ö.; Bozkurt, M.F. Anti-inflammatory and antioxidative activities of safranal in the reduction of renal dysfunction and damage that occur in diabetic nephropathy. Inflammation 2015, 38, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Bo-Qiang, L.; Si-Tong, Z.; Zu-Yuan, L.; Wan-Yun, N.; Bin, C.; Yuan, L.; Xuyun, L.; Liangen, M.; You-Chao, C.; Xin-Zhen, Y. Safranal carried by nanostructured lipid vehicles inhibits generalized epilepsy in mice. Die Pharm.-Int. J. Pharm. Sci. 2018, 73, 207–212. [Google Scholar]
- Pitsikas, N. Constituents of saffron (Crocus sativus L.) as potential candidates for the treatment of anxiety disorders and schizophrenia. Molecules 2016, 21, 303. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.A.; Malik, A.H.; Wani, Z.A.; Mohiuddin, T.; Shah, Z.; Abbas, N.; Ashraf, N. Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. S. Afr. J. Bot. 2015, 99, 80–87. [Google Scholar] [CrossRef]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Rahimi, M. Chemical and medicinal properties of saffron. Bulletin of Environment. Pharmacol. Life Sci. 2015, 4, 69–81. [Google Scholar]
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main chemical compounds and pharmacological activities of stigmas and tepals of ‘red gold’ saffron. Trends Food Sci. Technol. 2016, 58, 69–78. [Google Scholar] [CrossRef]
- Rios, J.; Recio, M.; Giner, R.; Manez, S. An update review of saffron and its active constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- D’Archivio, A.A.; Maggi, M.A. Geographical identification of saffron (Crocus sativus L.) by linear discriminant analysis applied to the UV-visible spectra of aqueous extracts. Food Chem. 2017, 219, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Jadouali, S.M.; Atifi, H.; Mamouni, R.; Majourhat, K.; Bouzoubâ, Z.; Laknifli, A.; Faouzi, A. Chemical characterization and antioxidant compounds of flower parts of Moroccan Crocus sativus L. J. Saudi Soc. Agric. Sci. 2019, 18, 476–480. [Google Scholar] [CrossRef]
- Hashemi, P.; Erim, F.B. Analysis of vitamin B2 in saffron stigmas (Crocus sativus L) by capillary electrophoresis coupled with laser-induced fluorescence detector. Food Anal. Methods 2016, 9, 2395–2399. [Google Scholar] [CrossRef]
- Khare, C. Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Zheng, C.J.; Li, L.; Ma, W.H.; Han, T.; Qin, L.P. Chemical constituents and bioactivities of the liposoluble fraction from different medicinal parts of Crocus sativus. Pharm. Biol. 2011, 49, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.H.; Shoyama, Y. New minor glycoside components from saffron. J. Nat. Med. 2013, 67, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhou, J.; Yan, X. Encyclopedia of traditional Chinese medicines: Molecular structures, pharmacological activities. In Natural Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Giorgi, A.; Pentimalli, D.; Giupponi, L.; Panseri, S. Quality traits of saffron (Crocus sativus L.) produced in the Italian Alps. Open Agric. 2017, 2, 52–57. [Google Scholar] [CrossRef]
- Lage, M.; Cantrell, C.L. Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci. Hortic. 2009, 121, 366–373. [Google Scholar] [CrossRef]
- Bhandari, P.R. Crocus sativus L. (saffron) for cancer chemoprevention: A mini-review. J. Tradit. Complement. Med. 2015, 5, 81–87. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Hum. Psychopharmacol. 2014, 29, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Ishisaka, M.; Tsujii, S.; Tsuruma, K.; Shimazawa, M.; Kubo, K.; Umigai, N.; Iwawaki, T.; Hara, H. Crocetin protects ultraviolet A-induced oxidative stress and cell death in skin in vitro and in vivo. Eur. J. Pharmacol. 2016, 789, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Liu, C.; Fang, C. Protective effects of crocetin pretreatment on myocardial injury in an ischemia/reperfusion rat model. Eur. J. Pharmacol. 2014, 741, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C. Herbal Medicine in Depression: Traditional Medicine to Innovative Drug Delivery; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Samarghandian, S.; Afshari, R.; Sadati, A. Evaluation of lung and bronchoalveolar lavage fluid oxidative stress indices for assessing the preventing effects of safranal on respiratory distress in diabetic rats. Sci. World J. 2014, 2014, 251378. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007, 100, 1126–1131. [Google Scholar] [CrossRef]
- Singla, R.K.; Bhat, G. Crocin: An overview. Indo Glob. J. Pharm. Sci. 2011, 1, 281–286. [Google Scholar] [CrossRef]
- Tong, Y.; Zhu, X.; Yan, Y.; Liu, R.; Gong, F.; Zhang, L.; Hu, J.; Fang, L.; Wang, R.; Wang, P. The influence of different drying methods on constituents and antioxidant activity of saffron from China. Int. J. Anal. Chem. 2015, 2015, 953164. [Google Scholar] [CrossRef] [PubMed]
- Tarantilis, P.A.; Polissiou, M.G. Isolation and identification of the aroma components from saffron (Crocus sativus). J. Agric. Food Chem. 1997, 45, 459–462. [Google Scholar] [CrossRef]
- García-Rodríguez, M.V.; López-Córcoles, H.; Alonso, G.L.; Pappas, C.S.; Polissiou, M.G.; Tarantilis, P.A. Comparative evaluation of an ISO 3632 method and an HPLC-DAD method for safranal quantity determination in saffron. Food Chem. 2017, 221, 838–843. [Google Scholar] [CrossRef]
- Mounira, L.; Bernardo, M.; Luigi, C.P.; Guido, F.; Fatima, G.; Khadija, B.; Abdelmjid, Z.; Luisa, P. Phytochemical composition of Moroccan saffron accessions by headspacesolid-phase-microextraction. Am. J. Essent. Oils Nat. Prod. 2015, 2, 1–7. [Google Scholar]
- Hosseinzadeh, H.; Talebzadeh, F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia 2005, 76, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Shakib, S.S.; Sameni, A.K.; Taghiabadi, E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res. IJPR 2013, 12, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.M.; Abdel-Latif, G.A.; Abbas, S.S.; El Magdoub, H.M.; Schaalan, M.F. Neuroprotective effect of crocin against rotenone-induced Parkinson’s disease in rats: Interplay between PI3K/Akt/mTOR signaling pathway and enhanced expression of miRNA-7 and miRNA-221. Neuropharmacology 2020, 164, 107900. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Park, Y.-M.; Jung, H.-J.; Lee, J.Y.; Min, B.D.; Park, S.-U.; Jung, W.-S.; Cho, K.-H.; Park, J.-H.; Kang, I. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010, 648, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Mizuma, H.; Tanaka, M.; Nozaki, S.; Mizuno, K.; Tahara, T.; Ataka, S.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Daily oral administration of crocetin attenuates physical fatigue in human subjects. Nutr. Res. 2009, 29, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W.; Gao, S. A perspective on Crocus sativus L.(saffron) constituent crocin: A potent water-soluble antioxidant and potential therapy for Alzheimer’s disease. J. Agric. Food Chem. 2017, 65, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Shimeno, H.; Mishima, K.I.; Iwasaki, K.; Fujiwara, M.; Tanaka, H.; Shoyama, Y.; Toda, A.; Eyanagi, R.; Soeda, S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2007, 1770, 578–584. [Google Scholar] [CrossRef]
- Cao, W.; Cui, J.; Li, S.; Zhang, D.; Guo, Y.; Li, Q.; Luan, Y.; Liu, X. Crocetin restores diabetic endothelial progenitor cell dysfunction by enhancing NO bioavailability via regulation of PI3K/AKTeNOS and ROS pathways. Life Sci. 2017, 181, 9–16. [Google Scholar] [CrossRef]
- Ghofrani, S.; Joghataei, M.-T.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Crocin, a bioactive constituent of Crocus sativus, alleviates trimethyltin-induced cognitive deficits through down-regulation of hippocampal apoptosis and oxidative stress. J. Basic. Clin. Pathophysiol. 2022, 10, 38–44. [Google Scholar]
- Tashakori, A.; Hassanpour, S.; Vazir, B. Protective effect of crocin on the cuprizone-induced model of multiple sclerosis in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1713–1725. [Google Scholar] [CrossRef]
- Bandegi, A.R.; Rashidy-Pour, A.; Vafaei, A.A.; Ghadrdoost, B. Protective effects of Crocus sativus L. extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv. Pharm. Bull. 2014, 4, 493–499. [Google Scholar] [PubMed]
- Bao, X.; Hu, J.; Zhao, Y.; Jia, R.; Zhang, H.; Xia, L. Advances on the anti-tumor mechanisms of the carotenoid Crocin. PeerJ 2023, 11, e15535. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Zare, M.; Badie, M.R.; Watson, R.R.; Talebnejad, M.R.; Afarid, M. Crocin as a vision supplement. Clin. Exp. Optom. 2023, 106, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem Aljumaily, S.A.; Demir, M.; Elbe, H.; Yigitturk, G.; Bicer, Y.; Altinoz, E. Antioxidant, anti-inflammatory, and anti-apoptotic effects of crocin against doxorubicin-induced myocardial toxicity in rats. Environ. Sci. Pollut. Res. 2021, 28, 65802–65813. [Google Scholar] [CrossRef] [PubMed]
- Shafei, M.N.; Faramarzi, A.; Rad, A.K.; Anaeigoudari, A. Crocin prevents acute angiotensin II-induced hypertension in anesthetized rats. Avicenna J. Phytomedicine 2017, 7, 345–352. [Google Scholar]
- Sadeghnia, H.; Cortez, M.; Liu, D.; Hosseinzadeh, H.; Snead, O.C. Antiabsence effects of safranal in acute experimental seizure models: EEG and autoradiography. J. Pharm. Pharm. Sci. 2008, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rafieipour, F.; Hadipour, E.; Emami, S.A.; Asili, J.; Tayarani-Najaran, Z. Safranal protects against beta-amyloid peptide-induced cell toxicity in PC12 cells via MAPK and PI3 K pathways. Metab. Brain Dis. 2019, 34, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fotoohi, A.; Moloudi, M.R.; Hosseini, S.; Hassanzadeh, K.; Feligioni, M.; Izadpanah, E. A novel pharmacological protective role for safranal in an animal model of Huntington’s disease. Neurochem. Res. 2021, 46, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Sadeghnia, H.R.; Shaterzadeh, H.; Forouzanfar, F.; Hosseinzadeh, H. Neuroprotective effect of safranal, an active ingredient of Crocus sativus, in a rat model of transient cerebral ischemia. Folia Neuropathol. 2017, 55, 206–213. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; O’Callaghan, Y.C.; Galvin, K.; Tsimidou, M.Z.; O’Brien, N.M. Cellular transport and bioactivity of a major saffron apocarotenoid, picrocrocin (4-(β-D-glucopyranosyloxy)-2, 6, 6-trimethyl-1-cyclohexene-1-carboxaldehyde). J. Agric. Food Chem. 2015, 63, 8662–8668. [Google Scholar] [CrossRef]
- Hoshyar, R.; Bathaie, S.Z.; Ashrafi, M. Interaction of safranal and picrocrocin with ctDNA and their preferential mechanisms of binding to GC- and AT-rich oligonucleotides. DNA Cell Biol. 2008, 27, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, E.; Kadoglou, N.P.E.; Kostomitsopoulos, N.; Valsami, G. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Hosseinzadeh, H. Saffron as an antidote or a protective agent against natural or chemical toxicities. DARU J. Fac. Pharm. Tehran Univ. Med. Sci. 2015, 23, 31. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Ansari, M.A.; Ahmad, M.; Saleem, S.; Yousuf, S.; Hoda, M.N. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005, 81, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wu, T.S. Constituents of the stigmas of Crocus sativus and their tyrosinase inhibitory activity. J. Nat. Prod. 2002, 65, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Termentzi, A.; Kokkalou, E. LC-DAD-MS (ESIþ) analysis and antioxidant capacity of Crocus sativus petal extracts. Planta Medica 2008, 74, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Bodeker, G.; Ong, C.K.; Grundy, C.; Burford, G.; Shein, K. WHO Global Atlas of Traditional, Complementary and Alternative Medicine; WHO Centre for Health Development: Kobe, Japan, 2005. [Google Scholar]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A review on anti-nutritional factors: Unraveling the natural gateways to human health. Front. Nutr. 2023, 31, 1215873. [Google Scholar] [CrossRef] [PubMed]
- Tariq, L.; Bhat, B.A.; Hamdani, S.S.; Mir, R.A. Phytochemistry, pharmacology and toxicity of medicinal plants. In Medicinal and Aromatic Plants: Healthcare and Industrial Applications; Springer: Cham, Switzerland, 2021; pp. 217–240. [Google Scholar]
- Zargar, S.M.; Hami, A.; Manzoor, M.; Mir, R.A.; Mahajan, R.; Bhat, K.A.; Gani, U.; Sofi, N.R.; Sofi, P.A.; Masi, A. Buckwheat OMICS: Present status and future prospects. Crit. Rev. Biotechnol. 2023, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Mir, R.A.; Farooq, A.; Hami, A.; Pakhtoon, M.M.; Sofi, S.A.; Malik, F.A.; Hussain, K.; Bhat, M.A.; Sofi, N.R.; et al. Shifting archetype to nature’s hidden gems: From sources, purification to uncover the nutritional potential of bioactive peptides. 3 Biotech 2023, 13, 252. [Google Scholar] [CrossRef]
- José Bagur, M.; Alonso Salinas, G.L.; Jimenez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An old medicinal plant and a potential novel functional food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Razak, S.I.A.; Anwar Hamzah, M.S.; Yee, F.C.; Kadir, M.R.A.; Nayan, N.H.M. A review on medicinal properties of saffron toward major diseases. J. Herbs Spices Med. Plants 2017, 23, 98–116. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. BioMed Res. 2014, 2014, 920857. [Google Scholar] [CrossRef] [PubMed]
- Ghadrdoost, B.; Vafaei, A.A.; Rashidy-Pour, A.; Hajisoltani, R.; Bandegi, A.R.; Motamedi, F.; Haghighi, S.; Sameni, H.R.; Pahlvan, S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 2011, 667, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, M.; Aghili Moghaddam, N.S.; Nosrati, M.; Tousi, M.; Avan, A.; Ryzhikov, M.; Parizadeh, M.R.; Fiuji, H.; Rajabian, M.; Bahreyni, A.; et al. Saffron against components of metabolic syndrome: Current status and prospective. J. Agric. Food Chem. 2017, 65, 10837–10843. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Bathaie, S.Z.; Abroun, S.; Azizian, M. Effect of crocin on cell cycle regulators in N-nitroso-N-methylurea-induced breast cancer in rats. DNA Cell Biol. 2015, 34, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.H.; Wang, C.Z.; Ni, M.; Fishbein, A.; Mehendale, S.R.; Xie, J.T.; Shoyama, C.Y.; Yuan, C.S. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp. Oncol. 2007, 29, 175–180. [Google Scholar] [PubMed]
- Kawabata, K.; Tung, N.H.; Shoyama, Y.; Sugie, S.; Mori, T.; Tanaka, T. Dietary crocin inhibits colitis and colitis-associated colorectal carcinogenesis in male ICR mice. Evid. Based Complement. Alternat Med. 2012, 2012, 820415. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Dong, A.; Wang, R.; Shi, A. Crocetin treatment inhibits proliferation of colon cancer cells through downregulation of genes involved in the inflammation. Saudi J. Biol. Sci. 2018, 25, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Si, P.; Wang, H.; Tahir, U.; Chetn, K.; Xialo, J.; Duan, X.; Huang, R.; Xiang, G. Crocetin induces apoptosis of BGC-823 human gastric cancer cells. Mol. Med. Rep. 2014, 9, 521–526. [Google Scholar] [CrossRef]
- Amin, A.; Hamza, A.A.; Bajbouj, K.; Ashraf, S.S.; Daoud, S. Saffron: A potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology 2011, 54, 857–867. [Google Scholar] [CrossRef]
- Kim, B.; Park, B. Saffron carotenoids inhibit STAT3 activation and promote apoptotic progression in IL-6-stimulated liver cancer cells. Oncol. Rep. 2018, 39, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Mehta, S.; Dhar, G.; Dhar, K.; Banerjee, S.; Van Veldhuizen, P.; Campbell, D.R.; Banerjee, S.K. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol. Cancer Ther. 2009, 8, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Kumar, D.; Bansal, N.; Narasimhan, B.; Marwaha, R.K.; Sharma, P.C. Understanding mechanistic aspects and therapeutic potential of natural substances as anticancer agents. Phytomedicine Plus 2023, 3, 100418. [Google Scholar] [CrossRef]
- Cui, H.; Miao, S.; Esworthy, T.; Zhou, X.; Lee, S.-J.; Liu, C.; Yu, Z.-X.; Fisher, J.P.; Mohiuddin, M.; Zhang, L.G. 3D bioprinting for cardiovascular regeneration and pharmacology. Adv. Drug Deliv. Rev. 2018, 132, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yuan, C.; Wang, L.; Chen, R.; Li, X.; Zhang, Y.; Liu, C.; Liu, X.; Liang, W.; Xing, Y. The beneficial effects of saffron extract on potential oxidative stress in cardiovascular diseases. Oxidative Med. Cell. Longev. 2021, 2021, 6699821. [Google Scholar] [CrossRef] [PubMed]
- Chahine, N.; Hanna, J.; Makhlouf, H.; Duca, L.; Martiny, L.; Chahine, R. Protective effect of saffron extract against doxorubicin cardiotoxicity in isolated rabbit heart. Pharm. Biol. 2013, 51, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Mashmoul, M.; Azlan, A.; Yusof, B.N.M.; Khaza’ai, H.N.; Mohtarrudin, N.; Boroushaki, M.T. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. J. Funct. Foods 2014, 8, 180–187. [Google Scholar] [CrossRef]
- Hofer, S.E.; Raile, K.; Reiterer, E.F.; Kapellen, T.; Dost, A.; Rosenbauer, J.; Grulich-Henn, J.; Holl, R.W. Tracking of metabolic control from childhood to young adulthood in Type 1 diabetes. J. Pediatr. 2014, 165, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Qian, Z.; Zheng, S.; Xi, L. Mechanism of hypolipidemic effect of crocin in rats: Crocin inhibits pancreatic lipase. Eur. J. Pharmacol. 2006, 543, 116–122. [Google Scholar] [CrossRef]
- Zheng, S.; Qian, Z.; Tang, F.; Sheng, L. Suppression of vascular cell adhesion molecule-1 expression by crocetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochem. Pharmacol. 2005, 70, 1192–1199. [Google Scholar] [CrossRef]
- Zheng, S.; Qian, Z.; Sheng, L.; Wen, N. Crocetin attenuates atherosclerosis in hyperlipidemic rabbits through inhibition of LDL oxidation. J. Cardiovasc. Pharmacol. 2006, 47, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Abedimanesh, N.; Bathaie, S.Z.; Abedimanesh, S.; Motlagh, B.; Separham, A.; Ostadrahimi, A. Saffron and crocin improved appetite, dietary intakes and body composition in patients with coronary artery disease. J. Cardiovasc. Thorac. Res. 2017, 9, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Sanaie, S.; Nikanfar, S.; Kalekhane, Z.Y.; Azizi-Zeinalhajlou, A.; Sadigh-Eteghad, S.; Araj-Khodaei, M.; Ayati, M.H.; Andalib, S. Saffron as a promising therapy for diabetes and Alzheimer’s disease: Mechanistic insights. Metab. Brain Dis. 2023, 38, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Urbani, E.; Blasi, F.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Investigation on secondary metabolite content and antioxidant activity of commercial saffron powder. Eur. Food Res. Technol. 2016, 242, 987–993. [Google Scholar] [CrossRef]
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiù, V.; et al. The atlas of inflammation resolution (AIR). Mol. Asp. Med. 2020, 74, 100894. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; López-Villarín, N.; Salobrar-García, E.; López-Cuenca, I.; Licastro, E.; Inarejos-García, A.M.; Almodóvar, P.; Pinazo-Durán, M.D.; et al. Neuroprotective and anti-inflammatory effects of a hydrophilic saffron extract in a model of glaucoma. Int. J. Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.H.; Farkhondeh, T. Antiinflammatory, Antioxidant, and Immunomodulatory Effects of Crocus sativus L. and Its Main Constituents. Phytother. Res. 2016, 30, 1072–1094. [Google Scholar] [CrossRef]
- Xu, G.L.; Li, G.; Ma, H.P.; Zhong, H.; Liu, F.; Ao, G.Z. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J. Agric. Food Chem. 2009, 57, 8325–8330. [Google Scholar] [CrossRef] [PubMed]
- Kianbakht, S.; Hajiaghaee, R. Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. J. Med. Plants 2011, 10, 82–89. [Google Scholar]
- Karimi-Nazari, E.; Nadjarzadeh, A.; Masoumi, R.; Marzban, A.; Mohajeri, S.A.; Ramezani-Jolfaie, N.; Salehi-Abargouei, A. Effect of saffron (Crocus sativus L.) on lipid profile, glycemic indices and antioxidant status among overweight/obese prediabetic individuals: A double-blinded, randomized controlled trial. Clin. Nutr. ESPEN 2019, 34, 130–136. [Google Scholar] [CrossRef]
- Maeda, A.; Kai, K.; Ishii, M.; Ishii, T.; Akagawa, M. Safranal, a novel protein tyrosine phosphatase 1 B inhibitor, activates insulin signaling in C 2 C 12 myotubes and improves glucose tolerance in diabetic KK-Ay mice. Mol. Nutr. Food Res. 2014, 58, 1177–1189. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, X.; Liu, D.; Deng, Z.; Hu, W.; Li, Z.; Li, Y. The hypoglycemic and renal protection properties of crocin via oxidative stress-regulated NF-κB Signaling in db/db Mice. Front. Pharmacol. 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, V.; Mohammadi, M.; Mohaddes, G.; Darishnejad, H.; Chodari, L. Effect of crocin and voluntary exercise on P53 protein in pancreas of type2 diabetic rats. Pharm. Sci. 2017, 23, 182–188. [Google Scholar] [CrossRef]
- Garabadu, D.; Agrawal, N.; Sharma, A.; Sharma, S. Mitochondrial metabolism: A common link between neuroinflammation and neurodegeneration. Behav. Pharmacol. 2019, 30, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.A.; Almilaibary, A.; Mir, R.A.; Aljarallah, B.M.; Mir, W.R.; Ahmad, F.; Mir, M.A. Natural Therapeutics in Aid of Treating Alzheimer’s Disease: A Green Gateway Toward Ending Quest for Treating Neurological Disorders. Front. Neurosci. 2022, 16, 884345. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Kiasalari, Z.; Rahmati, B.; Narenjkar, J. Behavioral and histological analysis of Crocus sativus effect in intracerebroventricular streptozotocin model of Alzheimer disease in rats. Iran. J. Pathol. 2010, 5, 27–33. [Google Scholar]
- Baghishani, F.; Mohammadipour, A.; Hosseinzadeh, H.; Hosseini, M.; Ebrahimzadeh-Bideskan, A. The effects of tramadol administration on hippocampal cell apoptosis, learning and memory in adult rats and neuroprotective effects of crocin. Metab. Brain Dis. 2018, 33, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, B.; Cheng, B.; Liu, Y.; Zhang, B.; Wang, X.; Gong, G. Crocin Alleviates Myocardial Ischemia/Reperfusion Induced Endoplasmic Reticulum Stress via Regulation of miR-34a/Sirt1/Nrf2 Pathway. Shock 2019, 51, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, Y.S.; Bharate, S.S.; Kumar, V.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B.; Kaddoumi, A. Crocus sativus extract tightens the blood-brain barrier, reduces amyloid β load and related toxicity in 5XFAD mice. ACS Chem. Neurosci. 2017, 8, 1756–1766. [Google Scholar] [CrossRef]
- Heidari, S.; Mehri, S.; Hosseinzadeh, H. Memory enhancement and protective effects of crocin against D-galactose aging model in the hippocampus of Wistar rats. Iran. J. Basic. Med. Sci. 2017, 20, 1250–1259. [Google Scholar]
- Chu, W.Z.; Qian, C.Y. Expressions of Abeta1–40, Abeta1–42, tau202, tau396 and tau404 after intracerebroventricular injection of streptozotocin in rats. Acad. J. First Med. Coll. PLA 2005, 73, 168–170. [Google Scholar]
- Ghahghaei, A.; Bathaie, S.Z.; Kheirkhah, H.; Bahraminejad, E. The protective effect of crocin on the amyloid fibril formation of Aβ42 peptide in vitro. Cell. Mol. Biol. Lett. 2013, 18, 328–339. [Google Scholar] [CrossRef]
- Karakani, A.M.; Riazi, G.; Mahmood Ghaffari, S.; Ahmadian, S.; Mokhtari, F.; Jalili Firuzi, M.; Zahra Bathaie, S. Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro. Iran. J. Basic. Med. Sci. 2015, 18, 485–492. [Google Scholar]
- Marx, W.; Lane, M.; Rocks, T.; Ruusunen, A.; Loughman, A.; Lopresti, A.; Marshall, S.; Berk, M.; Jacka, F.; Dean, O.M. Effect of saffron supplementation on symptoms of depression and anxiety: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hatami, H.; Dehghan, G. The Effect of Ethanolic the effect of ethanolic extract of Saffron (Crocus sativus L.) on improving the spatial memory parameters in the experimental models of Parkinson disease in male rats. J. Adv. Biomed. Sci. 2015, 5, 534–541. [Google Scholar]
- Pan, P.K.; Qiao, L.Y.; Wen, X.N. Safranal prevents rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson’s disease through regulating Keap 1/Nrf2 signaling pathway. Cell. Mol. Biol. 2016, 62, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Inoue, E.; Shimizu, Y.; Masui, R.; Hayakawa, T.; Tsubonoya, T.; Hori, S.; Sudoh, K. Effects of saffron and its constituents, crocin-1, crocin-2, and crocetin on α-synuclein fibrils. J. Nat. Med. 2018, 72, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Squitieri, F.; Frati, L.; Ciarmiello, A.; Lastoria, S.; Quarrell, O. Juvenile Huntington’s disease: Does a dosage-effect pathogenic mechanism differ from the classical adult disease? Mech. Ageing Dev. 2006, 127, 208–212. [Google Scholar] [CrossRef]
- Bahramikia, S.; Yazdanparast, R. Anti-amyloidogenic and fibril-destabilizing effects of two manganese-salen derivatives against hen egg-white lysozyme aggregation. Int. J. Biol. Macromol. 2012, 50, 187–197. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Dang, Z.; Su, S.; Li, Z.; Lu, D. Cognitive protective mechanism of crocin pretreatment in rat submitted to acute high-altitude hypoxia exposure. BioMed Res. Int. 2020, 2020, 3409679. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Eskin, N.M.; Iranshahy, M.; Fazly Bazzaz, B.S. Phytochemicals: A promising weapon in the arsenal against antibiotic-resistant bacteria. Antibiotics 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Lachguer, K.; El Merzougui, S.; Boudadi, I.; Laktib, A.; Ben El Caid, M.; Ramdan, B.; Serghini, M.A. Major Phytochemical Compounds, In Vitro Antioxidant, Antibacterial, and Antifungal Activities of Six Aqueous and Organic Extracts of Crocus sativus L. Flower Waste. Waste Biomass Valorization 2023, 14, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Primavilla, S.; Pagano, C.; Roila, R.; Branciari, R.; Ranucci, D.; Valiani, A.; Perioli, L. Antibacterial Activity of Crocus sativus L. Petals Extracts against Foodborne Pathogenic and Spoilage Microorganisms, with a Special Focus on Clostridia. Life 2022, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Asgarpanaha, J.; Darabi-Mahbouba, E.; Mahboubib, A.; Mehrabb, R.; Hakemivalac, M. In-Vitro Evaluation of Crocus sativus L. Petals and Stamens as Natural Antibacterial Agents against Food-Borne Bacterial Strains. Iran. J. Pharm. Sci. 2013, 9, 69–82. [Google Scholar]
- Wali, A.F.; Alchamat, H.A.A.; Hariri, H.K.; Hariri, B.K.; Menezes, G.A.; Zehra, U.; Ahmad, P. Antioxidant, antimicrobial, antidiabetic and cytotoxic activity of Crocus sativus L. petals. Appl. Sci. 2020, 10, 1519. [Google Scholar] [CrossRef]
- Muzaffar, S.; Rather, S.A.; Khan, K.Z. In vitro bactericidal and fungicidal activities of various extracts of saffron (Crocus sativus L.) stigmas from Jammu & Kashmir, India. Cogent Food Agric. 2016, 2, 1158999. [Google Scholar]
- Serrano-Díaz, J.; Sánchez, A.M.; Alvarruiz, A.; Alonso, G.L. Preservation of saffron floral bio-residues by hot air convection. Food Chem. 2013, 141, 1536–1543. [Google Scholar] [CrossRef]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Lizard, G. Saffron (Crocus sativus L.): A source of nutrients for health and for the treatment of neuropsychiatric and age-related diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef]
- Zhao, K.; Rhee, S.Y. Omics-guided metabolic pathway discovery in plants: Resources, approaches, and opportunities. Curr. Opin. Plant Biol. 2022, 67, 102222. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Metabolite-based genome-wide association studies in plants. Curr. Opin. Plant Biol. 2015, 24, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Im, E. Heterologous expression of heat shock proteins confers stress tolerance in Escherichia coli, an industrial cell factory: A short review. Biocatal. Agric. Biotechnol. 2020, 29, 101833. [Google Scholar] [CrossRef]
- Goyal, S.; Arora, S.; Sharma, A.; Joshi, S.; Ray, R.; Bhatia, J.; Kumari, S.; Arya, D. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine 2011, 17, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.T.; Moallem, S.A.; Mahmoudi, M.; Hosseinzadeh, H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine 2011, 18, e499–e504. [Google Scholar] [CrossRef] [PubMed]
- Hesari, A.K.; Shahrooz, R.; Ahmadi, A.; Malekinejad, H.; Saboory, H. Crocin prevention of anemia-induced changes in structural and functional parameters of mice testes. J. Appl. Biomed. 2015, 13, e213–e223. [Google Scholar] [CrossRef]
- Razavi, B.M.; Hosseinzadeh, H.; Movassaghi, A.R.; Imenshahidi, M.; Abnous, K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem.-Biol. Interact. 2013, 203, e547–e555. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Xu, G.; Zheng, S.; Sun, S.; Wen, N.; Sheng, L.; Shi, Y.; Zhang, Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J. Nutr. Biochem. 2007, 18, e64–e72. [Google Scholar] [CrossRef]
- Yamauchi, M.; Tsuruma, K.; Imai, S.; Nakanishi, T.; Umigai, N.; Shimazawa, M.; Hara, H. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur. J. Pharmacol. 2011, 650, e110–e119. [Google Scholar] [CrossRef]
- Berger, F.; Hensel, A.; Nieber, K. Saffron extract and trans-crocetin inhibit glutamatergic synaptic transmission in rat cortical brain slices. Neuroscience 2011, 180, e238–e247. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant effect of Crocus sativus L. extracts and their constituents, Crocin and safranal, in mice. J. Med. Plants 2004, 3, 48–58. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghnia, H.R. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia-induced oxidative damage in rat hippocampus. Pharm. Pharm. Sci. 2005, 8, e394–e399. [Google Scholar]
- Modaghegh, M.H.; Shahabian, M.; Esmaeili, H.A.; Rajbai, O.; Hosseinzadeh, H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine 2008, 15, e1032–e1037. [Google Scholar] [CrossRef] [PubMed]
- Shamsa, A.; Hosseinzadeh, H.; Molaei, M.; Shakeri, M.T.; Rajabi, O. Evaluation of Crocus sativus L. (saffron) on male erectile dysfunction: A pilot study. Phytomedicine 2009, 16, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Sabet, M.S.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Shastry, R.P.; Rekha, P.D. Bacterial cross-talk with gut microbiome and its implications: A short review. Folia Microbiol. 2021, 66, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Guan, X.; Chen, D.; Ma, W. The Th17/Treg Cell Balance: A Gut Microbiota-Modulated Story. Microorganisms 2019, 7, 583. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Brim, H.; Haileselassie, Y.; Varma, S.; Habtezion, A.; Rashid, M.; Sinha, S.R.; Ashktorab, H. Microbiomic and Metabolomic Analyses Unveil the Protective Effect of Saffron in a Mouse Colitis Model. Curr. Issues Mol. Biol. 2023, 30, 5558–5574. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Connell, E.; Le Gall, G.; Pourtau, L.; Gaudout, D.; Angeloni, C.; Zallocco, L.; Ronci, M.; Giusti, L.; Müller, M.; et al. Saffron extract (Safr’Inside™) improves anxiety related behaviour in a mouse model of low-grade inflammation through the modulation of the microbiota and gut derived metabolites. Food Funct. 2022, 13, 12219–12233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).